Dual nature of radiation and matter Class 12th Notes - Free NCERT Class 12 Physics Chapter 11 Notes - Download PDF
Have you ever seen solar panels that are placed on the roof of a house, turning the sunlight directly into electricity? This amazing process is the photoelectric effect, to be precise. The photoelectric effect is a phenomenon that you will find in the NCERT Class 12 Physics Chapter 11 Notes Dual nature of radiation and matter. The chapter explains the failure of the wave theory of light to provide experimental results like the photoelectric effect and Compton effect; thus, it introduces the character of photons, which is a new concept of the light-matter interaction.
This Story also Contains
- NCERT Class 12 Physics Chapter 11 Note: Download PDF
- NCERT Notes for Class 12 Physics Chapter 11
- Dual Nature of Radiation and Matter: Previous Year Question and Answer
- Importance of Dual Nature of Radiation and Matter Class 12 Notes
- NCERT Class 12 Notes Chapterwise
- NCERT Books and Syllabus
Class 12 Physics NCERT Notes are a great help while studying. They cover topics like the failure of wave theory, Einstein’s quantum explanation, photon energy, and the wave nature of matter, which is the main reason for the electron microscope. The NCERT Class 12 Physics Chapter 11 Notes Dual nature of radiation and matter contain simplified explanations, the main formulas, and solved examples, and they are also illustrated with clear diagrams. They are perfect for revising for the CBSE board or JEE and NEET preparation. Students can study the Dual Nature of Matter and Radiation Class 12 Notes PDF to learn the complex quantum concepts quickly, get an effective revision, and thus, their foundation in modern physics will be stronger.
Also, students can refer,
NCERT Class 12 Physics Chapter 11 Note: Download PDF
NCERT Class 12 Physics Chapter 11 Notes PDF is an insightful material that aids students in grasping the concepts related to the dual nature of light and matter. Topics covered in the chapter include the photoelectric effect, photon theory, and the wave nature of particles. Besides, this free PDF serves as the first source for last-minute revision, board exam preparation, and competitive exams like JEE & NEET. It consists of short explanatory texts, formulas, and solved examples, which make it an easy reference.
NCERT Notes for Class 12 Physics Chapter 11
NCERT Physics Class 12 notes for chapter 11 explain the intriguing concept of the dual nature of light and matter, along with the photoelectric effect, photon theory, and wave nature of particles. These notes with diagrams, key formulas, and solved examples are an excellent choice for CBSE board, JEE, and NEET preparation as they simplify difficult concepts.
Electron Emission
The attraction forces of the ions keep free electrons inside the metal surface. The electrons can only get out if it has enough energy to overcome the attractive pull. An electron must be supplied with a certain amount of energy in order to be pulled away from the metal's surface. The work function of a metal is the minimum energy required for an electron to escape from its metal surface.
The different types of Electron Emission are-
Thermionic emission:-
The free electrons can be given enough thermal energy to allow them to come out of the metal by adding heat.
Field emission:-
Electrons can be drawn out of metal by applying a very powerful electric field (of the order of 108 V m–1), as in a spark plug.
Photo-electric emission:-
Electrons are emitted when an appropriate frequency of light strikes a metal surface. Photoelectrons are electrons that are created by light.
Photoelectric Effect
Electrons are emitted from metallic surfaces when they are irradiated by electromagnetic radiation. This phenomenon is known as The Photoelectric Effect.
The electrons in the metal escape the pull of ions in the metal by absorbing energy from incident electromagnetic radiation.
Hertz’s Observation
Heinrich Hertz discovered in 1887 that when light strikes a metal surface, some electrons close to the surface acquire enough energy from the incident radiation to overcome the pull of the positive ions in the surface's substance. He did this in his study of the production of EM waves by spark discharge. He observed that the sparks were enhanced when the emitter plate was illuminated by UV light.
He concluded that the electrons escape from the metal's surface into the surrounding space after obtaining enough energy from the incoming light.
Hallwachs’ and Lenard’s Observations
Hallwachs and Lenard studied the photoelectric effect in detail between the years 1886 and 1902.
Lenard observed that when light was shown on an emitter plate between two electrodes, current started to flow. Both of them studied the variation of this photocurrent with collector plate potential, intensity of light and frequency of light. Furthermore, Hallwach observed that zinc plates became positively charged when exposed to UV light. This confirmed that negatively charged particles were emitted.
Through their study, they discovered that there is a minimum frequency of light, named the threshold frequency, below which no electrons are emitted, through a series of tests. This frequency varied from metal to metal.
Experimental Study of Photoelectric Effect
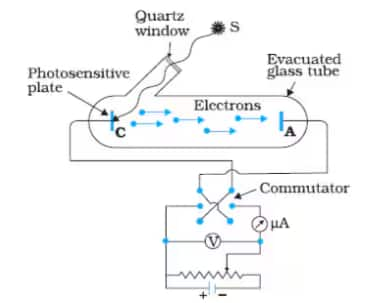
The following items make up the experimental setup:
The photosensitive plate (emitter) and the metal plate (collector) in an evacuated tube allow electrons to flow easily from emitter to collector without encountering any air resistance.
To absorb visible light and emit electrons, a photosensitive plate (emitter) is used.
A metal plate (collector) receives electrons released by the emitter, forming a photoelectric current flow from the collector to the emitter plate (opposite to the flow of electrons)
Short-wavelength monochromatic light (meaning high frequency)
Through a potential difference, a battery is used to accelerate emitted electrons.
Photoelectric current flow causes a potential difference between the emitter and collector plates, which can be measured with a voltmeter.
Photoelectric current is measured with an ammeter.
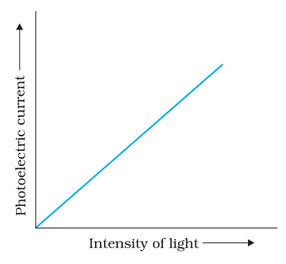
Effect of Intensity of Light on Photocurrent
The number of photoelectrons emitted per second is directly proportional to the photocurrent. This means that the rate at which photoelectrons are emitted is proportional to the intensity of incident energy.
Effect of Potential on Photoelectric Current
The stopping potential of incident radiation is independent of its intensity for a given frequency. In other words, photoelectrons' maximum kinetic energy is dependent only on the light source and the material of the emitter plate.
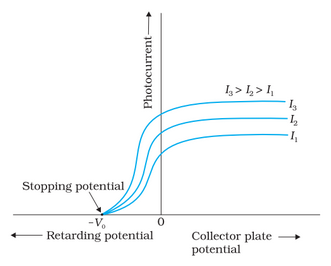
The cut-off or stopping potential in the above figure for a certain frequency of incident radiation is the least negative (retarding) potential V0 applied to plate A for which the photocurrent ends or becomes zero.
Effect of Frequency of Incident Radiation on Stopping Potential
The higher the frequency of incident light, the higher the photoelectrons' maximum kinetic energy. As a result, we'll need more retarding power to completely stop them.
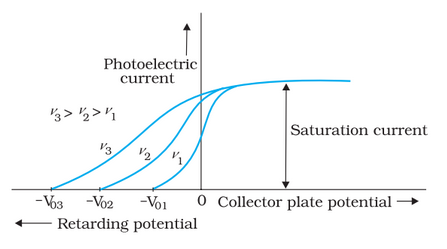
The graphic above depicts the variation of photoelectric current with collector plate potential for various frequencies of incoming radiation.
Above a threshold frequency, the stopping potential is proportional to the incident frequency of light. The following graph shows the variation of stopping potential V0 with incident radiation frequency v for different photosensitive materials.
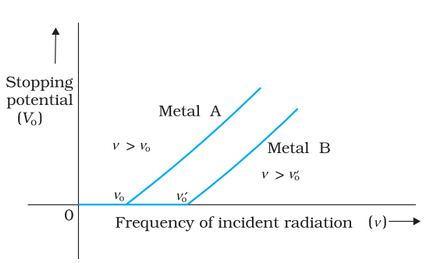
We can make the following conclusions from these observations-
- The maximum kinetic energy of electrons varies linearly with frequency and is independent of intensity.
- There exists a minimum frequency below which no photoelectric effect emission occurs.
Photoelectric Effect and Wave Theory of Light
The wave picture of light was used to explain interference, diffraction, and polarisation.
The experimental investigation of the photoelectric effect, on the other hand, cannot be described using the wave theory of light. According to the wave theory, electrons should continuously absorb radiant energy. The more the intensity, more is the amplitude of electric and magnetic fields and hence a greater kinetic energy of electrons.
The wave model fails to describe the most fundamental characteristics of photoelectric emission.
As a result, a new hypothesis called the photon picture of light was presented to explain the photoelectric effect.
Einstein’s Photoelectric Equation: Energy Quantum of Radiation
To explain the photoelectric effect, Albert Einstein presented a radical new model of electromagnetic radiation (quanta of energy of radiation) in 1905. The energy of each quantum of radiant energy is hv, where h is Planck's constant and v is the light frequency.
An electron absorbs a quantum of energy (hv) of radiation in the photoelectric effect.
If the amount of energy absorbed is more than the minimal amount of energy required for the electron to escape from the metal surface, the electron is expelled with the maximal kinetic energy given by
This is known as Einstein's Photoelectric Equation.
The value of the work function for a given material is constant and is determined by the substance's nature.
Kinetic Energy of Photoelectron
As
Threshold frequency
Since kinetic energy cannot be negative, for a given photosensitive material, there is a minimum cut-off frequency vo for which the stopping potential is zero. This minimum cut-off frequency vo is referred to as the threshold frequency.
Stopping Potential
This is the minimum reverse potential to stop the flow of electrons.
From the photoelectric equation,
From the above equation, we can conclude,
Intensity of Light
The amount of photons present in light determines its intensity. The frequency of incident light has no bearing on it.
Photoelectric Current and Frequency of Incident Light
Photoelectric Current is the rate at which photoelectrons are emitted from a metal surface. It has a direct relationship with the amount of incident radiation.
The photoelectrons emitted in the photoelectric effect are solely dependent on the intensity of light. If
Particle Nature of Light: The Photon
The Photon Theory of Light, proposed by Albert Einstein in the early 20th century, posits that light is composed of discrete packets of energy known as photons. This theory was a significant departure from the classical wave theory of light, which described light as a continuous wave.
- A photon is a packet of energy or particles of light that travels at the speed of light in a straight line.
- The energy of a photon is given as
- The momentum of the photon is given as
- All photons of light of a given frequency or wavelength have the same energy or momentum, irrespective of light intensity.
- Photons are electrically neutral and not affected by electric and magnetic fields.
- Photons travel at the speed of light,
- Photons can interact with other particles like electrons(Compton effect) and transfer its energy. During the photon-electron collision, the momentum and total energy are conserved.
- The dynamic mass of the photon is m = E/c2, where E is the energy of the photon
Wave Nature of Matter
Interference, diffraction, and polarisation are all examples of light's wave nature. Radiation also behaves as if it were made up of a bunch of particles – the photons – in the photoelectric and Compton effects, which involve energy and momentum transfer.
A logical question arises: If radiation has a dual (wave-particle) nature, don't natural particles (electrons, protons, etc.) have wave-like properties as well?
Matter, according to the de Broglie hypothesis, has a wave-like quality.
If radiation has two sides, then matter should as well.
According to De Broglie, a moving material particle can be associated with a wave. De Broglie proposed that the wavelength,
The above equation shows the wave nature of matter.
Furthermore, we can write the De-Broglie wavelength as
Where,
Davisson and Germer’s Experiment
The Davisson-Germer Experiment provides experimental proof of the concept of the material particle-wave nature.
Davisson and Germer's experimental setup is schematically depicted in the diagram below:
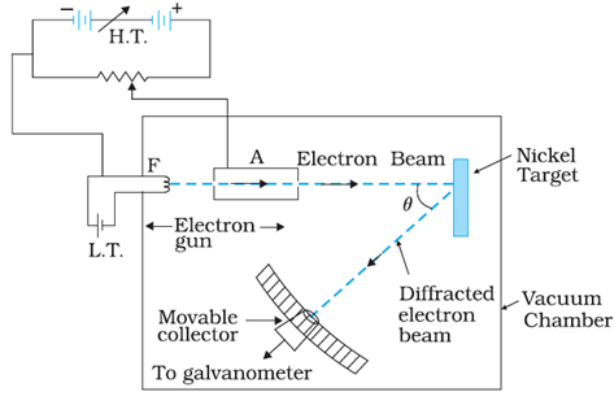
An electron gun was utilised to create a beam of electrons in the experiment.
This tiny beam of accelerated electrons was directed into a nickel crystal, where the crystal's atoms scattered the electrons in various ways.
A detector measured the intensity of the electron beam dispersed in a certain direction.
On a circular scale, this detector was designed to rotate.
As a result of the experiment, scientists examined the intensity of scattered electron beams at various latitude angles or scattering angles, which are the angles between the incident and scattered electron beams. The accelerating voltage was varied from 44 to 68 V during the experiment.
For an accelerating voltage of 54V and a scattering angle of θ=500, a high peak in the intensity (I) of the scattered electron was observed.
The constructive interference of electrons scattered from distinct layers of the regularly spaced atoms of the crystals causes the peak to appear in a specific direction.
The wavelength of matter waves was determined by electron diffraction studies to be 0.165 nm.
Dual Nature of Radiation and Matter: Previous Year Question and Answer
Q.1 Using a photon picture of light, show how Einstein’s photoelectric equation can be established. Write two features of the photoelectric effect that cannot be explained by wave theory.
Answer:
In the photoelectric effect, electrons absorb a quantum of energy
Wave theory cannot explain
i) that the photoelectric effect is instantaneous
ii) that the maximum kinetic energy is independent of the intensity of the incident light.
Q.2 The maximum kinetic energy of the photoelectrons emitted is doubled when the wavelength of light incident on the photosensitive surface changes from
Answer:
Q.3 The work function of aluminium is 4·2 eV. If two photons, each of energy 2·5 e,V are incident on its surface, will the emission of electrons take place? Justify your answer.
Answer:
If the two photons, each of energy 2.5 eV, are incident on the given surface, then the emission of electrons will not take place. This is because the energy
If
Importance of Dual Nature of Radiation and Matter Class 12 Notes
Foundation of Modern Physics
- These notes allow students to comprehend how light and matter show the characteristics of both particles and waves, which is the main idea of quantum mechanics.
Photoelectric Effect Simplified
- Deals with an easy explanation of the photoelectric effect by Einstein with simple examples, really important for Class 12 board exams, JEE, and NEET.
Applications in Real Life
- Mentions the practical side of the electron microscope, solar cells, and photocells to make it easier for the students to link the theory with the actual devices.
Concise Key Formulas
- The notes are packed with all the necessary formulas like de Broglie wavelength, photon energy, and work function, which are the main ones for solving numerical problems.
Quick Revision Resource
- The well-structured text, figures, and the process of the derivations in these notes are the last-minute revision very handy for CBSE Class 12 Physics exams.
Boosts Competitive Exam Preparation
- The Dual Nature topics are the core of modern physics questions in JEE Main, JEE Advanced, NEET, and other entrance exams.
Easy-to-Understand Language
- The content is presented in a simple and student-friendly language, which makes even the most difficult concepts of wave-particle duality quite understandable.
NCERT Class 12 Notes Chapterwise
NCERT Class 12 Notes Chapterwise links provide direct access to well-structured study material for every subject and chapter. These notes are designed for quick revision, exam preparation, and competitive exams like JEE & NEET, making learning easier and more effective.
|
NCERT Class 12 Physics Chapter 11 Notes |
Subject Wise NCERT Exemplar Solutions
- NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12 Maths
- NCERT Exemplar Class 12 Physics
- NCERT Exemplar Class 12 Chemistry
- NCERT Exemplar Class 12 Biology
Subject Wise NCERT Solutions
NCERT Books and Syllabus
Frequently Asked Questions (FAQs)
There is a minimum cut off frequency vo for a certain photosensitive material for which the stopping potential is zero; this minimum cut off frequency vo is known as the threshold frequency.
The amount of photons present in light determines its intensity. The frequency of incident light has no bearing on it.
Photons are energy packets or quanta connected with electromagnetic radiation. E = h v gives the energy of a photon, where v is the frequency associated with the photon and h is Planck's constant.
Photoelectric Current is the rate at which photoelectrons are emitted from a metal surface. It has a direct relationship with the amount of incident radiation.
From the notes for Class 12 Physics chapter 11, students should expect 4 to 6 mark questions, and they can use this note for quick revision to help them improve their grades.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
