These class 12 chemistry chapter 5 coordination compounds solutions offer detailed step by step answers to all the exercise questions from the chapter on coordination compounds. They help students strengthen their understanding of key concepts, practise problem-solving, and prepare thoroughly for board and competitive exams.
NCERT solutions for class 12 chemistry chapter 5 Coordination Compounds
Do you know why blood appears red and emerald appears deep blue, what makes medicines effective against certain diseases, and how vitamin B12 helps in metabolism? The answers to these numerous questions lie in coordination compounds. These compounds are made up of a central metal atom or ion bonded to surrounding molecules or ions, known as ligands. These ligands define their structure and reactivity in fascinating ways.
Electronic gadgets or devices such as Mobile phones, smartwatches, calculators, and more are not allowed inside the CBSE 2026 exam hall.

In this article, students can find the NCERT Class 12 Chemistry Solutions. Our subject experts design these solutions to provide a systematic and structured approach that helps students to understand concepts clearly, solve questions easily, and build confidence. These NCERT solutions are a valuable resource to enhance candidates performance in board exams as well as in competitive exams like NEET, JEE, etc. The coordination compounds ncert solutions is important from the exam point of view as it carries good weightage in CBSE board exams. Below, we have provided selected HOTS questions as well. It will enhance conceptual clarity and problem-solving ability.
NCERT Solutions for Class 12 Chemistry Chapter 5: Download PDF
Students can download the class 12 chemistry chapter 5 coordination compounds solutions pdf for free by clicking on the provided link. These solutions are designed to help you understand the fundamental concepts and solve textbook questions with ease.
Also Read,
NCERT Solutions for Class 12 Chemistry Chapter 5 Coordination Compounds (Intext Questions From 5.1 to 5.10 )
These solutions will help in better concept clarity and exam preparation. These class 12 chemistry chapter 5 coordination compounds question answer provide clear and accurate solutions, helping students understand the fundamental concepts of coordination chemistry. These solutions of NCERT simplify complex topics like nomenclature, bonding, and isomerism, making it easier to grasp and apply in exams.
Page 125
Question 5.1(i) Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt(III) chloride
Answer :
The chemical formula for the coordination compound Tetraamminediaquacobalt(III) chloride is
$[CO(H_2O)_2(NH_3)_4]Cl_3$
Question 5.1(ii) Write the formulas for the following coordination compounds:
(ii) Potassium tetracyanidonickelate(II)
Answer :
The formula for the coordination compound Potassium tetracyanidonickelate II is :
$K_2[Ni(CN)_4]$
Question 5.1(iii) Write the formulas for the following coordination compounds:
(iii) Tris(ethane–1,2–diamine) chromium(III) chloride
Answer :
The formula for the coordination compound Tris(ethane–1,2–diamine) chromium(III) chloride is :
$[Cr(en)_3]Cl_3$
Question 5.1(iv) Write the formulas for the following coordination compounds:
(iv) Amminebromidochloridonitrito-N-platinate(II)
Answer :
The formula for the coordination compound Amminebromidochloridonitrito-N-platinate(II) :
$[Pt(NH_3)BrCl(NO_2)]^{-}$
Question 5.1(v) Write the formulas for the following coordination compounds:
(v) Dichloridobis(ethane–1,2–diamine)platinum(IV) nitrate
Answer :
The chemical formula for the coordination compounds: Dichloridobis(ethane–1,2–diamine)platinum(IV) nitrate
$[PtCl_2(en)_2](NO_3)_2$
Question 5.1(vi) Write the formulas for the following coordination compounds:
(vi) Iron(III) hexacyanidoferrate(II)
Answer :
The formula for the coordination compound Iron(III) hexacyanidoferrate(II):
$Fe_4[Fe(CN)_6]_3$
Page 125
Question 5.2(i) Write the IUPAC names of the following coordination compounds:
$(i)\left [ Co\left ( NH_{3} \right )_{6} \right ]Cl_{3}$
Answer :
The IUPAC name of the coordination compound $\left [ Co\left ( NH_{3} \right )_{6} \right ]Cl_{3}$ is :
Hexaamminecobalt(III) chloride
Question 5.2(ii) Write the IUPAC names of the following coordination compounds:
$(ii) [Co(NH_{3})_{5}Cl]Cl_{2}$
Answer :
The IUPAC name of the coordination compound $[Co(NH_{3})_{5}Cl]Cl_{2}$ is
Pentaamminechloridocobalt(III) chloride
Question 5.2(iii) Write the IUPAC names of the following coordination compounds:
Answer :
The IUPAC name of the coordination compound $K_{3}[Fe(CN)_{6}]$ is
Potassium hexacyanoferrate(III).
Question 5.2(iv) Write the IUPAC names of the following coordination compounds:
$(iv)K_{3}[Fe(C_{2}O_{4})_{3}]$
Answer :
The IUPAC name of the following coordination compound $K_{3}[Fe(C_{2}O_{4})_{3}]$ is
Potassium trioxalatoferrate(III)
Question 5.2(v) Write the IUPAC names of the following coordination compounds:
Answer :
The IUPAC names of the coordination compound $K_{2}[PdCl_{4}]$ is
Potassium tetrachloridopalladate(II).
Question 5.2(vi) Write the IUPAC names of the following coordination compounds:
$(vi)[Pt(NH_{3})_{2}Cl(NH_{2}CH_{3})]Cl$
Answer :
The IUPAC name of the following coordination compound $[Pt(NH_{3})_{2}Cl(NH_{2}CH_{3})]Cl$ is :
Diamminechlorido(methylamine)platinum(II) chloride.
Page 128
Question 5.3(i) Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
$(i) K[Cr (H_{2}O)_{2}(C_{2}O_{4})_{2}$
Answer :
Both geometrical (cis-, trans-) and optical isomers exists for the given compound.
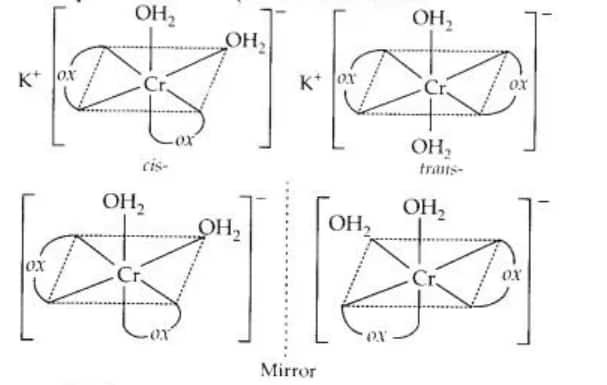
Question 5.3(ii) Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
Answer :
Two optical isomers can exist, Dextro and Laevo.
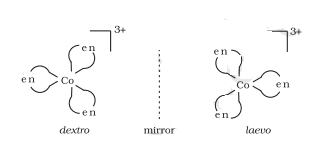
Question 5.3(iii) Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
$(iii) [Co(NH_{3})_{5}(NO_{2})](NO_{3})_{2}$
Answer :
$[Co(NH_{3})_{5}(NO_{2})](NO_{3})_{2}$ There are ionisation and linkage isomers possible.
Ionization isomerism
$[Co(NH_{3})_{5}(NO_{2})](NO_{3})_{2}$ AND $[Co(NH_{3})_{5}(NO_{3})](NO_{3})(NO_2)$
It can also show linkage isomerism
$[Co(NH_{3})_{5}(NO_{2})](NO_{3})_2$ AND $[Co(NH_{3})_{5}(ONO)](NO_{3})_2$
Question 5.3(iv) Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
$(iv)\; [Pt(NH_{3})(H_{2}O)Cl_{2}]$
Answer :
Geometrical (cis-, trans-) isomers can exists for $\; [Pt(NH_{3})(H_{2}O)Cl_{2}]$
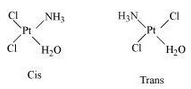
Page 128
Answer :
The ionisation isomers dissolve in water to yield different ions and thus react differently to various reagents:
$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{SO}_4+\mathrm{Ba}^{2+} \rightarrow \mathrm{BaSO}_{4(s)}$ (white ppt.)
$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{SO}_4\right] \mathrm{Cl}+\mathrm{Ba}^{2+} \rightarrow$ No reaction
$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{SO}_4+\mathrm{Ag}^{+} \rightarrow$ No reaction
$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{SO}_4\right] \mathrm{Cl}+\mathrm{Ag}^{+} \rightarrow \mathrm{AgCl}_{(\mathrm{s})}$ (white ppt.)
Page 135
Answer :
In both compounds, the oxidation state of Nickel is +2. So it has d8 configuration.
Now, on the basis of ligand pairing of electrons will occur. Since CN - is a strong ligand so pairing will occur, but in case of Cl - pairing will not be there as it is a weak ligand. So, the configuration of both the compounds looks like :-
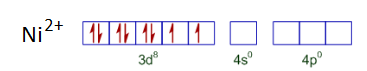
Thus $[Ni(CN)_{4}]^{2-}$ is a square planer and diamagnetic and $[NiCl_{4}]^{2-}$ has tetrahedral geometry and is paramagnetic.
Page 135
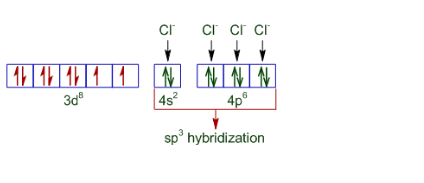
Question 5.6 $[NiCl_{4}]^{2-}$ is paramagnetic while $[Ni(CO)_{4}]$ is diamagnetic though both are tetrahedral. Why?
Answer :
The difference in the magnetic behaviour is due to the nature of ligands present. In case of $[NiCl_{4}]^{2-}$ the oxidation state of nickel is +2 and also Cl - is a weak ligand. Thus its configuration becomes:-
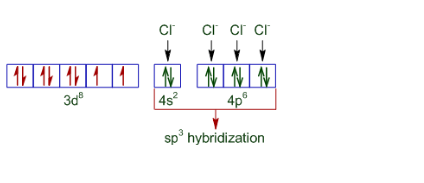
So it is paramagnetic and tetrahedral in nature.
In the case of $[Ni(CO)_{4}]$ , the oxidation state of nickel is 0. So its configuration is 3d 8 4s2 . We also know that CO is a strong ligand, thus the configuration of nickel becomes:-
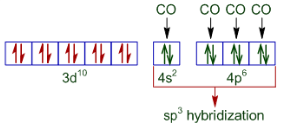
Hence the given compound is diamagnetic but tetrahedral in nature.
Page 135
Question 5.7 $[Fe(H_{2}O)_{6}]^{3+}$ is strongly paramagnetic whereas $[Fe(CN)_{6}]^{3-}$ is weakly paramagnetic. Explain.
Answer :
In both the compounds Fe has a +3 oxidation state i.e., d5 configuration.
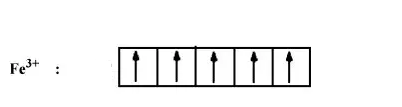
In the case of strong ligand $\mathrm{CN}^{-}$, the pairing of electron will occur. So number of electrons left unpaired will be 1.
In case of weak ligand ($\mathrm{H}_2 \mathrm{O}$), pairing of electron will not there. Thus number of electrons unpaired will be 5.
We know that paramagnetic strength is directly proportional to the number of unpaired electrons.
Hence paramagnetism will be more in case of $[Fe(H_{2}O)_{6}]^{3+}$
Page 135
Answer :
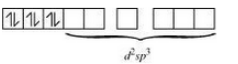
Firstly consider $[Co(NH_{3})_{6}]^{3+}$ :-
The oxidation state of cobalt is +3. So the electronic configuration of it will be d6.
Since (NH3) is a strong ligand so the pairing of electron will be there.
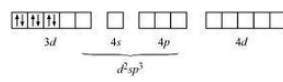
So, it has d2 sp3 hybridisation and an inner orbital complex.
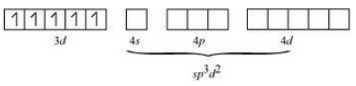
Now consider, $[Ni(NH_{3})_{6}]^{2+}$
The oxidation state of nickel is +3. So its electronic configuration will be d8 .
Also (NH 3 ) is a strong ligand so pairing of electrons will be seen.
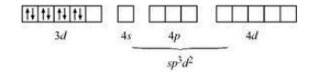
Thus $[Ni(NH_{3})_{6}]^{2+}$ is an outer orbital complex.
Page 135
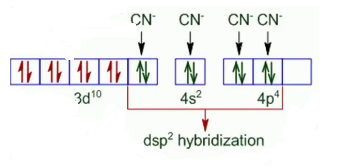
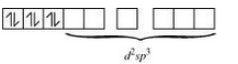
Question 5.9 Predict the number of unpaired electrons in the square planar $[Pt(CN)_{4}]^{2-}$ ion.
Answer :
The oxidation state of Pt in the given compound is +2. Also, it is given that the compound has square planar geometry, i.e., it has dsp2 hybridisation (d8).
CN - is a strong ligand so the pairing of electron will occur.
So there are no unpaired electrons in the given compound.
Page 135
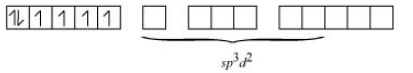
Answer :
Consider Hexaqua manganese (II) :- In this compound, the oxidation state of Mn is +2, and its electronic configuration is $d^5$.
$\mathrm{H}_2 \mathrm{O}$ is a weak ligand and the crystal field is octahedral, so the arrangement of electrons will be$t_{2 g}{ }^3 e g^2$.
So the total number of unpaired electrons is 5.
Now consider hexacyanoion:- In this compound, the oxidation state of Mn is +2. It is surrounded by the strong ligands CN-, so pairing will be there.
Its arrangement will be $t_{2 g}{ }^5 e g^0$ .
Thus, the number of unpaired electrons will be 1.
NCERT Solutions for Class 12 Coordination Compounds (Exercise Questions )
Question 5.1 Explain the bonding in coordination compounds in terms of Werner’s postulates.
Answer :
Werner his theory of coordination compounds and gave some postulates. The main postulates are:
1. In coordination compounds, metals show two types of linkages or valences, namely primary valency and secondary valency.
2. The primary valences are generally ionisable and are satisfied or balanced by negative ions.
3. The secondary valences are non-ionisable. These are satisfied by either neutral molecules or by negative ions. The secondary valence is equal to the coordination number (No. of atoms surrounding the metal) and is constant for a metal.
4. According to different coordination numbers, the ions/groups bound by the secondary linkages to the metal have characteristic spatial arrangements.
Answer : $\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4+\mathrm{FeSO}_4+6 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{FeSO}_4 \cdot\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \cdot 6 \mathrm{H}_2 \mathrm{O} \quad$ Mohr's Salt
$\mathrm{CuSO}_4+4 \mathrm{NH}_3+5 \mathrm{H}_2 \mathrm{O} \longrightarrow\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right] \mathrm{SO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O} \quad \begin{aligned} & \text { Tetraaminocopper (II) } \\ & \text { Sulphate }\end{aligned}$
The major difference between both the compounds is that the first compound is a salt and the other one is a coordination compound. In case of double salt compounds (Mohr's salt), the compound breaks into its constituent ions when dissolved in water, therefore it gives a positive test for the presence of Fe+2. But in case of coordination compounds, they maintain their identity in both solid and dissolved state. Thus the individual property of each constituent is lost. And therefore it doesn't give a positive test for Cu+2.
Answer :
(i) Coordination entity:- It is an electrically charged species carrying either a positive charge or a negative charge. In a coordination entity, the central atom or ion is surrounded by some number of neutral molecules or negative ions ( called ligands) accordingly.
$E.g\ \left [ PtCl_4 \right ]^{2-}\ and\ \left [ Ag(CN)_2 \right ]^{-}$
(ii) Ligand:- Ligands are the neutral molecules or negatively charged ions that surround the metal atom in a coordination entity according to the holding capacity of central metal ion are known as ligands. NH3 and H2O are two neutral ligands.
(iii) Coordination number:- The total number of metals that surrounds the central metal ion is known as coordination number.
For e.g,(a) In case of $\left [ PtCl_6 \right ]^{2-}$ six chlorine atoms are attached to Pt, thus the coordination number of the given compound is 6.
(b) In case of $\left [ Ni(NH_3)_4 \right ]^{2-}$ the central metal ion Ni is surrounded by 4 atoms of ligand, so its coordination number is 4.
(iv) Coordination polyhedron:- It is defined as the spatial arrangement of the ligands which are directly attached to the central metal ion/atom.
E.g. square planar, tetrahedral.
(v) Homoleptic:- Homoleptic compounds are defined as the compounds in which the donor/ligand attached to central metal atom/ion is of one kind.
E.g. $\left [ PtCl_6 \right ]^{2-}$ and $\left [ Ni(NH_3)_4 \right ]^{2-}$
(vi) Heteroleptic:- These are the coordination compounds in which central atoms are attached with more than one type of ligand.
E.g. $\left [ Co(NH_3)_5Cl \right ]^{2+}$ and $\left [ Co(NH_3)_4 Cl_2\right ]^{+1}$
Question 5.4 What is meant by unidentate, didentate and ambidentate ligands? Give two examples for each.
Answer :
Unidentate ligands:- The ligand which has only one donor site are known as unidentate ligands. E.g. Cl- , NH3
Bidentate(or didentate):- The ligands which have two donor sites are known as bidentate ligands. E.g. Ethane-1,2-diamine, Oxalate ion
Ambidentate ligands:- The ligands which can attach themselves to the central metal through two different atoms. E.g. NO2 , SCN
Question 5.5 (i) Specify the oxidation numbers of the metals in the following coordination entities:
$(i)[Co(H_{2}O)(CN)(en)_{2}]^{2+}$
Answer :
Let us assume that the coordination number of cobalt is x.
$x\ +\ 0 +(-1)\ + 2(0)\ =\ +2$
Thus $x = 3$
Hence coordination number of cobalt is +3.
Question 5.5(ii) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let us assume that the coordination number of cobalt is x.
Then, according to the question :
$x\ + 2(-1)\ +\ 2(0)\ =\ 1$
Thus $x = 3$
Hence, the coordination number of cobalt is 3.
Question 5.5(iii) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let us assume the oxidation state of Pt to be x.
Then the equation becomes:-
$\begin{gathered}x+4(-1)=-2 \\ x-4=-2 \\ x=-2+4 \\ x=2\end{gathered}$
Hence, the oxidation state of Pt metal is 2.
Question 5.5(iv) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let the oxidation state of Fe be x.
Then the equation will be:-
$\\x\ +\ 6(-1)\ =\ -3\\x=+3$
Thus oxidation state of Fe in this coordination compound is 3.
Question 5.5(v) Specify the oxidation numbers of the metals in the following coordination entities:
Answer :
Let us assume the oxidation state of Cr to be x.
Then, according to the question we get,
$x\ +\ 3(0)\ +\ 3(-1)\ =\ 0$
$x=+3$
So the oxidation state of Cr will be 3.
Question 5.6(i) Using IUPAC norms write the formulas for the following:
(i) Tetrahydroxidozincate(II)
Answer :
Using the IUPAC rules:-
$\left [ Zn(OH)_4 \right ]^{2-}$
Question 5.6(ii) Using IUPAC norms write the formulas for the following:
(ii) Potassium tetrachloridopalladate(II)
Answer :
The required compound is:-
$K_2\left [ PdCl_4 \right ]$
Question 5.6(iii) Using IUPAC norms write the formulas for the following:
(iii) Diamminedichloridoplatinum(II)
Answer :
The required chemical formula of compound is :-
$\left [ Pt(NH_3)_2Cl_2 \right ]$
Question 5.6(iv) Using IUPAC norms write the formulas for the following:
(iv) Potassium tetracyanidonickelate(II)
Answer :
The required compound is:-
$K_2\left [ Ni(CN)_4 \right ]$
Question 5.6(v) Using IUPAC norms write the formulas for the following:
(v) Pentaamminenitrito-O-cobalt(III)
Answer :
The compound is:-
$\left [ Co(ONO)(NH_3)_5 \right ]^{2+}$
Question 5.6(vi) Using IUPAC norms write the formulas for the following:
(vi) Hexaamminecobalt(III) sulphate
Answer :
The required compound is:-
$\left [ Co(NH_3)_6 \right ](SO_4)_3$
Question 5.6(vii) Using IUPAC norms write the formulas for the following:
(vii) Potassium tri(oxalato)chromate(III)
Answer :
The required compound is:-
$K_3\left [ Cr(C_2O_4)_3 \right ]$
Question 5.6(viii) Using IUPAC norms write the formulas for the following:
(viii) Hexaammineplatinum(IV)
Answer :
The required compound is :- $\left [ Pt(NH_3)_6 \right ]^{4+}$
Question 5.6(ix) Using IUPAC norms write the formulas for the following:
(ix) Tetrabromidocuprate(II)
Answer :
The required compound is $\left [ Cu(Br)_4 \right ]^{2-}$
Question 5.6(x) Using IUPAC norms write the formulas for the following:
(x) Pentaamminenitrito-N-cobalt(III)
Answer :
The required compound is:-
$\left [ Co(NO_2)(NH_3)_5 \right ]^{2+}$
Question 5.7(i) Using IUPAC norms write the systematic names of the following:
Answer :
Using IUPAC norms, the name of the given compound is:-
Hexaamminecobalt(III) chloride
Question 5.7(ii) Using IUPAC norms write the systematic names of the following:
$(ii) \; [Pt(NH_{3})_{2}Cl(NH_{2}CH_{3})]Cl$
Answer :
According to the IUPAC norms :-
Diamminechlorido(methylamine) platinum(II) chloride
Question 5.7(iii) Using IUPAC norms write the systematic names of the following:
$(iii)\; [Ti(H_{2}O)_{6}]^{3+}$
Answer :
Using the nomenclature rules, the required name of the compound is:- Hexaquatitanium(III) ion
Question 5.7(iv) Using IUPAC norms write the systematic names of the following:
$(iv)\; [Co(NH_{3})_{4}Cl(NO_{2})]Cl$
Answer :
Using IUPAC norms, the name of the given compound is :-
Tetraamminichloridonitrito-N-Cobalt(III) chloride
Question 5.7(v) Using IUPAC norms write the systematic names of the following:
Answer :
The IUPAC name of the givne compound is :-
Hexaquamanganese(II) ion
Question: 5.7(vi) Using IUPAC norms write the systematic names of the following:
Answer :
According to the IUPAC norms, the systematic name of the given compound is:- Tetrachloridonickelate(II) ion
Question 5.7(vii) Using IUPAC norms write the systematic names of the following:
Answer :
The IUPAC name of the given compound is:- Hexaamminenickel(II) chloride
Question 5.7(viii) Using IUPAC norms write the systematic names of the following:
Answer :
The name of the given compound is :- Tris(ethane-1, 2-diammine) cobalt(III) ion
Question 5.7(ix) Using IUPAC norms write the systematic names of the following:
Answer :
The IUPAC name of the given compound is :- Tetracarbonylnickel(0)
Question 5.8 List various types of isomerism possible for coordination compounds, giving an example of each.
Answer :
Two main types of isomerism are known in case of coordination compounds which can be further divided into subgroups:-
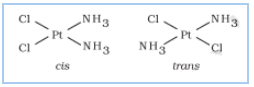
(a) Stereoisomerism:-
(i) Geometrical isomerism E.g
. 
(ii) Optical isomerism
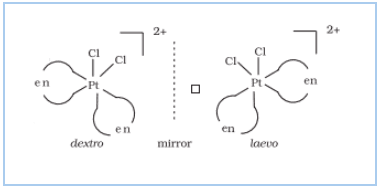
(b) Structural isomerism:-
(i) Linkage isomerism E.g $\left [ Co(NH_3)_5(NO_2) \right ]Cl_2$ and $\left [ Co(NH_3)_5(ONO) \right ]Cl_2$
(ii) Ionisation isomerism E.g $\left [ Co(NH_3)_5SO_4 \right ]Br$ and $\left [ Co(NH_3)_5Br\right ]SO_4$
(iii) Coordination isomerism E.g $\left [ Co(NH_3)_6 \right ]\left [ Cr(CN)_6 \right ]$ and $\left [ Cr(NH_3)_6 \right ]\left [ Co(CN)_6 \right ]$
(iv) Solvate isomerism E.g $\left [ Cr(H_2O)_6 \right ]Cl_3$ and $\left [ Cr(H_2O)_5Cl \right ]Cl_2.H_2O$
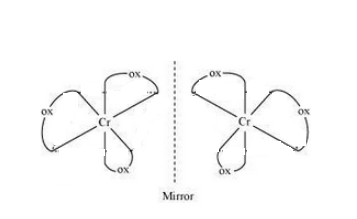
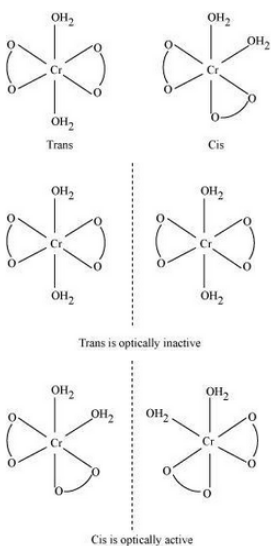
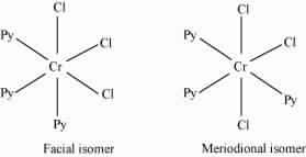
Question : 5.9(i) How many geometrical isomers are possible in the following coordination entities?
$(i)\; [Cr(C_{2}O_{4})_{3}]^{3-}$
Answer:
No geometrical isomers are possible since the given compound is a bidentate compound.
Question 5.9(ii) How many geometrical isomers are possible in the following coordination entities?
Answer :
The facial (fac) and meridional (mer) isomers are possible for the given compound.
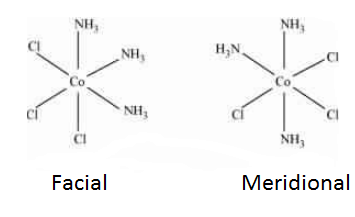
Question 5.10(i) Draw the structures of optical isomers of:
$(i); [Cr(C_{2}O_{4})_{3}]^{3-}$
Answer :
The optical isomers of the given compound are:-
Question 5.10(ii) Draw the structures of optical isomers of:
Answer :
The optical isomers of the given compound are given below :-
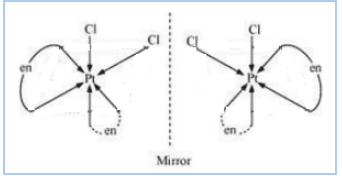
Question 5.10(iii) Draw the structures of optical isomers of:
$(iii)\; [Cr(NH_{3})_{2}Cl_{2}(en)]^{+}$
Answer :
The optical isomers of the given compound are:-
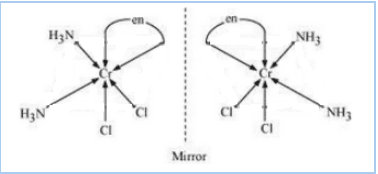
Question 5.11(i) Draw all the isomers (geometrical and optical) of:
Answer :
The configurational isomers are:-
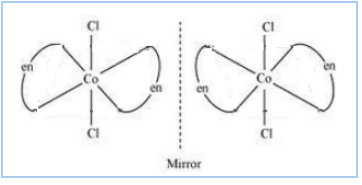
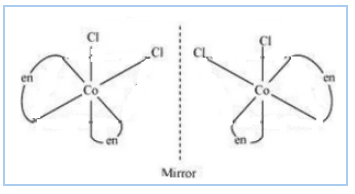
Question 5.11(ii) Draw all the isomers (geometrical and optical) of:
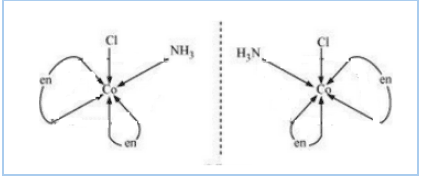
$(ii) [Co(NH_{3})Cl(en)_{2}]^{2+}$
Answer :
The isomers of the given compound are :-
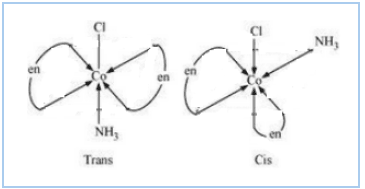
Question 5.11(iii) Draw all the isomers (geometrical and optical) of:
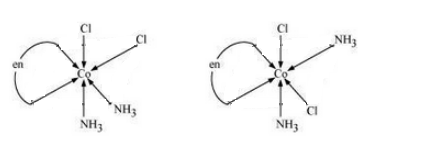
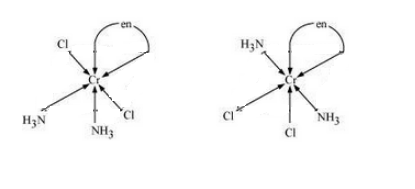
$(iii) [Co(NH_{3})_{2}Cl_{2}(en)]^{+}$
Answer :
The possible isomers of the given compound are as follows :-
Question 5.12 Write all the geometrical isomers of $[Pt(NH_{3})(Br)(Cl)(py)]$ and how many of these will exhibit optical isomers?
Answer :
The geometrical isomers of the compound are given below:-
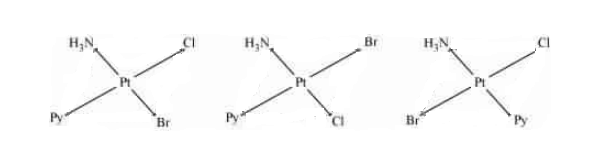
We know that the given compound has tetrahedral geometry, so it can be optically active only when it has unsymmetric chelating agents. Hence the given compound doesn't have any optically active isomer.
Question 5.13 Aqueous copper sulphate solution (blue in colour) gives:
(i) a green precipitate with aqueous potassium fluoride and
(ii) a bright green solution with aqueous potassium chloride. Explain these experimental results.
Answer :
We know that strong ligands can replace weak ligands from its solution.
(i) In this case F - ions can replace H 2 O from aqueous copper sulphate solution.
$\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_4\right]^{2+}+4 \mathrm{~F} \longrightarrow\left[\mathrm{Cu}(\mathrm{F})_4\right]^{2-}+4 \mathrm{H}_2 \mathrm{O}$
$\left[\mathrm{Cu}(\mathrm{F})_4\right]^{2-}$ is of green.
(ii) In this compound, Cl - being stronger ligand will replace H2O and give a bright green solution
$\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_4\right]^{2+}+4 \mathrm{Cl}^{-} \longrightarrow\left[\mathrm{CuCl}_4\right]^{2-}+4 \mathrm{H}_2 \mathrm{O}$
$\left[\mathrm{CuCl}_4\right]^{2-}$ is of bright green.
Answer :
When KCN is passed through an aqueous solution of copper sulphate, CN - being a strong ligand, will replace water and form $K_2\left [ Cu(CN)_4 \right ]$ .
It is known that in stable coordination compounds, the individual identity of each constituent is lost i.e., Cu +2 is not available freely.
Thus no precipitate of copper sulphide is obtained in the given conditions.
Question 5.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
Answer :
In the given compound oxidation state of Fe is +2. The electronic configuration of this compound is 3d6 .
Also CN - is a strong field ligand so it will cause the pairing of electrons.
Therefore the complex is diamagnetic and its geometry is octahedral.
Question 5.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
Answer :
(ii) In the given complex the oxidation state of Fe is +3. Its electronic configuration is d 5 .
Also, the F - ions are weak field ligands, therefore, the pairing of electrons will not occur.
Thus its geometry is octahedral and it is paramagnetic in nature.
Question 5.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
$(iii)[Co(C_{2}O_{4})_{3}]^{3-}$
Answer:
(iii) In the given compound, the oxidation state of Co is +3. Its electronic configuration thus becomes d 6 .
Also, oxalate is a weak field ligand therefore pairing of electrons will not occur.
Hence the complex is octahedral and paramgnetic in nature.
Question 5.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
Answer :
(iv) The oxidation state of Co in the given comound is +3. The electronic configuration of the compound is d6.
Also, F - is a weak field ligand, so no pairing of electrons will occur.
nbsp;
Hence the geometry of compound is octahedral and it is paramagnetic in nature.
Question 5.16 Draw figure to show the splitting of d orbitals in an octahedral crystal field.
Answer :
The splitting of d orbital is shown below:-
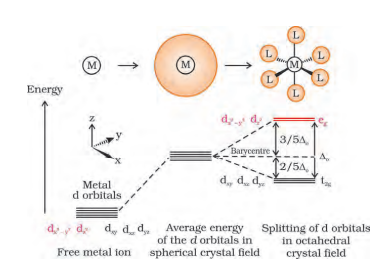
In this splitting dx2 y2 and dz2 experience a rise in energy and make the eg level, while dxy , dyz and dzx experience a fall in energy and generate the t2g level.
Question 5.17 What is the spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Answer :
The arrangement of ligands in the increasing order of their crystal-field splitting energy (CFSE) values is known as the spectrochemical series. $\begin{aligned} \mathrm{I}^{-}<\mathrm{Br}^{-}<\mathrm{SCN}^{-}<\mathrm{Cl}^{-}<\mathrm{S}^{2-}<\mathrm{F}^{-} & <\mathrm{OH}^{-}<\mathrm{C}_2 \mathrm{O}_4{ }^{2-}<\mathrm{H}_2 \mathrm{O}<\mathrm{NCS}^{-} \\ & <\text {edta }^{4-}<\mathrm{NH}_3<\text { en }<\mathrm{CN}^{-}<\mathrm{CO}\end{aligned}$
The ligands on the right side of the series, strong field ligands are present, whereas on the left-hand side, weak field ligands are present.
The strong field ligands are capable of splitting d orbitals to a higher extent as compared to weak field ligands.
Answer :
It is known that the degenerated d-orbitals split into two levels - $\mathrm{e}_{\mathrm{g}}$ and $\mathrm{t}_{2 \mathrm{~g}}$ . The splitting of the degenerate levels due to the presence of ligands is called the crystal-field splitting and the energy difference between the two levels ($\mathrm{e}_{\mathrm{g}}$ and $\mathrm{t}_{2 \mathrm{~g}}$ ) is called the crystal-field splitting energy (CFSE).
The CFSE is denoted by $\Delta_{\mathrm{o}}$ .
After splitting of orbitals, the filling of the electrons starts. After 1 electron has been filled in each of the three $\mathrm{t}_{2 \mathrm{~g}}$ orbitals, the fourth electron can enter the eg orbital ($t_{2 g}{ }^3 e_g{ }^1$ like electronic configuration) or the pairing of the electrons can take place in the $\mathrm{t}_{2 \mathrm{~g}}$ orbitals ($t_{2 g}{ }^4 e_g{ }^0$ like electronic configuration).
If the CFSE value or $\Delta_{\mathrm{o}}$ value of a ligand is less than the pairing energy (P), then the electrons enter into the eg orbital. And, if the $\Delta_{\mathrm{o}}$ value of a ligand is more than the pairing energy (P), then the electrons will enter the $\mathrm{t}_{2 \mathrm{~g}}$ orbital.
Question 5.19 $[Cr(NH_{3})_{6}]^{3+}$ is paramagnetic while $[Ni(CN)_{4}]^{2-}$ is diamagnetic. Explain why?
Answer :
In $[Cr(NH_{3})_{6}]^{3+}$ the oxidation state of the compound is +3. Its electronic configuration is d3 . Also, NH3 is a weak field ligand so the pairing of electrons will not occur.
So this compound is paramagnetic in nature.
In $[Ni(CN)_{4}]^{2-}$ the oxidation state of the Ni is +2. Its electronic configuration is d8. Also, CN - is a strong field ligand so the pairing of electrons will occur.

Hence, the above compound is diamagnetic in nature.
Question 5.20 A solution of $[Ni(H_{2}O)_{6}]^{2+}$ is green but a solution of $[Ni(CN)_{4}]^{2-}$ is colourless. Explain.
Answer :
In the case of $[Ni(CN)_{4}]^{2-}$ , we have CN - as a strong field ligand. So, the pairing of electrons will occur. The electronic configuration of Ni+2 is d6. Since all the electrons will be paired thus d-d electronic transition is not possible on this case. Whereas in case of $[Ni(H_{2}O)_{6}]^{2+}$ we have a weak field ligand (H2O). The pairing of electrons will not occur. Thus electrons from a lower state of energy can transit to a higher state of energy and thus will give some colour.
Question 5.21 $[Fe(CN)_{6}]^{4-}$ and $[Fe(H_{2}O)_{6}]^{2+}$ are of different colours in dilute solutions. Why?
Answer :
In both compounds, the oxidation state of Fe is +2. Also, in $[Fe(H_{2}O)_{6}]^{2+}$ we have weak field ligand whereas in $[Fe(CN)_{6}]^{4-}$ we have strong field ligand. So there is a difference in CFSE value in both compounds. As a result, the colour shown by both compounds is different.
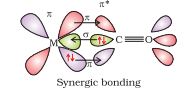
Question 5.22 Discuss the nature of bonding in metal carbonyls.
Answer :
The metal-carbon bond in metal carbonyls has both σ and π character. Basically the M–C σ bond is generated due to the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal. Whereas the M–C π bond is formed due to the donation of a pair of electrons from a filled d orbital of metal into the vacant/empty antibonding π* orbital of carbon monoxide. As a result, this metal-to-ligand bonding leads to a synergic effect which strengthens the bond between CO and the metal.
Question 5.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
$(i) K_{3}[Co(C_{2}O_{4})_{3}]$
Answer :
Coordination number $=6$.
We know,
The oxidation state is:
$
\begin{aligned}
& x-6=-3 \\
& x=+3
\end{aligned}
$
The d orbital occupation: $\mathrm{t}_{2 \mathrm{~g}}{ }^6 \mathrm{eg}^0$.
The oxidation state of Co is +3 and its oxidation number is 6. The d orbital occupation of the given central metal ion is t2g6 eg0.
Question 5.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
$(ii)cis-[CrCl _{2}(en)_{2}]Cl$
Answer :
Central metal ion : Cr.
Coordination number $=6$.
We know,
The oxidation state is:
$
\begin{aligned}
& x+2(0)+2(-1)=+1 \\
& x-2=-1 \\
& x=+3
\end{aligned}
$
The d orbital occupation: $\mathrm{t}_{2 \mathrm{~g}}{ }^3$.
The oxidation state of Cr in the given complex is +3. The coordination number of Cr is 6. The d orbital occupation for the central metal ion Cr3+ is t2g3.
Question 5.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
Answer :
Central metal : Co
Coordination number $=4$.
We know,
The oxidation state is:
$
\begin{aligned}
& x-4=-2 \\
& x=+2
\end{aligned}
$
The $d$ orbital occupation: $e_g{ }^4 t_{2 g}{ }^3$.
The oxidation state of Co in the given coordination compound is +2. Also, the coordination number of Co is 4. The d orbital occupation for the central metal ion Co 2+ is eg4 t2g3 .
Question 5.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
Answer :
Central metal ion: Mn .
Coordination number $=6$.
We know,
The oxidation state is :
$
\begin{aligned}
& x+0=2 \\
& x=+2
\end{aligned}
$
The d orbital occupation: $\mathrm{t}_{2 \mathrm{~g}}{ }^3 \mathrm{eg}^2$.
In the given complex compound the oxidation state of Mn is +2. Also, the coordination number of Mn is 6. The d orbital occupation for the central metal ion Mn+2 is t2g3 eg2.
$(i) K[Cr(H_{2}O)_{2}(C_{2}O_{4})_{2}].3H_{2}O$
Answer :
The IUPAC name of the given compound is Potassium diaquadioxalatochromate (III) trihydrate.
The oxidation state of Cr in this compound is +3.
The electronic configuration is 3d3.
The coordination number of this compound is 6.
The magnetic moment of a compound is given by :
$=\ \sqrt{n(n+2)}$ Here n is the number of the unpaired electrons.
or $=\ \sqrt{3(3+2)}\ =\ \sqrt{15}BM$
Stereochemistry:-
$(ii) [Co(NH_{3})_{5}Cl_{-}]Cl_{2}$
Answer :
The IUPAC name of the given compound is Pentaamminechloridocobalt(III) chloride.
The oxidation state of Co in this compound is +3.
The electronic configuration is d6.
The coordination number of this compound is 6.
The magnetic moment of a compound is given by :
$=\ \sqrt{n(n+2)}$ Here n is the number of the unpaired electrons.
or $=\ \sqrt{0(0+2)}\ =\ 0BM$
Stereochemistry:-
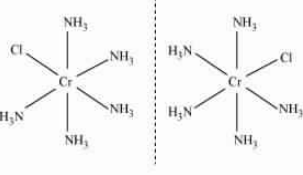
Answer :
The IUPAC name of the given compound is Trichloridotripyridinechromium (III).
The oxidation state of Cr in this compound is +3.
The electronic configuration is d3.
The coordination number of this compound is 6.
The magnetic moment of a compound is given by :
$=\ \sqrt{n(n+2)}$ Here n is the number of the unpaired electrons.
or $=\ \sqrt{3(3+2)}\ =\ \sqrt{15}BM$
Stereochemistry:-
Answer :
The IUPAC name of the given compound is Caesium tetrachloroferrate (III).
The oxidation state of Cs in this compound is +3.
The electronic configuration is d5.
The coordination number of this compound is 4.
The magnetic moment of a compound is given by :
$=\ \sqrt{n(n+2)}$ Here n is the number of the unpaired electrons.
or $=\ \sqrt{5(5+2)}\ =\ \sqrt{35}BM=5.92$
Stereochemistry:- Optically inactive.
Answer :
The IUPAC name of the given compound is Potassium hexacyanomanganate(II).
The oxidation state of Mn in this compound is +2.
The electronic configuration is d5.
The coordination number of this compound is 6.
The magnetic moment of a compound is given by :
$=\ \sqrt{n(n+2)}$ Here n is the number of the unpaired electrons.
or $\\=\ \sqrt{1(1+2)}\ =\ \sqrt{3}BM\\=1.72BM$
Stereochemistry:- Optically inactive.
Question 5.25 Explain the violet colour of the complex $[Ti(H_{2}O)_{6}]^{3+}$ on the basis of crystal field theory.
Answer :
In the ground state, Ti has 23 electrons with electronic configuration 3d3 4s2.
The oxidation state of Ti in the given compound is +3.
Hence, it will now have the configuration 3d2. Since it has 2 unpaired electrons and has the ability to undergo d-d transition, the given complex gives violet colour.
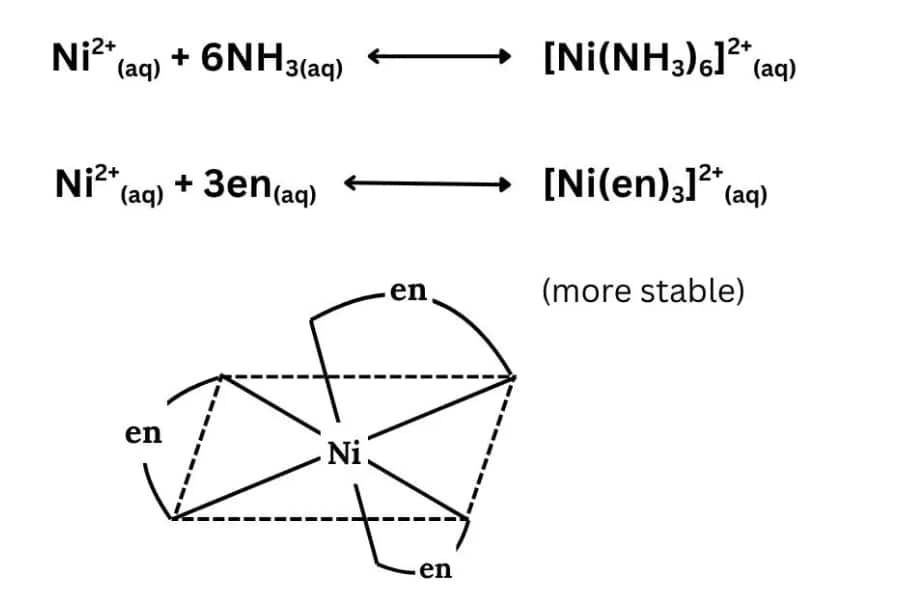
Question 5.26 What is meant by the chelate effect? Give an example.
Answer :
When a ligand is attached to the metal ion in such a manner that it forms a ring-like structure, then the metal-ligand bond is found to be more stable, i.e., complexes containing chelate rings are more stable than complexes without rings. The formation of such rings is known as the chelate effect.
Question 5.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(i) Biological systems
Answer :
Coordination compounds play a great role in biological systems. The pigment which is responsible for photosynthesis, chlorophyll, is a coordination compound of magnesium. Haemoglobin (which acts as oxygen carrier) the red pigment of blood is a coordination compound of iron. Vitamin B 12, and cyanocobalamine, the anti-pernicious anaemia factor, are few coordination compounds of cobalt which have biological importance.
Question 5.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(ii) medicinal chemistry
Answer :
The role of coordination compounds in the medicine industry is very significant such as the use of chelate therapy in medicinal chemistry. The excess of copper and iron is removed by the chelating ligands D–penicillamine and desferrioxamine B via the formation of coordination compounds. Nowadays, some coordination compounds of platinum (such as cis–platin and related compounds) effectively inhibit the growth of tumours.
Question 5.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(iii) analytical chemistry
Answer :
(iii) In analytical chemistry, the familiar colour reactions given by metal ions with a number of ligands, generally chelating ligands. The formation of coordination entities gives the basis for their detection and estimation by classical and instrumental methods of analysis.
Question 5.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(iv) extraction/metallurgy of metals
Answer :
In the metal extraction process of metals, like silver and gold, make use of complex formation. For example, gold combines with cyanide in the presence of oxygen and water to form the coordination entity [Au(CN)2] in aqueous solution, which can be further separated by addition of zinc.
Question 5.28 How many ions are produced from the complex $Co(NH_{3})_{6}Cl_{2}$ in solution?
(i) 6 (ii) 4 (iii) 3 (iv) 2
Answer :
Total of 3 ions will be produced.
2 of Cl- and one ion of [Co(NH3)6]+ will be produced.
Question 5.29 Amongst the following ions which one has the highest magnetic moment value?
$(i)[Cr(H_{2}O)_{6}]^{3+}$ $(ii)[Fe(H_{2}O)_{6}]^{2+}$ $(iii)[Zn(H_{2}O)_{6}]^{2+}$
Answer :
(i) No. of unpaired electrons in $[Cr(H_{2}O)_{6}]^{3+}$ is 3.
The magnetic moment is given by :
$=\ \sqrt{n(n+2)}$
or $=\ \sqrt{3(3+2)}\ =\ \sqrt{15}BM$
(ii) Similarly in $[Fe(H_{2}O)_{6}]^{2+}$ the number of unpaired electrons is 4.
So the magnetic moment :
$=\ \sqrt{4(4+2)}\ =\ \sqrt{24}BM$
(iii) In the case of $[Zn(H_{2}O)_{6}]^{2+}$ the number of unpaired electrons is 0. So its magnetic moment is also zero.
Thus $[Fe(H_{2}O)_{6}]^{2+}$ has the highest dipole moment among all.
Question 5.30 Amongst the following, the most stable complex is
Answer :
We know that due to the chelation effect stability of the chelating compound is more than the simple compound. Thus it is easy to notice that $[Fe(C_{2}O_{4})_{3}]^{3-}$ is most stable among all given compounds.
Question 5.31 What will be the correct order for the wavelengths of absorption in the visible region for the following:
$[Ni(NO_{2})_{6}]^{4-}, [Ni(NH_{3})_{6}]^{2+}, [Ni(H_{2}O)_{6}]^{2+} ?$
Answer :
The order of the wavelength of absorption will be decided from the order of their CFSE values.
The CFSE values increase in the order :- H2O < NH3 < NO2 -
Hence, the order of wavelengths of absorption in the visible region is : [Ni(H2O) 6 ] 2+ > [Ni(NH3)6]2+ > [Ni(NO2)6]4-
Class 12 Chemistry NCERT Chapter 5: Higher Order Thinking Skills (HOTS) Questions
These coordination compounds class 12 question answer are designed to challenge students analytical and application skills beyond basic textbook knowledge. NCERT Solutions for Class 12 encourage critical thinking and help students tackle complex problems confidently in exams.
Question 1. Given below are two statements :
Statement (I) : In octahedral complexes, when $\Delta_{\mathrm{o}}<\mathrm{P}$ high spin complexes are formed. When $\Delta_{\mathrm{o}}>\mathrm{P}$ low spin complexes are formed.
Statement (II) : In tetrahedral complexes because of $\Delta_t<\mathrm{P}$, low spin complexes are rarely formed.
In the light of the above statements, choose the most appropriate answer from the options given below :
1) Statement I is correct but Statement II is incorrect.
2) Both Statement I and Statement II are incorrect
3) Statement I is incorrect but Statement II is correct
4) Both Statement I and Statement II are correct
Answer:
In octahedral complex $(\mathrm{CN}=6)$
If $\Delta_0<$ P.E. , then high spin complexes are formed
If $\Delta_0>$ P.E. , then low spin complexes are formed
But in tetrahedral complex $(\mathrm{CN}=4)$
$\Delta_{\mathrm{t}}<$ P.E. , then mainly high spin complexes are formed and rarely low spin complexes are formed.
Hence, the correct answer is option (4).
Question 2. The number of paramagnetic complex among $\left[\mathrm{FeF}_6\right]^3,\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-},\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-},\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}$, $\left[\mathrm{MnCl}_6\right]^{3-}$ and $\left[\mathrm{CoF}_6\right]^{3-}$, which involved $\mathrm{d}^2 \mathrm{sp}^3$ hybridization is ____________
Answer:
The octahedral complexes with strong field ligands generally form inner orbital complexes.
Complex Nature Hybridisation
| $\left[\mathrm{FeF}_6\right]^{3-}$ | Paramagnetic | $\mathrm{sp}^3 \mathrm{~d}^2$ |
| $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$ | Paramagnetic | $\mathrm{d}^2 \mathrm{sp}^3$ |
| $\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$ | Paramagnetic | $\mathrm{d}^2 \mathrm{sp}^3$ |
| $\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}$ | Diamagnetic | $\mathrm{d}^2 \mathrm{sp}^3$ |
| $\left[\mathrm{MnCl}_6\right]^{3-}$ | Paramagnetic | $\mathrm{sp}^3 \mathrm{~d}^2$ |
| $\left[\mathrm{CoF}_6\right]^{3-}$ | Paramagnetic | $\mathrm{sp}^3 \mathrm{~d}^2$ |
Only $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$ and $\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-} \quad$ are paramagnetic and $\mathrm{d}^2 \mathrm{sp}^3$ hybridisation of metal.
Hence, the answer is 2.
Question 3. Given below are two statements :
Statement I: A homoleptic octahedral complex, formed using monodentate ligands, will not show stereoisomerism.
Statement II : cis- and trans- platin are heteroleptic complexes of Pd.
In light of the above statements, choose the correct answer from the options given below.
(1) Both statement I and Statement II are false.
(2) Statement I is false but Statement II is true.
(3) Both statement I and Statement II are true.
(4) Statement I is true but Statement II is false.
Answer:
Homolytic cleavage: Bond breaks evenly, each atom gets one electron, forming free radicals.
Heterolytic cleavage: Bond breaks unevenly, one atom gets both electrons, forming ions.
[Ma6] type complex will not show stereoisomerism, where $a$ is monodentate ligand
Cis and trans-platin are heterolytic complexes of $\mathrm{Pt}($ Platinum $)$. Formula is $\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]$
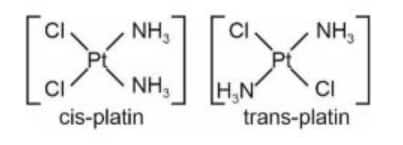
Hence, the correct answer is option (4).
Question 4. The number of species from the following that are involved in $\mathrm{sp}^3 \mathrm{~d}^2$ hybridization is
$\begin{aligned}
& {\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}, \mathrm{SF}_6,\left[\mathrm{CrF}_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}} \\
& \text { and }\left[\mathrm{MnCl}_6\right]^{3-}
\end{aligned}$
(1) 5
(2) 6
(3) 4
(4) 3
Answer:
Hence, the correct answer is option (4).
Question 5. The number of paramagnetic metal complex species among $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}, \quad\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}$, $\left[\mathrm{MnCl}_6\right]^{3-},\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$ and $\left[\mathrm{FeF}_6\right]^{3-}$ with same number of unpaired electrons is___________.
Answer:
Number of paramagnetic complexes with same number of unpaired electrons = 2 pairs (each pair has 2 complexes)
- 0 unpaired electrons- 2 complexes are
$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+},\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}$
Now,
$\left[\mathrm{MnCl}_6\right]^{3-}$
$\mathrm{Cl}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Mn}=+3$
$M n$ atomic number $=25 \rightarrow \mathrm{Mn}^{3+}=3 \mathrm{~d}^4$
$\mathrm{Cl}^{-}$is a weak-field ligand $\rightarrow$ high-spin
$3 d^4$ high-spin $\rightarrow 4$ unpaired electrons
$\rightarrow$ Paramagnetic, 4 unpaired
$\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$
$\mathrm{CN}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Mn}=+3$
$\mathrm{Mn}^{3+}=3 \mathrm{~d}^4$
$\mathrm{CN}^{-}=$strong field $\rightarrow$ low-spin
$3 \mathrm{~d}^4$ low-spin $\rightarrow 2$ unpaired electrons
$\rightarrow$ Paramagnetic, 2 unpaired
$\left[\mathrm{CoF}_6\right]^{3-}$
$\mathrm{F}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Co}=+3$
$\mathrm{Co}^{3+}=3 \mathrm{~d}^6$
$\mathrm{F}^{-}=$weak field $\rightarrow$ high-spin
$3 d^6$ high-spin $\rightarrow 4$ unpaired electrons
$\rightarrow$ Paramagnetic, 4 unpaired
$\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$
$\mathrm{CN}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Fe}=+3$
Fe atomic number $=26 \rightarrow \mathrm{Fe}^{3+}=3 \mathrm{~d}^5$
$\mathrm{CN}^{-}=$strong field $\rightarrow$ low-spin
$3 d^5$ low-spin $\rightarrow 1$ unpaired electron
$\rightarrow$ Paramagnetic, 1 unpaired
$\left[\mathrm{FeF}_6\right]^{3-}$
$\mathrm{F}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Fe}=+3$
$\mathrm{Fe}^{3+}=3 \mathrm{~d}^5$
$\mathrm{F}^{-}=$weak field $\rightarrow$ high-spin
$3 \mathrm{~d}^5$ high-spin $\rightarrow 5$ unpaired electrons $\rightarrow$ Paramagnetic, 5 unpaired
2 paramagnetic species have the same number of unpaired electrons. $\left[\mathrm{Mn}(\mathrm{Cl})_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-}$
No other pairs have the same number.
Hence, the answer is 2.
Question 6: Match the LIST-I with LIST-II
| LIST-I (Complex/Species) | LIST-II (Shape & magnetic moment) | ||
| A. | $\left[\mathrm{Ni}(\mathrm{CO})_4\right]$ | I. | Tetrahedral, 2.8 BM |
| B. | $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ | II. | Square planar, 0 BM |
| C. | $\left[\mathrm{NiCl}_4\right]^2$ | III. | Tetrahedral, 0 BM |
| D. | $\left[\mathrm{MnBr}_4\right]^{2-}$ | IV. | Tetrahedral, 5.9 BM |
Choose the correct answer from the options given below :
(1) A-III, B-IV, C-II, D-I
(2) A-I, B-II, C-III, D-IV
(3) A-III, B-II, C-I, D-IV
(4) A-IV, B-I, C-III, D-II
Answer:
A) $\mathrm{Ni}(\mathrm{CO})_4 \rightarrow \mathrm{Ni}(0) \Rightarrow \mathrm{sp}^3$, tetrahedral, 0 BM
$\left(3 d^{10}\right)$ (pairing)
B) $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-} \rightarrow \mathrm{Ni}^{2+} \Rightarrow \mathrm{dsp}^2$, square planar, 0 BM
$\left(3 \mathrm{~d}^8\right)$ (pairing)
C) $\left[\mathrm{NiCl}_4\right]^{2-} \rightarrow \mathrm{Ni}^{2^{+}}$(no pairing) $\Rightarrow \mathrm{sp}^3$, tetrahedral,
2 unpaired electrons therefore, $2.8 \text { BM }$
D) $\left[\mathrm{MnBr}_4\right]^{2^{-}} \Rightarrow \mathrm{Mn}^{2+} \Rightarrow 3 \mathrm{~d}^5$ (no pairing)
Hence, the correct answer is option (3).
Question 7: Which of the following statements is incorrect regarding the Crystal Field Theory?
(1) CFT is successful in explaining the color and magnetic properties of coordination compounds.
(2) CFT is successful in explaining why anionic ligands lying at the lower end of the spectrochemical series.
(3) CFT does not take into account the covalent character of bonding between the ligand and the central atom.
(4) Ligands are treated as point charges in case of anions or point dipoles in case of neutral molecules.
Answer:
This is a limitation of CFT as it can't explain any anionic ligands lying at the lower end of the spectrochemical series.
Hence, the correct answer is option (3).
Question 8: When the metal ion $\mathbf{C o}^{\mathbf{2 +}}$ in the complex $\left.\left[\mathrm{NH}_3\right)_2\right]$ is replaced by $\mathbf{R h}^{\mathbf{3 +}}$ to form $\left.\left[\mathrm{NH}_3\right)_2\right]^{+}$, which of the following properties are expected to change?
A. Hybridisation of the metal ion
B. Nature of magnetic behaviour
C. Possibility of geometrical isomerism
D. Type of bonding (inner orbital / outer orbital complex)
Choose the correct option:
(1) A, B
(2) B and C
(3) A, B and D only
(4) A, B, C and D
Answer:
- $\quad \mathrm{Co}^{2+}(3 \mathrm{~d})$ vs $\mathrm{Rh}^{3+}(4 \mathrm{~d}) \rightarrow$ pairing tendency increases
- Geometry, magnetic behaviour, hybridisation all may change
- Geometrical isomerism depends on coordination \& geometry
- Bonding nature differs for 3d vs 4d metals
Hence, the correct answer is option (4).
NCERT Solutions for Class 12
- NCERT Solutions for Class 12 Biology
- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 12 Physics
Approach to Solve Questions of Chapter 5 Coordination Compounds
Sometimes, problems related to class 12 chemistry chapter 5 coordination compounds solutions seem difficult, but once we understand the basic formulas, it becomes very easy to solve all the questions. We can follow the steps given below to solve the questions based on this chapter.
1. This is the basic yet crucial step. Some of the important concepts are listed below-
- Ligands and coordination number
- Nomenclature rules Bonding theories
- Isomerism
- Stability and applications of coordination compounds
2. Follow a stepwise strategy to solve the questions
A. Nomenclature questions
Identify the ligands, metal and their oxidation states and follow IUPAC rules.
B. Structure and formula
Determine oxidation number and coordination number and predict the geometry based on the hybridization. Use VBT or CFT to justify geometry and magnetic properties.
C. Bonding and Hybridization
Use Valence Bond Theory to determine inner or outer orbital complex and hybridization then apply Crystal Field Theory for splitting of d-orbitals in octahedral/tetrahedral fields and magnetic behavior (low spin vs high spin)
D. Magnetic Moment and Color
Apply formula to get the magnetic moment of the compound $\mu=\sqrt{n(n+2)} \mathrm{BM}$ where n = number of unpaired electrons.
Relate unpaired electrons to color and magnetism.
3. Go through the coordination compounds ncert solutions as they illustrate standard methods. Use these methods to solve the questions.
4. Attempt in-text questions after each concept and solve all the exercises. You can also prepare tables and charts to revise the concepts.
NCERT Books and NCERT Syllabus:
Topics of NCERT Syllabus Class 12 Chemistry Coordination Compounds
This section outlines all the important topics covered under the class 12 chemistry chapter 5 coordination compounds solutions. The topics given in this chapter are given below:
5.1 Werner's Theory
5.2 Definitions of Some Important Terms Pertaining to Coordination Compounds
5.3 Nomenclature of Coordination Compounds
5.4 Isomerism In Coordination Complexes
5.5 Bonding in Coordination Compounds
5.6 Bonding in Metal Carbonyls
5.7 Importance and Applications of Coordination Compounds
NCERT Exemplar Solutions
What Extra Should Students Study Beyond NCERT for JEE/NEET?
Along with the NCERT solutions for class 12 chemistry chapter 5 Coordination Compounds students preparing for JEE/NEET should explore advanced reference books and additional practice materials. The table below will help you classify the topics based on board exams and competitive exams.
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 5 Coordination Compounds
These class 12 chemistry coordination compound question answer help students understand the core principles of coordination chemistry in a structured way. Given below some points on key learnings of this chapter:
- Here students will learn about how to name coordination entities according to IUPAC rules.
- Basic theories of coordination and types of linkages are explained well in these solutions.
- These coordination compounds class 12 question answer help students to identify ligands, their denticity, and determine coordination numbers.
- These NCERT Solutions for Class 12 explain different types of isomerism such as structural and stereoisomerism.
- Valence Bond Theory and Crystal Field Theory are explained well in these solutions.
- Stability constants and factors affecting the stability of complexes are explained in detail using these solutions through a series of solved examples.
Importance of Class 12 Chemistry Chapter 5 Coordination Compounds Solutions
The coordination compounds class 12 question answer are important as it connects concepts from inorganic and physical chemistry. Given below some points on importance of these solutions:
- These solutions explain the structure, bonding, and properties of coordination compounds.
- These compounds play vital roles in medicines and metallurgy.
- The class 12 chemistry coordination compound question answer is important for both board exams and competitive exams like JEE and NEET.
- It provides a base for studying bioinorganic chemistry, organometallics, and coordination catalysis.
NCERT Solutions Class 12 Chemistry Chapterwise
Apart from the class 12 chemistry chapter 5 coordination compounds solutions , students can also explore the links provided below for solutions to other chapters.
Frequently Asked Questions (FAQs)
Class 12 Chemistry Chapter 5 NCERT solutions provide step by step answers to all the textbook questions from the Coordination Compounds chapter. They help students understand concepts such as nomenclature, isomerism, bonding theories, and the applications of coordination compounds.
Students can access Class 12 Chemistry Chapter 5 NCERT solutions from trusted educational websites, learning platforms, or reference books that provide solved NCERT questions.
The crystal field splitting energy is the energy difference between the d orbitals of a metal ion in a coordination compound, caused by the presence of ligands. It arises due to the interaction between the metal ion's d electrons and the ligand's electric field.
A unidentate ligand is a ligand that binds to a metal ion through a single donor atom, while a multidentate ligand is a ligand that binds to a metal ion through multiple donor atoms. For example, ammonia is a unidentate ligand, while ethylenediamine is a bidentate ligand.
The magnetic moment of a coordination compound is a measure of its magnetic properties, which depends on the number and arrangement of unpaired electrons in the compound. It is expressed in units of Bohr magneton (μB).
Coordination compounds are chemical compounds in which a central metal atom or ion is bonded to surrounding molecules or ions, called ligands, through coordinate covalent bonds. These compounds are also known as complex compounds, and they play an important role in biological systems, industrial processes, and analytical chemistry.
Studying coordination compounds is crucial because they play significant roles in various fields, including biochemistry, industrial processes, and materials science.
The coordination number of a central metal atom in a coordination compound is determined by the number of ligand atoms that are directly bonded to it. It reflects the geometric arrangement of the ligands around the metal and can vary depending on the size and electronic configuration of the metal and ligands involved.
Ligands can be identified by their ability to donate a pair of electrons to the central metal ion. They can be classified as monodentate, bidentate, or polydentate. Identifying ligands often involves recognizing the presence of lone pairs of electrons that can be shared with the metal ion.
In coordination compounds, a sigma bond is formed when a ligand donates a pair of electrons to the central metal ion, resulting in a strong bond. A pi bond, on the other hand, involves the overlap of p-orbitals and is typically found in the bonding of ligand systems with delocalized electrons, such as in some aromatic compounds. While sigma bonds are generally stronger and more stable, pi bonds can contribute to the overall bonding characteristics of the complex.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters


