This chapter covers important concepts related to electrochemistry ncert solutions, including their types, properties, and concentration terms. Topics covered in this chapter are given below:
NCERT Solutions for Class 12 Chemistry Chapter 2 - Electrochemistry
Do you know how the batteries work, why some metals conduct electricity better than others and how corrosion damages iron? The answer to all these questions lies in class 12 chemistry chapter 2 electrochemistry. This chapter explains the connection between chemical energy and electrical energy. It discusses how chemical reactions can produce electrical energy, as seen in batteries, and how electrical energy can initiate non spontaneous chemical reactions, such as in electrolysis.
The Central Board of Secondary Education (CBSE) will release the answer key soon for CBSE Class 12 Psychology paper. Students will be able to use it to cross-check their answers and estimate scores before the official results are declared.
This Story also Contains
- NCERT Solutions for Class 12 Chemistry Chapter 2: Download PDF
- NCERT Solutions Of Class 12 Chemistry Chapter 2 (Intext Questions 2.1 to 2.15)
- NCERT Solutions for Class 12 (Exercise Questions)
- Class 12 Chemistry NCERT Chapter 2: Higher Order Thinking Skill (HOTS) Questions
- Approach to Solve Questions of Chapter 2 Class 12 Chemistry
- Topics of NCERT Class 12 Chemistry Chapter 2
- What Extra Should Students Study Beyond NCERT for JEE?
- NCERT Class 12 Chemistry Chapter 2 Electrochemistry Formulas
- What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 2 Electrochemistry
- NCERT Solutions for Class 12 Chemistry

NCERT solutions for class 12 chemistry are designed by our subject experts to offer a systematic and structured approach to solve questions and help students to develop a clear understanding of concepts through the series of solved questions and conceptual explanations. Students can refer to these NCERT Solutions to strengthen their concept and problem solving ability. In this article, we have included Higher Order Thinking Skills questions that helps students gain a clearer understanding of how to learn the chapter effectively.
NCERT Solutions for Class 12 Chemistry Chapter 2: Download PDF
Students can download the class 12 chemistry chapter 2 electrochemistry solutions pdf for free. These solutions are designed to help you understand the fundamental concepts and solve textbook questions with ease. These solutions of NCERT cover in text questions, exercises, and important numerical problems for thorough practice.
Also Read,
NCERT Solutions Of Class 12 Chemistry Chapter 2 (Intext Questions 2.1 to 2.15)
Here are detailed and accurate class 12 chemistry chapter 2 electrochemistry question answer. These solutions will help in better concept clarity and exam preparation. They are designed to simplify complex topics and assist in exam preparation effectively.
Page 36
Question 2.1 How would you determine the standard electrode potential of the system $Mg^{2+} | Mg$ ?
Answer:
To determine the standard electrode potential of the given system, we need to use a hydrogen electrode. In the setup, we shall put a hydrogen electrode as the cathode and Mg | MgSO4 as the anode.
$\mathrm{Mg}\left|\mathrm{Mg}^{2+}(\mathrm{aq}, 1 \mathrm{M})\right|\left|\mathrm{H}^{+}(\mathrm{aq}, 1 \mathrm{M})\right| \mathrm{H}_2(\mathrm{~g}, \mathrm{I}$ bar $), \mathrm{Pt}_{(\mathrm{s})}$
Now we will measure the emf of the cell. This emf will be the standard electrode potential of the magnesium electrode.
E°cell = E° right – E°left
E°left =0 ( The standard hydrogen electrode is always zero)
Hence
$E^{o}cell = E^{o} Mg|Mg^{2+}$
Question 2.2 Can you store copper sulphate solutions in a zinc pot?
Answer:
The standard electrode potential of Zinc is - 0.76 whereas that of Copper is 0.34. So Zinc will reduce copper into the lower state.
It is known that zinc is more reactive than copper. Thus if we will store copper sulphate solution in zinc pot then zinc will displace copper from its solution.
The following reaction will take place:-
$Zn \:+\:CuSO_{4}\rightarrow ZnSO_{4}\:+\:Cu$
Answer:
The oxidising strength of elements increases as the standard electrode potential increases.
So all the elements having greater standard potential than iron can oxidise it to a higher state.
Few such elements are $\mathrm{F}_2, \mathrm{Cl}_2, \mathrm{Br}_2, \mathrm{Ag}^{+1}$ etc.
Page 41
Question 2.4 Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Answer:
It is given that pH of the solution is 10,i.e., the hydrogen ion concentration in the solution is 10 -10 M.
$\mathrm{H}^{+}+\mathrm{e}^{-} \longrightarrow \frac{1}{2} \mathrm{H}_2$
By Nernst equation we have :-
$E_{Cell} = E_{cell}^{\circ}\ - \frac{RT}{2F}ln \frac{1}{\left [ H^+ \right ]}$
So, $= 0 - \frac{0.0591}{1}log \frac{1}{\left [ 10^{-10} \right ]}$
or $= -\ 0.591\ V$
So the required potential is - 0.591 V.
Answer:
Here we can directly apply the Nernst equation :-
$E_{Cell} = E_{cell}^{\circ}\ - \frac{0.0591}{n}log \frac{[Ni^{+2}]}{\left [ Ag^+ \right ]^2}$
Putting the value in this equation :-
$= 1.05\ - \frac{0.0591}{2}log \frac{0.160}{(0.002)^2}$
$= 1.05\ - 0.02955\ log (4\times10^4)$
$= 0.914\ V$
Hence the required potential is 0.914 V.
Answer:
For finding Gibbs free energy we know the relation :-
$\Delta G_r^{\circ} = -\ nFE_{cell}^{\circ}$
$= -\ 2\times96487\times 0.236$
$= -\ 45541.864\ J\ mol^{-1}$
$= -\ 45.54\ KJ\ mol^{-1}$
Now, for equilibrium constant we will use :-
$\Delta G_r^{\circ} = -2.303\ RTlog\ K_c$
So, $logK_c = -\frac{-45.54\times10^3}{2.303\times8.314\times298}$
$logK_c = 7.981$
$K_c = 9.57\times10^7$
Page 51
Question 2.7 Why does the conductivity of a solution decrease with dilution?
Answer:
The conductivity of a solution depends upon the number of ions and the distance between them. In the process of dilution, we don't increase the number of ions in the solution instead we increase the distance between them. So the conductivity of the solution decreases due to dilution.
Question 2.8 Suggest a way to determine the $\Lambda ^{0}_{m}$ value of water.
Answer:
We know :
$\Lambda _m=\Lambda _m^{\circ} - A c^{\frac{1}{2}}$
If we draw a straight line between $\Lambda _m$ and $\sqrt c$ , its slope will be -A and the intercept on the y-axis will be $\Lambda _m^{\circ}$ .
In this way, we can obtain the value of limiting molar conductivity.
Answer:
We know that :-
$\Lambda _m = \lambda^{\circ}(H^+) + \lambda^{\circ}(HCOO^-)$
$= 349.6 +54.6$
$= 404.2\ Scm^2\ mol^{-1}$
For degree of dissociation, we have :-
$\alpha = \frac{\Lambda _m(HCOOH)}{\Lambda ^{\circ}(HCOOH)}$
or $\alpha = \frac{46.1}{404.2} = 0.114$
For dissociation constant, we have :-
$K_a = \frac{c\alpha ^2}{1-\alpha }$
or $K_a = \frac{0.025\times(0.114)^2}{1-0.114 }$
or $= 3.67\times 10^{-4}\ mol\ L^{-1}$
Page no 54
Question 2.10 If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Answer:
Firstly we will find total charge flown through the wire then we will calculate number of electrons.
We are given :- I = 0.5 A, Time = 2 hours = 7200 seconds.
We have, Q = I.t
= (0.5)7200 = 3600 C.
Now we will convert charge into number of electrons.
We know that $96487\ C = 6.023\times10^{23}\ No.\ of\ electrons$
So toal number of electrons :
$=\frac{3600}{96487}\times 6.023\times10^{23}$
or $=2.25\times 10^{22}$ no. of electrons will flow through wire.
Question 2.11 Suggest a list of metals that are extracted electrolytically.
Answer:
Metals like Na, Mg, Al, etc. are produced on a large scale by electrochemical reduction of their respective cations or by the process electrolysis because there are no suitable reducing agents available for this purpose.
Answer:
It is clear from the given reaction that reduction of 1 mol of $\mathrm{Cr}_2 \mathrm{O}_7^{2-}$ will be
= 6 F (as 6 electrons are required to balance the reaction; Charge required = nF)
$= 6\times96500$
$= 579000\ C$
Thus 578922 C charge is required for reduction of 1 mol of $\mathrm{Cr}_2 \mathrm{O}_7^{2-}$.
Page no 58
Answer:
The lead storage battery can be recharged by reversing the direction of current passing through it.
For recharging $\mathrm{PbSO}_4$ is converted into Pb at the anode and into $\mathrm{PbO}_2$ at the cathode.
The chemical reactions are as follows

Question 2.14 Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Answer:
The two materials are methane and methanol that can be used as fuels in fuel cells.
Question 2.15 Explain how rusting of iron is envisaged as setting up of a electrochemical cell.
Answer:
The chemistry of corrosion is quite complex but it can be understood by considering it as an electrochemical phenomenon. Consider a particular spot on an object where corrosion takes place. At here oxidation takes place and this spot behaves as the anode. The released electrons at anodic spot go through the metal and go to another spot on the metal and reduction of oxygen takes place in the presence of H+. This spot behaves as a cathode with the reaction. In this way this analogy is possible.
NCERT Solutions for Class 12 (Exercise Questions)
Here are detailed and accurate NCERT solutions for exercise questions. These class 12 chemistry chapter 2 electrochemistry solutions offer detailed answers to all exercise questions enabling students to practice effectively and strengthen their understanding of important concepts.
Answer:
The order in which metals displace each other from the solution of their salts can be given with the help of their standard electrode potential. Since magnesium has the least standard electrode potential so it is the most strong reducing agent. So the required order we get is:-
$M g>A l>Z n>F e>C u$
Question 2.2 Given the standard electrode potentials,
$K^{+}/K=-2.93V , Ag^{+}/Ag=0.80V,$
$Mg^{2+}/Mg=-2.37V, Cr^{3+}/Cr =-0.74 V$
Arrange these metals in their increasing order of reducing power.
Answer:
Elements with reducing power or reducing agents have least/minimum standard electrode potential i.e., reducing power increases with a decrease in standard electrode potential. So the result obtained is:-
K > Mg > Cr > Hg > Ag
Question 2.3 Depict the galvanic cell in which the reaction
$Zn(s)+2Ag^{+}(aq)\rightarrow Zn^{2+}(aq) +2Ag(s))$ takes place. Further show
(i) Which of the electrode is negatively charged?
Answer:
The galvanic cell of the given reaction is depicted below:-
$\mathrm{Zn}(\mathrm{s})\left|\mathrm{Zn}^{+2}(\mathrm{aq}) \| \mathrm{Ag}^{+}(\mathrm{aq})\right| \mathrm{Ag}(\mathrm{s})$
Clearly Zn electrode is negatively charged.
Question 2.3 Depict the galvanic cell in which the reaction
$Zn(s)+2Ag^{+}(aq)\rightarrow Zn^{2+}(aq)+2Ag(s)$ takes place.
(ii) The carriers of the current in the cell.
Answer:
The carriers of current in the cell are ions. and current flows from silver to zinc in the external circuit.
Question 2.3 Depict the galvanic cell in which the reaction $Zn(s)+2Ag^{2+}(aq)\rightarrow Zn^{2+}(aq)+2Ag(s)$ takes place.
(iii) Individual reaction at each electrode.
Answer:
The reactions taking place at both cathode and anode are shown below:-
(i) Cathode reaction:-
$Ag^+_{(aq)} + e^- \rightarrow Ag_{(s)}$
(ii) Anode reaction :-
$Zn_{(s)}\ \rightarrow Zn^{2+}_{(aq)}\ +\ 2 e^-$
Question 2.4 Calculate the standard cell potentials of galvanic cell in which the following reactions take place:
(i) $2Cr(s)+3Cd^{2+}(aq)\rightarrow 2Cr^{3+}(aq)+3Cd$
Calculate the $\Delta _{r}G^{0}$ and equilibrium constant of the reactions.
Answer:
The galvanic cell of the given reaction is shown below:-
$\mathrm{Cr}_{(s)}\left|\mathrm{Cr}_{(\mathrm{aq})}^{3+} \| \mathrm{Cd}_{(\mathrm{aq})}^{2+}\right| \mathrm{Cd}_{(s)}$
The standard electrode potential of Cr and Cd can be found in table of standard electrode potential.
So, we get :
$E^{\circ} = E^{\circ}_R\ -\ E^{\circ} _L$
$= -0.40\ -\ (-0.74)$
$=\ 0.34\ V$
Now
$\Delta G_r^{\circ}\ = -\ nFE_{cell}^{\circ}$
Putting values :
$\Delta G_r^{\circ}\ = -\ 6\times96487\times 0.34$
$= -196.83\ KJ\ mol^{-1}$
Now for finding equlilibrium constant we have :
$log\ k = \frac{-\Delta G _r^{\circ}}{2.303\times R\times T}$
or $log\ k = 34.496$
or $K = 3.13\times10^34$
Question 2.4 Calculate the standard cell potentials of galvanic cell in which the following reactions take place:
(ii). $Fe^{2+}(aq)+Ag^{+}(aq)\rightarrow Fe^{3+}(aq)+Ag(s)$
Calculate the $\Delta _{r}G^{e}$ and equilibrium constant of the reactions.
Answer:
The galvanic cell of the given reaction is shown below :-
$\mathrm{Fe}_{(a q)}^{2+}\left|\mathrm{Fe}_{(a q)}^{3+}\right|\left|\mathrm{Ag}_{(a q)}^{+}\right| \mathrm{Ag}_{(s)}$
We can know about the electrode potential of Fe and Ag with the help of table of standard electrode potential.
We have : $E_{(cell)}^{\circ} = E_{R}^{\circ} - E_{L}^{\circ}$
or $= 0.80 - 0.77$
or $= 0.03\ V$
Now consider : $\Delta G_r^{\circ} = -\ nFE_{(cell)}^{\circ}$
or $= -1\times96487\times0.03$
$= -2.89\ KJ\ mol^{-1}$
Now for equilibrium constant :
$log\ K =\ -\frac{\Delta G_r^{\circ}}{2.303\times RT}$
or $=\ -\frac{-2894.61}{2.303\times 8.314\times298}$
or $=\ 0.5073$
Thus $k\ \approx \ 3.2$
Question 2.5 Write the Nernst equation and emf of the following cells at 298 K:
(i) $Mg(s)| Mg^{2+}(0.001M)|| Cu^{2+}(0.000.1M)|Cu(s)$
Answer:
The nernst equation gives :
$E_{Cell} = E_{cell}^{\circ}\ - \frac{0.059}{n}log \frac{[Mg^{2+}]}{\left [ Cu^{2+} \right ]}$
This gives,
$= {0.34 - (-2.36)} - \frac{0.059}{2}log \frac{0.001}{ 0.0001}$
$= 2.7 - 0.02955$
$= 2.67\ V$
So the emf of the cell is 2.67 V.
Question 2.5 Write the Nernst equation and emf of the following cells at 298 K:
(ii) $Fe(s)|Fe^{2+}(0.001M)||H^{+}(1M)|H_{2}(g)(1 bar)|Pt(s)$
Answer:
The nernst equation for this gives :
$E_{Cell} = E_{cell}^{\circ}\ - \frac{0.0591}{n}log \frac{[Fe^{+2}]}{\left [ H^+ \right ]^2}$
This gives : $= 0 - (-0.44)- \frac{0.0591}{2}log \frac{0.001}{1^2}$
or $= 0.44 - 0.02955(-3) = 0.53\ V$
Thus the emf of the given galvanic cell is 0.53 V.
Question 2.5 Write the Nernst equation and emf of the following cells at 298 K:
(iii) $Sn(s)|Sn^{2+}(0.050M)||H^{+}(0.020M)|H_{2}(g)(1 bar)Pt(s)$
Answer:
The nernst equation for this reaction gives :-
$E_{Cell} = E_{cell}^{\circ}\ - \frac{0.0591}{n}log \frac{[Sn^{+2}]}{\left [ H^+ \right ]^2}$
Now for emf, just put all the values.
$E_{Cell} =0 - (-0.14) - \frac{0.0591}{2}log \frac{0.050}{0.020^2}$
or $= 0.14 - 0.0295 \times log125$
or $= 0.14 - 0.062 = 0.078\ V$
Thus emf of the cell is 0.078 V.
Question 2.5 Write the Nernst equation and emf of the following cells at 298 K:
(iv) $Pt(s)|Br^{-}(0.010M)|Br_{2}(1)||H^{+}(0.030 M)| H_{2}(g)(I bar)Pt(s)$
Answer:
The Nernst equation of the given reaction gives :
$E_{Cell} = E_{cell}^{\circ}\ - \frac{0.0591}{n}log \frac{1}{\left[Br^-]^2 [ H^+ \right ]^2}$
or $=(0-1.09)\ - \frac{0.0591}{2}\ log \frac{1}{(0.010)^2 (0.030)^2}$
or $=-1.09\ - 0.02955\times\ log(1.11\times10^7)$
or $=-1.09\ - 0.208 =\ -1.298\ V$
So the required emf of the cell is -1.298 V.
Question 2.6 In the button cells widely used in watches and other devices the following reaction takes place:
$Zn (s)+ Ag_{2}O(s)+H_{2}O(l)\rightarrow Zn^{2+}(aq)+2Ag(s)+2OH^{-}(aq)$
Determine $\Delta_{r}G^{e}$ and $E^{e}$ for the reaction.
Answer:
The given reaction is obtained from :-
$\mathrm{Zn}_{(s)} \longrightarrow \mathrm{Zn}_{(a q)}^{2+}+2 \mathrm{e}^{-} ; E^{\ominus}=0.76 \mathrm{~V}$
$\mathrm{Ag}_2 \mathrm{O}_{(s)}+\mathrm{H}_2 \mathrm{O}_{(t)}+2 \mathrm{e}^{-} \longrightarrow 2 \mathrm{Ag}_{(s)}+2 \mathrm{OH}_{(a q)}^{-} ; E^{\ominus}=0.344 \mathrm{~V}$
So the E o cell can be obtained directly.
$E_{cell}^{\circ} = 0.76 - (-0.344) = 1.104\ V$
Now for free energy calculation, we have :-
$\Delta G_r^{\circ} = -nFE^{\circ}_{cell}$
or $= -2\times96487\times1.04$
or $= - 213043.29\ J$
or $= - 213.04\ KJ$
Question 2.7 Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Answer:
Conductivity(k) or specific conductance of a solution is defined as the inverse of resistivity.
Mathematically, it can be written as:-
$G = \kappa \frac{A}{L}$
In the above equation is $\kappa$ the conductivity of a solution. Thus the definition of conductivity becomes as the conductance of a substance which is 1 cm long and has 1 sq. cm of cross-sectional area.
With dilution conductivity of a solution decreases due to an increase in distance between ions.
Molar conductivity: - It is defined as the conductivity of a solution per unit concentration
i.e., $\Lambda _M\ =\ \frac{\kappa }{C}$
It is clear from the above mathematical expression of the molar conductivity that, if we dilute the solution or decrease its concentration then molar conductivity increases. This is because, on dilution of a solution, a decrease in is $\kappa$ more than compensated by the increase in its volume.
Question 2.8 The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm-1. Calculate its molar conductivity.
Answer:
We know that the molar conductivity of a solution is defined as:-
$\Lambda _M\ = \frac{\kappa }{C}$
Putting the value of conductivity and concentration in the above equation:-
$\Lambda _M\ = \frac{0.0248\times1000 }{0.20} = 124\ Scm^2\ mol^{-1}$
Answer:
We are given with conductivity of cell $\kappa =$ $0.146\times10^{-3}\ S cm^{-1}$ and resistance R = 1500 $\Omega$ .
Also, Cell constant = $\kappa\times R$
or = $0.146\times 10^{-3}\times 1500$
or = $0.219\ cm^{-1}$
Question 2.10 The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
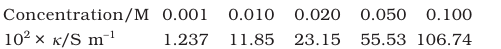
Calculate $\Lambda_m$ for all concentrations and draw a plot between $\Lambda_m$ and $\mathrm{c}^{1 / 2}$. Find the value of $\Lambda_m^0$.
Answer:
Given,
$
\kappa=1.237 \times 10^{-2} \mathrm{~S} \mathrm{~m}^{-1}, c=0.001 \mathrm{M}
$
Then, $\kappa=1.237 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}, \mathrm{c}^{1 / 2}=0.0316 \mathrm{M}^{1 / 2}$
$
\begin{aligned}
& \therefore \Lambda_m=\frac{k}{c} \\
& =\frac{1.237 \times 10^{-4} \mathrm{Scm}^{-1}}{0.001 \mathrm{molL}^{-1}} \times \frac{1000 \mathrm{~cm}^3}{L} \\
& =123.7 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}
\end{aligned}
$
Given,
$
\kappa=11.85 \times 10^{-2} \mathrm{~S} \mathrm{~m}^{-1}, c=0.010 \mathrm{M}
$
Then, $\kappa=11.85 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}, \mathrm{c}^{1 / 2}=0.1 \mathrm{M}^{1 / 2}$
$
\begin{aligned}
& \therefore \Lambda_m=\frac{k}{c} \\
& =\frac{11.85 \times 10^{-4} \mathrm{Scm}^{-1}}{0.010 \mathrm{~mol} \mathrm{~L}^{-1}} \times \frac{1000 \mathrm{~cm}^3}{L} \\
& =118.5 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}
\end{aligned}
$
Given,
$
\kappa=23.15 \times 10^{-2} \mathrm{~S} \mathrm{~m}^{-1}, c=0.020 \mathrm{M}
$
Then, $\kappa=23.15 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}, \mathrm{c}^{1 / 2}=0.1414 \mathrm{M}^{1 / 2}$
$
\begin{aligned}
& \therefore \Lambda_m-\frac{k}{c} \\
& =\frac{23.15 \times 10^{-4} \mathrm{Scm}^{-1}}{0.020 \mathrm{~mol} \mathrm{~L}^{-1}} \times \frac{1000 \mathrm{~cm}^3}{L} \\
& =115.8 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}
\end{aligned}
$
Given,
$
\begin{aligned}
& \kappa=55.53 \times 10^{-2} \mathrm{~S} \mathrm{~m}^{-1}, \mathrm{c}=0.050 \mathrm{M} \\
& \text { Then, } \kappa=55.53 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}, \mathrm{c}^{1 / 2}=0.2236 \mathrm{M}^{1 / 2} \\
& \therefore k=\frac{k}{c} \\
& =\frac{55.53 \times 10^{-4} \mathrm{Scm}^{-1}}{0.050 \mathrm{~mol} \mathrm{~L}^{-1}} \times \frac{1000 \mathrm{~cm}^3}{L} \\
& =111.11 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}
\end{aligned}
$
Given,
$
\kappa=106.74 \times 10^{-2} \mathrm{~S} \mathrm{~m}^{-1}, c=0.100 \mathrm{M}
$
Then, $\kappa=106.74 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}, \mathrm{c}^{1 / 2}=0.3162 \mathrm{M}^{1 / 2}$
$
\begin{aligned}
& \therefore \Lambda_m=\frac{k}{c} \\
& =\frac{106.74 \times 10^{-4} \mathrm{~S} \mathrm{~cm}^{-1}}{0.100 \mathrm{~mol} \mathrm{~L}^{-1}} \times \frac{1000 \mathrm{~cm}^3}{L} \\
& =106.74 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}
\end{aligned}
$
Now, we have the following data:
| $c^{1 / 2} / m^{1 / 2}$ | 0.0316 | 0.1 | 0.1414 | 0.2236 | 0.3162 |
| $\Lambda_{\mathrm{m}}\left(\mathrm{Scm}^2 \mathrm{~mol}^{-1}\right)$ | 123.7 | 118.5 | 115.8 | 111.1 | 106.74 |
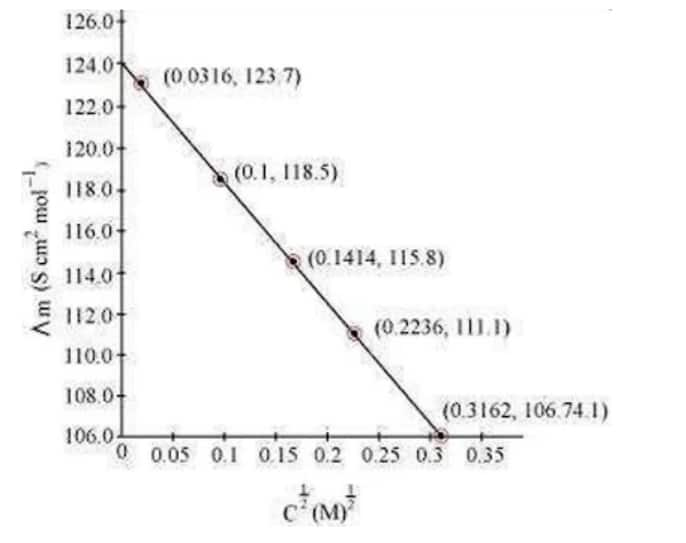
Since the line interrupts $\Lambda \mathrm{m}$ at $124.0 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}, \Lambda^{\circ}{ }_{\mathrm{m}}=124.0 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$.
Question 2.11 Conductivity of 0.00241 M acetic acid is $7.896\times 10^{-5} \: S cm^{-1}$ . Calculate its molar conductivity. If $\Delta _{m}^{0}$ for acetic acid is $390.5 \: S cm^{2} mol^{-1}$ , what is its dissociation constant?
Answer:
Molar conductivity of a solution is given by :-
$\Lambda _M = \frac{\kappa }{C}$
So, $= \frac{7.896\times10^{-5}}{0.00241}\times1000$
or $= 32.76\ Scm^2\ mol^{-1}$
Also, it is given that $\Lambda _m^{\circ}= 390.5\ Scm^2\ mol^{-1}$ .
$\alpha = \frac{\Lambda _m}{\Lambda _m^{\circ}}$
or $\alpha = \frac{32.76}{390.5}$
$\alpha = 0.084$
For dissociation constant we have,
$K_d\ = \frac{c\alpha ^2}{(1-\alpha )}$
so, $= \frac{0.00241\times0.084^2}{(1-0.084 )}$
or $= 1.86\times 10^{-5}\ mol\ L^{-1}$
Question 2.12 How much charge is required for the following reductions:
(i) $1\ mol\ of\ Al^{3+}\ to\ Al\ ?$
Answer:
The equation becomes:-
$Al^{+3}\ + 3e^-\ =\ Al$
So required charge is 3F.
Q = n*96500
Q = 3*96500 = 289500 C
Question 2.12 How much charge is required for the following reductions:
(ii) $1\ mol \ of\ Cu^{2+}\ to\ Cu?$
Answer:
The equation can be written as:-
$Cu^{2+}\ +\ 2e^-\ =\ Cu$
Thus charge required is $=\ 2F$
$= 2(96500) = 193000\ C$
Question 2.12 How much charge is required for the following reductions:
(iii) $MnO_{4}^{-}\ to\ Mn^{2+}\ ?$
Answer:
The given reaction can be written as:-
$Mn^{+7}\ +\ 5e^- =\ Mn^{+2}$
Thus charge required in above equation $=\ 5F$
$= 5(96500)$
$= 482500\ C$
Question 2.13 How much electricity in terms of Faraday is required to produce
(i) 20.0 g of Ca from molten $CaCl_{2}$ ?
Answer:
The equation for the question is given by :-
$Ca^{2+}\ +\ 2e^-\ =\ Ca$
In this equation, for 1 mol of Ca, 2F charge is required or we can say that for 40 g of Ca charge required is 2F.
So, for 20 g of Ca charge required will be = F = 96500 C.
Question 2.13 How much electricity in terms of Faraday is required to produce
(ii) 40.0 g of AI from molten $Al_{2}O_{3}$ ?
Answer:
The equation for the given question is :-
$Al^{+3}\ + 3e^-\ =\ Al$
Thus for 1 mol of Al, charge required is 3F.
So the required amount of electricity in terms of charge will be :-
$=\ \frac{3}{27}\times40F = 4.44F$
Question 2.14 How much electricity is required in coulomb for the oxidation of
(i) 1 mol of $H_{2}O\ to\ O_{2}$ ?
Answer:
According to question the equation of oxidation will be :-
$O^{2-}\ \rightarrow \ \frac{1}{2}O_2\ +\ 2e^-$
Thus, for oxidation of O 2- , 2F charge is required.
$= 2\times96500\ C$
$= 193000\ C$
Question 2.14 How much electricity is required in coulomb for the oxidation of
(ii) 1 mol of $FeO\ to\ Fe_{2}O_{3}$ ?
Answer:
The oxidation equation for the given reaction will be :-
$Fe^{+2}\ \rightarrow\ Fe^{+3}\ +\ e^-$
So for oxidation of 1 mol $Fe^{+2}$ charge required $= 1F$
$= 96500\ C$
Answer:
We are given:
I = 5A
and t = 20(60) = 1200 sec.
So total charge = 5(1200) = 6000 C.
The equation for nickel deposition will be:-
$Ni^{+2}\ +\ 2e^-\ \rightarrow\ Ni$
Thus, from 2F charge 58.7 g of nickel deposition takes place.
i.e., $2(96487)\ C \rightarrow 58.7\ g\ Ni$
So for 6000 C charge total nickel deposition will be:-
$= \frac{58.7}{2\times96487}\times6000$
or $= 1.825\ g$
Hence 1.825 g Ni will be deposited in the given conditions.
Answer:
Since the cells are connected in series so the current passing through each cell will be equal.(1.5 A)
Now we are given that 1.45 g of silver is deposited. So firstly we will consider the cell containing silver.
$Ag^+\ +\ e^-\ \rightarrow Ag$
Since for deposition of 108 g silver 96487 C charge is required, thus for 1.45 g deposition of silver charge required will be:-
$= \frac{96487}{108}\times1.45$ $= 1295.43\ C$
Now we can find the time taken by 1.5 A current to deposit 1.45 g silver.
$Time\ taken = \frac{1295.43}{1.5} \approx 864\ sec.$
For copper:-
$Cu^{+2}\ + 2e^-\ =\ Cu$
Since 2F charge will deposit 63.5 g of Cu, then deposition by 1295.43 C will be:-
$= \frac{63.5}{2\times96487}\times1295.43$ $= 0.426\ g$
Hence 0.426 g of copper will be deposited.
For zinc:-
$Zn^{+2}\ +\ 2e^-\ \rightarrow\ Zn$
Since 2F charge will deposit 65.4 g of Zn, then deposition by 1295.43 C will be:-
$= \frac{65.4}{2\times96487}\times1295.43$ $= 0.439\ g$
Hence 0.439 g of zinc will be deposited.
Question 2.17 Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(i) $Fe^{3+}_{aq}\ and\ I^{-}_{aq}$
Answer:
The concept used here will be that a reaction is feasible only if $E_{cell} ^{\circ}$ is positive.
Anode and cathode reactions will be as follows:-
$Fe^{3+}\ +\ e^-\ =\ Fe^{2+}$ $E^{\circ}\ = 0.77\ V$
$2I^{-}\ =\ I_2\ +\ 2e^-$ $E^{\circ}\ = -0.54\ V$
So $E_{cell} ^{\circ} = 0.77 - 0.54 = 0.23\ V$
So this reaction is feasible.
Question 2.17 Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(ii) $Ag^{+}_{aq}\ and\ Cu_{(s)}$
Answer:
A reaction is feasible only if $E_{cell} ^{\circ}$ is positive.
So, anode and cathode reactions will be as follows :-
$(Ag^{+}\ +\ e^-\ =\ Ag)\times 2$ $E^{\circ}\ = 0.80\ V$
$Cu\ =\ Cu^{+2}\ +\ 2e^-$ $E^{\circ}\ = -0.34\ V$
and $E_{cell} ^{\circ} = 0.80 - 0.34 = 0.46\ V$
So this reaction is feasible.
Question 2.17 Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(iii) $Fe^{3+}_{aq}\: and\ Br^{-}_{aq}$
Answer:
A reaction is feasible only if $E_{cell} ^{\circ}$ is positive.
So, anode and cathode reactions will be as follows :-
$(Fe^{+3}\ +\ e^-\ =\ Fe^{+2})\times 2$ $E^{\circ}\ = 0.77\ V$
$2Br^-\ =\ Br_2\ +\ 2e^-$ $E^{\circ}\ = -1.09\ V$
and $E_{cell} ^{\circ} = 0.77 - 1.09 = -0.32\ V$
So this reaction is not feasible.
Question 2.17 Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(iv) $Ag_{s}\ and\ Fe^{3+}_{aq}$
Answer:
A reaction is feasible only if $E_{cell} ^{\circ}$ is positive.
So, anode and cathode reactions will be as follows:-
$Ag\ =\ Ag^{+}\ +\ e^-$ $E^{\circ}\ = -0.80\ V$
$Fe^{+3}\ +\ e^-\ =\ Fe^{+2}$ $E^{\circ}\ = 0.77\ V$
and $E_{cell} ^{\circ} = -0.80 + 0.77 = -0.03\ V$
So this reaction is not feasible.
Question 2.17 Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(v) $B r_{2(a q)}$ and $F e_{a q}^{2+}$
Answer:
A reaction is feasible only if $E_{cell} ^{\circ}$ is positive.
So, anode and cathode reactions will be as follows :-
$Br_2\ +\ 2e^-\ =\ 2Br^{-}$ $E^{\circ}\ = 1.09\ V$
$Fe^{2+}\ =\ Fe^{3+}\ +\ e^-$ $E^{\circ}\ = -0.77\ V$
and $E_{cell} ^{\circ} = 1.09 - 0.77 = 0.32\ V$
So this reaction is feasible.
Question 2.18 Predict the products of electrolysis in each of the following:
(i) An aqueous solution of $AgNO_{3}$ with silver electrodes.
Answer:
For the given solution :
At cathode:- Reaction with greater E0 will take place.
$Ag^+\ +\ e^-\ =\ Ag_{(s)}$
At anode :-
$Ag\ +\ NO^{3-}\ =\ AgNO_3\ +\ e^-$
Hence, silver will get deposited at the cathode, and it will be dissolved at anode.
Question 2.18 Predict the products of electrolysis in each of the following:
(ii) An aqueous solution of $AgNO_{3}$ with platinum electrodes.
Answer:
For the given solution :
At cathode :- Reaction with greater E0 will take place.
$Ag^+\ +\ e^-\ =\ Ag_{(s)}$
At anode :- Self ionisation will take place due to the presence of water.
$H_2O\ \rightarrow\ 2H^+\ + \frac{1}{2}O_2\ +\ 2e^-$
Hence, silver will get deposited at the cathode and O2 will be produced from anode.
Question 2.18 Predict the products of electrolysis in each of the following:
(iii) A dilute solution of $H_{2}SO_{4}$ with platinum electrodes.
Answer:
For the given solution:
At cathode :- Reaction with a greater E0 will take place.
$\mathrm{H}^{+}+\mathrm{e}^{-} \rightarrow 1 / 2 \mathrm{H}_2$
At anode :- Self ionisation of water will take place due to the presence of platinum electrode.
$H_2O\ \rightarrow\ 2H^+\ + \frac{1}{2}O_2\ +\ 2e^-$
Hence, H2 gas will be generated at cathode and O2 will be produced from anode.
Question 2.18 Predict the products of electrolysis in each of the following:
(iv) An aqueous solution of $CuCI_{2}$ with platinum electrodes.
Answer:
For the given solution :
At cathode :- Reaction with greater E0 will take place.
$\mathrm{Cu}^{2+}+2 e^{-} \rightarrow \mathrm{Cu}(\mathrm{s})$
At anode :-
$2Cl^-\ =\ Cl_2\ +\ 2e^-$
Hence, Cu will get deposited at cathode and Cl2 will be produced from anode.
Class 12 Chemistry NCERT Chapter 2: Higher Order Thinking Skill (HOTS) Questions
These electrochemistry class 12 question answer helping students develop analytical and problem-solving skills beyond the textbook, they encourage deeper understanding and application of concepts in new scenarios. These NCERT Solutions for Class 12 provides concept, accurate answers and they are very effective for board as well as competitive exams like NEET and JEE preparation.
Question 1. The standard cell potential $\left(\mathrm{E}_{\text {cell }}^{\ominus}\right)$ of a fuel cell based on the oxidation of methanol in air that has been used to power television relay station is measured as 1.21 V . The standard half cell reduction potential for $\mathrm{O}_2\left(\mathrm{E}_{\mathrm{O}_2 / \mathrm{H}_2 \mathrm{O}}^{\mathrm{0}}\right)$ is 1.229 V.
Choose the correct statement:
(1) The standard half cell reduction potential for the reduction of $\mathrm{CO}_2\left(\mathrm{E}_{\mathrm{CO}_2 / \mathrm{CH}_3 \mathrm{OH}}^0\right)$ is 19 mV
(2) Oxygen is formed at the anode.
(3) Reactants are fed at one go to each electrode.
(4) Reduction of methanol takes place at the cathode.
Answer:
Fuel cell reaction
$\mathrm{CH}_3 \mathrm{OH}(\mathrm{I})+\frac{3}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{I})$
Here $\mathrm{O}_2$ reduces to $\mathrm{H}_2 \mathrm{O}$ and $\mathrm{CH}_3 \mathrm{OH}$ oxidises to $\mathrm{CO}_2$.
Standard cell potential:
$
E_{\text {cell }}^{\circ}=E_{\text {cathode }}^{\circ} \quad-E_{\text {anode }}^{\circ}
$
Given:
$
\begin{aligned}
& E_{\text {cell }}^{\circ}=1.21 \mathrm{~V} \\
& E_{0_2 / \mathrm{H}_2 \mathrm{O}}^{\circ}=1.229 \mathrm{~V}
\end{aligned}
$
Substituting the values,
$
1.21=1.229-E_{\text {anode }}^{\circ}
$
Solving for $E_{\text {anode }}^{\circ}$ :
$
E_{\text {anode }}^{\circ}=1.229-1.21=0.019 \mathrm{~V}
$
0.019 V convert into mV multiply by 1000 = 0.019 * 1000 = 19mV
Hence, the correct answer is option (1).
Question 2. The molar conductance of an infinitely dilute solution of ammonium chloride was found to be $185 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$ and the ionic conductance of hydroxyl and chloride ions are 170 and $70 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^1$, respectively. If molar conductance of 0.02 M solution of ammonium hydroxide is $85.5 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$, its degree of dissociation is given by $\mathrm{x} \times 10^{-1}$. The value of $x$ is _______ (Nearest integer)
(1) 3
(2) 2
(3) 4
(4) 1
Answer:
$\begin{aligned}
& \lambda_{\mathrm{m}}^{\prime \prime} \text { of } \mathrm{NH}_4 \mathrm{Cl}=185 \\
& \left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4}+\left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{Cl}^{-}}=185 \\
& \left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4}=185-70=115 \mathrm{Scm}^2 \mathrm{~mol}^{-1} \\
& \left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4 \mathrm{OH}}=\left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4^{\circ}}+\left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{OH}^{-}} \\
& =115+170 \\
& \left(\lambda_{\mathrm{m}}^0\right)_{\mathrm{NH}_4 \mathrm{OH}}=285 \mathrm{Scm}^2 \mathrm{~mol}^{-1} \\
& \text { degree of dissociation }=\frac{\left(\lambda_{\mathrm{m}}\right)_{\mathrm{NH}_4 \mathrm{OH}}}{\left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4 \mathrm{OH}}} \\
& =\frac{85.5}{285} \\
& =0.3 \\
& =3 \times 10^{-1}
\end{aligned}$
Hence, the correct answer is option (1).
Question 3. The standard reduction potential values of some of the p-block ions are given below. Predict the one with the strongest oxidising capacity.
(1) $\mathrm{E}_{\mathrm{Sn}^{4+} / \mathrm{Sn}^{2+}}^{\ominus}=+1.15 \mathrm{~V}$
(2) $\mathrm{E}_{\mathrm{Tl} ^{3+} / \mathrm{T} l}^{\ominus}=+1.26 \mathrm{~V}$
(3) $\mathrm{E}_{\mathrm{Al}^{3+} / \mathrm{Al}}^{\ominus}=-1.66 \mathrm{~V}$
(4) $\mathrm{E}_{\mathrm{Pb}^{4+} / \mathrm{Pb}^{2+}}^{\ominus}=+1.67 \mathrm{~V}$
Answer:
Standard reduction potential value $(+\mathrm{ve})$ increases oxidising capacity increases.
From the given options, the higher standard reduction potential is for lead $(\mathrm{Pb}) .(+1.67)$.
so, the p-block ion with strongest oxidizing capacity is Pb (lead).
Hence, the correct answer is option (4).
Question 4. Consider the following half cell reaction
$\mathrm{Cr}_2 \mathrm{O}_7^{2-}(\mathrm{aq})+6 \mathrm{e}^{-}+14 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+7 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
The reaction was conducted with the ratio of $\frac{\left[\mathrm{Cr}^{3+}\right]^2}{\left[\mathrm{Cr}_2 \mathrm{O}_7^{2-}\right]}=10^{-6}$. The pH value at which the EMF of the half cell will become zero is _______ . (nearest integer value)
[Given : standard half cell reduction potential
$\left.\mathrm{E}_{\mathrm{C}_2 \mathrm{O}_{-}^2+\mathrm{H}^* / \mathrm{Cr}^{3+}}^{\mathrm{o}}=1.33 \mathrm{~V}, \frac{2.303 \mathrm{RT}}{\mathrm{~F}}=0.059 \mathrm{~V}\right]$
Answer:
$\mathrm{Cr}_2 \mathrm{O}_7^{2-}(\mathrm{aq})+6 \mathrm{e}^{-}+14 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+7 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
Using Nernst equation
$\mathrm{E}_{\mathrm{Cr}_2 \mathrm{O}_7^{2-} / \mathrm{H}^{+} / \mathrm{Cr}^{3+}}=\mathrm{E}_{\mathrm{Cr}_2 \mathrm{O}_7^{2-} / \mathrm{H}^{+} / \mathrm{cr}^{3+}}^0-\frac{2.303 \mathrm{RT}}{6 \mathrm{~F}} \log \frac{\left[\mathrm{Cr}^{3+}\right]^2}{\left[\mathrm{Cr}_2 \mathrm{O}_7^{2-}\right]\left[\mathrm{H}^{+}\right]^{14}} $
$0=\mathrm{E}_{\mathrm{Cr}_2 \mathrm{O}_7^{2-} \mathrm{H}^{+} / \mathrm{C}^{3+}}^0-\frac{0.059}{6} \log \left(10^{-6}\left[\mathrm{H}^{+}\right]^{-14}\right)$
$0=1.33-\frac{0.059}{6}(-6+14 \cdot \mathrm{pH})$
$1.33=\frac{0.059}{6}(14 \cdot \mathrm{pH}-6)$
$1.33=0.00983(14 \cdot \mathrm{pH}-6)$
$\frac{1.33}{0.00983}=14 \cdot \mathrm{pH}-6 \Rightarrow 135.3 \approx 14 \cdot \mathrm{pH}-6$
$141.3=14 \cdot \mathrm{pH} \Rightarrow \mathrm{pH}=\frac{141.3}{14} = 10.1$
Hence, the answer is 10.
Question 5. Given below are two statements :
Statement I : Mohr's salt is composed of only three types of ions-ferrous, ammonium, and sulphate.
Statement II : If the molar conductance at infinite dilution of ferrous, ammonium and sulphate ions are $\mathrm{x}_1, \mathrm{x}_2$ and $\mathrm{x}_3 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$, respectively then the molar conductance for Mohr's salt solution at infinite dilution would be given by $\mathrm{x}_1+\mathrm{x}_2+2 \mathrm{x}_3$
In the light of the given statements, choose the correct answer from the options given below :
(1) Both statements I and Statement II are false
(2) Statement I is false but Statement II is true
(3) Statement I is true but Statement II are false
(4) Both statements I and Statement II are true
Answer:
Mohr's salt : $\mathrm{FeSO}_4 \cdot\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \cdot 6 \mathrm{H}_2 \mathrm{O}$
Mohr's salt dissociates into one Fe ion, two ammonium ions and two sulphate ions. Therefore, using Kohlrausch's law we get
$\lambda_{\mathrm{m}}^*(\text { Mohr's salt })=\mathrm{x}_1+2 \mathrm{x}_2+2 \mathrm{x}_3$
Hence, the correct answer is option (3).
Question 6: Which of the below quantity will increase on dilution of the solution?
(1) Specific conductance (k) of strong electrolyte
(2) Specific conductance (k) of weak electrolyte
(3) Molar conductance ($\Lambda_m$) of a strong electrolyte
(4) Limiting molar conductance ($\Lambda_m^{\infty}$) of a strong electrolyte
Answer:
The molar conductance of a strong electrolyte. Increases due to a decrease in interionic attraction forces on dissolution of the solution.
Hence, the correct answer is option (3).
NCERT Exemplar Class 12 Solutions Subject-wise
- NCERT Exemplar Class 12 Chemistry Solutions
- NCERT Exemplar Class 12 Mathematics Solutions
- NCERT Exemplar Class 12 Biology Solutions
- NCERT Exemplar Class 12 Physics Solutions
Approach to Solve Questions of Chapter 2 Class 12 Chemistry
To master class 12 chemistry chapter 2 electrochemistry question answer it is essential to understand core concepts of Electrochemistry. The approaches given below will help you solve class 12 chemistry electrochemistry question answer.
1. Before jumping into problems, make sure you have understood the topics clearly. Some important concepts are redox reactions and their balancing, electrochemical cells, standard electrode potentials, Nernst Equation, Conductance and Kohlrausch’s Law, Electrolysis and Faraday’s laws.
2. Memorize and understand how to apply the formulas in the question; some of them are given here,
Nernst equation-
$
E=E^{\circ}-\frac{0.0591}{n} \log Q
$
Cell potential-
$
E_{\text {cell }}=E_{\text {cathode }}^{\circ}-E_{\text {anode }}^{\circ}
$
Faraday's Laws-
First law: $m=\frac{Z I t}{1000}$
Second law relates mass of different substances
3. First identify the type of questions asked in electrochemistry class 12 question answer and then try to apply the formulas or the concepts related to the topic. Also check if the question requires graphical topics like conductivity vs. dilution.
4. Read the question carefully and note the given values. Solve the question in stepwise manner and do put correct units. Check electrode potentials like what is cathode or anode in the cell.
5. Solve the class 12 chemistry chapter 2 electrochemistry solutions and refer to the solved examples. You can also attempt previous years’ board questions for better learning. Make the use of concept maps or flashcards for definitions and laws.
Topics of NCERT Class 12 Chemistry Chapter 2
2.1 Electrochemical Cells
2.2 Galvanic Cells
2.2.1 Measurement of Electrode Potential
2.3 Nernst Equation
2.3.1 Equilibrium Constant from Nernst Equation
2.3.2 Electrochemical Cell and Gibbs Energy of the Reaction
2.4 Conductance of Electrolytic Solutions
2.4.1 Measurement of the Conductivity of Ionic Solutions
2.4.2 Variation of Conductivity and Molar Conductivity
2.5 Electrolytic Cells and Electrolysis
2.5.1 Products of Electrolysis
2.6 Batteries
2.6.1 Primary Batteries
2.6.2 Secondary Batteries
2.7 Fuel Cells
2.8 Corrosion
NCERT Books and NCERT Syllabus:
What Extra Should Students Study Beyond NCERT for JEE?
Along with class 12 chemistry chapter 2 electrochemistry solutions students should explore advanced reference books and practice higher level numerical problems. Focusing on previous years JEE papers, mock tests, and conceptual clarity will also help them excel in competitive exams.Here is a comparison table highlighting what to study beyond NCERT for JEE.
NCERT Class 12 Chemistry Chapter 2 Electrochemistry Formulas
This section provides all the important formulas from the class 12 chemistry electrochemistry question answer, helping students quickly revise and apply them while solving numerical problems. Learning these formulas thoroughly is essential for scoring well in board exams and competitive tests. Given below are important formulas from this chapter:
1. Conductance(G) is the reciprocal of resistance (R), and specific conductance or conductivity(k) isthe inverse of resistivity $(\rho )$
$\\G=\frac{1}{R}=\frac{1}{\rho }\left ( \frac{a}{l} \right )\\k=G\left ( \frac{l}{a} \right )$
2. l/a is called the cell constant of the conductivity cell.
3. Equivalent Conductivity is defined as the conductance of a solution containing 1g of an electrolyte.
$\\ \Lambda _{eq}=K\times V\\$
4. Nernst equation
aA+bB $\rightarrow$ cC+dD
$E_{cell} = E_{cell}^{o}-\frac{0.0591}{n}log\frac{\left [ C \right ]^{c}\left [ D \right ]^{d}}{\left [ A \right ]^{a}\left [ B \right ]^{b}}$
NCERT Solutions for Class 12
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 2 Electrochemistry
These class 12 chemistry chapter 2 electrochemistry question answer help students build a clear understanding of electrochemical concepts. Given below some key learning from this chapter:
- They help students to understand the construction and working of electrochemical cells.
- These class 12 chemistry chapter 2 electrochemistry solutions help students to learn about the Nernst equation and its application in calculating electrode and cell potentials.
- Using these solutions students gain knowledge about electrochemical series and its role in predicting the feasibility of redox reactions.
- Important topics like conductance, specific conductance, molar conductance are well explained in these solutions.
- Here students will learn about Faraday’s laws of electrolysis and their numerical applications
NCERT Solutions for Class 12 Chemistry
Along with the class 12 chemistry electrochemistry question answer, here are the links to chapter wise NCERT Class 12 Chemistry solutions. These solutions provides clear explanations for quick revision and effective exam preparation.
Frequently Asked Questions (FAQs)
Electrochemistry is the branch of chemistry that deals with the interconversion of chemical energy and electrical energy. It involves the study of chemical reactions that occur in electrochemical cells, where oxidation and reduction processes take place. This field finds applications in batteries, fuel cells, and corrosion science.
NCERT Solutions for Class 12 Chemistry Chapter 2 are detailed answers to all the questions given in the Electrochemistry chapter of the NCERT textbook. They help students understand key concepts, practise numerical problems, and prepare thoroughly for exams.
Electrochemistry is important because it explains how chemical reactions produce electrical energy and how electricity can drive chemical changes. It has wide applications in batteries, fuel cells, electroplating, corrosion prevention, and industrial chemical production.
The standard electrode potential is a measure of the inherent tendency of a half-cell to be reduced under standard conditions: 1 M concentration, 1 atm pressure, and 25°C. It allows us to predict the direction of spontaneous reactions, determine cell voltages, and compare the reactivity of different electrodes.
Conductance refers to the ability of a solution to conduct electric current. It is influenced by the concentration of ions present in the solution. In electrochemistry, conductance plays a critical role in determining the efficiency of electrochemical reactions, as higher ionic concentration typically leads to better conductivity and faster reaction rates.
Electrochemical cells are devices that convert chemical energy into electrical energy or vice versa. They consist of two electrodes immersed in an electrolyte.
The Nernst equation relates the cell potential of an electrochemical cell to the concentration of the reactants and products involved. It is significant because it allows us to calculate the cell potential under non-standard conditions.
Galvanic cells generate electrical energy from spontaneous chemical reactions and have a positive cell potential. In contrast, electrolytic cells require an external source of electrical energy to drive non-spontaneous reactions and have a negative cell potential.
Conducting solutions, or electrolytes, contain ions that facilitate the flow of electric current. The extent of conduction depends on the concentration of ions, temperature, and the nature of the solvent.
Electrochemistry has numerous applications, including in batteries, fuel cells, electroplating, corrosion prevention, purification of metals, and in various analytical methods such as potentiometry.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
