NCERT Syllabus for Class 12 (2026) – Download Subject-Wise Updated Syllabus PDF
To make students' preparation more effective and focused, the National Council of Educational Research and Training (NCERT) has published the updated NCERT syllabus for Class 12 for the academic year 2025–26. It includes a well-structured syllabus for Science, Arts, and Commerce streams so that students can understand the important concepts of each subject clearly. The NCERT syllabus for Class 12 can be downloaded in PDF format from the official website, CBSE Academic. With a planned routine, students can make their studies and preparation go in the right direction throughout the year.
This Story also Contains
- NCERT Class 12 Syllabus 2025-26 PDF Download
- NCERT Syllabus for Class 12 English
- NCERT Syllabus for Class 12 Physics
- NCERT Syllabus for Class 12 Maths
- NCERT Syllabus for Class 12 Chemistry
- NCERT Syllabus for Class 12 Biology
- How to download the NCERT Syllabus for Class 12?
- How to Use the CBSE Class 12 Syllabus Effectively?
- Benefits of Knowing the NCERT Class 12 Syllabus
The NCERT class 12th syllabus provides subject-wise topics, unit-wise weightage, theory, and practical section division wherever required. Along with NCERT Books and Solutions, it provides a complete framework for students to build a strong understanding of concepts and do great in academics. The syllabus is essential not just for board exams but also for competitive exams like NEET, JEE, CUET, and other entrance exams. It is widely used by boards of education like CBSE and is aligned with national standards of education.
NCERT Class 12 Syllabus 2025-26 PDF Download
According to the recent CBSE instructions and curriculum framework, the NCERT Class 12 Syllabus 2025-26 has been revised, and the students will be tested in a systematic and concept-oriented manner. Each subject involves a list of chapters, learning outcomes and assessment patterns, which are provided in the syllabus. The NCERT Class 12 syllabus PDF is easily accessible by students; therefore, they can strategise their learning and increase their preparation for the board exams. This allows adhering to a systematic study strategy during the academic year.
| Subjects | Links |
| NCERT Class 12 English syllabus | Click here |
| NCERT Class 12 Maths syllabus | Click here |
| NCERT Class 12 Physics syllabus | Click here |
| NCERT Class 12 Chemistry syllabus | Click here |
| NCERT Class 12 Biology syllabus | Click here |
| NCERT Class 12 History syllabus | Click here |
| NCERT Class 12 Psychology syllabus | Click here |
| NCERT Class 12 Accountancy syllabus | Click here |
| NCERT Class 12 Political Science syllabus | Click here |
| NCERT Class 12 Geography syllabus | Click here |
| NCERT Class 12 Sociology syllabus | Click here |
| NCERT Class 12 Economics syllabus | Click here |
| NCERT Class 12 Business Studies syllabus | Click here |
NCERT Syllabus for Class 12 English
The NCERT Class 12 English syllabus (2025-26) is structured to improve students' reading, writing, and literary abilities, developing them for board exams as well as competitive examinations. Divided into the topics of Reading Comprehension, Writing Skills, and Literature, the syllabus deals with Flamingo and Vistas, providing a combination of prose, poetry, and short stories. To perform well, students must practice reading comprehension, become proficient in writing formats, and study literature thoroughly.
NCERT English Books for Class 12th | Chapters | |
| Flamingo - Prose | 1. The Last Lesson | |
| 2. Lost Spring | ||
3. Deep Water | ||
4. The Rattrap | ||
5. Indigo | ||
| 8. Going Places | ||
| Flamingo - Poetry | ||
| 5. Aunt Jennifer’s Tigers | ||
| Vistas - Supplementary Reader | ||
4. The Enemy | ||
| 6. Memories of Childhood | ||
| Kaleidoscope | SHORT STORIES – INTRODUCTION | |
2. Eveline | ||
4. Tomorrow | ||
| 5. One Centimetre | ||
POETRY – INTRODUCTION | ||
3. Poems by black | ||
4. Kubla Khan | ||
5. Trees | ||
| 8. Blood | ||
NON-FICTION – INTRODUCTION | ||
1. Freedom | ||
3. Film making | ||
| 6. On Science Fiction | ||
DRAMA - INTRODUCTION | ||
1. Chandalika | ||
| 2. Broken Images | ||
NCERT Syllabus for Class 12 Physics
The NCERT Physics Class 12 syllabus for 2025-26 is intended to build a strong foundation of concepts, which is essential for CBSE board exams, JEE, and NEET. The theory examination (70 marks) has important topics such as Electrostatics, Current Electricity, Magnetism, Optics, Modern Physics, and Electronics, whereas the practical exam (30 marks) has experimental practice with circuits, optics, and semiconductors. Balanced by theory principles and actual application, concept clarity, numerals-based problem solving, and experiential learning must be prioritised for the students.
NCERT Syllabus for Class 12 - Physics (Theory)
NCERT Syllabus for Class 12 - Physics (Practicals)
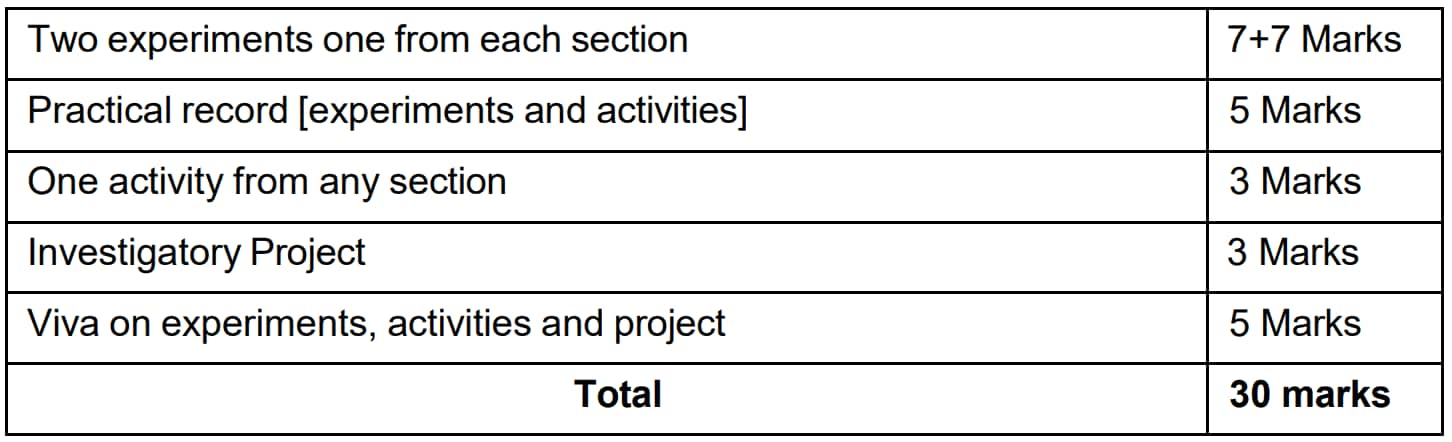
The practical section consists of 30 marks and the period of time totals to 3 hours. It includes two experiments (7+7 marks), a practical report, an activity, an investigatory project, and a viva. This applied test makes sure that students learn how to be competent in the aspects of experimentation, data recording, and conceptual knowledge.
NCERT Syllabus for Class 12 Maths
The mathematics syllabus of 12th NCERT is segregated into theory and internal assessment. The question paper of NCERT is 80 marks, wherein the internal assessment will be of 20 marks. The questions asked in the examination will be very short, short, and long answer types. Check the NCERT syllabus for class 12 for mathematics from the table below.
Unit Name | Chapter No. | Chapter Name | Topics |
Unit I: Relations and Functions | 1 | Relations and Functions | Introduction, Types of Relations, Types of Functions, Composition of Functions and Invertible Functions |
2 | Inverse Trigonometric Functions | Introduction, Basic Concepts, Properties of Inverse Trigonometric Functions | |
Unit II: Algebra | 3 | Matrices | Introduction, Matrix, Types of Matrices, Operations on Matrices, Transpose of a Matrix, Symmetric and Skew-Symmetric Matrices, Invertible Matrices |
4 | Determinants | Introduction, Determinant, Area of a Triangle, Minors and Cofactors, Adjoint and Inverse of a Matrix, Applications of Determinants and Matrices | |
Unit III: Calculus | 5 | Continuity and Discontinuity | Introduction, Continuity, Differentiability, Exponential and Logarithmic Functions, Logarithmic Differentiation, Derivatives of Functions in Parametric Forms, Second Order Derivative |
6 | Application of Derivatives | Introduction, Rate of Change of Quantities, Increasing and Decreasing Functions, Maxima and Minima | |
7 | Integrals | Introduction, Integration as an Inverse Process of Differentiation, Methods of Integration, Integrals of Some Particular Functions, Integration by Partial Functions, Integration by Parts, Definite Integral, Fundamental Theorem of Calculus, Evaluation of Definite Integrals by Substitution, Some Properties of Definite Integrals | |
8 | Application of Integrals | Introduction, Area under Simpler Curves | |
9 | Differential Equations | Introduction, Basic Concepts, General and Particular Solutions of a Differential Equation, Methods of Solving First-Order, First-Degree Differential Equations | |
Unit IV: Vectors and 3D Geometry | 10 | Vector Algebra | Introduction, Some Basic Concepts, Types of Vectors, Addition of Vectors, Multiplication of Vectors, Product of Two Vectors |
11 | Three-Dimensional Geometry | Introduction, Direction Cosines and Direction Ratios of a Line, Equation of a Line in Space, Angle between Two Lines, Shortest Distance between Two Lines | |
Unit V: Linear Programming | 12 | Linear Programming | Introduction, Linear Programming Problem and Its Mathematical Formulation |
Unit VI: Probability | 13 | Probability | Introduction, Conditional Probability, Multiplication Theorem on Probability, Independent Events, Bayes’ Theorem |
NCERT Syllabus for Class 12 Chemistry
The NCERT Class 12 Chemistry syllabus (2025-26) offers an overview of physical, organic, and inorganic chemistry, which is necessary for CBSE board exams, JEE, NEET, and other competitive exams. The theory paper (70 marks) includes major topics such as Solid State, Solutions, Electrochemistry, Chemical Kinetics, Coordination Compounds, Organic Reactions, and Biomolecules, whereas the practical exam (30 marks) encompasses titrations, analysis of salts, and organic compounds. Focused on conceptual clarity, reaction mechanisms, and numerical problems, students ought to practice repeatedly for good basics and success in examinations. The evaluation scheme for the chemistry practical examination is given in the second table.
NCERT Syllabus for Class 12 - Chemistry
| Chapter No. | Chapter Name | Important Topics |
| 1 | 1.2 Expressing the concentration of solutions 1.3 Solubility 1.4 Vapour pressure of liquid solutions 1.5 Ideal and Non-Ideal Solutions 1.6 Colligative properties and determination of molar mass 1.7 Abnormal molar mass | |
| 2 | 2.1 Electrochemical cells 2.2 Galvanic cells 2.3 Nernst equation 2.4 Conductance of Electrolytic Solutions 2.5 Electrolytic Cells and Electrolysis 2.6 Batteries 2.7 Fuel Cells 2.8 Corrosion | |
| 3 | 3.1 Rate of a chemical reaction 3.2 Factors Influencing the Rate of a Reaction 3.3 Integrated Rate Equations 3.4 Temperature Dependence of the Rate of a Reaction 3.5 Collision Theory of Chemical Reactions | |
| 4 | 4.1 Position in the Periodic Table 4.2 Electronic Configurations of the d-Block Elements 4.3 General Properties of the Transition Elements (d-Block) 4.4 Some Important Compounds of Transition Elements 4.5 The Lanthanoids 4.6 The Actinoids 4.7 Some Applications of d- and f-Block Elements | |
| 5 | 5.1 Werner’s theory of coordination compounds 5.2 Definitions of Some Important Terms On Coordination Compound 5.3 Nomenclature of Coordination Compounds 5.4 Isomerism in Coordination Compounds 5.5 Bonding in Coordination Compounds 5.6 Bonding in Metal Metal Carbonyls 5.7 Importance and Applications of of Coordination Compounds | |
| 6 | 6.1 Classification 6.2 Nomenclature 6.3 Nature of C-X Bond 6.4 Methods of preparation of haloalkanes 6.6 Physical Properties 6.7 Chemical Reactions 6.8 Polyhalogen Compounds | |
| 7 | 7.1 Classification 7.2 Nomenclature 7.3 Structures of Functional Groups 7.4 Alcohols and Phenols 7.5 Some Commercially Important Alcohols 7.6 Ethers | |
| 8 | 8.1 Nomenclature, the structure of the carbonyl group 8.2 Preparation of Aldehydes and Ketones 8.3 Physical Properties 8.5 Uses of Aldehydes and Ketones 8.6 Nomenclature and Structure of Carboxyl Group 8.7 Methods of Preparation of Carboxylic Acids 8.8 Physical Properties 8.9 Chemical Reactions 8.10 Uses of Carboxylic Acids | |
| 9 | 9.1 Structure of amines 9.2 Classification 9.3 Nomenclature 9.5 Physical Properties 9.6 Chemical Reactions 9.7 Method of Preparation of Diazoniun Salts 9.8 Physical Properties 9.9 Chemical Reactions 9.10 Importance of Diazonium Salts in Synthesis of Aromatic Compounds | |
| 10 | 10.1 Carbohydrates 10.2 Proteins 10.3 Enzymes 10.4 Vitamins 10.5 Nucleic Acids 10.6 Hormones |
NCERT Syllabus for Class 12 - Chemistry Practical
Practicals | Marks |
Volumetric Analysis | 8 |
Salt Analysis | 8 |
Content-based Experiment | 6 |
Project Work | 4 |
Class record and viva | 4 |
Total | 30 |
NCERT Syllabus for Class 12 Biology
NCERT Class 12 Biology syllabus is framed for enhancing the conceptual base of human physiology, genetics, biotechnology, and ecology to make it a must for CBSE board exams, NEET, and other medical entrance examinations. Theory paper (70 marks) includes important chapters such as Reproduction, Evolution, Human Health & Disease, Biotechnology, and Environmental Issues, whereas the practical exam (30 marks) consists of microscopy, slide preparation, plant and animal specimen work, and investigatory projects. Emphasising diagrams, scientific vocabulary, and application-oriented learning, students must revise regularly for improved retention and high marks.
NCERT Syllabus for Class 12 - Biology (Theory)
| Chapter | Chapter Name | Topic Name |
| Chapter 1 | Sexual Reproduction in Flowering Plants |
|
| Chapter 2 | Human Reproduction |
|
| Chapter 3 | Reproductive Health |
|
| Chapter 4 | Principles of Inheritance and Variation |
|
| Chapter 5 | Molecular Basis of Inheritance |
|
| Chapter 6 | Evolution |
|
| Chapter 7 | Human Health and Disease |
|
| Chapter 8 | Microbes in Human Welfare |
|
| Chapter 9 | Biotechnology: Principles and Processes |
|
| Chapter 10 | Biotechnology and its Applications |
|
| Chapter 11 | Organisms and Population |
|
| Chapter 12 | Ecosystem |
|
| Chapter 13 | Biodiversity and Conservation |
|
Also Read
NCERT Syllabus for Class 12 - Biology (Practicals)
Particulars | Marks |
One Major Experiment | 5 |
One Minor Experiment | 4 |
Slide Preparation | 5 |
Spotting | 7 |
Practical Record/Project record + Viva Voce | 4 |
| Investigatory Project and its Project Record + Viva Voce | 5 |
Total | 30 |
How to download the NCERT Syllabus for Class 12?
The candidates can download the NCERT Syllabus for Class 12 using the following steps given below.
Step 1: Click on the link https://cbseacademic.nic.in/curriculum_2026.html.
Step 2: A page will open on the screen. Click on "Senior Secondary Curriculum Class (XI-XII).
Step 3: Then click on 'Languages- (Group - L)/ Academic Disciplines (Group- A)' and click on the required subject.
Step 4: The NCERT Class 12 Syllabus will be displayed on the screen.
How to Use the CBSE Class 12 Syllabus Effectively?
CBSE Class 12 syllabus is an effective instrument that enables students to plan their preparation for board exams in an intelligent, systematic manner. It is a clear demonstration of the chapters, weightage, question pattern, and competence needed in each subject. When the syllabus is properly used, students will be able to study productively, they will not waste time on irrelevant topics, and they will improve their overall grades. With a properly organised strategy depending on the syllabus, there will be clarity, confidence and improved outcomes in the board exams.
- Read the whole syllabus to get the clue of all chapters, topics, and subtopics. This will give you a clear idea of what will be required in the exam, and there will be no need to study irrelevant material. It is also important to go through the syllabus closely and identify high-weightage units early.
- Break down all the subjects and topics to study to avoid being bored. Shorter learning blocks enhance retention and ensure steady progress. It also assists you in the process of having a realistic daily or weekly study schedule.
- Basing your study on the syllabus, assign each unit a specific number of days or weeks. This guarantees that you will address all the subjects in an orderly fashion and will not be under last-minute stress. A timetable based on syllabuses makes revision more organised and predictable as well.
- Spend more time on those chapters that have greater marks as per the official weightage distribution. This will make you more efficient as you prepare smartly and get maximum marks. The weightage analysis is also useful in the process of planning the revision cycles.
- To keep yourself motivated and organised, tick off the topics that you have completed. This assists you in pictorially perceiving your readiness and discovering areas that require additional preparation. Exam anxiety is also reduced with a progress-tracking habit.
- Check every subject in your NCERT textbook to be sure that you are studying what is needed. This will eliminate time wasted on getting irrelevant or old information. It also enhances the conceptual accuracy that is given attention by CBSE.
- Attempt past papers and sample papers through comparison with the existing syllabus. This will assist you in knowing the question pattern, the difficulty level and the critical areas. It is also possible to examine the areas that are regularly included in examinations.
- The syllabus should be used as a checklist in the revision to be sure that no topic is omitted. Organise your revision unit-by-unit to cover all. The revision with a syllabus is useful in long-term memorisation and increased accuracy in exams.
- There are a lot of students who study excessive material and hence become confused. The syllabus provides clear lines and helps you in studying what is important. This spares time and puts your preparation time on scoring areas.
Benefits of Knowing the NCERT Class 12 Syllabus
The NCERT Class 12 syllabus is important to students who plan to prepare well for board exams and competition exams such as JEE, NEET, CUET, and others. The syllabus gives a clear formula of all chapters, topics and weightage, and this will assist students to plan their studies strategically. The structured roadmap will allow students to spend time more efficiently, work on the essential concepts and improve the results during the examinations. It also makes the students follow the updated academic trend of CBSE in the year 2025-26.
Students understand what topics they need to study in Class 12.
It helps them plan their studies properly and stay organised.
They can manage time better and finish the syllabus before exams.
The board exam paper is made from the syllabus, so it's important to follow it.
It helps students keep a check on what they’ve completed and what’s left.
NCERT Books for class 12 all Subjects
Frequently Asked Questions (FAQs)
There are 13 chapters in Class 12 Maths as per the latest NCERT textbook, grouped under six units:
Relations and Functions
Algebra
Calculus
Vectors and 3D Geometry
Linear Programming
Probability
Yes, the English syllabus is common across all streams. Students choose between:
English Core (Code 301) – most common
English Elective (Code 001) – more in-depth and literature-focused
Yes, the NCERT syllabus is followed by all CBSE-affiliated schools, and it forms the official basis for the CBSE Class 12 board exams.
Yes, CBSE board exams are based entirely on the NCERT syllabus. The questions are usually either directly from or inspired by NCERT content.
Yes, NCERT books are more than enough for board exams. However, students aiming for competitive exams like JEE or NEET may need reference books for deeper understanding and practice.
You can download the official and updated syllabus from:
NCERT official website
careers360 platform
The NCERT Class 12 syllabus includes core subjects and stream-specific subjects, such as:
Science: Physics, Chemistry, Biology, Mathematics, Computer Science
Commerce: Accountancy, Business Studies, Economics, Mathematics
Arts/Humanities: History, Geography, Political Science, Sociology, Psychology
Languages: English Core, English Elective, Hindi Core, Hindi Elective, and other regional/foreign languages
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
