It is a downloadable resource containing advanced practice questions and solutions for Chapter 3 Electrochemistry, designed to improve conceptual clarity and exam readiness.
NCERT Exemplar Class 12 Chemistry Chapter 3 Electrochemistry
Do you know how the batteries work, why some metals conduct electricity better than others and how corrosion damages iron? The answer to all these questions lies in Class 12 Chemistry Chapter 3. This chapter explains the connection between chemical energy and electrical energy. It discusses how chemical reactions can produce electrical energy, as seen in batteries, and how electrical energy can initiate non-spontaneous chemical reactions, such as in electrolysis.
Electronic gadgets or devices such as Mobile phones, smartwatches, calculators, and more are not allowed inside the CBSE 2026 exam hall.
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: Long Answer Type
- Class 12 Chemistry NCERT Chapter 3: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 3 Electrochemistry.
- Formulas Of NCERT Class 12 Chemistry Chapter 3
- Advantages of Using NCERT Exemplar Class 12 Chemistry Solutions Chapter 3
- Topics of NCERT Exemplar Solutions Class 12 Electrochemistry
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Exemplar Class 12 Solutions Subject-wise
- NCERT Solution subject-wise
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus

Our subject experts design the NCERT Exemplar Solutions to offer a systematic and structured approach to important concepts and help students to develop a clear understanding of critical concepts through the series of solved examples and conceptual explanations. Students can refer to NCERT Exemplar Class 12 Chemistry Solutions to strengthen their concept and problem-solving ability for other chapters. In this article, we have included Higher Order Thinking Skills (HOTS) questions along with a structured approach to solving the class 12 chemistry chapter 3 questions . This helps students gain a clearer understanding of how to learn the chapter effectively. The HOTS questions also provide insight into the challenging and thought-provoking questions they may face. Also, check the NCERT Solutions for Class 12 for all the chapters.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: MCQ (Type 1)
The MCQ questions are covered in Electrochemistry to enhance your knowledge. To answer these questions concepts are explained in detail in Electrochemistry class 12 notes available on our website
Question1: Which cell will measure standard electrode potential of copper electrode?
$(i) \; Pt (s)| H_{2}(g,0.1 bar)| H^+ (aq.,1 M)|| Cu^{2+}(aq.,1M) |Cu$
$(ii) \; Pt (s) |H_{2}(g,1 bar) |H^+ (aq.,1 M)|| Cu^{+2}(aq.,2M)| Cu$
$(iii) \; Pt (s) | H_{2}(g,1 bar)| H^+ (aq.,1 M) || Cu^{2+}(aq.,1M) |Cu$
$(iv) \; Pt (s) |H_{2}(g,1 bar) |H^+ (aq.,0.1 M) ||Cu^{2+}(aq.,1M)| Cu$
Answer:
The answer is option (iii). On connecting the copper electrode to a standard hydrogen electrode, it acts as the cathode, and its standard electrode potential can be measured.
$E^{0}=E_{R}^{0}-E_L^0=E_R^0-0=E_R^0$
$Pt (s)\left | H_{2}(g,1\; bar) \right |H^{+}(aq., 1 M)\parallel Cu^{2+}(aq., 1\; M)\mid Cu$
will measure the standard electrode potential of the copper electrode.
The standard electrode potential is measured for a given cell by coupling it with the standard hydrogen electrode, where the pressure of hydrogen gas is maintained at one bar. The concentration of the H+ ion in the solution is one molar, and so is the concentration of the oxidized and reduced forms of the species.
The answer is the option (ii) $E_{\frac{Mg2+}{Mg}}=E_{{\frac{Mg^{2+}}{Mg}}}^o-\frac{0.059}{2}log[Mg^{2+}]$
The given equation is that of a straight line with a positive slope and a non-zero intercept
Question 3: Which of the following statements is correct?
(i) $\mathrm{E}_{\text {Cell }}$and $\Delta_{\mathrm{r}} \mathrm{G}$ of the cell reaction are both extensive properties.
(ii) $\mathrm{E}_{\text {Cell }}$ and $\Delta_{\mathrm{r}} \mathrm{G}$ of the cell reaction are both intensive properties.
(iii) $\mathrm{E}_{\text {Cell }}$ is an intensive property, while? $\Delta_{\mathrm{r}} \mathrm{G}$ of the cell reaction is an extensive property.
(iv) $\mathrm{E}_{\text {Cell }}$ is an extensive property, while Δ$\Delta_{\mathrm{r}} \mathrm{G}$ of the cell reaction is an intensive property.
Answer:
The answer is option (iii). While $\mathrm{E}_{\text {Cell }}$ is independent of the mass of species or the number of particles, it is an intensive property, whereas $\Delta_{\mathrm{r}} \mathrm{G}$ depends on the number of particles and is an extensive property.
Question 4: The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called ___________.
(i) Cell potential
(ii) Cell emf
(iii) Potential difference
(iv) Cell voltage
Answer:
The answer is the option (ii) When no current is drawn through the cell, the difference between the electrode potentials of two electrodes is EMF.
Question 5: Which of the following statement is not correct about an inert electrode in a cell?
(i) It does not participate in the cell reaction.
(ii) It provides a surface either for oxidation or for the reduction reaction.
(iii) It provides a surface for the conduction of electrons.
(iv) It provides a surface for a redox reaction.
Answer:
The answer is the option (iv) Inert electrodes act only as source or sink for electrons. They do not undergo redox reactions and merely provide the surface for the reaction.
Question 6: An electrochemical cell can behave like an electrolytic cell when ____________.
(i) $\mathrm{E}_{\text {Cell }}$ = 0
(ii) $\mathrm{E}_{\text {Cell }}$ > $\mathrm{E}_{\text {ext }}$
(iii) $\mathrm{E}_{\text {ext }}$ > $\mathrm{E}_{\text {Cell }}$
(iv) $\mathrm{E}_{\text {Cell }}$l = $\mathrm{E}_{\text {ext }}$
Answer:
The answer is the option (iii) An electrochemical cell can behave like an electrolytic cell when there is an application of an external opposite potential on the galvanic cell and the reaction is not inhibited until the opposing voltage reaches the value 1.1 V. No current flows through the cell when this happens. Reaction will function in the opposite direction on increasing the external potential any further.
Question 7: Which of the statements about solutions of electrolytes is not correct?
(i) The conductivity of the solution depends upon the size of ions.
(ii) Conductivity depends upon the viscosity of the solution.
(iii) Conductivity does not depend upon the solvation of ions present in the solution.
(iv) The conductivity of the solution increases with temperature.
Answer:
The answer is option (iii) Conductivity decreases with an increasing salvation of ions.
Question 8: Use the data given below to find out the strongest reducing agent.
$E^{\circleddash }_{Cr_{2}O{_{7}}^{2-}/Cr^{3+}}=1.33 \; V$
$E^{\circleddash }_{Cl_{2}/Cl^{-}}=1.36 \; V$
$E^{\circleddash }_{MnO{_{4}}^{-}/Mn^{2+}}=1.51 \; V$
$E^{\circleddash }_{Cr^{3+}/Cr}=-0.74\; V$
$(i)\; Cl^{-}$
$(ii)\; Cr$
$(iii)\; Cr^{3+}$
$(iv)\; Mn^{+2}$
Answer:
The answer is that option $(ii)\; Cr$ is the strongest reducing agent as it is the only one with a negative standard reduction potential
Question 9: Use the data given in Q.8 and find out which of the following is the strongest oxidizing agent.
$(i) \; Cl^{-}$
$(ii) \: Mn^{2+}$
$(iii) \; MnO^{-}_{4}$
$(iv) Cr^{3+}$
Answer:
The answer is the option (iii). Oxidizing capacity increases with a positive increase in the standard reduction potential. $MnO^{4-}$ with the highest standard reduction potential is the strongest oxidizing agent.
Question10: Using the data given in Q.8 find out in which option the order of reducing power is correct.
$(i) Cr^{3+} < Cl^{-} < Mn^{2+} < Cr$
$(ii) Mn^{2+} < Cl^{-}< Cr^{+3} < Cr$
$(iii) Cr^{3+} < Cl^{-}< Cr_{2}O_{7}^{2-} < MnO^{-}_{4}$
$(iv) Mn^{2+} < Cr^{3+} < Cl^{-}< Cr$
Answer:
The answer is option (ii). A decrease in reduction potential corresponds with an increase in reduction power. The order of reducing power is $(ii) Mn^{2+} < Cl^{-}< Cr^{+3} < Cr$
Question 11: Use the data given in Q.8 and find out the most stable ion in its reduced form.
$(i) \; Cl^{-}$
$(ii) \; Cr^{3+}$
$(iii) \; Cr$
$(iv)\; Mn^{2+}$
Answer:
The answer is option (iv). Due to a higher $E^{o}$ value, $Mn^{2+}$ is most stable in its reduced form.
Question 12: Use the data of Q.8 and find out the most stable oxidized species.
$(i) \; Cr^{3+}$
$(ii) \; MnO^{-}_{4}$
$(iii)\; Cr_{2}O_{7}^{2-}$
$(iv) \; Mn^{2+}$
Answer:
The answer is option (i). Due to the lowest $E^{o}$value, $Cr^{3+}$ is most stable in its oxidized form.
Question 13: The quantity of charge required to obtain one mole of aluminium from $Al_{2}O_{3}$ is ___________.
(i) 1F
(ii) 6F
(iii) 3F
(iv) 2F
Answer:
The answer is the option (iii) $Al_{2}O_{3}\rightarrow 2Al^{3+}+ 3O^{2-}$
$Al^{3+}+3e^{-} \rightarrow Al$ (for 1 mole)
To obtain 1 mole $Al$ from $Al_{2}O_{3}$, we require 3F charge
Question 14: The cell constant of a conductivity cell _____________.
(i) changes with the change of electrolyte.
(ii) changes with the change of concentration of electrolyte.
(iii) changes with the temperature of the electrolyte.
(iv) remains constant for a cell.
Answer:
The answer is option (iv). The cell constant of a conductivity cell remains constant for a cell.
Question 15: While charging the lead storage battery ______________.
(i) $PbSO_{4}$ anode is reduced to $Pb$
(ii) $PbSO_{4}$ cathode is reduced to $Pb$
(iii) $PbSO_{4}$ cathode is oxidised to $Pb$
(iv) $PbSO_{4}$ anode is oxidised to $PbO_{2}$
Answer:
The answer is the option (i)
Reduction at Anode: $PbSO_4 (s)+2e^-\rightarrow Pb(s)+SO_{4}^{2-} (aq) (Reduction)$
Oxidation at Cathode: $PbSO_4 (s)+2H_2 O\rightarrow PbO_2 (s)+SO_{4}^{2-}+4H^++2e^-$
Overall reaction : $2PbSO_4 (s)+2H_2 O\rightarrow Pb(s)+PbO_2 (s)+4H^++2SO_{4}^{2-}$
Question 16: $\wedge ^{0}_{m(NH_{4}OH)}$ is equal to ______________.
$(i) \wedge ^{0}_{m(NH_{4}OH)} + \wedge ^{0}_{m(NH_{4}Cl)}- \wedge ^{0}_{(HCl)}$
$(ii) \wedge ^{0}_{m(NH_{4}Cl)} + \wedge ^{0}_{m(NaOH)}- \wedge ^{0}_{(NaCl)}$
$(iii) \wedge ^{0}_{m(NH_{4}Cl)} + \wedge ^{0}_{m(NaCl)}- \wedge ^{0}_{(NaOH)}$
$(iv) \wedge ^{0}_{m(NaOH)} + \wedge ^{0}_{m(NaCl)}- \wedge ^{0}_{(NH_{4}Cl)}$
Answer:
The answer is the option (ii) $NH_{4}Cl\rightleftharpoons NH_4^++Cl^-$
$NaCl\rightleftharpoons Na^{+}+Cl^{-}$
$NaOH\rightleftharpoons Na^{+}+OH^{-}$
$NH_{4}OH\rightleftharpoons NH_{4}^{+}+OH^{-}$
So
$\wedge ^{0}_{m(NH_{4}Cl)} + \wedge ^{0}_{m(NaOH)}- \wedge ^{0}_{(NaCl)}$
Question 17: In the electrolysis of aqueous sodium chloride solution which of the half-cell reactions will occur at the anode?
$(i) Na^{+}(aq) + e^{-} \rightarrow Na (s); E^{o}_{Cell} = -2.71V$
$(ii) 2H_{2}O (l) \rightarrow O_{2}(g) + 4H^+(aq) + 4e^{-} ; E^{o}_{Cell} = 1.23V$
$(iii) H^{+}(aq) + e^{-}\rightarrow \frac{1}{2}H_{2} (g); E^{o}_{Cell} = 0.00V$
$(iv) Cl^{-}(aq) \rightarrow \frac{1}{2} Cl_{2}(g) + e^{-} ; E^{o}_{Cell} = 1.36V$
Answer:
The answer is option (ii). Upon electrolysis, H2O gives-
$2H_2 O\rightarrow O_2+4H^++4e^-;E_{cell}^{0}=1.23V$
Since, $E_{cell}^{0}=1.23V$. Hence, this half-cell reaction occurs at the anode.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: MCQ (Type 2)
The Class 12 Chemistry Chapter 3 Electrochemistry solutions are provided here with simple explanations. Learn more through these advanced MCQs.
Question 18: The positive value of the standard electrode potential of $\mathrm{Cu}{ }^{2+} / \mathrm{Cu}$ indicates that $\qquad$
(i) this redox couple is a stronger reducing agent than the $\mathrm{H}^{+} / \mathrm{H}_2$ couple.
(ii) this redox couple is a stronger oxidising agent than $\mathrm{H}^{+} / \mathrm{H}_2$.
(iii) Cu can displace $\mathrm{H}_2$ from acid.
(iv) Cu cannot displace $\mathrm{H}_2$ from acid.
Answer:
The answer is option (ii, iv). A decrease in E° indicates an increase in reduced power
$\\Cu^{2+}+2e^-\rightarrow Cu ; \; E^0=0.34V\\ 2H^++2e^-\rightarrow H_2 ;\; E^0=0.00V$
As, E° value for $Cu^{2+}$is higher -
(i) It is the stronger oxidizing agent
(ii) It can't displace $H_{2}$
(i) In dilute sulphuric acid solution, hydrogen will be reduced at the cathode.
(ii) In concentrated sulphuric acid solution, water will be oxidized at the anode.
(iii) In dilute sulphuric acid solution, water will be oxidized at the anode.
(iv) In dilute sulphuric acid solution, $SO_{4}^{2-}$ ion will be oxidized to tetrathionate ion at the anode.
Answer:
The answer is the option (ii, iii) In dilute sulphuric acid solution -
Reduction at cathode: $H^++e^-\rightarrow \frac{1}{2} H_2$
Oxidation at anode : $2H_2 O \rightarrow O_2+4H^++4e^-$
In concentrated sulphuric acid solution, sulphate $(SO{_{4}}^{2-})$ ions oxidize to form tetrathionate $(S_{2}O{_{8}}^{2-})$ ions.
Question 20: $E^{\Theta }_{Cell} = 1.1V$ for Daniel cell. Which of the following expressions are correct description of state of equilibrium in this cell?
$(i) \; 1.1 = K_{c}$
$(ii) \;\frac{2.303RT}{2 F} logK_{c} = 1.1$
$(iii)\; log K_{c} = 2.2/0.059$
$(iv) log K_{c} = 1.1$
Answer:
The answer is option (ii,iii)
$E_{cell}^{0}=\frac{2.303\; RT}{nF} logK_{c}$
$=\frac{0.059}{2}logK_{c}$
$1.1=\frac{0.059}{2}logK_{c}$
$logK_{c}=\frac{2.2}{0.059}$
Question 21: The conductivity of an electrolytic solution depends on ____________.
(i) nature of electrolyte.
(ii) the concentration of electrolyte.
(iii) power of the AC source.
(iv) distance between the electrodes.
Answer:
The answer is the option (i, ii). Mobile ions are responsible for the conductivity of electrolyte solution and this phenomenon is termed ionic conductance. It depends only on the following factors–
-
The nature of the electrolyte added
-
size of the ion produced and its solvation
-
concentration of electrolyte
-
Nature of the solvent and its viscosity
-
temperature
Question 22: $\wedge _{m(H_{2}O)}^{0}$ is equal to ______________.
$(i) \wedge_{m(HCl)}^{0} + \wedge _{m(NaOH)}^{0} - \wedge_{m(NaCl)}^{0}$
$(ii) \wedge_{m(HNO_{3})}^{0} + \wedge _{m(NaNO_{3})}^{0} - \wedge_{m(NaOH)}^{0}$
$(iii) \wedge_{m(HNO_{3})}^{0} + \wedge _{m(NaOH)}^{0} - \wedge_{m(NaNO_{3})}^{0}$
$(iv) \wedge_{m(NH_{4}OH)}^{0} + \wedge _{m(HCl)}^{0} - \wedge_{m(NH_{4}Cl)}^{0}$
Answer:
The answer is option (i,iv)
$\wedge _{m(H_{2}O)}^{0}=\wedge _{m(HCl)}^{0}+\wedge _{m(NaOH)}^{0}-\wedge _{m(NaCl)}^{0}$
$\wedge_{m(NH_{4}OH)}^{0} + \wedge _{m(HCl)}^{0} - \wedge_{m(NH_{4}Cl)}^{0}= \wedge _{m(H_{2}O)}^{0}$
The molar conductivity of water is equal to the sum of the molar conductivities of constituent ions. However, $NH_{4}OH$ doesn’t undergo complete decomposition as it is a weak electrolyte.
Question 23: What will happen during the electrolysis of an aqueous solution of $CuSO_{4}$ by using platinum electrodes?
(i) Copper will deposit at the cathode.
(ii) Copper will deposit at the anode.
(iii) Oxygen will be released at the anode.
(iv) Copper will dissolve at the anode.
Answer:
The answer is option (i,iii)
$CuSO_4\rightleftharpoons Cu^{2+}+SO_{4}^{2-}$
$H_{2}O\rightleftharpoons H^{+}+OH^{-}$
At cathode :
$Cu^{2+}+2e^{-}\rightarrow Cu; E_{cell}^{0}=0.34 \; V$
$H^{+}+e^{-}\rightarrow\; \frac{1}{2} H_{2}; E_{cell}^{0}=0.00 \; V$
At cathode, the reaction with higher $E^{0}$ is preferred
At anode :
$2SO_{4}^{2-}-2e^{-}\rightarrow S_{2}O_{8}^{2-}; E_{cell}^{0}=1.96 V$
$2H_{2}O\rightarrow O_{2}+4H^{+}+4e^{-};E_{cell}^{0}=1.23V$
At the anode, the reaction with lower $E^{o}$ is preferred.
Question 24: What will happen during the electrolysis of an aqueous solution of $CuSO_{4}$ in the presence of $Cu$ electrodes?
(i) Copper will deposit at the cathode.
(ii) Copper will dissolve at the anode.
(iii) Oxygen will be released at the anode.
(iv) Copper will deposit at the anode.
Answer:
The answer is option (i,ii)
At Cathode :
$Cu^{2+}+2e^{-}\rightarrow Cu(s)$
At anode :
$Cu(s)\rightarrow Cu^{2+}+2e^{-}$
The cathode will witness deposition of $Cu$ and the Anode will witness its dissolution
Question 25: Conductivity κ , is equal to ____________.
$(i) \frac{1}{R} \frac{l}{A}$
$(ii) \frac{G^{*}}{R}$
$(iii) \wedge_{m}$
$(iv) \frac{l}{A}$
Answer:
The answer is option (i,ii)
$(i) \frac{1}{R} \frac{l}{A}$
$(ii) \frac{G^{*}}{R}$
Question 26: Molar conductivity of ionic solution depends on ___________.
(i) temperature.
(ii) distance between electrodes.
(iii) the concentration of electrolytes in solution.
(iv) the surface area of electrodes.
Answer:
The answer is option (i, iii). Temperature and concentration of electrolytes determine the molar conductivity of an ionic solution
Question 27: For the given cell, $Mg\left | Mg^{2+} \right |\left | Cu^{2+} \right |Cu$
(i) $Mg$ is cathode
(ii) $Cu$ is cathode
(iii) The cell reaction is $Mg + Cu^{2+} \rightarrow Mg^{2+} + Cu$
(iv) Cu is the oxidizing agent
Answer:
The answer is the option (ii, iii). At the cathode, Cu is reduced and at the anode, Mg is oxidized.
(ii) $Cu$ is cathode
(iii) The cell reaction is $Mg + Cu^{2+} \rightarrow Mg^{2+} + Cu$
NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: Short Answer Type
Chapter 3 short-answer type questions are also given here. This section contains Class 12 Chemistry Electrochemistry questions and answers that are asked in the exams. Practice short answer types from the questions below.
Question 28: Can the absolute electrode potential of an electrode be measured?
Answer:
No, the absolute electrode potential of an electrode cannot be measured.
Question 29: Can $E^{\Theta }_{cell}$ or $\Delta _{r}G^{\Theta }$ for cell reaction ever be equal to zero?
Answer:
$E^{o}$and $\Delta G^{o}$ can never be zero for a cell reaction.
Question 30: Under what condition is $E_{cell} = 0$ or $\Delta _{r}G= 0 \;?$
Answer:
Upon completely discharging or at equilibrium, $E_{cell} = 0$ and $\Delta _{r}G= 0$.
Question 31: What does the negative sign in the expression $E^{\Theta }_{Zn^{2+}/Zn}=-0.76\; V$ mean ?
Answer:
$E^{\Theta }_{Zn^{2+}/Zn}=-0.76\; V$
Zinc has a higher reducing power than Hydrogen it has a negative $E^{0}$ while it is zero for Hydrogen
$Zn+H_{2}SO_{4}\rightarrow ZnSO_{4}+H_{2}$
Answer:
According to Faraday's second law of electrolysis amount of different substances liberated by the same quantity or electricity that passes through an electrolyte solution is directly proportional to their chemical equivalent weight.
W1/W2=E1/E2
where E1 and E2 have different values depending on a number of electrons required to reduce the metal ion.
Hence, the masses of Cu and Ag deposited will be different.
Question 33: Depict the galvanic cell in which the cell reaction is $Cu + 2Ag^+\rightarrow 2Ag + Cu^{2+}$
Answer:
$Cu\left | Cu^{2+} \right |\left | Ag^{+} \right |Ag$
Answer:
Under the conditions of electrolysis of aqueous sodium chloride, oxidation of water at the anode requires overpotential hence Cl- is oxidized instead of water
Question 35: What is electrode potential?
Answer:
The electrical potential difference set up between the metal and its solution is called the electrode potential.
Question 36: Consider the following diagram in which an electrochemical cell is coupled to an electrolytic cell. What will be the polarity of electrodes ‘A’ and ‘B’ in the electrolytic cell?
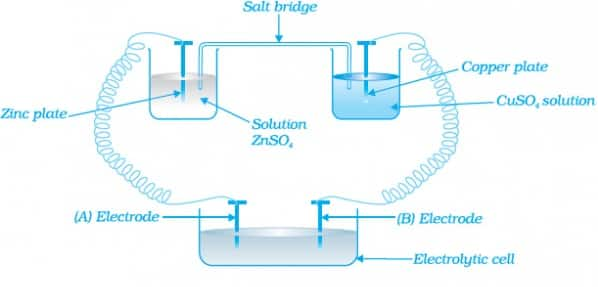
Answer:
‘A’ has negative and ‘B’ has positive polarity.
Question 37: Why is alternating current used for measuring the resistance of an electrolytic solution?
Answer:
Direct current will lead to electrolysis of the solution, which will change the concentration of ions in the solution. The use of an Alternating current will prevent this from happening.
Answer:
Cell reaction ceases if the opposing potential is equal to the electrical potential.
Question 39: How will the pH of brine (aq. NaCl solution) be affected when it is electrolyzed?
Answer:
Upon electrolysis of Brine solution, the following reactions take place:
Cathode :$2H^{+}+2e^{-}\rightarrow H_{2}$
Anode : $2Cl^{-}\rightarrow Cl_{2}+2e^{-}$
The remaining $Na^{+}$ and $OH^{-}$ ions are responsible for turning the solution basic and thus increasing the pH.
Question 40: Unlike dry cells, the mercury cell has a constant cell potential throughout its useful life. Why?
Answer:
The mercury cell has a constant cell potential throughout its useful life because the Ions are not involved in the overall cell reaction of mercury cells.
Answer:
The lower increase of $\wedge _{m}$ for Electrolyte B is due to the complete ionization of the same. Hence, It is the stronger electrolyte.
Answer:
The following reaction occurs:
At Anode : $2H_{2}O\rightarrow O_{2}+4H^{+}+4e^{-}$
At cathode : $4H^{+}+4e^{-}\rightarrow 2H_{2}(\uparrow)$
As the concentration of H+ ions is maintained, there will be no change in pH.
Question 43: In an aqueous solution, how does the specific conductivity of electrolytes change with the addition of water?
Answer:
The concentration of ions will decrease in the addition of water, which in turn will reduce the electrical conductivity.
Question 44: Which reference electrode is used to measure the electrode potential of other electrodes?
Answer:
The standard hydrogen electrode is used as the reference electrode. For other electrodes, we measure the electrode potential considering the electrode potential for standard hydrogen electrodes to be zero.
Answer:
The given cell is :
$Cu (s)\left | Cu^{2+} \right |\left | Cl^{-} \right |Cl_{2}(pt)$
Anode : $Cu (s)\rightarrow Cu^{2+}+2e^{-}$
Cathode : $Cl_{2}(g)+2e^{-}\rightarrow 2Cl^{-}$
Answer:
Daniel Cell :
$Zn\; (s)\left |Zn^{2+} \right |\left | Cu^{2+} \right |Cu\; (s)$
Anode :
$Zn(s)\rightarrow Zn^{2+}+2e^{-}$
Cathode :
$Cu^{2+}+2e^{-}\rightarrow Cu (s)$
Overall cell reaction :
$Zn (s)+Cu^{2+}\rightleftharpoons Zn^{2+}+Cu (s)$
$Q=\frac{[Zn^{2+}]}{[Cu^{2+}]}$
$E_{cell}=E_{cell}^{0}-\frac{0.059}{2}log \; \frac{[Zn^{2+}]}{[Cu^{2+}]}$
Therefore, with increasing concentration of $Zn^{2+}$, the cell potential decreases.
Question 47: What advantage do the fuel cells have over primary and secondary batteries?
Answer:
Primary batteries come with a limited number of reactants and can’t be reused once they are discharged. Secondary batteries take a considerable amount of time to recharge. Fuel cells is superior to both primary and secondary batteries as they operate without any breaks as long as you keep supplying the reactants
Answer:
During Discharge: $Pb+PbO_2+2H_2 SO_4\rightarrow 2PbSO_4+2H_2 O$
As water is formed during the discharge process, the concentration of electrolyte reduces.
Answer:
For weak electrolytes like $CH_{3}COOH,$ on dilution, the concentration of ions increases due to the increase in the degree of dissociation, but for strong electrolytes like $CH_{3}COONa$, the number of ions remains constant upon dilution
NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: Matching Type
Matching Type questions are generally asked in exams to test your knowledge. These are quite helpful for competitive exams.
Question 50: Match the terms given in Column I with the units given in Column II.
|
Column I |
Column II |
|
(i) $\wedge _{m}$ |
(a) S cm-1 |
|
(ii) $E _{cell}$ |
(b) m-1 |
|
(iii) $\kappa$ |
(c) S cm2 mol -1 |
|
(iv) $G^{*}$ |
(d) V |
Answer:
$(\mathrm{i} \rightarrow \mathrm{c}),(\mathrm{ii} \rightarrow \mathrm{d}),(\mathrm{iii} \rightarrow \mathrm{a}),(\mathrm{iv} \rightarrow \mathrm{b})$
Question 51: Match the terms given in Column I with the items given in Column II.
|
Column I |
Column II |
|
(i) $\wedge _{m}$ |
(a) intensive property |
|
(ii) $E_{cell}^{\Theta }$ |
(b) depends on the number of ions/volume |
|
(iii) $\kappa$ |
(c) extensive property |
|
(iv) $\Delta_{r} G_{cell}$ |
(d) increases with dilution |
Answer:
$(\mathrm{i} \rightarrow \mathrm{d}),(\mathrm{ii} \rightarrow \mathrm{a}),(\mathrm{iii} \rightarrow \mathrm{b}),(\mathrm{iv} \rightarrow \mathrm{c})$
Question 52: Match the items of Column I and Column II.
|
Column I |
Column II |
|
(i) Lead storage battery |
(a) maximum efficiency |
|
(ii) Mercury cell |
(b) Prevented by galvanization |
|
(iii) Fuel cell |
(c) gives steady potential |
|
(iv) Rusting |
(d) Pb is anode, PbO2 is cathode |
Answer:
$(\mathrm{i} \rightarrow \mathrm{d}),(\mathrm{ii} \rightarrow \mathrm{c}),(\mathrm{iii} \rightarrow \mathrm{a}),(\mathrm{iv} \rightarrow \mathrm{b})$
Question 53: Match the items of Column I and Column II.
|
Column I |
Column II |
Answer:
$(\mathrm{i} \rightarrow \mathrm{d}),(\mathrm{ii} \rightarrow \mathrm{c}),(\mathrm{iii} \rightarrow \mathrm{b}),(\mathrm{iv} \rightarrow \mathrm{a})$
Question 54: Match the items of Column I and Column II.
|
Column I |
Column II |
|
i) Lechlanche cell |
(a) cell reaction $2H_{2}+O_{2}\rightarrow 2H_{2}O$ |
|
ii) Ni-Cd cell |
(b) does not involve any ion in solution and is used in hearing aids. |
|
iii) Fuel cell |
(c) rechargeable |
|
iv) Mercury cell |
(d) reaction at anode, $Zn\rightarrow Zn^{2+}+2e^{-}$ |
|
|
(e) converts the energy of combustion into electrical energy. |
Answer:
$(\mathrm{i} \rightarrow \mathrm{d}),(\mathrm{ii} \rightarrow \mathrm{c}),(\mathrm{iii} \rightarrow \mathrm{a,e}),(\mathrm{iv} \rightarrow \mathrm{b})$
|
Column I |
Column II |
|
(a) metal is the strongest reducing agent | |
|
(b) metal ion which is the weakest oxidizing agent | |
|
(c) non-metal which is the best oxidizing agent | |
|
(d) unreactive metal | |
|
(e) anion that can be oxidized by $Au^{3+}$ | |
|
(f) Anion which is the weakest reducing Agent | |
|
(g) metal ion, which is an oxidizing agent |
Answer:
$(\mathrm{i} \rightarrow \mathrm{c}),(\mathrm{ii} \rightarrow \mathrm{a}),(\mathrm{iii} \rightarrow \mathrm{g}),(\mathrm{iv} \rightarrow \mathrm{e}),(\mathrm{v} \rightarrow \mathrm{d}),(\mathrm{vi} \rightarrow \mathrm{b}), (\mathrm{vii} \rightarrow \mathrm{g,f})$
NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: Assertion and Reason Type
Assertion and reason type questions of Chapter 3 are discussed below. These Class 12 Chemistry Electrochemistry questions and answers will improve your critical thinking. The most typical and important section for exams.
Question 56: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion:$Cu$ is less reactive than hydrogen.
Reason:$E_{Cu^{2+}/Cu}^{\circleddash }$ is negative
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true and the Reason is not the correct explanation for the Assertion
(iii) The assertion is true, but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false, but the Reason is true.
Answer:
The answer is option (iii) As $E_{Cu^{2+}/Cu}^{\circleddash }$ is positive, Copper is less reactive than hydrogen.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true and the Reason is not the correct explanation for the Assertion
(iii) The assertion is true but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false, but the Reason is true.
Answer:
The answer is the option (iii) $E_{cell}=E_{cathode}-E_{anode}$. To have a positive value of $E_{cell}, E_{cathode}>E_{anode}$
Question 58: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion: Conductivity of all electrolytes decreases on dilution.
Reason: On dilution number of ions per unit volume decreases.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true and the Reason is not the correct explanation for the Assertion
(iii) The assertion is true, but the Reason is false.
(iv) Both Assertion and Reason are false.
(v)The Assertion is false, but the Reason is true.
Answer:
The answer is option (I). Upon dilution, the concentration of ions decreases, and hence, conductivity also decreases.
Question 59: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion: $\wedge _{m}$ for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
Reason: For weak electrolytes degree of dissociation increases with a dilution of the solution.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true and the Reason is not correct explanation for the Assertion
(iii) The assertion is true, but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false, but the Reason is true.
Answer:
The answer is option (I). Degree of dissociation of weak electrolytes increases on dilution, which results in a sharp increase in $\wedge _{m}$ values.
Question 60: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion: Mercury cell does not give steady potential.
Reason: In the cell reaction, ions are not involved in the solution.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true and the Reason is not the correct explanation for the Assertion
(iii) The assertion is true, but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false butthe Reason is true.
Answer:
The answer is option (v). Mercury cell maintains a constant cell potential because the electrolyte isn’t consumed in the cell process
Question 61: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion: Electrolysis of $NaCl$ solution gives chlorine at anode instead of $O_{2}$.
Reason: Formation of oxygen at the anode requires overvoltage.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true, and the Reason is not the correct explanation for the Assertion
(iii) The assertion is true but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false but Reason is true.
Answer:
The answer is the option (i). Though the $E^{o}$ value for the formation of oxygen is lower than that for the formation of chlorine, it is not formed because it requires overvoltage.
Question 62: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion: For measuring the resistance of an ionic solution an AC source is used.
Reason: The concentration of ionic solution will change if a DC source is used.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true and the Reason is not the correct explanation for the Assertion
(iii) The assertion is true but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false but the Reason is true.
Answer:
The answer is option (i). Direct current will lead to electrolysis of the solution, which will change the concentration of ions in the solution. Use of an Alternating current will prevent this from happening
Question 63: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion: Current stops flowing when $E_{cell}=0.$
Reason: Equilibrium of the cell reaction is attained.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true and the Reason is not the correct explanation for the Assertion
(iii) The assertion is true, but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false, but Reason is true.
Answer:
The answer is the option (i) At equilibrium, $E_{cell}=0$ and no current flows.
Question 64: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion:$E_{Ag^{+}/Ag}$ increases with increase in concentration of $Ag^{+}$ ions.
Reason:$E_{Ag^{+}/Ag}$ has a positive value.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for the Assertion.
(ii) Both Assertion and Reason are true and the Reason is not correct explanation for the Assertion
(iii) The assertion is true but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false but Reason is true.
Answer:
The answer is option (ii) Both Assertion and Reason are true and the Reason is not the correct explanation for Assertion
Question 65: In the following question, a statement of Assertion (i) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices:
Assertion: Copper sulfate can be stored in a zinc vessel.
Reason: Zinc is less reactive than copper.
(i) Both Assertion and Reason are true and the Reason is the correct explanation for Assertion.
(ii) Both Assertion and Reason are true and the Reason is not the correct explanation for Assertion
(iii) Assertion is true but the Reason is false.
(iv) Both Assertion and Reason are false.
(v) Assertion is false but Reason is true.
Answer:
The answer is option (iv) As Zinc is more reactive than Copper, Zinc dissolves in $CuSO_{4}$ solution.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 3: Long Answer Type
The following are long-answer type questions of Electrochemistry solutions that require in-depth understanding and thorough practice. These are important questions from NCERT Chemistry chapter 3 that are frequently asked in examinations.
Answer:
(i) As the potential of ‘B’ is lower than the potential of ‘A’, it will act as the electrolytic cell. The reactions at electrode ‘B’ are shown below:
Cathode : $Zn^{2+}+2e^{-}\rightarrow Zn(s)$
Anode: $Cu (s)\rightarrow Cu^{2+}+2e^{-}$
(ii) At higher potential, Cell ‘B’ acts as a galvanic cell and the reactions will be:
Anode :$Zn(s)\rightarrow Zn^{2+}+2e^{-}$
Cathode : $Cu^{2+}+2e^{-}\rightarrow Cu(s)$
(i) The cell reaction can be summarised as:
$Zn(s)\left | Zn^{+2} \right |\left | Ag^{+} \right |Ag$
Electrons move from Zn to Ag.
(ii) Due to a higher standard reduction potential, Silver will act as a Cathode and in the external circuit, electrons will flow from the zinc anode to the silver cathode.
(iii) Removal of the salt bridge will lead to a sudden drop in the potential to zero.
(iv) If the potential reaches zero (or the cell is discharged), all reactions will cease and the cell will stop functioning.
(v) Nernst equation for the cell is: 0.059,
$E=E^{0}-\frac{0.059}{2}log\frac{[Zn^{2+}]}{[Ag^{+}]^{2}}$
With the increase in the concentration of $[Zn^{+2}]$, cell potential will decrease, and with an increase in the concentration of $[Ag^{+}]$, cell potential will increase.
(vi) At equilibrium (discharged state, potential drop to zero), the concentration of $[Zn^{+2}]$ and $[Ag^{+}]$ will not change.
Answer:
The required relationship between Gibbs free energy and the emf in a galvanic cell is $\Delta G=-nFE$
The maximum work will be obtained from a galvanic cell $W_{max}=nFE^{0}$ where,
E=cell potential
$E^{0}$ = Standard emf of the cell
Class 12 Chemistry NCERT Chapter 3: Higher Order Thinking Skills (HOTS) Questions
The NCERT class 12 chemistry chapter 3 questions are given below that will help you tackle complex problems. Students can follow Electrochemistry class 12 notes to learn the concepts in detail.
Question 1: Given below are two statements :
1 M aqueous solution of each of $\mathrm{Cu}\left(\mathrm{NO}_3\right)_2$, $\mathrm{AgNO}_3, \mathrm{Hg}_2\left(\mathrm{NO}_3\right)_2, \mathrm{Mg}\left(\mathrm{NO}_3\right)_2$ are electrolysed using inert electrodes,
Given : $\mathrm{E}_{\mathrm{Ag}^* / \mathrm{Ag}}^0=0.80 \mathrm{~V}, \mathrm{E}_{\mathrm{Hg}_2^{2+} / \mathrm{Hg}}^0=0.79 \mathrm{~V}$, $\mathrm{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^0=0.24 \mathrm{~V}$ and $\mathrm{E}_{\mathrm{Mg}^{2+} / \mathrm{Mg}}^0=-2.37 \mathrm{~V}$
Statement (I) : With increasing voltage, the sequence of deposition of metals on the cathode will be $\mathrm{Ag}, \mathrm{Hg}$ and Cu
Statement (II) : Magnesium will not be deposited at cathode instead oxygen gas will be evolved at the cathode.
In the light of the above statement, choose the most appropriate answer from the options given below
1) Both statement I and statement II are incorrect
2) Statement I is correct but statement II is incorrect
3) Both statement I and statement II are correct
4) Statement I is incorrect but statement II is correct
Answer:
Due to higher Standard reduction potential, Ag will deposit first followed by Hg and Cu . Magnesium will not deposit, rather $\mathrm{H}_2 \mathrm{O}$ will get reduced due to higher SRP and $\mathrm{H}_2$ gas will be produced.
$2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-} \rightleftharpoons \mathrm{H}_2+2 \mathrm{OH}^{-} \mathrm{E}^{\circ}=-0.827 \mathrm{~V}$
Hence, the correct answer is option (2).
Question 2: $\mathrm{O}_2$ gas will be evolved as a product of electrolysis of:
(A) an aqueous solution of $\mathrm{AgNO}_3$ using silver electrodes.
(B) an aqueous solution of $\mathrm{AgNO}_3$ using platinum electrodes.
(C) a dilute solution of $\mathrm{H}_2 \mathrm{SO}_4$ using platinum electrodes.
(D) a high concentration solution of $\mathrm{H}_2 \mathrm{SO}_4$ using platinum electrodes.
Choose the correct answer from the options given below :
1) (B) and (C) only
2) (A) and (D) only
3) (B) and (D) only
4) (A) and (C) only
Answer:
During the electrolysis of an aqueous $\mathrm{AgNO}_3$ solution using silver electrodes, silver ions $\left(\mathrm{Ag}^{+}\right)$from the solution are reduced at the cathode, depositing silver metal. At the anode, the silver electrode itself undergoes oxidation, releasing $\mathrm{Ag}^{+}$ions into the solution instead of producing oxygen gas. As a result, $\mathrm{O}_2$ gas is not evolved in this case because the anode dissolves rather than oxidizing water. On the other hand, in the electrolysis of a dilute $\mathrm{H}_2 \mathrm{SO}_4$ solution using platinum electrodes, hydrogen gas $\left(\mathrm{H}_2\right)$ is released at the cathode due to the reduction of $\mathrm{H}^{+}$ions. At the anode, water molecules undergo oxidation, producing oxygen gas $\left(\mathrm{O}_2\right)$ and releasing protons into the solution. This means that $\mathrm{O}_2$ gas is evolved at the anode in the electrolysis of dilute sulfuric acid with platinum electrodes.
Hence, the correct answer is option (1).
Question 3: If the molar conductivity $\left(\Lambda_{\mathrm{m}}\right)$ of a $0.050 \mathrm{~mol} \mathrm{~L}^{-1}$ solution of a monobasic weak acid is $90 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$, its extent (degree) of dissociation will be [Assume $A_{+}^{\circ}=349.6 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$ and $A_{-}^{\circ}=50.4 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$]
(1) 0.215
(2) 0.115
(3) 0.125
(4) 0.225
Answer:
To calculate the degree of dissociation ( $\alpha$ ) of the weak monobasic acid, we use the formula:
$
\alpha=\frac{\Lambda_m}{\Lambda_m^{\circ}}
$
Given that
$\begin{aligned} & \Lambda_m=90 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1} \\ & \Lambda_m^{\circ}=A_{+}^{\circ}+A_{-}^{\circ}=349.6+50.4=400 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\end{aligned}$
so put the value in $
\alpha=\frac{\Lambda_m}{\Lambda_m^{\circ}}
$
$\alpha=\frac{90}{400}=0.225$
Hence, the correct answer is option (4).
Question 4: For the cell, $\mathrm{Pt}\left|\mathrm{H}_2(\mathrm{~g})\right|$ solution $\mathrm{X} \| \mathrm{KCl}$ (saturated) $\left|\mathrm{Hg}_2 \mathrm{Cl}_2\right| \mathrm{Hg} \mid \mathrm{Pt}$, the observed EMF at $25^{\circ} \mathrm{C}$ was 600 mV . When solution X was replaced by a standard phosphate buffer with $\mathrm{pH}=$ 7.00, then EMF was 777 mV . The pH of solution X is $\left(u s e \frac{2.303 R T}{F}=0.059 \mathrm{~V}\right)$
Answer:
$\begin{aligned} & \mathrm{H}_2 \rightarrow 2 \mathrm{H}^{+}+2 e^{-} \\ & \mathrm{Hg}_2 \mathrm{Cl}_2+2 e^{-} \rightarrow 2 \mathrm{Hg}+2 \mathrm{Cl}^{-} \\ & \mathrm{H}_2+\mathrm{Hg}_2 \mathrm{Cl}_2 \rightarrow 2 \mathrm{H}^{+}+2 \mathrm{Cl}^{-}+2 \mathrm{Hg} \\ & E_{\text {initial }}=E^{\circ}+\frac{0.059}{2} \log \frac{1}{\left[\mathrm{H}^{+}\right]^2\left[\mathrm{Cl}^{-}\right]^2}=0.6 \\ & E_{\text {final }}=E^{\circ}+\frac{0.059}{2} \log \frac{1}{\left[10^{-7}\right]^2\left[\mathrm{Cl}^{-}\right]^2}=0.777\end{aligned}$
On subtracting we get, $0.177=0.059 \log \frac{\left[\mathrm{H}^{+}\right]}{10^{-7}}$
$\begin{aligned} & \Rightarrow \frac{\left[H^{+}\right]}{10^{-7}}=1000 \Rightarrow\left[H^{+}\right]=10^{-4} M \\ & \Rightarrow p H=4 .\end{aligned}$
Hence, the answer is 4.
Question 5: Molar conductivity of $0.1 \mathrm{M} \mathrm{CH}_3 \mathrm{COOH}$ at certain temperature is $256.62 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$. What is its pH ?
$\left[\lambda_{\mathrm{H}^{+}}^0=349.6 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}, \lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^0=78.1 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1} \text { and } \log (6)=0.78\right]$
(1) 3.21
(2) 1.22
(3) 2.31
(4) 4.73
Answer:
$\begin{aligned} & \alpha=\frac{\lambda_{\mathrm{m}}}{\lambda_{\mathrm{m}}^{\circ}}=\frac{256.62}{427.7}=0.6 \\ & {\left[\mathrm{H}^{+}\right]=\mathrm{C} \alpha=0.1 \times 0.6=0.06} \\ & \mathrm{pH}=-\log \left[\mathrm{H}^{+}\right]=1.22\end{aligned}$
Hence, the correct answer is option (2).
Approach to Solve Questions of Chapter 3 Electrochemistry.
To master Electrochemistry, it's essential to understand core concepts of Electrochemistry. The approaches given below will help you solve class 12 chemistry chapter 3 Electrochemistry questions with confidence.
1. Before jumping into problems, make sure you have understood the topics clearly. Some important concepts are
- Redox reactions and their balancing
- Electrochemical cells
- Standard electrode potentials
- Nernst Equation
- Conductance and Kohlrausch’s Law
- Electrolysis and Faraday’s laws
2. Memorize and understand how to apply the formulas in the question; some of them are given here,
(1). Nernst equation-
$
E=E^{\circ}-\frac{0.0591}{n} \log Q
$
(2). Cell potential-
$
E_{\text {cell }}=E_{\text {cathode }}^{\circ}-E_{\text {anode }}^{\circ}
$
(3). Faraday's Laws-
First law: $m=\frac{Z I t}{1000}$
Second law relates mass of different substances
3. First identify the type of questions asked and then try to apply the formulas or the concepts related to the topic. Also check if the question requires graphical topics like conductivity vs. dilution
4. Read the question carefully and note the given values. Solve the question in stepwise manner and do put correct units. Check electrode potentials like what is cathode or anode in the cell.
5. Solve the NCERT in-text and exercise questions and refer to the solved examples. You can also attempt previous years’ board questions for better learning. Make the use of concept maps or flashcards for definitions and laws.
Formulas Of NCERT Class 12 Chemistry Chapter 3
Below are the important formulas used in the NCERT Exemplar Class 12 Chemistry Solutions Chapter 3 Electrochemistry.
1. Conductance(G) is the reciprocal of resistance (R) and specific conductance or conductivity(k) is inverse of resistivity ( $\rho$ )
$
G=\frac{1}{R}=\frac{1}{\rho}\left(\frac{a}{l}\right) k=G\left(\frac{l}{a}\right)
$
2. I/a is called the cell constant of conductivity cell.
3. Equivalent Conductivity is defined as the conductance of a solution containing 1 g of an electrolyte.
$
\Lambda_{e q}=K \times V
$
4. Nernst equation
$
\mathrm{aA}+\mathrm{bB} \rightarrow \mathrm{cC}+\mathrm{dD}
$
$
E_{\text {cell }}=E_{\text {cell }}^o-\frac{0.0591}{n} \log \frac{[C]^c[D]^d}{[A]^a[B]^b}
$
Advantages of Using NCERT Exemplar Class 12 Chemistry Solutions Chapter 3
The NCERT Exemplar Class 12 Chemistry Solutions Chapter 3 Electrochemistry provide a clear explanation of concepts that helps in mastering topics and scoring well in board and competitive exams. The advantages of using these solutions are given below:
- These solutions are designed to simplify the concepts like redox reactions, electrode potential, and electrochemical cells using solved questions.
- Students can revise concepts and questions using these NCERT Exemplar Class 12 Solutions before exams.
- These solutions are prepared by experts in a very detailed manner and they also include diagrams and equations.
- Formulas for Nernst equation, EMF, and conductance are summarised in these Class 12 Chemistry Chapter 3 Electrochemistry solutions.
Topics of NCERT Exemplar Solutions Class 12 Electrochemistry
Below are the important topics covered in the Class 12 NCERT Chemistry chapter 3.
- Electrochemical Cells
- Galvanic Cells
- Nernst Equation
- Conductance of Electrolytic Solutions
- Electrolytic Cells and Electrolysis
- Products of Electrolysis
- Batteries
- Primary Batteries
- Secondary Batteries
- Fuel Cells
- Corrosion
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Exemplar Class 12 Solutions Subject-wise
Here is a list of NCERT Exemplar Solution Subject-wise :
NCERT Solution subject-wise
The hyperlinks of NCERT exemplar of class 12 are given below
NCERT Notes subject-wise
The hyperlinks of the NCERT solution of class 12 are given below:
NCERT Books and NCERT Syllabus
Students can refer to the links given below for the NCERT books and Syllabus:
Frequently Asked Questions (FAQs)
Electrochemistry is the branch of chemistry that studies the relationship between electrical energy and chemical reactions. It deals with the processes that involve the transfer of electrons, which can lead to the generation of electricity through spontaneous chemical reactions.
Electrolytes are substances that dissociate into ions when dissolved in water, allowing the solution to conduct electricity. Examples include salts, acids, and bases. Non-electrolytes, on the other hand, do not dissociate into ions in solution, and therefore do not conduct electricity.
Difference between a voltaic (galvanic) cell and an electrolytic cell
Voltaic (Galvanic) Cell: This type of cell uses a spontaneous redox reaction to generate electrical energy. It is the basis of batteries. In this cell, Delta G is negative.
Electrolytic Cell: This type of cell uses electrical energy to drive a non-spontaneous redox reaction. It is used in processes such as electroplating and the production of certain chemicals. In this cell, Delta G is positive.
Standard electrode potentials are measurements of the potential of half-cells against a standard hydrogen electrode at standard conditions. They are determined experimentally through electrochemical measurements and are crucial for predicting the direction and feasibility of redox reactions.
Class 12 Chemistry Chapter 3 Electrochemistry solutions are detailed, step-by-step answers to advanced questions , helping students strengthen concepts, practise higher-level problems, and prepare effectively for board and competitive exams.
You can download revision PDFs and solved questions from the official NCERT website or trusted educational platforms that provide free, chapter-wise study materials for Class 12 Chemistry.
It is a set of detailed answers to the higher-level questions in the NCERT Exemplar book, helping Class 12 students strengthen concepts, practise advanced problems, and prepare for board and competitive exams.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters



