These chemical kinetics ncert solutions include a wide range of topics that explain the movement of electrons in redox reactions and their practical applications. Understanding these topics will help students connect theoretical concepts with real-world electrochemical processes and devices.
NCERT Solutions for Class 12 Chemistry Chapter 3 - Chemical Kinetics
Have you ever thought why some medicines expire within months while others last for years? Why does milk spoil faster in summer than in winter? Why do fireworks explode in seconds but rusting of iron takes days? The chemical kinetics answers all these questions. It helps in understanding the rates at which chemical reactions occur and the factors that influence these rates. Chemical kinetics helps explain the speed at which reactions happen and the conditions under which the speed depends.
This Story also Contains
- NCERT Solutions for Class 12 Chemistry Chapter 3: Download PDF
- NCERT Solutions for Class 12 Chemistry Chemical Kinetics (Intext Questions)
- NCERT Solutions for class 12 Chemistry Chapter 3 (Exercise Questions)
- Class 12 Chemistry NCERT Chapter 3: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 3 Chemical Kinetics
- Topics and Subtopics Covered in the NCERT Class 12 Chemistry Chapter 3
- What Extra Should Students Study Beyond the NCERT for JEE/NEET?
- What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 3 Chemical Kinetics
- Importance of Class 12 Chemistry Chapter 3 Chemical Kinetics Solutions
- NCERT Solutions for Class 12 Chemistry Chapter-wise
.jpg)
NCERT Solutions Class 12 Chemistry are prepared by subject experts in a very clear and comprehensive manner. These NCERT Solutions cover all the chemical kinetics class 12 questions answers. This article includes some higher-order thinking skills (HOTS) questions that are beyond memorisation and promote conceptual understanding. This article also includes a comparison table in which you will get a comparative study of JEE and NCERT concepts. Also, you will get an effective approach to solving questions of chemical kinetics.
NCERT Solutions for Class 12 Chemistry Chapter 3: Download PDF
Students can download the Class 12 Chemistry Chapter 3 Chemical Kinetics Question Answer PDF for free. These solutions of NCERT are designed to help you understand the fundamental concepts and solve textbook questions with ease.
Also Read,
NCERT Solutions for Class 12 Chemistry Chemical Kinetics (Intext Questions)
These chemical kinetics ncert solutions provide step-by-step answers to all the in text questions from the NCERT textbook. These solutions will help students understand the underlying principles, practise numerical problems effectively, and strengthen their grasp of reaction rates and mechanisms.
Page no 66
Answer :
We know that,
The average rate of reaction = $\frac{-\Delta [R]}{\Delta t}$
= $-\frac{ [R]_{2}-[R]_{1}}{t_{2}-t_{1}}$
= $\frac{0.03-0.02}{25}Mmin^{-1}$
= $4\times 10^{-4}Mmin^{-1}$
In seconds, we need to divide it by 60. So,
= $\frac{4\times 10^{-4}}{60}Msec^{-1}$
= 6.67 $\times 10^{-6}Msec^{-1}$
Question 3.2 In a reaction, $2A\rightarrow Products$ P, the concentration of A decreases from $0.5 mol L^{-1}$ to $0.4 mol L^{-1}$ in 10 minutes. Calculate the rate during this interval?
Answer :
According to the formula of an average rate
= $-\frac{1}{2}\frac{\Delta[ A]}{\Delta t}$ (final concentration - initial conc.)/time interval
= $-\frac{1}{2}\frac{[ A]_{2}-[A]_{1}}{t_{2}-t_{1}}$
= $-\frac{1}{2}\frac{[ 0.4]-[0.5]}{10}$
= $\frac{0.1}{20}Mmin^{-1}$
= $5\times 10^{-3}Mmin^{-1}$
Page no 71
Question 3.3 For a reaction, $A+B\rightarrow product$ ; the rate law is given by, $r=k\left [ A \right ]^{1/2}\left [ B \right ]^{2}$ . What is the order of the reaction?
Answer :
Order of reaction = Sum of powers of concentration of the reactant in the rate law expressions
So, here the power of A = 0.5
and power of B = 2
order of reaction = 2+0.5 =2.5
Answer :
The order of a reaction means the sum of the powers of concentration of the reactants in the rate law expression.
So rate law expression for the second-order reaction is $R=k[x]^{2}$ here R = rate
if the concentration is increased to 3 times means $x^{'}_{new}=3x$
new rate law expression = $R^{'}=k[3x]^{2}$ = $9k[x]^{2}$ = 9R
The rate of formation of Y becomes 9 times faster than before
Page no 78
Question 3.5 A first-order reaction has a rate constant $1.15\times 10^{-3}s^{-1}$. How long will $5g$ of his reactant take to reduce to $3g$?
Answer :
Given data,
initial conc. = 5g
final conc. = 3g
rate const. for first-order = $1.15\times 10^{-3}s^{-1}$
We know that for the first-order reaction,
$t=\frac{2.308}{k}\log\frac{[R]_{0}}{[R]}$
$=\frac{2.308}{1.15\times 10^{-3}}\log\frac{5}{3}$ [log(5/3)= 0.2219]
= 444.38 sec (approx)
Answer :
We know that t(half ) for the first-order reaction is $t_{1/2}=\frac{0.693}{k}$
and we have given the value of half time $t_{1/2} = 60 min$
thus, $k = \frac{0.693}{60}min^{-1}$
= 0.01155 /min
OR = 1.1925 $\times 10^{-4}sec^{-1}$
Alternative method
we can also solve this problem by using the first-order reaction equation.
$k = \frac{2.303}{t}\log\frac{[R]_{0}}{[R]}$
put $[R]=[R_{0}]/2$
Page no 84
Question 3.7 What will be the effect of temperature on the rate constant?
Answer :
The rate constant of the reaction is nearly doubled with a rise of 10 degrees in 10-degree temperature. The Arrhenius equation depicts the relation between temperature and the rate constant.
$k = Ae^{-\frac{E_{a}}{RT}}$
A= Arrhenius factor
Ea = Activation energy
R = gas constant
T = temperature
Question 3.8 The rate of the chemical reaction doubles for an increase of $10K$ in absolute temperature from $298K$. Calculate $E_{a}$.
Answer :
Given data
$T_{1}$ (initial temperature) = 298K and $T_{2}$ (final temperature)= 308K
And we know that the rate of reaction is nearly doubled when the temperature rises by 10 degrees
So, $\frac{k_{2}}{k_{1}}=2$ and R = 8.314 J/mol/K
now, $\log\frac{k_{2}}{k_{1}}=\frac{ E_{a}}{2.303}[\frac{T_{2}-T_{1}}{T_{1}T_{2}}]$
On putting the value of the given data, we get,
$\log2=\frac{ E_{a}}{2.303}[\frac{10}{298\times 308}]$
Activation energy ( $E_{a}$ ) = $\frac{2.303\times 8.314\times 298\times 308\times \log2}{10}Jmol^{-1}$
=52.9 KJ/mol(approx)
Question 3.9 The activation energy for the reaction $2HI(g)\rightarrow H_{2}+I_{2}(g)$ is $209.5 KJ mol^{-1}$ at $518 K$ . Calculate the fraction of molecules of reactants having energy equal to or greater than the activation energy?
Answer :
We have
Activation energy = 209.5KJ/mol
temperature= 581K
R = 8.314J/mol/K
Now, the fraction of molecules of reactants having energy equal to or greater than the activation energy is given as
$x= e^{-E_{a}/RT}$
Taking the log of both sides, we get
$\log x = -\frac{E_{a}}{RT}$
$=\frac{209500Jmol^{-1}}{2.303\times 8.314Jmol^{-1}K^{-1}\times 581}$
= 18.832
x = antilog(18.832)
= 1.471 $\times 10^{-19}$
NCERT Solutions for class 12 Chemistry Chapter 3 (Exercise Questions)
All the exercise questions of the chapter on chemical kinetics with detailed solutions are given below. These class 12 chemistry chemical kinetics question answer are prepared as per the latest NCERT guidelines to help students understand concepts clearly and score better in exams.
Question 3.1(i) From the rate expression for the following reactions, determine their order of reaction and the dimensions of the rate constants.
$3NO(g)\rightarrow N_{2}O\: \: Rate= k\left [ NO \right ]^{2}$
Answer :
Given pieces of information
Rate = $k[NO]^{2}$
So the order of the reaction is 2
The dimension of k = $Rate/[NO]^{2}$
$=\mathrm{molL}^{-1} \mathrm{~s}^{-1} / \mathrm{mol}^2 L^{-2} $
$=L \mathrm{~mol}^{-1} \mathrm{~s}^{-1}$
Question 3.1(ii) From the rate expression for the following reactions, determine their order of reaction and the dimensions of the rate constants.
Answer :
Given rate = $k[H_{2}O_{2}][I^{-}]$
Therefore, the order of the reaction is 2
Dimension of k = $rate/[H_{2}O_{2}][I^{-}]$
$\\=mol\ L^{-1}\ s^{-1}/(mol\ L^{-1})(mol\ L^{-1})\\$
$=L\ mol^{-1}\ s^{-1}$
Question 3.1(iii) From the rate expression for the following reactions, determine their order of reaction and the dimensions of the rate constants.
$CH_{3}CHO(g)\rightarrow CH_{4}(g)+CO(g)\: \: Rate=k\left [ CH_{3} CHO\right ]^{3/2}$
Answer :
Given $Rate = k[CH_{3}CHO]^{3/2}$
Therefore the order of the reaction is 3/2
and the dimension of k $=Rate/[CH_{3}CHO]^{3/2}$
$\\=molL^{-1}s^{-1}/(molL^{-1})^{3/2}\\$
$=molL^{-1}s^{-1}/mol^{3/2}L^{-3/2}\\$
$ =L^{1/2}\ mol^{-1/2}\ s^{-1}$
Question 3.1(iv) From the rate expression for the following reactions, determine their order of reaction and the dimensions of the rate constants.
$C_{2}H_{5}Cl(g\rightarrow )C_{2}H_{4}(g)+HCL(g) \: Rate=k\left [ C_{2}H_{5}Cl \right ]$
Answer :
$rate = k[C_{2}H_{5}Cl]$
so the order of the reaction is 1
and the dimension of k = $rate/[C_{2}H_{5}Cl]$
$\\=molL^{-1}s^{-1}/mol L^{-1}\\ =s^{-1}$
Question 3.2 For the reaction:
$2A+B\rightarrow A_{2}B$
the rate = $k\left [ A \right ]\left [ B \right ]^{2}$ with $k=2.0\times 10^{-6}mol^{-2}L^{2}s^{-1}$ . Calculate the initial rate of the reaction when $\left [ A \right ]=0.1 mol L^{-1}\: \: ,\left [ B \right ]=0.2 mol L^{-1}$ . Calculate the rate of reaction after $\left [ A \right ]$ is reduced to $0.06molL ^{-1}$ .
Answer :
The initial rate of reaction =
$rate = k[A][B]^{2}$
Substitute the given values of [A], [B] and k,
rate = $2\times 10^{-6}\times 0.1\times (0.2)^{2}$
=8 $\times 10^{-9}mol^{-2}\ L^{2}\ s^{-1}$
When [A] is reduced from 0.1 mol/L to 0.06 mol/L $[A^{'}=0.06]$
So, conc. of A reacted = 0.1-0.06 = 0.04 mol/L
and conc. of B reacted = 1/2(0.04) = 0.02mol/L
conc. of B left = (0.2-0.02) = 0.18 mol/L $[B^{'}=0.18]$
Now, the rate of the reaction is (R) = $k[A^{'}][B^{'}]$
$=2\times 10^{-6}\times 0.06\times (0.18)^{2}$
= $3.89\times 10^{-6}$ $mol L^{-1}s^{-1}$
Answer :
The decomposition of $NH_{3}$ on the platinum surface reaction
$2NH_{3}(s)\overset{Pt}{\rightarrow}N_{2}(g)+3H_{2}(g)$
therefore,
Rate = $-\frac{1}{2}\frac{d[NH_{3}]}{dt}=\frac{d[N_{2}]}{dt}=\frac{1}{3}\frac{d[H_{2}]}{dt}$
For zero order reaction rate = k
therefore, $-\frac{1}{2}\frac{d[NH_{3}]}{dt}=\frac{d[N_{2}]}{dt}=\frac{1}{3}\frac{d[H_{2}]}{dt}=k$
So $\frac{d[N_{2}]}{dt}= 2.5\times 10^{-4}mol\ L^{-1}\ s^{-1}$
and the rate of production of dihydrogen $(H_{2})$ = 3 $\times$ (2.5 $\times 10^{-4}$ ) $mol\ L^{-1}\ s^{-1}$
= 7.5 $\times 10^{-4}$ $mol\ L^{-1}\ s^{-1}$
Answer :
Given that
$rate = k(P_{CH_{3}OCH_{3}})^{3/2}$
So, the unit of rate is bar/min .( $bar\ min^{-1}$ )
And thus the unit of k = unit of rate $/(bar)^{3/2}$
$\\=bar\ min^{-1}/(bar)^{3/2}\\ =bar^{-1/2}min^{-1}$
Question 3.5: Mention the factors that affect the rate of a chemical reaction.
Answer :
The following factors affect the rate of reaction-
- The concentration of reactants
- temperature, and
- presence of a catalyst
- Surface area of reactants
- Pressure
Question 3.6(i) A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is doubled
Answer :
Let us assume the concentration of the reactant is x
So, rate of reaction, $R =k[x]^{2}$
Now, if the concentration of the reactant is doubled, then $x\rightarrow 2x$. So the rate of reaction would be $R = k[2x]^{2} = 4kx^{2}=4R$
Hence, we can say that the rate of reaction increased by 4 times.
Question 3.6(ii) A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is reduced to half?
Answer :
Let us assume the concentration of the reactant is x
So, rate of reaction, R = $k[x]^{2}$
Now, if the concentration of the reactant is doubled, then $x\rightarrow \frac{x}{2}$ . So the rate of reaction would be $R = k[\frac{x}{2}]^{2} = \frac{kx^{2}}{4}=\frac{R}{4}$
Hence, we can say that the rate of reaction is reduced to 1/4 times.
Answer :
The rate constant is nearly double when there is a 10-degree rise in temperature in a chemical reaction.The
effect of temperature on the rate constant can be represented quantitatively by the Arrhenius equation.
$K =Ae^{-E_{a}/RT}$ where k is rate constant
A is the Arrhenius factor
R is the gas constant
T is temperature and
$E_{a}$ is the activation energy
Question 3.8 In pseudo-first-order hydrolysis of an ester in water, the following results were obtained:
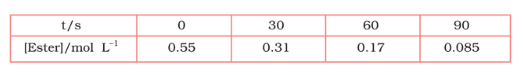
(i) Calculate the average rate of reaction between the time intervals 30 to 60 seconds.
Answer :
The average rate of reaction between 30 s and 60 s is expressed as
$R = \frac{d[ester]}{dt}$
$\\=(0.31-0.17)/60-30\\ $
$=0.14/30=4.67\times 10^{-3} mol\ L^{-}\ s^{-}$
Question 3.9(i) A reaction is first order in A and second order in B.
(i)Write the differential rate equation.
Answer :
The reaction is first order in A and second order in B. it means the power of A is one and the power of B is 2
The differential rate equation will be
$-\frac{d[R]}{dt}=k[A][B]^{2}$
Question 3.9(ii) A reaction is first order in A and second order in B.
(ii) How is the rate affected by increasing the concentration of B three times?
Answer :
If the concentration of [B] is increased by 3 times, then
$-\frac{d[R]}{dt}=k[A][3B]^{2}$
$=9k[A][B]^{2}$
Therefore, the rate of reaction will increase 9 times.
Question 3.9(iii) A reaction is first order in A and second order in B.
(iii) How is the rate affected when the concentrations of both A and B are doubled?
Answer :
If the concentration of [A] and[B] is increased by 2 times, then
$-\frac{d[R]}{dt}=k[2A][2B]^{2}$
$=8k[A][B]^{2}$
Therefore, the rate of reaction will increase 8 times.
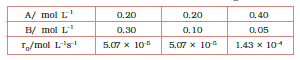
What is the order of the reaction with respect to A and B?
Answer :
we know that
rate law ( $r_{0}$ ) = $k[A]^{x}[B]^{y}$
As per data
$\\5.07\times 10^{-5} =k[0.2]^{x}[0.3]^{y}\\ 5.07\times 10^{-5}$
$=k[0.2]^{x}[0.1]^{y}\\ 1.43\times 10^{-4}$
$=k[0.4]^{x}[0.05]^{y}$
These are equations 1, 2 and 3, respectively
Now, divide eq.1 by equation 2, we get
$1= (0.3/0.1)^{y}$
from here, we calculate that y = 0
Again, dividing Eq. 2 by Eq. 3, we get
Since y =0, also substitute the value of y
So,
= $(\frac{1.43}{0.507})= (\frac{0.4}{0.2})^{x}$
= $2.821=2^{x}$
Taking the log of both sides, we get,
$x = \frac{\log2.821}{\log2}$
= 1.496
= approx 1.5
Hence the order of reaction w.r.t A is 1.5 and w.r.t B is 0(zero)
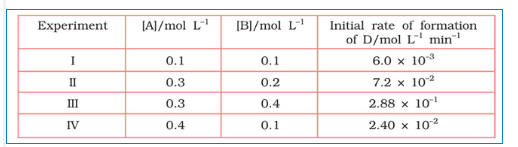
Question 3.11 The following results have been obtained during the kinetic studies of the reaction:
2A + B $\rightarrow$ C + D
Determine the rate law and the rate constant for the reaction .
Answer :
Let assume the rate of reaction wrt A is $x$ and wrt B is $y$ . So, the rate of reaction is expressed as-
Rate = $k[A]^{x}[B]^{y}$
According to given data,
$\\6\times 10^{-3}=k[0.1]^{x}[0.1]^{y}\\ 7.2\times 10^{-2}$
$=k[0.3]^{x}[0.2]^{y}\\ 2.88\times 10^{-2}$
$=k[0.3]^{x}[0.4]^{y}\\ 2.4\times 10^{-2}$
$=k[0.4]^{x}[0.1]^{y}$
These are equations 1, 2, 3 and 4, respectively
Now, divide the equation(iv) by (i) we get,
$4 = (0.4/0.1)^{x}$
from here, we calculate that $x =1$
Again, divide equation (iii) by (ii)
$4 =(0.4/0.2)^{y}$
from here, we can calculate that the value of y is 2
Thus, the rate law is now, $Rate = k[A][B]^{2}$
So, $k = rate/[A][B]^{2}$
$\\= 6\times 10^{-3}/(0.1)\times (0.1)^{2}\\$
$ =6\ L^{2}\ mol^{-2}\ min^{-1}$
Hence the rate constant of the reaction is $=6\ L^{2}\ mol^{-2}\ min^{-1}$
|
Experiment
|
[A]/molL-1
| [B]/molL-1 | Initial rate/ mol L-1 min-1 |
|
I
|
0.1
|
0.1
|
2*10-2
|
|
II
| - |
0.2
|
4*10-2
|
|
III
|
0.4
|
0.4
| - |
|
IV
|
0.2
|
2*10-2
|
Answer :
The given reaction is first order wrt A and zero order in wrt B. So, the rate of reaction can be expressed as;
$Rate = k[A][B]^{0}$
Rate = k[A]
from exp 1,
$2\times 10^{-2}=k(0.1)$
k = 0.2 per min.
from experiment 2nd,
$4\times 10^{-2}=0.2[A] \\$
[A] = $0.2\ mol/L$
from experiment 3rd,
$rate =(0.2\ min^{-1})\times (0.4\ mol/L)$
$= 0.08\ mol\ L^{-1}\ min^{-1}$
from the experiment 4th,
$2\times 10^{-2}=0.2*[A]$
from here [A] = 0.1 mol/L
Question 3.13 (1) Calculate the half-life of a first-order reaction from their rate constants given below:
$200 s^{-1}$
Answer :
We know that,
half-life ( $t_{1/2}$ ) for first-order reaction = $0.693/k$
= $0.693/200$
$\approx 3.4\times 10^{-3}s$
Question 3.13 (2) Calculate the half-life of a first-order reaction from their rate constants given below:
$2\: \: min ^{-1}$
Answer :
The half-life for the first-order reaction is expressed as ;
$t_{1/2}=0.693/k$
= 0.693/2
= 0.35 min (approx)
Question 3.13 (3) Calculate the half-life of a first-order reaction from their rate constants given below:
4 $years ^{-1}$
Answer :
The half-life for the first-order reaction is $t_{1/2}= 0.693/k$
= 0.693/4
= 0.173 year (approximately)
Answer :
Given,
half-life of radioactive decay = 5730 years
So, $t_{1/2}= 0.693/k$
$k = 0.693/5730$ per year
We know that, for a first-order reaction,
$t = \frac{2.303}{k} \log\frac{[R_{0}]}{[R]}$
$t = \frac{2.303}{.693/5730} \log\frac{100}{80}$
= 1845 years (approximately)
Thus, the age of the sample is 1845 years
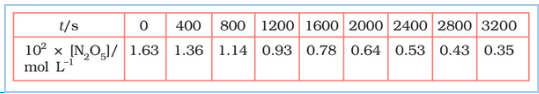
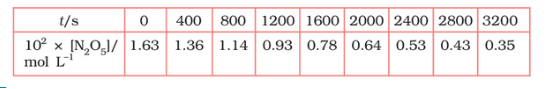
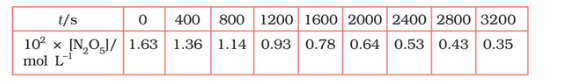
Question 3.15 (1) The experimental data for the decomposition of
$2 N_2 O_5 \rightarrow 4 NO_2 + O_2$
In the gas phase at 318K are given below:
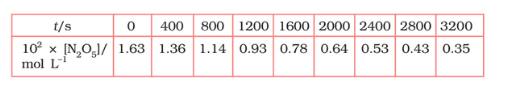
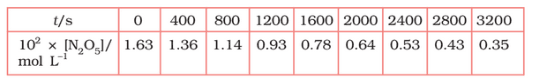
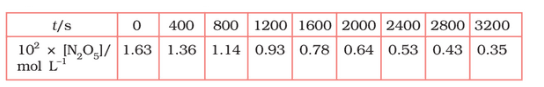
Plot $[N_2O_5]$ against t.
Answer :
On increasing time, the concentration of $N_{2}O_{5}$ gradually decreasing exponentially.
Question 3.15 (2) The experimental data for decomposition of $2 N_2O_5 \rightarrow 4 NO_2 + O_2$ in gas phase at 318K are given below:
Find the half-life period for the reaction.
Answer :
The half-life of the reaction is-
The time corresponding to the $1.63 \times 10^{2}/2$ mol/ L = 81.5 mol /L is the half-life of the reaction. From the graph, the answer should be in the range of 1400 s to 1500 s.
Question 3.15 (3) The experimental data for decomposition of $N_2O_5 [2N_2O_5 \rightarrow 4NO_2 + O_2]$ in the gas phase at 318K are given below:
Draw a graph between $\log [ N_2 O_5 ]$ and t.
Answer :
| $t/S$ | $10^{2}\times [N_{2}O_{5}]$ | $\log[N_{2}O_{5}]$ |
| 0 | 1.63 | -1.79 |
| 400 | 1.36 | -1.87 |
| 800 | 1.14 | -1.94 |
| 1200 | 0.93 | -2.03 |
| 1600 | 0.78 | -2.11 |
| 2000 | 0.64 | -2.19 |
| 2400 | 0.53 | -2.28 |
| 2800 | 0.43 | -2.37 |
| 3200 | 0.35 | -2.46 |
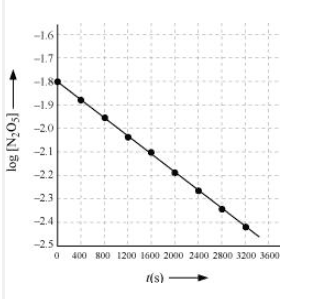
Question 3.15 (4) The experimental data for decomposition of $N_2O_5 [2N_2O_5 \rightarrow 4NO_2 + O_2]$ in the gas phase at 318K are given below:
Answer :
Here, the reaction is a first-order reaction because its log graph is linear.
Thus rate law can be expessed as $Rate = k[N_{2}O_{5}]$
Question 3.15 (5) The experimental data for decomposition of $N_2O_5 [2N_2O_5 \rightarrow 4NO_2 + O_2]$ in the gas phase at 318K are given below:
Answer :
From the log graph,
the slope of the graph is = $\frac{-2.46-1.79}{3200}$
= -k/2.303 ..(from log equation)
On comparing both the equation, we get,
$-k/2.303 = -0.67/3200$
$k= 3200\times (0.67/3200)$
$k= 4.82 \times 10^{-4}\ s^{-1}$
Question 3.15 (6) The experimental data for decomposition of $N_2O_5 [2N_2O_5 \rightarrow 4NO_2 + O_2]$ in the gas phase at 318K are given below:
Calculate the half-life period from k and compare it with (ii).
Answer :
The half life produce = $0.693/k$
= $=0.693/4.82 \times 10^{-4}$
$1.438\times 10^{3} s$
Answer :
We know that,
for a first-order reaction,
$t=\frac{2.303}{k}\log\frac{[R]_{0}}{[R]}$
$\\=\frac{2.303}{60}\log\frac{1}{1/16}\\ $
$=\frac{2.303}{60} \log2^{4}$
$=4.6 \times 10^{-2} s$ (nearly)
Hence the time required is $=4.6 \times 10^{-2} s$
Answer :
Given,
half life = 21.8 years
$\therefore\ k=0.693/t_{1/2}$
= 0.693/21.8
and, $t = \frac{2.303}{k}\log\frac{[R]_{0}}{[R]}$
by putting the value we get,
$10= \frac{2.303}{0.693/21.8}\log\frac{1}{[R]}$
$\log[R] = -\frac{10\times 0.693}{2.303\times 21.8}$
Taking antilog on both sides,
[R] = antilog(-0.1071)
= 0.781 $\mu g$
Thus, 0.781 $\mu g$ of ${Sr}^{90}$ will remain after 10 years.
Again,
$\begin{aligned} & t=\frac{2.303}{k} \log \frac{[\mathrm{R}]_0}{[\mathrm{R}]} \\ & \Rightarrow 60=\frac{2.303}{\frac{0.693}{28.1}} \log \frac{1}{[\mathrm{R}]} \\ & \Rightarrow \log [\mathrm{R}]=-\frac{60 \times 0.693}{2.303 \times 28.1} \\ & \begin{aligned} \Rightarrow[\mathrm{R}] & =\operatorname{antilog}(-0.6425) \\ & =\operatorname{antilog}(\overline{1} .3575) \\ & =0.2278 \mu \mathrm{~g}\end{aligned}\end{aligned}$
Thus 0.2278 $\mu g$ of ${Sr}^{90}$ will remain after 60 years.
Answer :
Case 1-
for 99% complition,
$t^{1} = \frac{2.303}{k}\log\frac{100}{100-99}$
$= \frac{2.303}{k}\log100$
$=2\times (\frac{2.303}{k})$
Case- II
for 90% completion,
$t^{2}=\frac{2.303}{k}\log\frac{100}{100-90}$
$=\frac{2.303}{k}\log10$
$=(\frac{t^{1}}{2})$
$t^{1}=2t^{2}$
Hence proved.
Question 3.19 A first-order reaction takes 40 min for 30% decomposition. Calculate $t_{1/2}$
Answer :
For the first-order reaction,
$t =\frac{2.303}{k}\log \frac{[R]_{0}}{[R]}$
$k =\frac{2.303}{40}\log \frac{100}{100-30}$ (30% already decomposed and remaining is 70%)
$=8.918\times 10^{-3} min^{-1}$
therefore half life = 0.693/k
= $0.693/8.918\times 10^{-3}$
= 77.7 (approx)
Question 3.20 For the decomposition of azoisopropane to hexane and nitrogen at 543K, the following data are obtained.
| t(sec) | P(mmHg) |
| 0 | 35.0 |
| 360 | 54.0 |
| 720 | 63.0 |
Calculate the rate constant.
Answer :
This decomposition is first-order, and in gas-phase kinetics, pressure can be used in place of concentration if temperature and volume are constant.
For a first-order reaction, the integrated rate law is:
$
k=\frac{1}{t} \ln \left(\frac{P_{\infty}-P_0}{P_{\infty}-P_t}\right)
$
Where:
- $P_0=35.0 \mathrm{mmHg}$ (initial pressure)
- $P_t$ is pressure at time $t$
- $P_{\infty}=2 P_0=70.0 \mathrm{mmHg}$ (since $1 \mathrm{~mol} \rightarrow 2 \mathrm{~mol}$ gases)
Step 2: Calculate rate constant using data at $t=360 \mathrm{~s}$
$
\begin{gathered}
k=\frac{1}{360} \ln \left(\frac{70-35}{70-54}\right)=\frac{1}{360} \ln \left(\frac{35}{16}\right)=\frac{1}{360} \ln (2.1875) \\
\ln (2.1875) \approx 0.7827 \\
k=\frac{0.7827}{360} \approx 2.174 \times 10^{-3} \mathrm{~s}^{-1}
\end{gathered}
$
$
k \approx 2.17 \times 10^{-3} \mathrm{~s}^{-1}
$
| Experiment | Time/s-1 | Total Pressure/atm |
| 1 | 0 | 0.5 |
| 2 | 100 | 0.6 |
Calculate the rate of the reaction when the total pressure is 0.65 atm.
Answer :
The thermal decomposition of $SO_{2}Cl_{2}$ is shown here;
$
\mathrm{SO}_2 \mathrm{Cl}_2 \rightarrow \mathrm{SO}_2+\mathrm{Cl}_2
$
After t time, the total pressure $p_{t}$ = $p_{0}-p+(p+p) = p_{0}+p$
So, $p = p_{t}-p_{0}$
thus, $p_{0}-p = 2p_{0}-p_{t}$
for a first-order reaction,
$k= \frac{2.303}{t}\log\frac{p_{0}}{p_{0}-p}$
$= \frac{2.303}{360}\log\frac{p_{0}}{2p_{0}-p_{t}}$
now putting the values of pressures, when t = 100s
$k = \frac{2.303}{100}\log\frac{0.5}{2*0.5-0.6}$
$= 2.231 \times 10^{-3}\ s^{-1}$
when $p_{t} = 0.65\ atm$
$p = p_{t}-p_{0}$
= 0.65 - 0.5
= 0.15 atm
So, $p(_{SO_{2}Cl_{2}}) = p_{0}-p$
= 0.5 - 0.15
= 0.35 atm
Thus, rate of reaction, when the total pressure is 0.65 atm
rate = k( $p(_{SO_{2}Cl_{2}})$ )
= $2.31\times 10^{-3}\times 0.35$
= 7.8 $\times 10^{-4}\ atm\ s^{-1}$
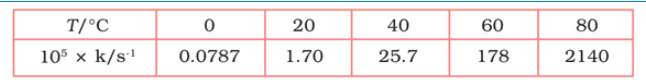
Question 3.22 The rate constant for the decomposition of $N_{2}O_{5}$ at various temperaturesis given below:
Draw a graph between ln k and 1/T and calculate the values of A and
$E_a$ . Predict the rate constant at 30° and 50°C.
Answer :
From the above data,
| T/ $C^{0}$ | 0 | 20 | 40 | 60 | 80 |
| T/K | 273 | 293 | 313 | 333 | 353 |
| $1/T$ $/$ $K^{-1}$ ( $\times 10^{-3}$ ) | 3.66 | 3.41 | 3.19 | 3.0 | 2.83 |
| $10^{5}*K/S^{-}$ | 0.0787 | 1.70 | 25.7 | 178 | 2140 |
| $ln\ K$ | -7.147 | -4.075 | -1.359 | -0.577 | 3.063 |
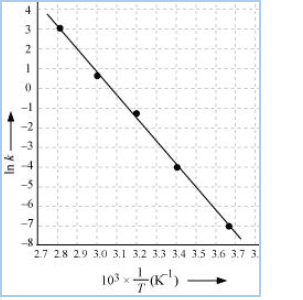
Slope of line = $\frac{y2-y2}{x2-x1} = -12.30\ K$
According to Arrhenius equations,
Slope = $-E_{a}/R$
$E_{a}=$ 12.30 $\times$ 8.314
= 102.27 $KJ mol^{-1}$
Again,
$
\begin{aligned}
& \ln k=\ln A-\frac{E_a}{\mathrm{R} T} \\
& \ln A=\ln k+\frac{E_a}{\mathrm{R} T}
\end{aligned}
$
When $T=273 \mathrm{~K}$,
$\ln k=-7.147$
When $T=273 \mathrm{~K}$,
$\ln k=-7.147$
Then, $\ln A=-7.147+\frac{102.27 \times 10^3}{8.314 \times 273}$
$=37.911$
Therefore, $A=2.91 \times 10^6$
When T = 30 +273 = 303 K and 1/T =0.0033K
$\ln k= -2.8$
$\therefore$ k = $6.08\times 10^{-2}\ s^{-1}$
When T = 50 + 273 = 323 K and 1/T = 3.1 $\times 10^{-3}$ K
$\ln k = -0.5$
$\therefore$ k = 0.607 per sec
Answer :
Given that,
k = $2.418 \times 10 ^{-5} s ^{-1}$
$E_{a}$ = 179.9 KJ/mol
T(temp) = 546K
According to Arrhenius equation,
$k=Ae^{-E_{a}/RT}$
taking log on both sides,
$\log k = \log A - \frac{E_{a}}{2.303 RT}$
$\log A =\log k + \frac{E_{a}}{2.303 RT}$
$=\log (2.418\times 10^{-5}) + \frac{179.9\times 10^{3}}{2.303 \times 8.314 \times 546}$
= (0.3835 - 5) + 17.2082
= 12.5917
Thus A = antilog (12.5917)
A = 3.9 $\times 10^{12}$ per sec (approx)
Answer :
Given that,
k = $=2\times 10^{-2}$
t = 100 s
$[A]_0= 1\ mol\ L^{-1}$
Here, the unit of k is in per sec, it means it is a first-order reaction.
therefore,
$k = \frac{2.303}{t}\log\frac{[A]_o}{[A]}$
$\\2\times 10^{-2} = \frac{2.303}{100}\log\frac{1}{[A]}\\ 2\times 10^{-2} $
$= -\frac{2.303}{100}\log[A]\\ \log[A]=-\frac{2}{2.303}\\$
$\\A = anti\log\frac{-2}{2.303}\\ A= 0.135 mol\ L^{-1}$
Hence, the concentration of the rest test sample is 0.135 mol/L
Answer :
For first-order reaction,
$k = \frac{2.303}{t}\log\frac{[R]_{0}}{[R]}$
given that half life = 3 hrs ( $t_{1/2}$ )
Therefore k = 0.693/half-life
= 0.231 per hour
Now,
$\\0.231 = \frac{2.303}{8}\log\frac{[R]_{0}}{[R]}\\ \log\frac{[R]_{0}}{[R]} $
$= 0.231\times \frac{8}{2.203}$
= antilog (0.8024)
= 6.3445
$[R]_{0}/[R] = 6.3445$
$[R]/[R]_{0} = 0.157$ (approx)
Therefore, the fraction of the sample of sucrose remains after 8 hrs is 0.157
Question 3.26 The decomposition of hydrocarbon follows the equation k=(4.51011s-1)e-28000K/T . Calculate $E_{a}$
Answer :
The Arrhenius equation is given by
$k=Ae^{-E_{a}/RT}$ .................................(i)
given equation,
A= 4.51011 per sec.............(ii)
by comparing equation (i) & (ii) we get,
A= 4.51011 per sec
$E_{a}/RT =28000/T$
Activation energy = 28000 $\times$ (R = 8.314)
= 232.792 KJ/mol
Calculate $E_a$ for this reaction and at what temperature will its half-period be 256 minutes?
Answer :
The Arrhenius equation is given by
$k=Ae^{-E_{a}/RT}$
Taking the log on both sides,
$\log k = \log A -\frac{E_{a}}{2.303RT}$ ....................(i)
given equation,
$\log k = 14.34 - 1.25 \times 10 ^ 4K/T$ .....................(ii)
On comparing both equation, we get,
$E_{a}/2.303R=1.25 \times 10^{4}$
activation energy
$\\=1.25 \times 10^{4} \times 2.303 \times 8.314\\ $
$=239.34\ KJ/mol$
half life ( $t_{1/2}$ ) = 256 min
k = 0.693/256
$k = 4.51\times 10^{-5} s^{-1}$
With the help of equation (ii),
$\log4.51\times 10^{-5} s^{-1} = 14.34-\frac{1.25\times 10^{4}}{T}$
$\frac{1.25\times 10^{4}}{T} = 18.686$
T = $\frac{1.25\times 10^{4}}{18.686}$
= 669 (approx)
Question 3.28 The decomposition of A into product has value of k as $4.5 \times 10 ^3 s ^{-1}$ at 10°Cand energy of activation 60 kJ $mol^{-1}$. At what temperature would k be $1.5 \times 10 ^4 s ^{-1}$ ?
Answer :
The decomposition of A into a product has a value of k as $4.5 \times 10 ^3 s ^{-1}$ at 10°C and energy of activation 60 kJ mol–1.
K1 = $4.5 \times 10 ^3 s ^{-1}$
K2 = $1.5 \times 10 ^4 s ^{-1}$
$E_a$ = 60 kJ mol–1
K2 = $1.5 \times 10 ^4 s ^{-1}$
$log\frac{K_2}{K_1}=\frac{E_a(T_2-T_1)}{2.303RT_1T_2}$
$log(\frac{1.5\times 10^{4}}{4.5\times 10^{3}})=\frac{60(T_2-283)}{2.303\times R\times 283\times T_2}$
$log(\frac{150}{45})=\frac{60(T_2-283)}{5418.61\times T_2}$
$log150-log45=\frac{60T_2-16980}{5418.61\times T_2}$
$2.176-1.653=\frac{60T_2-16980}{5418.61\times T_2}$
$0.5229=\frac{60T_2-16980}{5418.61\times T_2}$
$T_2=\frac{16980}{2733.4}$
$T_2=6.1K$
Answer :
We know that,
for a first order reaction-
$t = \frac{2.303}{k}\log\frac{a}{a-x}$
Case 1
At temp. = 298 K
$t = \frac{2.303}{k}\log\frac{100}{90}$
= 0.1054/k
Case 2
At temp = 308 K
$t' = \frac{2.303}{k}\log\frac{100}{75}$
= 2.2877/k'
As per the question
$t' = t$
K'/K = 2.7296
From the Arrhenius equation,
$\begin{aligned} & \log \frac{k^{\prime}}{k}=\frac{E_a}{2.303 \mathrm{R}}\left(\frac{T^{\prime}-T}{T T^{\prime}}\right) \\ & \log (2.7296)=\frac{E_a}{2.303 \times 8.314}\left(\frac{308-298}{298 \times 308}\right) \\ & E_a=\frac{2.303 \times 8.314 \times 298 \times 308 \times \log (2.7296)}{308-298}\end{aligned}$
= 76640.096 J /mol
=76.64 KJ/mol
k at 318 K
we have , T =318K
A= $4 \times 10^{10}$
Now $\log k = \log A- \frac{E_{a}}{2.303RT}$
After putting the value of the given variable, we get
$\log k = -1.9855$
On taking antilog we get,
k = antilog(-1.9855)
= 1.034 $\times 10^{-2}\ s^{-1}$
Answer :
From the Arrhenius equation,
$\log\frac{k_{2}}{k_{1}}=\frac{E_{a}}{2.303R}(\frac{T_{2}-T_{1}}{T_{1}T_{2}})$ ...................................(i)
it is given that $k_{2}=4k_{1}$
T1= 293 K
T2 = 313 K
Putting all these values in equation (i) we get,
$\log 4 =\frac{E_{a}}{2.303 \times 8.314}(\frac{313-293}{313 \times 293})$
$\begin{aligned} & \Rightarrow 0.6021=\frac{20 \times E_a}{2.303 \times 8.314 \times 293 \times 313} \\ & \Rightarrow E_a=\frac{0.6021 \times 2.303 \times 8.314 \times 293 \times 313}{20}\end{aligned}$
Activation Energy = 52.86 KJ/mol
This is the required activation energy
Class 12 Chemistry NCERT Chapter 3: Higher Order Thinking Skills (HOTS) Questions
These HOTS questions are designed to help students understand basic concepts and apply their knowledge of electrochemistry in complex situations. Solving class 12 chemistry chapter 3 chemical kinetics question answer will enhance analytical skills, conceptual understanding, and prepare students for competitive examinations based on the syllabus of NCERT.
Question 1. In a reaction $A+B \rightarrow C$, initial concentrations of $A$ and $B$ are related as $[A]_0=8[B]_0$. The half-lives of $A$ and $B$ are 10 min and 40 min, respectively. If they start to disappear at the same time, both following first-order kinetics, after how much time will the concentration of both the reactants be the same?
1) 60 min
2) 80 min
3) 20 min
4) 40 min
Answer:
$\begin{aligned} & \text { Given : }[\mathrm{A}]_0=8[\mathrm{~B}]_0 \\ & {\left[\mathrm{t}_{1 / 2}\right]_{\mathrm{A}}=10 \text { min. }} \\ & {\left[\mathrm{t}_{1 / 2}\right]_{\mathrm{B}}=40 \text { min. }} \\ & \mathrm{I}^{\mathrm{s}} \text { order kinetics } \\ & \mathrm{t}=? \\ & {[\mathrm{~A}]_{\mathrm{t}} \quad=[\mathrm{B}]_{\mathrm{t}}} \\ & -\mathrm{k}_{\mathrm{A}} \times \mathrm{t} \quad-\mathrm{k}_{\mathrm{B}} \times \mathrm{t} \\ & \Rightarrow \quad[\mathrm{A}]_0 \mathrm{e}=[\mathrm{B}]_0 \mathrm{e} \\ & \Rightarrow \quad \frac{[\mathrm{A}]_0}{[\mathrm{~B}]_0}=\mathrm{e}^{\left(\mathrm{k}_{\mathrm{A}}-\mathrm{k}_{\mathrm{B}}\right) \mathrm{t}} \\ & \Rightarrow \quad 8=\mathrm{e}^{\left(\mathrm{k}_{\mathrm{A}}-\mathrm{k}_{\mathrm{B}}\right) \times \mathrm{t}} \\ & \Rightarrow \quad \ell \mathrm{n} 8=\left(\mathrm{k}_{\mathrm{A}}-\mathrm{k}_{\mathrm{B}}\right) \times \mathrm{t}\end{aligned}$
$\ell \mathrm{n} 8=\ell \mathrm{n} 2\left(\frac{1}{\left(\mathrm{ta}_2\right)_{\mathrm{A}}}-\frac{1}{\left(\mathrm{ta}_2\right)_{\mathrm{B}}}\right) \times \mathrm{t}$
$t=\frac{3}{\left(\frac{1}{\left(t_{1 / 2}\right)_A}-\frac{1}{\left(t_{1 / 2}\right)_B}\right)}$
$
t=\frac{3}{\left(\frac{1}{10}-\frac{1}{40}\right)}
$
$
\frac{1}{10}-\frac{1}{40}=\frac{4-1}{40}=\frac{3}{40}
$
$
t=\frac{3}{\frac{3}{40}}=40 \text { minutes }
$
Hence, the correct answer is option (4).
Question 2. Drug X becomes ineffective after $50 \%$ decomposition. The original concentration of drug in a bottle was $ 16\mathrm {mg} / \mathrm{mL}$, which becomes 4 $\mathrm{mg} / \mathrm{mL}$ in 12 months. The expiry time of the drug in months is ________. Assume that the decomposition of the drug follows first-order kinetics.
Answer:
The decomposition of Drug $X$ follows first-order kinetics, meaning the rate of decomposition depends on the drug's concentration. The first-order rate equation is given by:
$ k=\frac{2.303}{t} \log \frac{[A]_0}{[A]} $
where: - $[A]_0=16 \mathrm{mg} / \mathrm{mL}$ (initial concentration), - $[\mathrm{A}]=\mathbf{4 \mathrm { mg }} / \mathrm{mL}$ (concentration after 12 months), - $\mathbf{t}=\mathbf{1 2}$ months (time taken for this change), - $\mathbf{k}$ is the rate constant.
$\begin{gathered}k=\frac{2.303}{12} \log \frac{16}{4} \\ k=\frac{2.303}{12} \log 4 \\ k=\frac{2.303}{12} \times 0.602 \\ k=0.1156 \text { month }^{-1}\end{gathered}$
The drug becomes ineffective after $50 \%$ decomposition, meaning when its concentration reduces to 8 $\mathrm{mg} / \mathrm{mL}$ (half of $16 \mathrm{mg} / \mathrm{mL}$ ). Using the first-order equation:
$ \begin{aligned} t_e & =\frac{2.303}{k} \log \frac{[A]_0}{[A]} \\ t_e & =\frac{2.303}{0.1156} \log \frac{16}{8} \\ t_e & =\frac{2.303}{0.1156} \times 0.301 \\ t_e & =6.01 \text { months } \end{aligned} $
Hence, the correct answer is option (4).
Question 3. Consider the following statements related to the temperature dependence of rate constants. Identify the correct statements,
A. The Arrhenius equation holds true only for an elementary homogeneous reaction.
B. The unit of A is the same as that of k in the Arrhenius equation.
C. At a given temperature, a low activation energy means a fast reaction.
D. A and Ea, as used in the Arrhenius equation, depend on temperature.
E. When Ea >> RT. A and Ea become interdependent.
Choose the correct answer from the options given below :
(i) A, C and D Only
(ii) B, D and E Only
(iii) B and C Only
(iv) A and B Only
Answer:
Arrhenius equation.
$
k=A e^{-\frac{E_a}{R T}}
$
A. Arrhenius holds true only for an elementary homogeneous reaction.
False, because it's an empirical law and is routinely used (and found valid) for complex, composite or even heterogeneous processes.
B. The unit of $A$ is the same as that of $k$.
True, since $e^{-E_a / R T}$ is dimensionless, $A$ must carry whatever units $k$ has.
C. At a given temperature, a low activation energy means a fast reaction.
True, because smaller $E_a$ makes $\exp \left(-E_a / R T\right)$ larger, hence a larger $k$.
D. A and $E_a$ as used in Arrhenius depend on temperature.
False, because in the simple Arrhenius model, both are treated as constants (over modest $T$ ranges).
E. When $E_a \gg R T, A$ and $E_a$ become interdependent.
False, because in some data sets you observe a correlation (compensation effect), the basic Arrhenius form still treats them as independent parameters.
Therefore, the only correct statements are B and C..
Hence, the correct answer is option (3)
Question 4. A first-order reaction is $50 \%$ completed in 20 min at $27^{\circ} \mathrm{C}$ and in 5 min at $47^{\circ} \mathrm{C}$. The energy of activation of the reaction is
(1) $43.85 \mathrm{~kJ} / \mathrm{mol}$
(2) $55.14 \mathrm{~kJ} / \mathrm{mol}$
(3) $110.28 \mathrm{~kJ} / \mathrm{mol}$
(4) $66.5 \mathrm{~kJ} / \mathrm{mol}$
Answer:
We know that half life for first order reaction is $\mathrm{t}_{1 / 2}=0.693 / \mathrm{k}$
So rate constant at $27^{\circ} \mathrm{C}, \mathrm{k}_1=0.69320 \mathrm{~min}^{-1}$
Rate constant at $47^{\circ} \mathrm{C}, \mathrm{k}_2=0.6935 \mathrm{~min}^{-1}$
$
\ln \frac{\mathrm{k}_1}{\mathrm{k}_2}=\frac{\mathrm{E}_{\mathrm{a}}}{\mathrm{R}}\left(\frac{\mathrm{~T}_1-\mathrm{T}_2}{\mathrm{~T}_1 \mathrm{~T}_2}\right)
$
On solving, we get:
$
\mathrm{Ea}=55.14 \mathrm{~kJ} / \mathrm{mol}
$
Hence, the answer is the option (2).
Question 5: In a first order decomposition reaction, the time taken for the decomposition of reactant to one fourth and one eighth of its initial concentration are $t_1$ and $t_2(\mathrm{~s})$, respectively. The ratio $t_1 / t_2$ will :
(1) $\frac{4}{3}$
(2) $\frac{3}{2}$
(3) $\frac{3}{4}$
(4) $\frac{2}{3}$
Answer:
$\begin{aligned} & \mathrm{t}_1=\mathrm{t}_{\frac{1}{4}}=\frac{1}{\mathrm{k}} \ln \frac{\mathrm{A}_0}{\frac{\mathrm{~A}_0}{4}}=\frac{1}{\mathrm{k}} \ln 4 \\ & \mathrm{t}_2=\mathrm{t}_{\frac{1}{8}}=\frac{1}{\mathrm{k}} \ln \frac{\mathrm{A}_0}{\frac{\mathrm{~A}_0}{8}}=\frac{1}{\mathrm{k}} \ln 8 \\ & \frac{\mathrm{t}_1}{\mathrm{t}_2}=\frac{\ln 4}{\ln 8}=\frac{2 \ln 2}{3 \ln 2}=\frac{2}{3}\end{aligned}$
Hence, the correct answer is option (4).
Question 6: If the rate of disappearance of hydrogen is $12 \mathrm{~g} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$.
$
-\frac{d\left[H_2\right]}{d t}=12 \mathrm{~g} L^{-1} \mathrm{~s}^{-1} .
$
Then, calculate the rate of disappearance of $\mathrm{N}_2$ gas for the reaction $\mathrm{N}_2+3 \mathrm{H}_2 \longrightarrow 2 \mathrm{NH}_3$.
(1) $4 \mathrm{gL}^{-1} \mathrm{~s}^{-1}$
(2) $28 \mathrm{~g} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
(3) $56 \mathrm{~g} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
(4) $2 \mathrm{~g} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$
Answer:
$\begin{aligned} & -\frac{d\left[H_2\right]}{d t}=12 g L^{-1} s^{-1}=6 \operatorname{mol} L^{-1} s^{-1} . \\ & \frac{-\frac{1}{3} \frac{d\left[H_2\right]}{d t}}{3}=-\frac{d\left[N_2\right]}{d t}=\frac{6}{3}=2 \operatorname{mol} L^{-1} s^{-1} .\end{aligned}$
$\begin{aligned} \frac{-d\left[N_2\right]}{d t} & =2 \mathrm{~mol} L^{-1} s^{-1} \\ \frac{-d\left[N_2\right]}{d t} & =2 \times 28 \mathrm{gL}^{-1} s^{-1}=56 \mathrm{gL}^{-1} s^{-1}\end{aligned}$
Hence, the correct answer is option (3).
Question 7: For the thermal decomposition of $\mathrm{N}_2 \mathrm{O}_5(\mathrm{~g})$ at constant volume, the following data table is obtained for the reaction: $2 \mathrm{~N}_2 \mathrm{O}_5(g) \rightarrow 2 \mathrm{~N}_2 \mathrm{O}_4(g)+\mathrm{O}_2(g)$
At a certain time $t$, the increase in partial pressure of $O_2$ is represented by $x$.
$
x=\ldots \times 10^{-3} \text { atm(nearest integer) }
$
Given that the rate constant for the reaction is: $=\ldots \times 10^{-3}$ and the initial pressure of $\mathrm{N}_2 \mathrm{O}_5$ was 0.400 atm .
Answer:
Reaction:
$2 \mathrm{~N}_2 \mathrm{O}_5(g) \rightarrow 2 \mathrm{~N}_2 \mathrm{O}_4(g)+\mathrm{O}_2(g)$
Initial pressure of $\mathrm{N}_2 \mathrm{O}_5$ :
$P_0=0.400 \mathrm{~atm}$
Rate constant:
$k=3.21 \times 10^{-2} S^{-1}$
First-order relation
$
\ln \frac{P_0}{P_t}=k t \ln \frac{0.400}{P_t}=3.21 \times 10^{-2} \times 10 \ln \frac{0.400}{P_t}=0.321 \frac{0.400}{P_t}=e^{0.321} \approx 1.38 P_t \approx 0.290 \mathrm{~atm}
$
Pressure decrease of $\mathrm{N}_2 \mathrm{O}_5$
$
\Delta P_{N_2 O_5}=0.400-0.290=0.110 \mathrm{~atm}
$
From stoichiometry:
$
2 \mathrm{~N}_2 \mathrm{O}_5 \rightarrow 1 \mathrm{O}_2 \mathrm{P}_{\mathrm{O}_2}=\frac{1}{2} \times 0.110=0.055 \mathrm{~atm}
$
$x=55 \times 10^{-3} \mathrm{~atm}$
Hence, the answer is $x=55 \times 10^{-3} \mathrm{~atm}$
NCERT Books and NCERT Syllabus
Approach to Solve Questions of Chapter 3 Chemical Kinetics
The approach should be such that it saves your time yet improves your accuracy. The following are some points that can help you build a good approach for the chemical kinetics ncert solutions:
1) While solving problems related to Chemical kinetics, students first need to understand the concept of the Rate of Reaction.
- Understanding the difference between the average rate, the instantaneous rate, and the initial rate.
- Rate law expression Rate $=k[A]^m[B]^n$
- Identification of the order of reaction.
2) Rate constant helps in understanding the speed and feasibility of the reaction under given conditions.
- Rate constant for zero order reaction: k= Rate
- Rate constant for first order reaction: $k=\frac{1}{t} \ln \left(\frac{[A]_0}{[A]}\right)$
- Rate constant for second order reaction: $k=\frac{1}{t}\left(\frac{1}{[A]}-\frac{1}{[A]_0}\right)$
3) To identify the order of a reaction and analyse its kinetics, graphical methods are important. Refer to these chemical kinetics class 12 question answers for a better understanding of concepts
- Zero-order reaction: Concentration vs time
- First-order reaction: ln[A] vs time
- Second-order reaction: Plot $\frac{1}{[A]}$ vs. time
4) Questions related to half-life are often asked in exams, as they help in understanding reaction kinetics, mathematical application of rate equations, and their ability to interpret the behaviour of reactants over time.
- For Zero order reaction: $t_{1 / 2}=\frac{[A]_0}{2 k}$
- For first order of reaction: $t_{1 / 2}=\frac{0.693}{k}$
- For second order reaction: $t_{1 / 2}=\frac{1}{k[A]_0}$
5) Start with the class 12 chemistry chapter 3 chemical kinetics solutions and practice all the NCERT exercise questions. You can also solve previous years' board questions for extra practice and feasible learning.
NCERT Exemplar Solution
Topics and Subtopics Covered in the NCERT Class 12 Chemistry Chapter 3
3.1 Rate of a Chemical Reaction
3.2 Factors Influencing the Rate of a Reaction
3.2.1 Dependence of Rate on Concentration
3.2.2 Rate Expression and Rate Constant
3.2.3 Order of a Reaction
3.2.4 Molecularity of a Reaction
3.3 Integrated Rate Equations
3.3.1 Zero Order Reactions
3.3.2 First Order Reactions
3.3.3 Half-Life of a Reaction
3.4 Temperature Dependence of the Rate of a Reaction
3.4.1 Effect of Catalyst
3.5 Collision Theory of Chemical
NCERT Solutions for Class 12
What Extra Should Students Study Beyond the NCERT for JEE/NEET?
Apart from class 12 chemistry chapter 3 chemical kinetics solutions, students should focus on practising advanced problems, exploring detailed reference books, and learning shortcuts and tricks to solve questions faster, which helps in scoring better in competitive exams. Here is a list of topics that are prominently asked in JEE:
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 3 Chemical Kinetics
These Class 12 chemistry chapter 3 chemical kinetics solutions help students understand the fundamental concepts of chemical kinetics. Below are some key points that students learn with the help of these solutions:
- Here, students will understand the concepts of reaction rates and the factors affecting them.
- These chemical kinetics ncert solutions help students to derive and apply rate laws and determine the order of reactions.
- Integrated rate equations for zero, first, and second-order reactions are explained well in these solutions through a series of solved examples
- Some important concepts, like half-life and its significance in chemical reactions, are explained here in a structured way.
- Using these chemical kinetics class 12 question answers, students will learn about collision theory and activation energy to understand reaction mechanisms.
Importance of Class 12 Chemistry Chapter 3 Chemical Kinetics Solutions
The class 12 chemistry chapter 3 chemical kinetics question answer helps students understand how fast or slow chemical reactions occur and the factors influencing their rates.
- These NCERT Class 12 Solutions explain the pathways through which chemical reactions proceed.
- The concept of Rate Laws are also explained here.
- Catalysts and temperature effects are covered in these class 12 chemistry chemical kinetics question answer.
NCERT Solutions for Class 12 Chemistry Chapter-wise
Along with the NCERT Solutions for Class 12 Chemistry Chapter 3 Chemical Kinetics, here are the clickable links to chapter-wise NCERT Class 12 Solutions:
Frequently Asked Questions (FAQs)
Chapter 3, Chemical Kinetics in NCERT 2025-26 focuses on the study of the speed or rate of chemical reactions and the factors that influence them. It covers concepts like rate laws, order of reactions, integrated rate equations, half-life, activation energy, and reaction mechanisms.
The NCERT solutions for Class 12 Chemistry Chapter 3 cover key topics such as the rate of chemical reactions, factors affecting reaction rates, rate laws, and the order of reactions. They also explain integrated rate equations, half-life, collision theory, activation energy, reaction mechanisms, and provide guidance on solving numerical problems related to these concepts.
The rate of reaction shows how fast reactants are used or products are formed over time. The rate constant (k) is a fixed value at a given temperature that links the rate with reactant concentrations in the rate law.
The rate law is a mathematical expression showing how the rate depends on reactant concentrations.
The Rate-Determining Step is the slowest elementary step in a reaction mechanism, and its rate determines the overall rate of the reaction. The rate law for the overall reaction is usually governed by the stoichiometry and concentrations involved in the rate-determining step.
Catalysts are substances that increase the rate of a reaction without being consumed in the process. They achieve this by providing an alternative pathway with a lower activation energy, making it easier for reactants to transform into products.
Activation energy is the minimum energy required for a reaction to occur. It acts as a barrier that reactants must overcome for the conversion to products. Understanding activation energy is essential for manipulating reaction conditions and predicting how different factors like temperature and catalysts will influence reaction rates.
First-order reactions depend linearly on the concentration of one reactant, meaning if you double the concentration, the rate will also double. In contrast, second-order reactions depend on either the concentration of one reactant squared or the product of two reactants' concentrations. The distinction is important for correctly applying integrated rate laws and predicting reaction behavior.
The main factors affecting reaction rates include concentration of reactants, temperature, the presence of catalysts, surface area of solid reactants, and the nature of the reactants. Higher concentrations and temperatures generally increase reaction rates, while catalysts can lower activation energy, speeding up reactions.
The order of a reaction can be determined by methodically varying the concentrations of the reactants and measuring the effect on the rate. This can be done through techniques like the method of initial rates or integrated rate laws, which compare concentration changes over time to derive the reaction order.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters