Apply to Aakash iACST Scholarship Test 2024
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with
Plan, Prepare & Make the Best Career Choices
NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions- In NCERT Class 12 Chemistry chapter 2 Solutions, there are direct answers to the 41 questions which are there in the chapter exercise. The students will be able to find step-by-step NCERT solutions for Class 12 Chemistry Chapter 2 Solutions which will eventually help you to write good answers and get good marks in the CBSE exam. The NCERT solutions which are provided here are free of cost and are easily accessible. By referring to the NCERT solutions for class 12, students can understand all the important concepts and practice questions well.
Latest: JEE Main high scoring chapters
Don't Miss: JEE Main 10 year's papers
Recommended: NEET high scoring chapters
Suggested: NEET question papers with solutions
In our daily life, we come across various mixtures like soft drinks, syrups, and air. All of them are mixtures of two or more pure substances like air is a mixture of mainly nitrogen and oxygen etc. Also, you know about various types of mixtures or solutions like gaseous solutions, liquid solutions, and solid solutions. These topics of NCERT solutions for Class 12 Chemistry Chapter 2 Solutions are not only important for the CBSE 12th exam but also for the various competitive exams like JEE Mains, NEET, BITSAT, VITEEE, etc.
Also Read,
NCERT Exemplar Solutions Class 12 Chemistry Chapter 2
NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions
NCERT chemistry class 12 intext questions solutions chapter 2: Exercise 2.1 to 2.12
Answer :
We know that solute and solvent forms solution.
So mass percentage of benzene (solute) :-
Similarly mass percentage of CCl 4 :-
Question 2.2 Calculate the mole fraction of benzene in solution containing by mass in carbon tetrachloride.
Answer :
For calculating mole fraction, we need moles of both the compounds.
It is given that benzene is in the solution by mass.
So if we consider 100g of solution then 30g is benzene and 70g is CCl 4 .
Similarly moles of benzene :
So mole fraction of benzene is given :
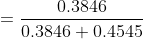
Question 2.3(a) Calculate the molarity of each of the following solutions:
of
in
of solution
Answer :
For finding molarity we need the moles of solute and volume of solution.
So moles of solute :
Now,
Question 2.3(b) Calculate the molarity of each of the following solutions:
of
diluted to
.
Answer :
By conservation of moles we can write :
M 1 V 1 = M 2 V 2
Given that M 1 = 0.5 M and V 1 = 30 ml ; V 2 = 500 ml
Question 2.4 Calculate the mass of urea required in making
of
aqueous solution.
Answer :
Let us assume that the mass of urea required be x g.
So moles of urea will be :
we get x = 37
Thus mass of urea required = 37 g.
Question 2.5 Calculate
(a) molality
(b) molarity and
(c) mole fraction
of KI if the density of (mass/mass) aqueous KI is
.
Answer :
If we assume our solution is 100 g. Then according to question, 20 g KI is present and 80 g is water.
So moles of KI :
(a) Molality :-
(b) Molarity :-
(c) Mol fraction :- Moles of water :-
So, mol fraction of KI :-
Answer :
For finding Henry's constant we need to know about the mole fraction of H 2 S.
Solubility of H 2 S in water is given to be 0.195 m .
i.e., 0.195 moles in 1 Kg of water.
So
At STP conditions, pressure = 1 atm or 0.987 bar
Equation is :
So we get :
Question 2.7 Henry’s law constant for in water is
at
Calculate the quantity of
in
of soda water when packed under
pressure at
Answer :
We know that ,
Pressure of CO 2 = 2.5 atm
We know that :
So, Pressure of CO 2 = Pa
By Henry Law we get,
Taking density of soda water = 1 g/ml
We get mass of water = 500 g.
So, Moles of water :
Also,
So, moles of CO 2 = 0.042 mol
Using relation of mole and given mass, we get
Mass of CO 2 = 1.848 g.
Answer :
Let the composition of liquid A (mole fraction) be x A .
So mole fraction of B will be x B = 1 - x A .
Given that,
Using Raoult’s law ,
Putting values of p total and vapour pressure of pure liquids in the above equation, we get :
600 = 450.x A + 700.(1 - x A )
or 600 - 700 = 450x A - 700x A
or x A = 0.4
and x B = 0.6
Now pressure in vapour phase :
= 450(0.4) = 180 mm of Hg
= 700(0.6) = 420 mm of Hg
And mole fraction of liquid B = 0.70
Question 2.9 Vapour pressure of pure water at is
of urea
is dissolved in
of water. Calculate the vapour pressure of water for this solution and its relative lowering.
Answer :
Given that vapour pressure of pure water,
Moles of water :
Moles of urea :
Let the vapour pressure of water be p w .
By Raoult's law, we get :
or
or p w = 23.4 mm of Hg.
Relative lowering :- Hence, the vapour pressure(v.P) of water in the solution = 23.4 mm of Hg
and its relative lowering = 0.0173.
Question 2.10 Boiling point of water at is
. How much sucrose is to be added to
of water such that it boils at
Answer :
Here we will use the formula :
Elevation in temperature = 100 - 99.63 = 0.37
K b = 0.52 ;
Putting all values in above formula, we get :
Thus 121.67 g of sucrose needs to be added.
Question 2.11 Calculate the mass of ascorbic acid (Vitamin C, ) to be dissolved in
of acetic acid to lower its melting point by
.
Answer :
Elevation in melting point = 1.5 degree celsius.
Here we will use the following equation :
Putting given values in the above equation :
Thus 5.08 ascorbic acid is needed for required condition.
Question 2.12 Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving of polymer of molar mass
in
of water at
Answer :
We know that :
We are given with :-
Volume, V = 0.45 L
Thus osmotic pressure :
NCERT solutions for class 12 chemistry chapter 2 Solutions : Exercises
Solution :- A solution is a homogeneous mixture of two or more non-reacting substances. It has two components :- solute and solvent.
Types of solutions are given below :-
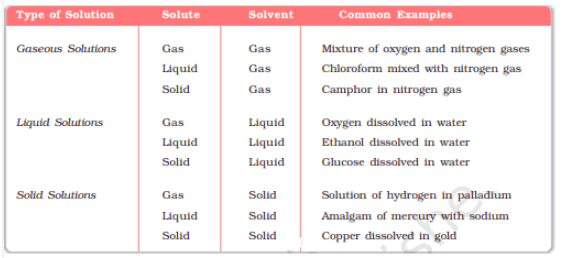
![]()
Q.2.2 Give an example of a solid solution in which the solute is a gas.
Answer:
Solution of hydrogen in palladium is such an example in which solute is a gas and solvent is solid.
Q.2.3(i) Define the following terms:
Mole fraction
Answer :
Mole fraction is defined as the ratio of number of moles of a component and total number of moles in all components.
i.e.,
Q.2.3(ii) Define the following terms:
Molality
Answer :
It is defined as the number of moles of solute dissolved per kg (1000g) of solvent
i.e.,
It is independent of temperature.
Q2.3(iii) Define the following terms:
Molarity
Answer :
Molarity is defined as number of moles of solute dissolved per litre(or 1000ml) of solution.
i.e.,
It depends on temperature because volume is dependent on temperature.
Q2.3(iv) Define the following terms:
Mass percentage.
Answer :
Mass percentage is defined as the percentage ratio of mass of one component to the total mass of all the components.
i.e.,
Answer :
According to given question, in 100 g of solution 68 g is nitric acid and rest is water.
So moles of 68 g HNO 3 :-
Density of solution is given to be 1.504.
So volume of 100 g solution becomes :-
Thus, molarity of nitric acid is :
Answer :
According to question, mass percentage means in 100 g of solution 10 g glucose is dissolved in 90 g water.
Molar mass of glucose (C 6 H 12 O 6 ) =
So moles of glucose are :
Mole fraction :-
Molarity :- Volume of 100 g solution :
Answer :
Total amount of mixture of Na 2 CO 3 and NaHCO 3 = 1 g.
Let the amount of Na 2 CO 3 be x g.
So the amount of NaHCO 3 will be equal to (1 - x) g.
Now it is given that it is an equimolar mixture.
So, Moles of Na 2 CO 3 = Moles of NaHCO 3 .
or
or x = 0.558 g
So
and
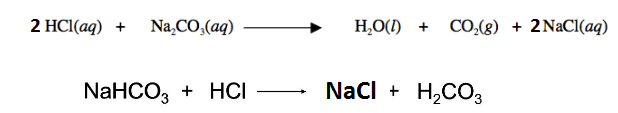
It is clear that for 1 mol of Na 2 CO 3 2 mol of HCl is required, similarly for 1 mol of NaHCO 3 1 mol of HCl is required.
So number of moles required of HCl = 2(0.00526) + 0.0053 = 0.01578 mol
It is given that molarity of HCl is 0.1 which means 0.1 mol of HCl in 1l of solution.
Thus required volume :
Answer :
According to question we have 2 solute,
Solute 1. : of 300 g gives :
Solute 2. : of 400 g gives :
So total amount of solute = 75 + 160 = 235 g.
Thus mass percentage of solute is :
and mass percentage of water
Answer :
For finding molality we need to find the moles of ethylene glycol.
Moles of ethylene glycol :
We know that :
Now for molarity :-
Total mass of solution = 200 + 222.6 = 422.6 g
Volume of solution
So molarity :-
express this in percent by mass
Answer :
We know that 15 ppm means 15 parts per million.
Required percent by mass :
determine the molality of chloroform in the water sample.
Answer :
Moles of chloroform :
Mass of water is . (Since contamination is 15 ppm)
So molality will be :
Q2.10 What role does the molecular interaction play in a solution of alcohol and water?
Answer :
Both alcohol and water individually have strong hydrogen bonds as their force of attraction. When we mix alcohol with water they form solution due to the formation of hydrogen bonds but they are weaker as compared to hydrogen bonds of pure water or pure alcohol.
Thus this solution shows a positive deviation from the ideal behaviour.
Q2.11 Why do gases always tend to be less soluble in liquids as the temperature is raised?
Answer:
It is known that dissolution of gas in a liquid is an exothermic process. So, by Le Chatelier principle we know that equilibrium shifts backwards as we increase temperature in case of exothermic process. Thus gases always tend to be less soluble in liquids as the temperature is raised.
Q2.12 State Henry’s law and mention some important applications.
Answer :
According to Henry's law at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of solution or liquid.
i.e., p = k h x, Here k h is Henry’s law constant.
Some of its applications are as follows:-
(a) We can increase the solubility of CO 2 in soft drinks, the bottle is sealed under high pressure.
(b) To avoid bends (due to blockage of capillaries) and the toxic effects of high concentrations of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
(c) The partial pressure of oxygen is less at high altitudes than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues climbers. Due to low blood oxygen, climbers become weak and unable to think clearly which are symptoms of a condition known as anoxia.
Answer :
Using Henry's Law we can write,
Putting value in this equation, we get :
So, the magnitude of k is .
Now, we will again use the above equation for .
So the required partial pressure is :-
or
Answer :
Positive and negative deviation: - A non-ideal solution is defined as a solution which does not obey Raoult’s law over the entire range of concentration i.e., and
. The vapour pressure of these solutions is either higher or lower than that expected by Raoult’s law. If vapour pressure is higher, the solution shows a positive deviation and if it is lower, it shows a negative deviation from Raoult’s law.
Enthalpy relation to positive and negative deviation can be understood from the following example:-
Consider a solution made up of two components - A and B. In the pure state the intermolecular force of attraction between them are A-A and B-B. But when we mix the two, we get a binary solution with molecular interaction A-B.
If A-B interaction is weak than A-A and B-B then enthalpy of reaction will be positive thus reaction will tend to move in a backward direction. Hence molecules in binary solution will have a higher tendency to escape. Thus vapour pressure increases and shows positive deviation from the ideal behaviour.
Similarly, for negative deviation, A-B interaction is stronger than that of A-A and B-B.
Answer :
It is given that of aq. solution. This means 2 g of non-volatile solute in 98 g of H 2 O.
Also the vapour of water at normal boiling point = 1.013 bar.
Using Raoult's law :
So we get :
Thus the molar mass of non-volatile solute is 41.35 unit.
Answer :
Vapour pressure of heptane =
and vapour pressure of octane =
Firstly we will find moles of heptane and octane so that we can find vapour pressure of each.
Molar mass of heptane = 7(12) + 16(1) = 100 unit.
and molar mass of octane = 8(12) + 18(1) = 114 unit.
So moles of heptane :
and moles of octane :
Mole fraction of heptane = 0.456 and mole fraction of octane = 0.544
Now we will find the partial vapour pressure:-
(i) of heptane :-
(ii) of octane :-
So total pressure of solution =
= 47.97 + 25.46 = 73.43 KPa
Answer :
It is asked the vapour pressure of 1 molal solution which means 1 mol of solute in 1000 g H 2 O.
Moles in 1000g of water = 55.55 mol. (Since the molecular weight of H 2 O is 18)
Mole fraction of solute :
Applying the equation :
or
or
Thus the vapour pressure of the solution is 12.083 KPa
Answer :
Let the initial vapour pressure of octane = .
After adding solute to octane, the vapour pressure becomes :
Moles of octane :
Using Raoult's law we get :
or
or
Thus required mass of non-volatile solute = 10g.
molar mass of the solute
Answer :
In this question we will find molar mass of solute by using Raoult's law .
Let the molar mass of solute is M.
Initially we have 30 g solute and 90 g water.
Moles of water :
By Raoult's law we have :-
or
or ------------------------------ (i)
Now we have added 18 g of water more, so the equation becomes:
Moles of H 2 O :
Putting this in above equation we obtain :-
or -----------------------------------(ii)
From equation (i) and (ii) we get
M = 23 u
So the molar mass of solute is 23 units.
vapour pressure of water at 298 K.
Answer :
In the previous part we have calculated the value of molar mass the Raoul's law equation.
We had :-
Putting M = 23 u in the above equation we get,
or
Thus vapour pressure of water = 3.53 kPa.
Answer :
It is given that freezing point of pure water is 273.15 K.
So, elevation of freezing point = 273.15 - 271 = 2.15 K
solution means 5 g solute in 95 g of water.
Moles of cane sugar :
Molality :
We also know that -
or
Now we will use the above procedure for glucose.
of glucose means 5 g of gluocse in 95 g of H 2 O.
Moles of glucose :
Thus molality :
So, we can find the elevation in freezing point:
Thus freezing point of glucose solution is 273.15 - 4.09 = 269.06 K.
Answer :
In this question we will use the formula :
Firstly for compound AB 2 :-
or
Similarly for compound AB 4 :-
If we assume atomic weight of element A to be x and of element B to be y, then we have :-
x + 2y = 110.87 ----------------- (i)
x + 4y = 196 ----------------- (ii)
Solving both the equations, we get :-
x = 25.59 ; y = 42.6
Hence atomic mass of element A is 25.59u and atomic mass of element B is 42.6u.
Answer :
According to given conditions we have same solution under same temperature. So we can write :
So, if we put all the given values in above equation, we get
or
Hence the required concentration is 0.061 M.
Q2.23(i) Suggest the most important type of intermolecular attractive interaction in the following pairs.
n-hexane and n-octane
Answer :
Since both the compounds are alkanes so their mixture has van der Waal force of attraction between compounds.
Q2.23(ii) Suggest the most important type of intermolecular attractive interaction in the following pairs.
Answer :
The binary mixture of these compounds has van der Waal force of attraction between them.
Q2.23(iii) Suggest the most important type of intermolecular attractive interaction in the following pairs.
Answer :
The given compounds will have ion-dipole interaction between them.
Q2.23(iv) Suggest the most important type of intermolecular attractive interaction in the following pairs.
Answer :
Methanol has -OH group and acetone has ketone group. So there will be hydrogen bonding between them.
Q2.23(v) Suggest the most important type of intermolecular attractive interaction in the following pairs.
acetonitrile and acetone
Answer :
They will have dipole-dipole interaction since both are polar compounds.
Cyclohexane, KCl, CH3OH, CH3CN.
Answer :
The order will be : Cyclohexane > CH 3 CN > CH 3 OH > KCl
In this, we have used the fact that like dissolves like.
Since cyclohexane is an alkane so its solubility will be maximum.
phenol
Answer :
We know the fact that like dissolves in like.
Since phenol is had both polar and non-polar group so it is partially soluble in water.
toluene
Answer :
Since toluene is a non-polar compound i.e., it doesn't have any polar group so it is insoluble in water. (because water is a polar compound)
formic acid
Answer :
Since the -OH group in formic acid (polar) can form H-bonds with water thus it is highly soluble in water.
ethylene glycol
Answer :
Ethylene glycol is an organic compound but is polar in nature. Also, it can form H-bonds with water molecules, thus it is highly soluble in water.
chloroform
Answer :
Chloroform is a non-polar compound so it is insoluble in water.
pentanol
Answer :
Pentanol has both polar and non-polar groups so it is partially soluble in water.
Answer :
We know that, Molality :
So, for moles of solute we have :
Thus, molality :
Molality of Na + ions is 4m.
Q2.27 If the solubility product of is
, calculate the maximum molarity of
in aqueous solution.
Answer :
We are given,
The dissociation equation of CuS is given by :-
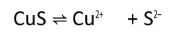
So, the equation becomes :-
or
or
Thus maximum molarity of solution is .
Q2.28 Calculate the mass percentage of aspirin in acetonitrile
when
of
is dissolved in
of
.
Answer :
Total mass of solution = Mass of aspirin + Mass of acetonitrile = 6.5 + 450 = 456.5 g.
We know that :
So,
Thus the mass percentage of aspirin is
Answer :
We are given with molality of the solution, so we need to find the moles of Nalorphene.
Molar mass of nalorphene = 19(12) + 21(1) + 1(14) + 3(16) = 311u.
So moles of nalorphene :
Molality :
or
or
So the required weight of water is 3.2 g.
Q2.30 Calculate the amount of benzoic acid required for preparing
of
solution in methanol.
Answer :
Molar mass of benzoic acid = 7(12) + 6(1) + 2(16) = 122u.
We are given with the molarity of solution.
or
or
So mass of benzoic acid :
Hence the required amount of benzoic acid is 4.575 g.
Answer :
We know that depression in freezing point of water will depend upon the degree of ionisation.
The degree of ionisation will be highest in the case of trifluoroacetic acid as it is most acidic among all three.
The order of degree of ionisation on the basis if acidic nature will be:- Trifluoroacetic acid > Trichloroacetic acid > Acetic acid.
So the depression in freezing point will be reverse of the above order.
Q2.32 Calculate the depression in the freezing point of water when of
is added to
of water.
Answer :
Firstly we will find the Vant's Hoff factor the dissociation of given compound.
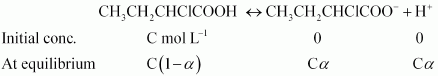
So we can write,
or
or
Putting values of K a and C in the last result, we get :
At equilibrium i = 1 - a + a + a = 1 + a = 1.0655
Now we need to find the moles of the given compound CH 3 CH 2 CHClCOOH.
So, moles =
Thus, molality of the solution :
Now we will use :
or
Answer :
Firstly we need to calculate molality in order to get vant's hoff factor.
So moles of CH 2 FCOOH :
We need to assume volume of solution to be nearly equal to 500 mL. (as 500 g water is present)
Now, we know that :
or
Now for dissociation constant :-
and,
Put values of C and a in the above equation, we get :
Answer :
Firstly we will find number of moles of both water and glucose.
Moles of glucose :
and moles of water :
Now,
or
or
Thus vapour pressure of water after glucose addition = 17.44 mm of Hg
Answer :
We know that :
We are given value of P and k, so C can be found.
Hence solubility of methane in benzene is .
Answer :
For calculating partial vapour pressure we need to calculate mole fractions of components.
So number of moles of liquid A :
and moles of liquid B :
Mole fraction of A (x A ) :
and mole fraction of B (x B ) :
Now, P total = P A + P B
or
or
or
Thus vapour pressure in solution due to A =
Plot this data also on the same graph paper. Indicate whether it has positive deviation or negative deviation from the ideal solution.
Answer :
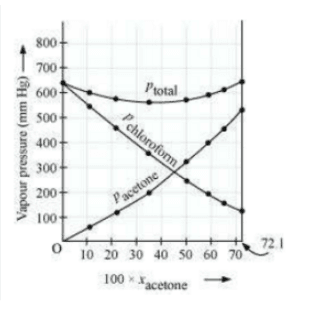
![]()
it has negative deviation from the ideal solution.
Answer :
Firstly, we will find the no. of moles of the given compounds.
No. of moles of benzene :
and the no. of moles of toluene :
.
Now we will find mol fraction of both:-
Mole fraction of benzene :-
and mole fraction of toluene :
Now,
P total = P b + P t
or
or
Hence mole fraction of benzene in vapour phase is given by :
Answer :
We have been given that the water is in equilibrium with air at a pressure of 10 atm or 7600 mm of Hg.
So the partial pressure of oxygen :
and partial pressure of nitrogen :
Now, by Henry's Law :
For oxygen :
For nitrogen :
Hence the mole fraction of nitrogen and oxygen in water is and
respectively.
Q2.41 Determine the amount of
dissolved in
of water such that its osmotic pressure is
at
.
Answer :
We know that osmotic pressure :
or
We have been given the values of osmotic pressure, V, i and T.
So the value of w can be found.
Hence 3.42 g CaCl 2 is required.
Answer :
Dissociation of K 2 SO 4 is as follows :-
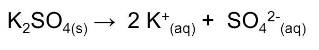 It is clear that 3 ions are produced, so the value of i will be 3.
It is clear that 3 ions are produced, so the value of i will be 3.
Molecular weight of K 2 SO 4 = 2(39) + 1(32) + 4(16) = 174u.
Putting all the values :-
More About Class 12 Chemistry Chapter 2 Solutions
NCERT Class 12 Chemistry chapter 2 solutions mainly discuss questions based on liquid solutions and their properties. The NCERT solutions for Class 12 Chemistry chapter 2 Solutions also cover other questions based on important concepts like types of solutions, Raoult's law and Henry's law, the concentration of solutions in different units, solubility, the vapour pressure of liquid solutions, ideal and non-ideal solutions, colligative properties, determination of molar mass and abnormal molar masses.
Topics and Sub-topics of NCERT Class 12 Chemistry Chapter 2 Solutions-
2.1Types of Solutions
2.2 Expressing Concentration of Solutions
2.3 Solubility
2.4 Vapour Pressure of Liquid Solutions
2.5 Ideal and Non-ideal Solutions
2.6 Colligative Properties and Determination of Molar Mass
2.7 Abnormal Molar Masses
This chapter of class 12 NCERT solutions is the second chapter of NCERT Class 12. It basically introduces basic concepts related to concentration, molarity, molality, mole fraction, Raoult's law, henry law, vapour pressure, colligative properties etc. NCERT solutions for Class 12 Chemistry Chapter 2 is an extension of chapter 1 class 11 NCERT also known as some basic concepts of chemistry. Class 12 NCERT solutions is very useful in the subsequent chapters like thermodynamics and Equilibrium etc. Ch 2 Chemistry Class 12 is very easy if basic concepts are understood well. Students can score decent marks in this chapter as most of the questions are form colligative properties and vapour pressure concepts which are easy to comprehend in the examination. Apart from NCERT, students can refer class notes for Chemistry Class 12 Chapter 2 to revise and score well in the final board examination as well as competitive exams.
Class 12 NCERT solutions has good amount of weightage in exams like NEET and JEE as well. Class 12 Chemistry Chapter 2 solutions is must read for the 12th class students. Most of the concepts like Molarity, molality, mole fraction, percentage composition etc. have been discussed in class 11 only, hence it is not very difficult to grasp the subsequent topics Henrys law, Colligative properties. Hence it is generally recommended to study class 11 chapters well before entering class 12th as heavy portion of syllabus is interlinked. Ch 2 Chemistry Class 12 solutions will take 8-10 hours to complete if all the concepts of 11th class are well read.
NCERT Exemplar Class 12 Solutions
NCERT solutions for class 12 chemistry
Chapter 1 | |
Chapter 2 | Solutions |
Chapter 3 | |
Chapter 4 | |
Chapter 5 | |
Chapter 6 | |
Chapter 7 | |
Chapter 8 | |
Chapter 9 | |
Chapter 10 | |
Chapter 11 | |
Chapter 12 | |
Chapter 13 | |
Chapter 14 | |
Chapter 15 | |
Chapter 16 |
NCERT solutions for class 12 subject wise
Benefits of NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
- Hope you have understood well with the help of the free NCERT class 12 Chemistry chapter 2 solutions provided here.
- After completing NCERT solutions for class 12 chemistry chapter 2 Solutions, students will be able to differentiate between the types of solutions characteristics of ideal and non-ideal solutions, define solubility and colligative properties, understand abnormal molar mass.
- NCERT class 12 Chemistry chapter 2 pdf which you read here will also help you in building your concepts as well as a strong base in the subject. These will also help you in various competitive exams.
- With the help of these NCERT class 12 Chemistry chapter 2 solutions pdf download, you will be able to write answers well.
Also Check NCERT Books and NCERT Syllabus here:
- NCERT Books Class 12 Chemistry
- NCERT Syllabus Class 12 Chemistry
- NCERT Books Class 12
- NCERT Syllabus Class 12
Happy Learning!!
Frequently Asked Question (FAQs)
- Henry’s law
- Solubility of gases in liquids
- Colligative properties
- Raoult's law
- Relative lowering of vapour pressure
- Elevation of boiling point
- Osmotic pressure
- Abnormal molecular mass
- Van't Hoff factor
For complete solutions : https://school.careers360.com/ncert/ncert-solutions-class-12-chemistry
4 marks questions can be expected. Practice JEE Main previous year papers to understand the question type asked.
Weightage of solutions is 5% . For good score follow NCERT textbook and solve NEET previous year papers.
Also Read
Articles
Certifications By Top Providers
Explore Top Universities Across Globe
Questions related to CBSE Class 12th
Hello aspirant,
The purpose of graphic design extends beyond the brand's look. Nevertheless, by conveying what the brand stands for, it significantly aids in the development of a sense of understanding between a company and its audience. The future in the field of graphic designing is very promising.
There are various courses available for graphic designing. To know more information about these courses and much more details, you can visit our website by clicking on the link given below.
https://www.careers360.com/courses/graphic-designing-course
Thank you
Hope this information helps you.
hello,
Yes you can appear for the compartment paper again since CBSE gives three chances to a candidate to clear his/her exams so you still have two more attempts. However, you can appear for your improvement paper for all subjects but you cannot appear for the ones in which you have failed.
I hope this was helpful!
Good Luck
Hello dear,
If you was not able to clear 1st compartment and now you giving second compartment so YES, you can go for your improvement exam next year but if a student receives an improvement, they are given the opportunity to retake the boards as a private candidate the following year, but there are some requirements. First, the student must pass all of their subjects; if they received a compartment in any subject, they must then pass the compartment exam before being eligible for the improvement.
As you can registered yourself as private candidate for giving your improvement exam of 12 standard CBSE(Central Board of Secondary Education).For that you have to wait for a whole year which is bit difficult for you.
Positive side of waiting for whole year is you have a whole year to preparing yourself for your examination. You have no distraction or something which may causes your failure in the exams. In whole year you have to stay focused on your 12 standard examination for doing well in it. By this you get a highest marks as a comparison of others.
Believe in Yourself! You can make anything happen
All the very best.
Hello Student,
I appreciate your Interest in education. See the improvement is not restricted to one subject or multiple subjects and we cannot say if improvement in one subject in one year leads to improvement in more subjects in coming year.
You just need to have a revision of all subjects what you have completed in the school. have a revision and practice of subjects and concepts helps you better.
All the best.
Hi,
You just need to give the exams for the concerned two subjects in which you have got RT. There is no need to give exam for all of your subjects, you can just fill the form for the two subjects only.
Popular CBSE Class 12th Questions
Colleges After 12th
Explore Career Options (By Industry)
Data Administrator
Database professionals use software to store and organise data such as financial information, and customer shipping records. Individuals who opt for a career as data administrators ensure that data is available for users and secured from unauthorised sales. DB administrators may work in various types of industries. It may involve computer systems design, service firms, insurance companies, banks and hospitals.
Bio Medical Engineer
The field of biomedical engineering opens up a universe of expert chances. An Individual in the biomedical engineering career path work in the field of engineering as well as medicine, in order to find out solutions to common problems of the two fields. The biomedical engineering job opportunities are to collaborate with doctors and researchers to develop medical systems, equipment, or devices that can solve clinical problems. Here we will be discussing jobs after biomedical engineering, how to get a job in biomedical engineering, biomedical engineering scope, and salary.
Ethical Hacker
A career as ethical hacker involves various challenges and provides lucrative opportunities in the digital era where every giant business and startup owns its cyberspace on the world wide web. Individuals in the ethical hacker career path try to find the vulnerabilities in the cyber system to get its authority. If he or she succeeds in it then he or she gets its illegal authority. Individuals in the ethical hacker career path then steal information or delete the file that could affect the business, functioning, or services of the organization.
GIS Expert
GIS officer work on various GIS software to conduct a study and gather spatial and non-spatial information. GIS experts update the GIS data and maintain it. The databases include aerial or satellite imagery, latitudinal and longitudinal coordinates, and manually digitized images of maps. In a career as GIS expert, one is responsible for creating online and mobile maps.
Data Analyst
The invention of the database has given fresh breath to the people involved in the data analytics career path. Analysis refers to splitting up a whole into its individual components for individual analysis. Data analysis is a method through which raw data are processed and transformed into information that would be beneficial for user strategic thinking.
Data are collected and examined to respond to questions, evaluate hypotheses or contradict theories. It is a tool for analyzing, transforming, modeling, and arranging data with useful knowledge, to assist in decision-making and methods, encompassing various strategies, and is used in different fields of business, research, and social science.
Geothermal Engineer
Individuals who opt for a career as geothermal engineers are the professionals involved in the processing of geothermal energy. The responsibilities of geothermal engineers may vary depending on the workplace location. Those who work in fields design facilities to process and distribute geothermal energy. They oversee the functioning of machinery used in the field.
Database Architect
If you are intrigued by the programming world and are interested in developing communications networks then a career as database architect may be a good option for you. Data architect roles and responsibilities include building design models for data communication networks. Wide Area Networks (WANs), local area networks (LANs), and intranets are included in the database networks. It is expected that database architects will have in-depth knowledge of a company's business to develop a network to fulfil the requirements of the organisation. Stay tuned as we look at the larger picture and give you more information on what is db architecture, why you should pursue database architecture, what to expect from such a degree and what your job opportunities will be after graduation. Here, we will be discussing how to become a data architect. Students can visit NIT Trichy, IIT Kharagpur, JMI New Delhi.
Remote Sensing Technician
Individuals who opt for a career as a remote sensing technician possess unique personalities. Remote sensing analysts seem to be rational human beings, they are strong, independent, persistent, sincere, realistic and resourceful. Some of them are analytical as well, which means they are intelligent, introspective and inquisitive.
Remote sensing scientists use remote sensing technology to support scientists in fields such as community planning, flight planning or the management of natural resources. Analysing data collected from aircraft, satellites or ground-based platforms using statistical analysis software, image analysis software or Geographic Information Systems (GIS) is a significant part of their work. Do you want to learn how to become remote sensing technician? There's no need to be concerned; we've devised a simple remote sensing technician career path for you. Scroll through the pages and read.
Budget Analyst
Budget analysis, in a nutshell, entails thoroughly analyzing the details of a financial budget. The budget analysis aims to better understand and manage revenue. Budget analysts assist in the achievement of financial targets, the preservation of profitability, and the pursuit of long-term growth for a business. Budget analysts generally have a bachelor's degree in accounting, finance, economics, or a closely related field. Knowledge of Financial Management is of prime importance in this career.
Data Analyst
The invention of the database has given fresh breath to the people involved in the data analytics career path. Analysis refers to splitting up a whole into its individual components for individual analysis. Data analysis is a method through which raw data are processed and transformed into information that would be beneficial for user strategic thinking.
Data are collected and examined to respond to questions, evaluate hypotheses or contradict theories. It is a tool for analyzing, transforming, modeling, and arranging data with useful knowledge, to assist in decision-making and methods, encompassing various strategies, and is used in different fields of business, research, and social science.
Underwriter
An underwriter is a person who assesses and evaluates the risk of insurance in his or her field like mortgage, loan, health policy, investment, and so on and so forth. The underwriter career path does involve risks as analysing the risks means finding out if there is a way for the insurance underwriter jobs to recover the money from its clients. If the risk turns out to be too much for the company then in the future it is an underwriter who will be held accountable for it. Therefore, one must carry out his or her job with a lot of attention and diligence.
Product Manager
A Product Manager is a professional responsible for product planning and marketing. He or she manages the product throughout the Product Life Cycle, gathering and prioritising the product. A product manager job description includes defining the product vision and working closely with team members of other departments to deliver winning products.
Operations Manager
Individuals in the operations manager jobs are responsible for ensuring the efficiency of each department to acquire its optimal goal. They plan the use of resources and distribution of materials. The operations manager's job description includes managing budgets, negotiating contracts, and performing administrative tasks.
Stock Analyst
Individuals who opt for a career as a stock analyst examine the company's investments makes decisions and keep track of financial securities. The nature of such investments will differ from one business to the next. Individuals in the stock analyst career use data mining to forecast a company's profits and revenues, advise clients on whether to buy or sell, participate in seminars, and discussing financial matters with executives and evaluate annual reports.
Researcher
A Researcher is a professional who is responsible for collecting data and information by reviewing the literature and conducting experiments and surveys. He or she uses various methodological processes to provide accurate data and information that is utilised by academicians and other industry professionals. Here, we will discuss what is a researcher, the researcher's salary, types of researchers.
Welding Engineer
Welding Engineer Job Description: A Welding Engineer work involves managing welding projects and supervising welding teams. He or she is responsible for reviewing welding procedures, processes and documentation. A career as Welding Engineer involves conducting failure analyses and causes on welding issues.
Transportation Planner
A career as Transportation Planner requires technical application of science and technology in engineering, particularly the concepts, equipment and technologies involved in the production of products and services. In fields like land use, infrastructure review, ecological standards and street design, he or she considers issues of health, environment and performance. A Transportation Planner assigns resources for implementing and designing programmes. He or she is responsible for assessing needs, preparing plans and forecasts and compliance with regulations.
Environmental Engineer
Individuals who opt for a career as an environmental engineer are construction professionals who utilise the skills and knowledge of biology, soil science, chemistry and the concept of engineering to design and develop projects that serve as solutions to various environmental problems.
Safety Manager
A Safety Manager is a professional responsible for employee’s safety at work. He or she plans, implements and oversees the company’s employee safety. A Safety Manager ensures compliance and adherence to Occupational Health and Safety (OHS) guidelines.
Conservation Architect
A Conservation Architect is a professional responsible for conserving and restoring buildings or monuments having a historic value. He or she applies techniques to document and stabilise the object’s state without any further damage. A Conservation Architect restores the monuments and heritage buildings to bring them back to their original state.
Structural Engineer
A Structural Engineer designs buildings, bridges, and other related structures. He or she analyzes the structures and makes sure the structures are strong enough to be used by the people. A career as a Structural Engineer requires working in the construction process. It comes under the civil engineering discipline. A Structure Engineer creates structural models with the help of computer-aided design software.
Highway Engineer
Highway Engineer Job Description: A Highway Engineer is a civil engineer who specialises in planning and building thousands of miles of roads that support connectivity and allow transportation across the country. He or she ensures that traffic management schemes are effectively planned concerning economic sustainability and successful implementation.
Field Surveyor
Are you searching for a Field Surveyor Job Description? A Field Surveyor is a professional responsible for conducting field surveys for various places or geographical conditions. He or she collects the required data and information as per the instructions given by senior officials.
Orthotist and Prosthetist
Orthotists and Prosthetists are professionals who provide aid to patients with disabilities. They fix them to artificial limbs (prosthetics) and help them to regain stability. There are times when people lose their limbs in an accident. In some other occasions, they are born without a limb or orthopaedic impairment. Orthotists and prosthetists play a crucial role in their lives with fixing them to assistive devices and provide mobility.
Pathologist
A career in pathology in India is filled with several responsibilities as it is a medical branch and affects human lives. The demand for pathologists has been increasing over the past few years as people are getting more aware of different diseases. Not only that, but an increase in population and lifestyle changes have also contributed to the increase in a pathologist’s demand. The pathology careers provide an extremely huge number of opportunities and if you want to be a part of the medical field you can consider being a pathologist. If you want to know more about a career in pathology in India then continue reading this article.
Gynaecologist
Gynaecology can be defined as the study of the female body. The job outlook for gynaecology is excellent since there is evergreen demand for one because of their responsibility of dealing with not only women’s health but also fertility and pregnancy issues. Although most women prefer to have a women obstetrician gynaecologist as their doctor, men also explore a career as a gynaecologist and there are ample amounts of male doctors in the field who are gynaecologists and aid women during delivery and childbirth.
Audiologist
The audiologist career involves audiology professionals who are responsible to treat hearing loss and proactively preventing the relevant damage. Individuals who opt for a career as an audiologist use various testing strategies with the aim to determine if someone has a normal sensitivity to sounds or not. After the identification of hearing loss, a hearing doctor is required to determine which sections of the hearing are affected, to what extent they are affected, and where the wound causing the hearing loss is found. As soon as the hearing loss is identified, the patients are provided with recommendations for interventions and rehabilitation such as hearing aids, cochlear implants, and appropriate medical referrals. While audiology is a branch of science that studies and researches hearing, balance, and related disorders.
Oncologist
An oncologist is a specialised doctor responsible for providing medical care to patients diagnosed with cancer. He or she uses several therapies to control the cancer and its effect on the human body such as chemotherapy, immunotherapy, radiation therapy and biopsy. An oncologist designs a treatment plan based on a pathology report after diagnosing the type of cancer and where it is spreading inside the body.
Anatomist
Are you searching for an ‘Anatomist job description’? An Anatomist is a research professional who applies the laws of biological science to determine the ability of bodies of various living organisms including animals and humans to regenerate the damaged or destroyed organs. If you want to know what does an anatomist do, then read the entire article, where we will answer all your questions.
Actor
For an individual who opts for a career as an actor, the primary responsibility is to completely speak to the character he or she is playing and to persuade the crowd that the character is genuine by connecting with them and bringing them into the story. This applies to significant roles and littler parts, as all roles join to make an effective creation. Here in this article, we will discuss how to become an actor in India, actor exams, actor salary in India, and actor jobs.
Acrobat
Individuals who opt for a career as acrobats create and direct original routines for themselves, in addition to developing interpretations of existing routines. The work of circus acrobats can be seen in a variety of performance settings, including circus, reality shows, sports events like the Olympics, movies and commercials. Individuals who opt for a career as acrobats must be prepared to face rejections and intermittent periods of work. The creativity of acrobats may extend to other aspects of the performance. For example, acrobats in the circus may work with gym trainers, celebrities or collaborate with other professionals to enhance such performance elements as costume and or maybe at the teaching end of the career.
Video Game Designer
Career as a video game designer is filled with excitement as well as responsibilities. A video game designer is someone who is involved in the process of creating a game from day one. He or she is responsible for fulfilling duties like designing the character of the game, the several levels involved, plot, art and similar other elements. Individuals who opt for a career as a video game designer may also write the codes for the game using different programming languages.
Depending on the video game designer job description and experience they may also have to lead a team and do the early testing of the game in order to suggest changes and find loopholes.
Radio Jockey
Radio Jockey is an exciting, promising career and a great challenge for music lovers. If you are really interested in a career as radio jockey, then it is very important for an RJ to have an automatic, fun, and friendly personality. If you want to get a job done in this field, a strong command of the language and a good voice are always good things. Apart from this, in order to be a good radio jockey, you will also listen to good radio jockeys so that you can understand their style and later make your own by practicing.
A career as radio jockey has a lot to offer to deserving candidates. If you want to know more about a career as radio jockey, and how to become a radio jockey then continue reading the article.
Choreographer
The word “choreography" actually comes from Greek words that mean “dance writing." Individuals who opt for a career as a choreographer create and direct original dances, in addition to developing interpretations of existing dances. A Choreographer dances and utilises his or her creativity in other aspects of dance performance. For example, he or she may work with the music director to select music or collaborate with other famous choreographers to enhance such performance elements as lighting, costume and set design.
Social Media Manager
A career as social media manager involves implementing the company’s or brand’s marketing plan across all social media channels. Social media managers help in building or improving a brand’s or a company’s website traffic, build brand awareness, create and implement marketing and brand strategy. Social media managers are key to important social communication as well.
Photographer
Photography is considered both a science and an art, an artistic means of expression in which the camera replaces the pen. In a career as a photographer, an individual is hired to capture the moments of public and private events, such as press conferences or weddings, or may also work inside a studio, where people go to get their picture clicked. Photography is divided into many streams each generating numerous career opportunities in photography. With the boom in advertising, media, and the fashion industry, photography has emerged as a lucrative and thrilling career option for many Indian youths.
Producer
An individual who is pursuing a career as a producer is responsible for managing the business aspects of production. They are involved in each aspect of production from its inception to deception. Famous movie producers review the script, recommend changes and visualise the story.
They are responsible for overseeing the finance involved in the project and distributing the film for broadcasting on various platforms. A career as a producer is quite fulfilling as well as exhaustive in terms of playing different roles in order for a production to be successful. Famous movie producers are responsible for hiring creative and technical personnel on contract basis.
Copy Writer
In a career as a copywriter, one has to consult with the client and understand the brief well. A career as a copywriter has a lot to offer to deserving candidates. Several new mediums of advertising are opening therefore making it a lucrative career choice. Students can pursue various copywriter courses such as Journalism, Advertising, Marketing Management. Here, we have discussed how to become a freelance copywriter, copywriter career path, how to become a copywriter in India, and copywriting career outlook.
Vlogger
In a career as a vlogger, one generally works for himself or herself. However, once an individual has gained viewership there are several brands and companies that approach them for paid collaboration. It is one of those fields where an individual can earn well while following his or her passion.
Ever since internet costs got reduced the viewership for these types of content has increased on a large scale. Therefore, a career as a vlogger has a lot to offer. If you want to know more about the Vlogger eligibility, roles and responsibilities then continue reading the article.
Publisher
For publishing books, newspapers, magazines and digital material, editorial and commercial strategies are set by publishers. Individuals in publishing career paths make choices about the markets their businesses will reach and the type of content that their audience will be served. Individuals in book publisher careers collaborate with editorial staff, designers, authors, and freelance contributors who develop and manage the creation of content.
Journalist
Careers in journalism are filled with excitement as well as responsibilities. One cannot afford to miss out on the details. As it is the small details that provide insights into a story. Depending on those insights a journalist goes about writing a news article. A journalism career can be stressful at times but if you are someone who is passionate about it then it is the right choice for you. If you want to know more about the media field and journalist career then continue reading this article.
Editor
Individuals in the editor career path is an unsung hero of the news industry who polishes the language of the news stories provided by stringers, reporters, copywriters and content writers and also news agencies. Individuals who opt for a career as an editor make it more persuasive, concise and clear for readers. In this article, we will discuss the details of the editor's career path such as how to become an editor in India, editor salary in India and editor skills and qualities.
Reporter
Individuals who opt for a career as a reporter may often be at work on national holidays and festivities. He or she pitches various story ideas and covers news stories in risky situations. Students can pursue a BMC (Bachelor of Mass Communication), B.M.M. (Bachelor of Mass Media), or MAJMC (MA in Journalism and Mass Communication) to become a reporter. While we sit at home reporters travel to locations to collect information that carries a news value.
Corporate Executive
Are you searching for a Corporate Executive job description? A Corporate Executive role comes with administrative duties. He or she provides support to the leadership of the organisation. A Corporate Executive fulfils the business purpose and ensures its financial stability. In this article, we are going to discuss how to become corporate executive.
Multimedia Specialist
A multimedia specialist is a media professional who creates, audio, videos, graphic image files, computer animations for multimedia applications. He or she is responsible for planning, producing, and maintaining websites and applications.
Welding Engineer
Welding Engineer Job Description: A Welding Engineer work involves managing welding projects and supervising welding teams. He or she is responsible for reviewing welding procedures, processes and documentation. A career as Welding Engineer involves conducting failure analyses and causes on welding issues.
Quality Controller
A quality controller plays a crucial role in an organisation. He or she is responsible for performing quality checks on manufactured products. He or she identifies the defects in a product and rejects the product.
A quality controller records detailed information about products with defects and sends it to the supervisor or plant manager to take necessary actions to improve the production process.
Product Manager
A Product Manager is a professional responsible for product planning and marketing. He or she manages the product throughout the Product Life Cycle, gathering and prioritising the product. A product manager job description includes defining the product vision and working closely with team members of other departments to deliver winning products.
QA Lead
A QA Lead is in charge of the QA Team. The role of QA Lead comes with the responsibility of assessing services and products in order to determine that he or she meets the quality standards. He or she develops, implements and manages test plans.
Structural Engineer
A Structural Engineer designs buildings, bridges, and other related structures. He or she analyzes the structures and makes sure the structures are strong enough to be used by the people. A career as a Structural Engineer requires working in the construction process. It comes under the civil engineering discipline. A Structure Engineer creates structural models with the help of computer-aided design software.
Process Development Engineer
The Process Development Engineers design, implement, manufacture, mine, and other production systems using technical knowledge and expertise in the industry. They use computer modeling software to test technologies and machinery. An individual who is opting career as Process Development Engineer is responsible for developing cost-effective and efficient processes. They also monitor the production process and ensure it functions smoothly and efficiently.
AWS Solution Architect
An AWS Solution Architect is someone who specializes in developing and implementing cloud computing systems. He or she has a good understanding of the various aspects of cloud computing and can confidently deploy and manage their systems. He or she troubleshoots the issues and evaluates the risk from the third party.
Azure Administrator
An Azure Administrator is a professional responsible for implementing, monitoring, and maintaining Azure Solutions. He or she manages cloud infrastructure service instances and various cloud servers as well as sets up public and private cloud systems.
Computer Programmer
Careers in computer programming primarily refer to the systematic act of writing code and moreover include wider computer science areas. The word 'programmer' or 'coder' has entered into practice with the growing number of newly self-taught tech enthusiasts. Computer programming careers involve the use of designs created by software developers and engineers and transforming them into commands that can be implemented by computers. These commands result in regular usage of social media sites, word-processing applications and browsers.
Product Manager
A Product Manager is a professional responsible for product planning and marketing. He or she manages the product throughout the Product Life Cycle, gathering and prioritising the product. A product manager job description includes defining the product vision and working closely with team members of other departments to deliver winning products.
Information Security Manager
Individuals in the information security manager career path involves in overseeing and controlling all aspects of computer security. The IT security manager job description includes planning and carrying out security measures to protect the business data and information from corruption, theft, unauthorised access, and deliberate attack
Automation Test Engineer
An Automation Test Engineer job involves executing automated test scripts. He or she identifies the project’s problems and troubleshoots them. The role involves documenting the defect using management tools. He or she works with the application team in order to resolve any issues arising during the testing process.
Also Read
Applications for Admissions are open.

Aakash iACST Scholarship Test 2024
ApplyGet up to 90% scholarship on NEET, JEE & Foundation courses

JEE Main Important Chemistry formulas
ApplyAs per latest 2024 syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters

SAT® | CollegeBoard
ApplyRegisteration closing on 19th Apr for SAT® | One Test-Many Universities | 90% discount on registrations fee | Free Practice | Multiple Attempts | no penalty for guessing

TOEFL ® Registrations 2024
ApplyThinking of Studying Abroad? Think the TOEFL® test. Register now & Save 10% on English Proficiency Tests with Gift Cards

Resonance Coaching
ApplyEnroll in Resonance Coaching for success in JEE/NEET exams

NEET 2024 Most scoring concepts
ApplyJust Study 32% of the NEET syllabus and Score upto 100% marks
