The key topics include in NCERT Exemplar Class 12 Chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids are nomenclature, structure and reactivity of carbonyl compounds, important reactions like nucleophilic addition, oxidation–reduction, and the preparation and properties of carboxylic acids.
NCERT Exemplar Class 12 Chemistry Solutions chapter 12 Aldehydes, Ketones and Carboxylic Acids
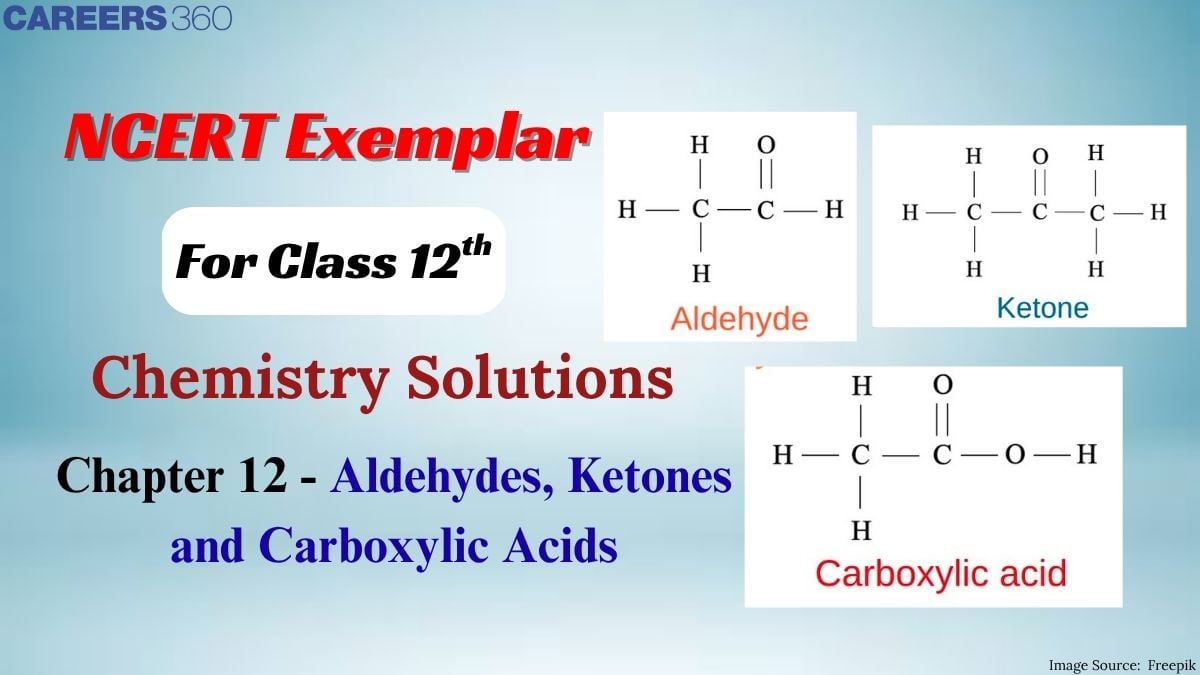
Have you ever heard of Vanilin? It is the aldehyde, present in Vanilla that gives the Vanilla its characteristic smell. Just like this, aldehyde, ketones, and carboxylic acid are building blocks of several important organic compounds that are utilized in biological systems, industries, and drugs. Aldehydes and Ketones contain a carbonyl group and carboxylic acid contains a carboxyl group that gives them their reactivity and characteristic properties. This chapter includes their structure, preparation methods, properties, and their role in organic synthesis.
Electronic gadgets or devices such as Mobile phones, smartwatches, calculators, and more are not allowed inside the CBSE 2026 exam hall.
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: Long Answer Type
- Class 12 Chemistry NCERT Chapter 12: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 12 Aldehydes, Ketones, and Carboxylic Acids
- Major Subtopics OF NCERT Exemplar Class 12 Chemistry Solutions Chapter 12
- Advantages of Using Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids NCERT Exemplar Solutions
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Exemplar Class 12 Solutions
- NCERT Solution subject-wise
- NCERT Notes subject-wise
To help students with the NCERT Exemplar Class 12 Chemistry Solutions of this chapter, our experts have carefully designed the solutions which have detailed explanations and a step-by-step problem-solving approach. These NCERT exemplar solutions are beneficial for boards as well as competitive exams. The pattern of the solutions aligns with the CBSE board exams and also ensures students gain a deep understanding of reaction mechanisms, which helps students in competitive examinations.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: MCQ (Type 1)
MCQ-type questions from NCERT Exemplar Class 12 Chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids are covered to improve your conceptual thinking. These questions also help in quick revision and enhance your problem-solving ability. Students can also access the NCERT Solutions for better understanding.
Answer:
The answer is the option (ii)
On addition of water to but-1-yne in the presence of H2SO4 and HgSO4 , 2-butanone is obtained (by Markovnikoff’s rule).
Question 2. Which of the following compounds is most reactive towards nucleophilic addition reactions?
Answer:
The answer is the option (i)
CH3CHO (an aldehyde) is generally considered more reactive than ketones towards nucleophilic addition reactions. Ketones have 2 large substituents compared to 1 in aldehydes, that hinder the nucleophilic approach. Aldehydes are also considered more reactive because the 2 alkyl groups lead to the reduction of electrophilicity of the carbonyl carbon of ketone by releasing electron in its direction.
The reactivity order can be given as:
Question 3. The correct order of increasing acidic strength is _____________.
(i) Phenol < Ethanol < Chloroacetic acid < Acetic acid
(ii) Ethanol < Phenol < Chloroacetic acid < Acetic acid
(iii) Ethanol < Phenol < Acetic acid < Chloroacetic acid
(iv) Chloroacetic acid < Acetic acid < Phenol < Ethanol
Answer:
The answer is the option (iii)
Due to formation of more stable conjugate base after removal of H+ phenol is more stable than the alcohol.
On the other hand, the stable conjugate base formed after removing H+ makes carboxylic acid more acidic than phenol.
In case chloroacetic acid, the presence of electron withdrawing chlorine group makes it more acidic compared to the acetic acid.
Question 4. Compound 
(i) Phenol and benzoic acid in the presence of NaOH
(ii) Phenol and benzoyl chloride in the presence of pyridine
(iii) Phenol and benzoyl chloride in the presence of ZnCl2
(iv) Phenol and benzaldehyde in the presence of palladium
Answer:
The answer is the option (ii)
The compound C6H5OCOC6H5 can be prepared by the given below reaction. In this, the phenol is made to react with acid chloride in presence of an alkali. The alkali used is pyridine and acid chloride is benzoyl chloride.
Question 5. The reagent which does not react with both, acetone and benzaldehyde.
(i) Sodium hydrogen sulphite
(ii) Phenyl hydrazine
(iii) Fehling’s solution
(iv) Grignard reagent
Answer:
The answer is the option (iii)
Fehling’s solution is the reagent that does not react with both acetone and benzaldehyde.
Question 6. Cannizaro’s reaction is not given by _____________.
Answer:
The answer is the option (iv)
CH3CHO contains 3 α-hydrogens while other three compounds have no any α -hydrogen. Hence, Cannizzaro’s reaction is given by them. Aldehydes without any alpha hydrogen undergo reduction and self-oxidation reaction when treated with conc. Alkali.
Question 7. Which product is formed when the compound
Answer:
The answer is the option (ii)
Benzaldehyde does not have any α hydrogen. When reacted with aqueous KOH solution, it gives Cannizzaro’s reaction.
Question 8.
Structure of ‘A’ and type of isomerism in the above reaction are respectively.
(i) Prop-1-en-2-ol, metamerism
(ii) Prop-1-en-1-ol, tautomerism
(iii) Prop-2-en-2-ol, geometrical isomerism
(iv) Prop-1-en-2-ol, tautomerism
Answer:
The answer is the option (iv)
A undergoes tautomerism to form acetone.
Question 9. Compounds A and C in the following reaction are __________.
(i) identical
(ii) positional isomers
(iii) functional isomers
(iv) optical isomers
Answer:
The answer is the option (ii) positional isomers
Thus, 
We know that acetaldehyde when treated with Grignard reagent followed by hydrolysis gives propane 2-ol.
Question 10. Which is the most suitable reagent for the following conversion?
(i) Tollen’s reagent
(ii) Benzoyl peroxide
(iii) I2 and NaOH solution
(iv) Sn and NaOH solution
Answer:
The answer is the option (iii)
Iodoform test is most suitable for the given reaction.
Question 11. Which of the following compounds will give butanone on oxidation with alkaline KMnO4 solution?
(i) Butan-1-ol
(ii) Butan-2-ol
(iii) Both of these
(iv) None of these
Answer:
The answer is the option (ii).
Aldehydes, when treated with common oxidising agents get readily oxidized to carboxylic acids.
Question 12. In Clemmensen Reduction carbonyl compound is treated with _____________.
(i) Zinc amalgam + HCl
(ii) Sodium amalgam + HCl
(iii) Zinc amalgam + nitric acid
(iv) Sodium amalgam + HNO3
Answer:
The answer is the option (i)
Clemmensen reduction is used in the conversion of carbonyl group to CH2 group. In this reaction, zinc amalgam and HCl act as reagent.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: MCQ (Type 2)
Class 12 Chemistry NCERT Exemplar Solutions Chapter 12 provides detailed explanations for all types of questions to strengthen student understanding. These solutions are highly useful for board exam preparation as well as competitive exams like NEET and JEE.
Question 13. Which of the following compounds do not undergo aldol condensation ?
Answer:
The answer is the option (ii, iv)
Aldol condensation takes place only if α hydrogen is present. In the reaction, aldehydes and ketones with 1 or more than 1 alpha hydrogen react in presence of dilute alkali to form aldol and ketol respectively.
Question 14. Treatment of compound 
(i) Phenol
(ii) Sodium phenoxide
(iii) Sodium benzoate
(iv) Benzophenone
Answer:
The answer is the option (ii, iii)
We get sodium phenoxide and sodium benzoate by nucleophilic substitution reaction.
Question 15. Which of the following conversions can be carried out by Clemmensen Reduction?
(i) Benzaldehyde into benzyl alcohol
(ii) Cyclohexanone into cyclohexane
(iii) Benzoyl chloride into benzaldehyde
(iv) Benzophenone into diphenyl methane
Answer:
The answer is the option (ii, iv)
Clemmensen reduction is used in the conversion of carbonyl group of aldehydes and ketones to CH2 group when treated with zinc amalgam and conc. HCl. It is used to convert cyclohexanone into cyclohexane and benzophenone into diphenyl methane.
Question 16. Through which of the following reactions number of carbon atoms can be increased in the chain?
(i) Grignard reaction
(ii) Cannizaro’s reaction
(iii) Aldol condensation
(iv) HVZ reaction
Answer:
The answer is the option (i,iii)
Grignard reaction is used to increase the number of carbon atom in chain. Aldol condensation increases the number of carbon atom in the chain for aldehydes and ketones.
Question 17. Benzophenone can be obtained by ____________.
(i) Benzoyl chloride + Benzene + AlCl3
(ii) Benzoyl chloride + Diphenyl cadmium
(iii) Benzoyl chloride + Phenyl magnesium chloride
(iv) Benzene + Carbon monoxide + ZnCl2
Answer:
The answer is the option (i,ii)
Benzophenone can be obtained by either of benzoyl chloride + diphenylcadmium or benzoyl chloride+ benzene+ AlCI3 . The latter is referred to as Friedel-Craft acylation reaction.
Question 18. Which of the following is the correct representation for intermediate of nucleophilic addition reaction to the given carbonyl compound (A) :
Answer:
The answer is the option (i,ii)
The 2 possible orientation of a planar molecule of carbonyl compound in case of nucleophilic attack are:
The nucleophililic attack can occur either from front or rear side.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: Short Answer Type
These Chapter 12 Aldehydes, Ketones and Carboxylic Acids short answer type questions help students practise precise explanations and improve their conceptual clarity. They also prepare students to write to-the-point answers in exams, saving time and boosting accuracy.
Question 19. Why is there a large difference in the boiling points of butanal and butan-1 -ol ?
Answer:
There is a huge difference in the boiling points of butanal and butan-1 -ol due to the presence of intermolecular hydrogen bonding between -OH groups in alcohol.
Question 20. Write a test to differentiate between pentan-2-one and pentan-3-one.
Answer:
The answer is Idoform test. There is a CH3CO group in Pentan-2-one and that is why it will give a positive iodoform test.
Question 21. Give the IUPAC names of the following compounds
Answer:
(i) 3-Phenylprop-2-en-1 -al
(ii) Cyclohexanecarbaldehyde
(iii) 3-Oxopentan-l-al
(iv) But-2-en-l-al
Question 23. Write IUPAC names of the following structures.
Answer:
(i) Ethane-1,2-dial
(ii) Benzene-1, 4-dicarbaldehyde
(iii) 3-Bromobenzaldehyde
Question 24. Benzaldehyde can be obtained from benzal chloride. Write reactions for obtaining benzal chloride and then benzaldehyde from it.
Answer:
Reactions for obtaining benzalchloride and benzaldehyde from it are:
Question 25. Name the electrophile produced in the reaction of benzene with benzoyl chloride in the presence of anhydrous AlCl3. Name the reaction also.
Answer:
The electrophile produced in the reaction of benzene with benzoyl chloride in the presence of anhydrous AlCl3 is benzoylium cation. The product formed in this reaction is benzophenone. This reaction is called Friedel Craft's acylation reaction.
Question 26. Oxidation of ketones involves carbon-carbon bond cleavage. Name the products formed on oxidation of 2, 5-dimethylhexan-3-one.
Answer:
The products formed on oxidation of 2, 5-dimethylhexan-3-one are: -
2-Methylpropanoic acid
Ethanoic acid and
Methanoic acid.
3-Methylbutanoic acid
Question 27. Arrange the following in decreasing order of their acidic strength and give the reason for your answer.
CH3CH2OH,CH3COOH,ClCH2COOH,FCH2COOH,C6H5CH2COOH
Answer:
FCH2COOH>ClCH2COOH>C6H5CH2COOH>CH3COOH>CH3CH2OH
The electron-withdrawing group increase the acidity of carboxylic acids since they stabilize the conjugate base by delocalization of charge. Whereas the electron donating group decrease the acidity as the resulting conjugate base is unstable.
Higher the electronegativity, greater the acidic strength.
(i.e,F>Cl>C6H5>H)
Question 28. What product will be formed on reaction of propanal with 2-methyl propanal in the presence of NaOH? What products will be formed? Write the name of the reaction also.
Answer:
On reaction of propanal with 2-methyl propanal in the presence of NaOH, aldehyde mixture is formed.
It is given that B and A on heating together in the presence of acid produces a fruity smelling compound which is ester.
-
Carboxylic Acid
-
Alcohol
-
Ester Group
Question 30. Arrange the following in decreasing order of their acidic strength. Give explanation for the arrangement.
C6H5COOH,FCH2COOH,NO2CH2COOH
Answer:
$\mathrm{NO}_2 \mathrm{CH}_2 \mathrm{COOH}>\mathrm{FCH}_2 \mathrm{COOH}>\mathrm{C}_6 \mathrm{H}_5 \mathrm{COOH}$
-NO2 and -F groups are electron withdrawing groups that increase the acidity due to stable nature of resulting conjugate base. Whereas the electron-donating group decrease the acidity as the resulting conjugate base is unstable.
Higher the electronegativity, greater the acidic strength.
Question 31. Alkenes

Answer:
In carbonyl groups, carbon acquires a partial positive charge because oxygen is more electronegative. Thus, carbonyl group is polar in nature and hence susceptible to nucleophilic addition reaction. Alkenes, on the other hand are non-polar and electron abundant; making them susceptible to electrophilic addition reaction.
Question 32. Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
Answer:
Carboxylic acid doesnt show nucleophilic substitution reaction as because of presence of lone pair atoms on O atom of OH group decreases electrophilic character of carbonyl carbon by resonance.. Hence partial positive charge decreases and therefore it doesnt show nucleophilic substitution reactions
Question 33. Identify the compounds A, B and C in the following reaction.

Answer:
-
Compound A is CH3−MgBr
-
Compound B is CH3−COOH
-
Compound C is CH3−COOCH3
Question 34. Why are carboxylic acids more acidic than alcohols or phenols although all of them have a hydrogen atom attached to an oxygen atom (−O−H)?
Answer:
Acidity depends upon the stability of the resulting conjugate base. In case of carboxylic acid, equivalent resonance structures stabilize the carboxylate ion as the negative charge is at the more electronegative oxygen atom. Phenoxide ion, conjugate base of phenol does not have equivalent resonance structure as the negative charge is on the less electronegative carbon atom.
Question 35. Complete the following reaction sequence.
Answer:
Answer:
The reason is formation of polysubstituted products. Friedel-craft’s acylation reaction can be used to avoid the formation of these products.
Question 37. Can Gatterman-Koch reaction be considered similar to Friedel Craft’s acylation? Discuss.
Answer:
Yes, the Gattermann-Koch reaction can be considered similar to Friedel Craft’s acylation reaction. In Gattermann-Koch reaction introduction of -CHO group to the benzene nucleus takes place by reacting CO with HCl gas. In Friedel-Crafts acylation reactions, treatment of benzene or any arene with an acid chloride in presence of anhydrous AlCl3 takes place. In this RCO group is introduced.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: Matching Type
Aldehydes, Ketones and Carboxylic Acids Class 12 Chemistry NCERT Exemplar Solutions Chapter 12 matching type questions are designed to test your understanding of the concepts . They enhance analytical skills and strengthen understanding for exams.
Question 38. Match the common names given in Column I with the IUPAC names given in Column II
|
Column I (Common names) |
Column II (IUPAC names) |
|
(i) Cinnamaldehyde |
(a) Pentanal |
|
(ii) Acetophenone |
(b) Prop-2-enal |
|
(iii) Valeraldehyde |
(c) 4-Methylpent -3-en-2-one |
|
(iv) Acrolein |
(d) 3-phenylprop-2-enal |
|
(v) Mesityl oxide |
(e) 1-Phenylethanone |
Answer:
(i → d), (ii → e), (iii → a), (iv → b), (v → c)
Question 39. Match the acids given in Column I with their correct IUPAC names given in Column II.
|
Column I (Acids) |
Column II(IUPAC names) |
|
(i) Phthalic acid |
(a) Hexane-1,6-dioic acid |
|
(ii) Oxalic acid |
(b) Benzene-1, 2-dicarboxylic acid |
|
(iii) Succinic acid |
(c) Pentane -1 , 5-dioic acid |
|
(iv) Adipic acid |
(d) Butane -1, 4-dioic acid |
|
(v) Glutaric acid |
(e) Ethane-1,2-dioic acid |
Answer:
(i → b), (ii → e), (iii → d), (iv → a), (v → c)
Question 40. Match the reactions given in Column I with the suitable reagents given in Column II.
|
Column I (Reactions) |
Column II (Reagents) |
|
(i) Benzophenone → Diphenylmethane |
(a) LiAIH4 |
|
(ii) Benzaldehyde → 1-Phenylethanol |
(b) DIBAL-H |
|
(iii) Cyclohexanone → Cyclohexanol |
(c) Zn (Hg)/Conc.HCl |
|
(iv) Phenyl benzoate → Benzaldehyde |
(d) CH3MgBr |
Answer:
(i → c), (ii → d), (iii → a), (iv → b)
Question 41. Match the example given in Column I with the name of the reaction in Column II
|
Column I (Examples) |
Column II (Reaction) | ||
|
(i) |
(a) |
Friedel-Crafts acylation | |
|
(ii) |
(b) |
HVZ reaction | |
|
(iii) |
(c) |
Aldol condensation | |
|
(iv) |
(d) |
Cannizzaro's reaction | |
|
(v) |
(e) |
Rosenmund's reduction | |
|
(vi) |
(f) |
Stephen's reaction | |
Answer:
(i → e), (ii → d), (iii → a), (iv →b), (v → f), (vi → c)
NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: Assertion and Reason Type
Assertion and Reason type questions of NCERT Class 12 chemistry Aldehydes, Ketones and Carboxylic Acids help students enhance knowledge and improve critical thinking. These are so important for exams and it will clear most of your doubts.
Question 42. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Formaldehyde is a planar molecule.
Reason: It contains sp2 hybridised carbon atom.
(i) Assertion and reason both are correct and the reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) The assertion is a correct statement but the reason is the wrong statement.
(iv) The assertion is a wrong statement but the reason is the correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct
explanation of assertion.
Answer:
The answer is the option (i). Formaldehyde contains sp2 hybridized carbon atom. That is why it is a planar molecule. Both the assertion and reason are correct, and reason is correct explanation of assertion.
Question 43. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Compounds containing -CHO group are easily oxidised to corresponding carboxylic acids.
Reason: Carboxylic acids can be reduced to alcohols by treatment with LiAlH4
(i) Assertion and reason both are correct and the reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) The assertion is a correct statement but the reason is the wrong statement.
(iv) The assertion is a wrong statement but the reason is the correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct
explanation of assertion
Answer:
The answer is the option (v). Due to high electronegativity of oxygen, carbonyl group in aldehydes are a strong electron-withdrawing group. This weakens the C−H bond in aldehydes. Even a weak oxidizing agent can oxidize the aldehydes into the corresponding carboxylic acids.
Question 44. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of the α-hydrogen atom is resonance stabilised.
(i) Assertion and reason both are correct and the reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) The assertion is a correct statement but the reason is the wrong statement.
(iv) The assertion is a wrong statement but the reason is the correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct
explanation of assertion
Answer:
The answer is the option (iv). The α -hydrogen atom in carbonyl compounds is more acidic. Carboxylic acids on dissociation in water give resonance stabilized carboxylate anions and hydronium ion.
Question 45. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Aromatic aldehydes and formaldehyde undergo Cannizaro reaction.
Reason: Aromatic aldehydes are almost as reactive as formaldehyde.
(i) Assertion and reason both are correct and the reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) The assertion is a correct statement but the reason is the wrong statement.
(iv) The assertion is a wrong statement but the reason is the correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct
explanation of assertion.
Answer:
The answer is the option (iii). Aromatic aldehydes and formaldehyde do not have a-hydrogen and thus undergo Cannizzaro’s reaction. Aldehydes with no alpha hydrogen atom undergo oxidation and reduction reaction when treated with conc. Alkali.
Question 46. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Aldehydes and ketones, both react with Tollen’s reagent to form a silver mirror.
Reason: Both, aldehydes and ketones contain a carbonyl group.
(i) Assertion and reason both are correct and the reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) The assertion is a correct statement but the reason is the wrong statement.
(iv) The assertion is a wrong statement but the reason is the correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct
explanation of assertion.
Answer:
The answer is the option (iv) Aldehyde and ketones both have carbonyl group but only the former undergoes reaction with Tollen’s reagent to give silver mirror.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 12: Long Answer Type
The long answer type questions of NCERT Exemplar Class 12 Chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids enhances students ability to present concepts systematically. They also help in strengthening theoretical understanding for board examinations.
As compound B turns up with a positive result for Fehling’s test, it is an aldehyde. It also gives a positive result to the iodoform test, which implies that it has a -COCH3 group. Compound C with a positive iodoform test and negative Fehling’s test, is a ketone.
Answer:
Molecular formula of the compound in question is C8H8O. It is given that ‘A’ does not give Tollens’ or Fehling’s test, making it a ketone. Since we get positive test with 2, 4-DNP and iodoform test, It means it is methyl ketone.
C3H6O will have following isomer.
Compound 1 (Propanal) is more susceptible to a reaction with HCN as carbon atom in carbonyl group acquires more positive charge than carbon atom in compound 2 (propan-2-one). Moreover, there is much less static hindrance in compound 1 compared to compound 2.
Equilibrium is established in this reaction as it is reversible in nature. Addition of an acid hinders the formation of CN- ion and thus, can stop the reaction.
Since liquid A forms a bright silver mirror when treated with ammoniacal AgNO3 solution it is an aldehyde.
In the same way, liquid ‘B’ must be a methyl ketone for it does not give test with ammoniacal AgNO3 solution.
Chemical equations for these reactions are :
Studying the chapter “Aldehydes, Ketones and Carboxylic Acids” from Class 12 Chemistry NCERT exemplar solutions chapter 12 will not only help you to write IUPAC names of different ketones, aldehydes, and carboxylic acids, but will also help you to remember these for future.
Class 12 Chemistry NCERT Chapter 12: Higher Order Thinking Skills (HOTS) Questions
NCERT Exemplar Class 12 Chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids Higher Order Thinking Skills (HOTS) Questionsare designed to enhance problem-solving. These questions are very helpful for competitive exams like JEE and NEET.
Question 1: An organic compound $(\mathrm{X})$ with molecular formula $\mathrm{C}_3 \mathrm{H}_6 \mathrm{O}$ is not readily oxidised. On reduction it gives $\left(\mathrm{C}_3 \mathrm{H}_8 \mathrm{O}(\mathrm{Y})\right.$ which reacts with HBr to give a bromide ( Z ) which is converted to Grignard reagent. This Grinard reagent on reaction with (X) followed by hydrolysis give 2,3-dimethylbutan-2-ol. Compounds $(\mathrm{X}),(\mathrm{Y})$ and $(\mathrm{Z})$ respectively are :
1) $\mathrm{CH}_3 \mathrm{COCH}_3, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}, \mathrm{CH}_3 \mathrm{CH}(\mathrm{Br}) \mathrm{CH}_3$
2) $\mathrm{CH}_3 \mathrm{COCH}_3, \mathrm{CH}_3 \mathrm{CH}(\mathrm{OH}) \mathrm{CH}_3, \mathrm{CH}_3 \mathrm{CH}(\mathrm{Br}) \mathrm{CH}_3$
3) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Br}$
4) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}, \mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}_2, \mathrm{CH}_3 \mathrm{CH}(\mathrm{Br}) \mathrm{CH}_3$
Answer:

Hence, the correct answer is option (2).
Question 2: Match List -I with List -II
| List-I | List-II |
 | (I) Etard reaction |
 | (II) Gatterman -Koch reaction |
 | (III) Rosenmund reduction |
 | (IV) Stephen reaction |
Choose the correct answer from the options given below :
1) (A)-(IV), (B)-(III), (C)-(I), (D)-(II)
2) (A)-(III), (B)-(IV), (C)-(II), (D)-(I)
3) (A)-(I), (B)-(III), (C)-(II), (D)-(IV)
4) (A)-(III), (B)-(IV), (C)-(I), (D)-(II)
Answer:
List-I
(A) $\mathrm{RCN} \xrightarrow[\text { (ii) } \mathrm{H}_3 \mathrm{O}^{+}]{\text {(i) } \mathrm{SnCl}_2, \mathrm{HCl}} \mathrm{RCHO}$
Stephen reaction
(B) 
Rosenmund reduction
Etard reaction
Gatterman -Koch reaction
Hence, the correct answer is option (1).
Question 3: Both acetaldehyde and acetone (individually) undergo which of the following reactions?
A. Iodoform Reaction
B. Cannizaro Reaction
C. Aldol condensation
D. Tollen's Test
E. Clemmensen Reduction
Choose the correct answer from the options given below :
(1) A, B and D only
(2) A, C and E only
(3) C and E only
(4) B, C and D only
Answer:
| Sr no |
Name of reaction |
Acetaldehyde
|
Acetone
|
| 1 | Iodoform reaction | $\oplus \mathrm{ve}$ | $\oplus \mathrm{ve}$ |
| 2 | Cannizaro | $\Theta \mathrm{ve}$ | $\Theta \mathrm{ve}$ |
| 3 | Aldol reaction | $\oplus \mathrm{ve}$ | $\oplus \mathrm{ve}$ |
| 4 | Tollen's test | $\oplus \mathrm{ve}$ | $\Theta \mathrm{ve}$ |
| 5 | Clemmensen reduction | $\oplus \mathrm{ve}$ | $\oplus \mathrm{ve}$ |
A, C, and E only
Hence, the correct answer is option (2).
Question 4:
When 
(1)
(2)
(3)
(4)
Answer:
The first step of the scheme shows aldol condensation in the presence of a base, resulting in the formation of $\beta$-hydroxy ketone with a 5-membered ring. On heating, water is eliminated and the product $\alpha, \beta$-Unsaturated cyclopentanone is obtained.
Hence, the correct answer is option (1).
Question 5: Aldol condensation is a popular and classical method to prepare $\alpha, \beta$-unsaturated carbonyl compounds. This reaction can be both intermolecular and intramolecular. Predict which one of the following is not a product of intramolecular aldol condensation? (1)

(2)

(3)

(4)

Answer:

The last reaction is intermolecular reaction as two different species are involved.
Hence, the correct answer is option (4).
Approach to Solve Questions of Chapter 12 Aldehydes, Ketones, and Carboxylic Acids
To solve the questions from Chemistry Class 12 NCERT Exemplar chapter 12 Aldehydes, Ketones and Carboxylic Acids, it is important to first understand the basic concepts thoroughly. Focus on practising different types of questions, revise key formulas, the approaches given below helps you to solve questions from Aldehydes, Ketones and Carboxylic Acids Class 12 Chemistry effectively.
1. It is easy yet crucial as there will be reactions on specific functional groups. To solve the question you also need to draw the structures of the given compound.
2. Know how each class is prepared from alcohols, nitriles/hydrolysis, acid chlorides, Grignard reagents, etc. Also learn to write balanced reactions with reagents and conditions.
3. For Aldehydes and Ketones-
- Nucleophilic addition reactions
- Reduction and oxidation reactions.
- Aldol condensation, Cannizzaro reaction.
For Carboxylic Acids-
- Acid-base reactions, decarboxylation, esterification
- Substitution on –COOH group, like Hell Volhard Zelinsky reaction.
For better understanding and conceptual clarity refer to NCERT notes for Aldehydes, Ketones and Carboxylic Acids
4. Learn the specifity of the reagents used and their reactivity order.
- Oxidizing agents- KMnO4, Tollen’s, Fehling’s.
- Reducing agents- LiAlH4, NaBH4, Clemmensen,Wolff-Kishner.
5. These are too important as most of the time they are asked directly in the exams.
- Aldol Condensation
- Cannizzaro Reaction
- Rosenmund Reduction
- Stephen Reduction
- Hell–Volhard–Zelinsky Reaction
7. Practice all sort of problems. You can refer to NCERT intext questions and solved examples. Also, try to solve the NCERT Exemplar and previous years questions.
Major Subtopics OF NCERT Exemplar Class 12 Chemistry Solutions Chapter 12
Following are the important topics from the Class 12 Chemistry chapter 12 Aldehydes, Ketones and Carboxylic Acids:
- Nomenclature and Structure of Carbonyl Group
- Preparation Of Aldehydes And Ketones
- Physical Properties of Aldehydes and Ketones
- Chemical Reactions of Aldehydes and Ketones
- Uses of Aldehydes and Ketones
- Structure of Carboxyl Group
- Nomenclature of Carboxyl Group
- Methods of Preparation of Carboxylic Acids
- Physical Properties of Carboxylic Acids
- Chemical Reactions of Carboxylic Acids
- Uses of Carboxylic Acids
Advantages of Using Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids NCERT Exemplar Solutions
Aldehydes, Ketones and Carboxylic Acids Class 12 Chemistry NCERT Exemplar Solutions Chapter 12 cover all topics from the NCERT book that helps students to understand complex reactions and mechanisms. The advantages of using these solutiond are given below:
- Students can use these solutions to get clear explanations of the structure, properties, and preparation methods of aldehydes, ketones, and carboxylic acids.
- These NCERT Exemplar Class 12 Solutions include detailed reaction mechanisms and important name reactions.
- NCERT Exemplar Class 12 Chemistry Solutions chapter 12 Aldehydes, Ketones and Carboxylic Acids contain summaries, formulas, and short tips.
- These solutions of NCERT are prepared by subject experts in a very clear and comprehensive way that helps students prepare for board and competitive exams.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Exemplar Class 12 Solutions
Make your preparation better by solving the NCERT exemplar solutions for other subjects as well.
NCERT Solution subject-wise
The NCERT subject-wise solutions will help you cover the intext and exercise questions. Follow the links below
NCERT Notes subject-wise
You can now do quick revision by following the NCERT notes. Click on the link given in the table
| NCERT Notes for Class 12 Physics |
| NCERT Notes for Class 12 Chemistry |
| NCERT Notes for Class 12 Maths |
| NCERT Notes for Class 12 Biology |
NCERT Books and NCERT Syllabus
Follow the links to the syllabus and books for the respective subjects.
Frequently Asked Questions (FAQs)
Students will learn about physical properties, uses and chemical reactions of very crucial hydrocarbons like Aldehydes, Ketones, and Carboxylic Acids alongside it’s nomenclatures and various structures of the carboxyl groups and the IUPAC names of different ketones, aldehydes, and carboxylic acids.
The carbonyl group is significant in aldehydes and ketones because it is a polar functional group that greatly affects the physical and chemical properties of these compounds. This polar nature allows them to form hydrogen bonds with water, making aldehydes and many ketones soluble in water.
A common test to distinguish between aldehydes and ketones is the Tollens' test. Aldehydes can reduce Tollens' reagent to form a silver mirror, indicating the presence of an aldehyde. Ketones, however, do not react with Tollens' reagent and thus will not produce a silver mirror.
Aldehydes and ketones can be synthesized using several methods. Aldehydes are commonly prepared by the oxidation of primary alcohols, while ketones can be formed by the oxidation of secondary alcohols. Another method is the Friedel-Crafts acylation, where an acyl group is added to an aromatic ring using an acid chloride in the presence of a Lewis acid catalyst.
Carboxylic acids can be converted into derivatives by reacting them with alcohols to form esters, a process known as esterification. This reaction typically requires an acid catalyst and can be reversible. Similarly, carboxylic acids can be converted into amides by reacting them with ammonia or amines.
Chemistry Class 12 NCERT Exemplar chapter 12 Aldehydes, Ketones and Carboxylic Acids help students by providing clear, step-wise explanations of reactions and mechanisms, making it easier to understand carbonyl chemistry and improve exam performance.
MCQ-type questions from NCERT Exemplar Class 12 Chemistry include concept-based, application-level, and numerical questions designed to test understanding of reactions, mechanisms, and problem-solving skills across each chapter.
Aldehydes are more reactive due to lesser steric hindrance and the presence of only one alkyl group, which makes the carbonyl carbon more electrophilic.
It is a reaction where a nucleophile attacks the electrophilic carbonyl carbon, followed by protonation to form an addition product.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters








































