Atoms Class 12th Notes - Free NCERT Class 12 Physics Chapter 12 Notes - Download PDF
Do you now how tube lights, neon signs, or fireworks can get such vivid colours? One word: atoms. The very structure of atoms and the way atoms emit light — it is through this concept that you get to NCERT Class 12 Physics Chapter 12 Notes Atoms.
This Story also Contains
- NCERT Class 12 Physics Chapter 12 Notes: Download PDF
- NCERT Class 12 Physics Chapter 12 Notes
- Atoms: Previous Year Question and Answer
- Importance of NCERT Class 12 Physics Chapter 12 Notes
- NCERT Notes Class 12 Chapterwise
- NCERT Books and Syllabus
Once you will be through the material, you will know about Rutherford’s Nuclear Model, Atomic Spectra, Bohr’s Model of Hydrogen Atom, and the De Broglie Hypothesis with NCERT Class 12 Physics Chapter 12 Notes Atoms. These NCERT notes state how the first discoveries led to the modern atomic theory along with strengths and weaknesses of the theory. The chapter has a very significant place in the CBSE board exams as well as the competitive exams like JEE Main and NEET, from where the numerical as well as theoretical questions are commonly asked. These Class 12 NCERT Notes cater to the needs of students by presenting complex concepts through clear diagrams, step-by-step explanations, and exam-focused summaries, thus making them a convenient tool for revision and last-minute preparation. These NCERT Class 12 Physics Chapter 12 Notes PDF prepared by experts help you to lay a solid foundation for modern physics and enhance your exam performance.
Also, students can refer,
NCERT Class 12 Physics Chapter 12 Notes: Download PDF
Download the NCERT Class 12 Physics Chapter 12 Notes Atoms PDF free of cost and have a quick revision of the class 12 physics chapter in a simplified manner. These notes deal with Rutherford’s Model, Bohr’s Theory, Atomic Spectra, and De Broglie Hypothesis with the help of easy explanations, diagrams, and important formulas. These are also very helpful to revise the whole syllabus quickly before CBSE board exams, JEE, and NEET.
NCERT Class 12 Physics Chapter 12 Notes
NCERT Class 12 Physics Chapter 12 Notes simplify the complicated concepts that atomic models, spectra, and quantum theory deal with, in very easy points to understand by students. The step-wise explanation of a concept, the provision of diagrams, and the use of formulae make these notes perfect for CBSE board exams as well as for other competitive exams like JEE and NEET.
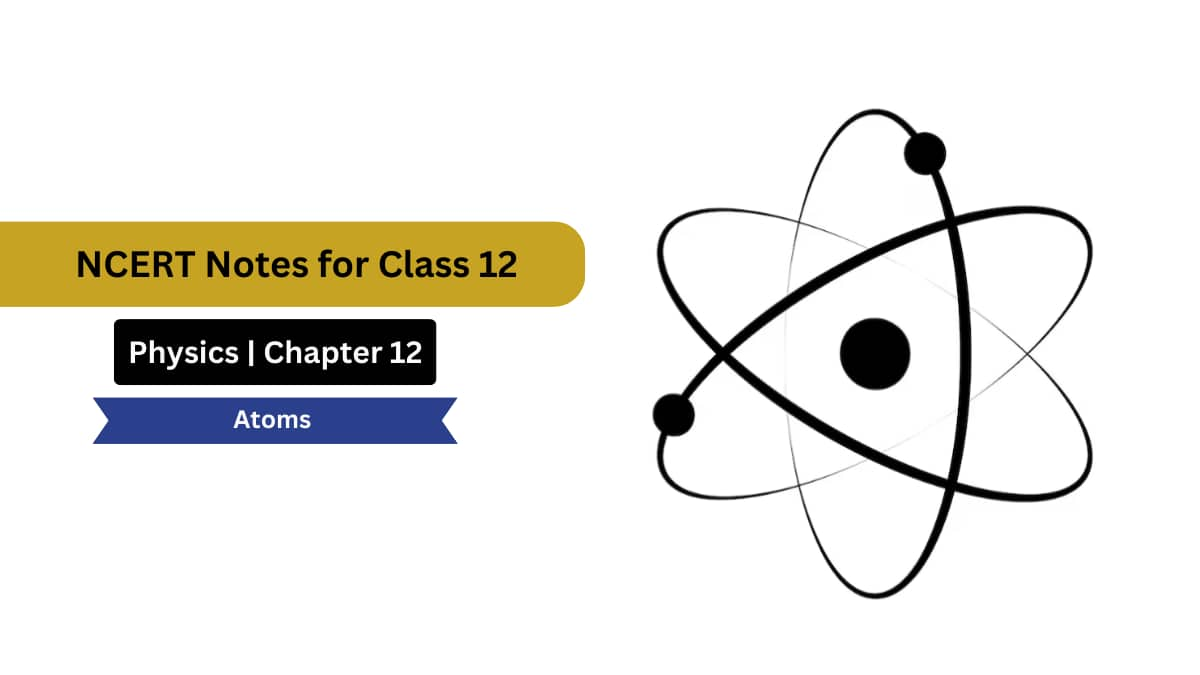
Thomson’s Atomic Model (Plum Pudding Model)
J. J. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. In Thomson’s model, the atom is composed of electrons surrounded by a soup of positive charge to balance the electrons’ negative charges, like negatively charged “plums” surrounded by positively charged “pudding”.
Drawbacks
- Failed to explain the results from the alpha-particle scattering experiment.
- Failed to explain atomic spectra.
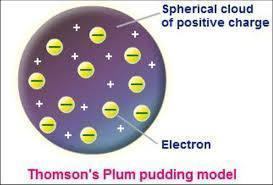
Alpha Particle Scattering and Rutherford’s Nuclear Model of the Atom
Rutherford and his colleagues, Geiger and Marsden bombarded a thin gold foil of thickness approximately
Observations:
- Most of the alpha particles behaved as expected, but there was a noticeable fraction of particles that got scattered by angles greater than 90 degrees.
- There were about 1 in every 2000 particles that got scattered by a full 180 degrees.
- The distribution of scattered α-particles was analysed and plotted as a graph as a function of scattering angle (θ).
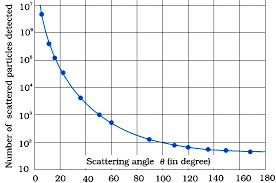
For a detector at a specific angle
Conclusion:
-
Most of the atom is empty space, since most particles pass undeflected.
-
The positive charge and mass of the atom are concentrated in a very small, dense region, which repelled the few alpha particles that were deflected. This region was named the nucleus.
-
Electrons revolve around the nucleus in circular orbits at very high speeds, like planets orbiting the Sun.
-
The atom remains electrically neutral as the positive charge of protons is balanced by the negative charge of electrons.
-
The electrostatic force of attraction between electrons and protons provides the centripetal force needed to keep the electrons in their orbits.
Limitations:
-
Could not explain the stability of the atom (electrons should spiral into the nucleus).
-
Did not explain the discrete spectral lines of hydrogen.
-
Could not describe atoms with more than one electron.
Alpha-particle Trajectory
We can understand the trajectory of alpha particles by calculating the distance of closest approach and the impact parameter.


Distance of Closest Approach
The distance of the closest approach in Rutherford scattering refers to the minimum distance between an incoming alpha particle and the nucleus of the target atom during a scattering event. At this distance, the entire initial kinetic energy has been converted into potential energy.
Impact Parameter
Impact parameter is defined as the perpendicular distance of the velocity of the alpha particle from the centre of the nucleus when it is far away from the atom. The shape of the trajectory of the scattered alpha particle depends on the impact parameter 'b' and the nature of the potential field. Rutherford deduced the following relationship between the impact parameter 'b' and the scattering angle
Electron Orbits
According to Rutherford, the electrostatic force of attraction provided the required centripetal force for orbit.
Here,
r=radius of orbit,
v = velocity of orbiting electron,
e = charge of an electron,
m = mass of an electron,
Z = atomic mass of the atom,
ε0= permittivity of free space
Kinetic Energy(K):
Potential Energy(U):
Using the electrostatic potential between 2 charged bodies, we get:
Here, negative signs show that there is a force of attraction, and energy has to be given to the system to overcome this force of attraction.
Total Energy(T):
T = U + K
Some important relations to note:
Kinetic energy (K) = -(1/2)Potential energy(U)
Kinetic energy (K) = -Total energy(T)
Potential energy(U) = 2×Total energy(T)
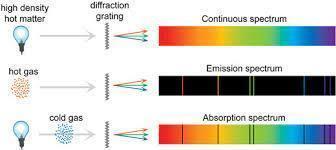
Atomic Spectra
- When electrons in an atom absorb energy, they jump to higher energy levels (excited state).
- When they return to lower energy levels (ground state), they emit energy in the form of light.
- This emitted light, when passed through a prism, shows discrete lines known as the line spectrum or atomic spectrum.
- Each element has a unique atomic spectrum.
- These spectral lines correspond to specific wavelengths (energies) of emitted radiation.
- The atomic spectrum of hydrogen is divided into series based on the final energy level.
Bohr Model of the Hydrogen Atom
Proposed by Niels Bohr in 1913, this model explained the stability of the atom and the line spectra of hydrogen, improving upon Rutherford’s nuclear model. By combining classical and early quantum concepts, Bohr's Model of the hydrogen atom has three postulates.
(1) Atoms have certain definite stable states in which they can exist, and each possible state has a definite total energy. These are called the stationary states of the atom. In these states, electrons don't radiate energy.
For electrons revolving in a stable orbit, the necessary centripetal force is provided by the coulomb's force
(2) This postulate states that the electron revolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of
(3). Bohr’s third postulate states that when an electron makes a transition from a higher to a lower energy orbit, it emits a photon with energy equal to the energy difference between the initial and final states. The frequency of the emitted photon is then given by
Using the first and second postulates, we can find the radius of the electron orbit.
We can find the energy of an electron in a stable orbit using Rutherford's expression for total energy.
By putting the value of Bohr’s radius, we get
By putting the values of electron mass (m), charge (e), the permittivity of free space (ε0), and Planck’s constant (h), we get,
Energy Levels
In Bohr's atomic model, the energy levels of an electron in an atom are quantised. This means that electrons can only occupy specific, discrete energy levels and cannot exist in between these levels. The potential energy is always negative, indicating that the electron is bound to the nucleus.
The energy of
- Energy of ground state
- Energy of first excited state
- Energy of the second excited state
- Energy of the third excited state
Line Spectra of Hydrogen Atom
According to Bohr, when an atom makes a transition from a higher energy level to a lower energy level, it emits a photon with energy equal to the energy difference between the initial and final levels. If Ei, is the initial energy of the atom before such a transition, Ef is its final energy after the transition, then conservation of energy gives the energy of the emitted photon.
where
The Line Spectra Of the Hydrogen Atom and its different series can be seen from the diagram below.

De-Broglie's Explanation of Bohr's Second Postulate
De-Broglie explanation of Bohr's Second Postulate show us clearly as to why the angular momentum of the revolving electron is the integral multiple of the
For an electron moving in
We also know,
From the above equations, we have,
This is the quantum condition proposed by Bohr for the angular momentum of the electron. Thus, De-Broglie's hypothesis confirms Bohr’s second postulate for the quantisation of angular momentum of the orbiting electron.
Limitations of Bohr's Model
-
The model works well only for hydrogen and other single-electron systems (like He⁺, Li²⁺). It fails for multi-electron atoms (like helium, lithium, etc.).
-
Could not explain the fine structure or small splitting of spectral lines seen in high-resolution spectra.
-
Failed to explain: Zeeman and Stark effect – splitting of spectral lines in a magnetic and electric fields.
Atoms: Previous Year Question and Answer
Q.1 Write the short comings of Rthe utherford atomic model. Explain how these were overcome by the postulates of Bohr’s atomic model.
Answer:
Rutherford’s Model
1. While the electrons are moving around the nucleus, they'll lose energy constantly. After losing energy, they should fall into the nucleus ultimately. Hence atom should collapse, but in reality, this does not happen.
2. He could not explain the spectrum of an atom.
Bohr’s Atomic Model postulates
(i) Explained the stability of the nucleus by postulating that the electron can orbit the nucleus in only those special orbits in which it does not radiate energy.
(ii) Explained the line spectrum by postulating that the electron emits photons of well-defined, unique energies when it undergoes a transition from one permitted orbit to another.
Q.2 Use the Bohr model of the hydrogen atom to calculate the speed of the electron in the first excited state.
Answer:
The speed of the electron
First existed a state
Q.3 How is the stability of the hydrogen atom in the Bohr model explained by de Broglie's hypothesis?
Answer:
The quantised electron orbits and energy states are due to the wave nature of the electron, and only resonant standing waves can persist. Thus, the De Broglie hypothesis gives an explanation for Bohr’s second postulate.
According to de Broglie's hypothesis
Importance of NCERT Class 12 Physics Chapter 12 Notes
Foundation of Modern Physics
- Knowledge of atomic structure is the basis of modern physics and quantum mechanics. It is very important for CBSE Class 12, JEE, NEET exam preparation.
Explains Atomic Spectra
- It is very helpful for students to understand emission and absorption spectra of elements, which is a very important part of practical experiments and competitive exams.
Basis for Bohr Model
- Through this students are given ideas about electron orbits, energy levels, and transitions, which eventually become the main concepts of atomic theory and quantum physics.
Supports Quantum Mechanics Concepts
- Just the de Broglie hypothesis and wave-particle duality can be understood by atomic models.
Important for Numerical Problem Solving
- Frequently used are key formulas related to atomic energy levels, radius, and spectra in CBSE numerical problems, JEE, and NEET questions.
Applicable in Real-life Technologies
- Atomic concepts explain the occurrence of lasers, neon lights, X-rays, and electron microscopes, thus providing a link between theory and practice.
Crucial for Advanced Studies
- Students possessing a deep understanding of atoms will be ready to take up physics, chemistry, and engineering at the higher level.
Enhances Conceptual Understanding
- Photoelectric effect and atomic transitions become easy to understand through simplification of complex phenomena, and thus, conceptual clarity gets enhanced.
NCERT Notes Class 12 Chapterwise
NCERT Class 12 Physics chapterwise notes are the thoughtfully designed materials that help in simplifying difficult concepts and give a clear idea of each topic. The notes include the whole Class 12 NCERT Physics syllabus with brief explanations, key formulas, diagrams, and stepwise solutions of examples. These are not only suitable for CBSE board exams but also for various competitive exams such as JEE and NEET, thus making revision more convenient and efficient.
|
NCERT Class 12 Physics Chapter 12 Notes |
Subject Wise NCERT Exemplar Solutions
- NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12 Maths
- NCERT Exemplar Class 12 Physics
- NCERT Exemplar Class 12 Chemistry
- NCERT Exemplar Class 12 Biology
Subject Wise NCERT Solutions
NCERT Books and Syllabus
Frequently Asked Questions (FAQs)
To ionize an atom, the electron must be taken out of the atom completely. A free electron has a minimum total energy equal to 0. The magnitude of ionization energy is thus equivalent to the energy of an electron in the ground state. For a hydrogen atom, this is equal to 13.6 eV.
According to classical theory, accelerating electrons should radiate energy as electromagnetic radiation. If this were true, electrons would fall into the nucleus and hence, atoms would be unstable. Rutherford's model failed to explain why electron orbits are stable in nature.
The major limitation of Bohr's atomic model is that it is not applicable to multi-electron atoms. It is also unable to explain phenomena such as Zeeman and Stark effect.
Energy levels denote the stable orbits of an electron in an atom. It is the total energy of an electron in that state.
The important topics of Class 12 Physics Chapter 12 Atoms include Rutherford's gold foil experiment, postulates of Bohr's model of the hydrogen atom, and the line spectra of the hydrogen atom.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
