D- and f-block elements are transition and inner-transition metals in the periodic table whose d or f orbitals are progressively filled, and they show properties like variable oxidation states, coloured ions, and complex formation.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 8 The d and f block elements
Did you know the middle of the periodic table has special metals known as d and f-block elements that are used in industries, colorful fireworks and important chemical reactions? This chapter features transition metals known as d-block elements and the inner transition metals known as f-block elements, as well as the principles and theories that govern their behaviour.The important topics like electronegativity, ionic size and colour in transition metal complexes are all discussed in these d and f-block elements Class 12 Chemistry Chapter 8 NCERT Exemplar Solutions.
As per the CBSE 2026 date sheet, the Class 12 board examinations will start on February 17 and conclude on April 9.
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: Long Answer Type
- NCERT Exemplar Class 12 Chemistry Chapter 8: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Class 12 Chemistry Chapter 8
- Topics and Subtopics Covered in the NCERT Exemplar Class 12 Chemistry Chapter 8
- NCERT Exemplar Class 12 Chemistry Chapter 8: Important Formulas and Key Points
- Advantages of Class 12 Chemistry Chapter 8 The d- and f-Block Elements NCERT Exemplar Solutions
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions subject-wise
- NCERT Exemplar Class 12 Solutions subject-wise
- NCERT Class 12 subject-wise notes
- NCERT Books and NCERT Syllabus

The NCERT Exemplar Class 12 Chemistry Solutions are designed to offer a systematic approach to help students develop a clear understanding of critical concepts through a series of solved examples and conceptual explanations. These NCERT Exemplar Solutions also provide a valuable resource to enhance performance in competitive exams like NEET, JEE Mains, etc. The higher-order thinking skills (HOTS) questions are added in this article to improve your analytical thinking. We have also added some important points that will help you build a good approach for NCERT Solutions.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: MCQ (Type 1)
The MCQ-type questions are covered in the NCERT Exemplar Solutions Class 12 Chemistry Chapter 8 to improve your conceptual thinking. These questions help students test their conceptual understanding and prepare effectively for exams.
Question 1. Electronic configuration of a transition element X in +3 oxidation state is [Ar]3d5. What is its atomic number?
(i) 25
(ii) 26
(iii) 27
(iv) 24
Answer:
The answer is option (ii).
Electronic configuration of a transition element X in +3 oxidation state is [Ar]3d5.
Atomic number = 26
Question 2. The electronic configuration of Cu(II) is 3d9 whereas that of Cu(I) is 3d10 . Which of the following is correct?
(i) Cu(II) is more stable
(ii) Cu(II) is less stable
(iii) Cu(I) and Cu(II) are equally stable
(iv) Stability of Cu(I) and Cu(II) depends on the nature of copper salts
Answer:
The answer is option (i).
The stability of Cu(II) is more due to greater effective nuclear charge of Cu(II)
|
Element Metallic radii/pm |
Fe |
CO |
Ni |
Cu |
(i) Fe
(ii) CO
(iii) Ni
(iv) Cu
Answer:
The answer is option (iv). In a periodic table, moving across the period from left to right, the atomic radii of elements decrease. This is the reason behind the increase in density as we move rightwards in a period.
Amongst all the options, Cu lies to the rightmost side of the Periodic Table and has the highest density.
Question 4. Generally, transition elements form coloured salts due to the presence of unpaired electrons. Which of the following compounds will be coloured in solid-state?
(i) Ag2SO4
(ii) CuF2
(iii) ZnF2
(iv) Cu2Cl2
Answer:
The answer is that option (ii) CuF2 has one unpaired electron, due to which it forms coloured salts in the solid state.
Question 5. On addition of a small amount of KMnO4 to concentrated H2SO4, a green oily compound is obtained which is highly explosive. Identify the compound from the following.
(i) Mn2O7
(ii) MnO2
(iii) MnSO4
(iv) Mn2O3
Answer:
The answer is option (i).
$2 \mathrm{KMnO}_4+2 \mathrm{H}_2 \mathrm{SO}_4($ Conc $) \rightarrow \mathrm{Mn}_2 \mathrm{O}_7+2 \mathrm{KHSO}_4+\mathrm{H}_2 \mathrm{O}$
Thus, we get $\mathrm{Mn}_2 \mathrm{O}_7$ which is highly explosive in nature.
Question 6. The magnetic nature of elements depends on the presence of unpaired electrons. Identify the configuration of the transition element which shows the highest magnetic moment.
(i) 3d7
(ii) 3d5
(iii) 3d8
(iv) 3d2
Answer:
The answer is option (ii).The value of the magnetic moment is directly proportional to the number of unpaired electrons. Therefore, 3d5 has the maximum number of unpaired electrons and the highest magnetic moment.
μ=5(5+2)
=35=5.95
Question 7. Which of the following oxidation state is common for all lanthanoids?
(i) +2
(ii) +3
(iii) +4
(iv) +5
Answer:
The answer is option (ii). +3 oxidation state is common for all lanthanoids. Although sometimes +2 and +4 ions are also obtained in solution or solid compounds.
Question 8. Which of the following reactions are disproportionation reactions?
(a) $\mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}$
(b) $3 \mathrm{MnO}_4^{-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O}$
(c) $2 \mathrm{KMnO}_4 \rightarrow \mathrm{~K}_2 \mathrm{MnO}_4+\mathrm{MnO}_2+\mathrm{O}_2$
(d) $2 \mathrm{MnO}_4^{-}+3 \mathrm{Mn}^{+2}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 5 \mathrm{MnO}_2+4 \mathrm{H}^{+}$
(i) a, b
(ii) a, b, c
(iii) b, c, d
(iv) a, d
Answer:
The answer is option (i). A disproportionation reaction is where an element is simultaneously oxidised and reduced.
(a) Cu+→Cu2++Cu
It is a disproportionation reaction, in the product:
- One Cu+ is oxidised to Cu2+ (+1 → +2).
- The other Cu+ is reduced to metallic Cu (+1 → 0).
(b) 3MnO4-+4H+→2MnO-4+MnO2+2H2O
It is also a disproportionation reaction, in the product:
- Some Mn in MnO-4 is reduced from +7 → +4 (forming MnO2).
- Some Mn is reduced from +7 → +6 (forming MnO42-).
Question 9. When KMnO4 solution is added to the oxalic acid solution, the decolourisation is slow in the beginning but becomes instantaneous after some time because
(i) CO2 is formed as the product.
(ii) The reaction is exothermic.
(iii) MnO-4 catalyses the reaction.
(iv) Mn2+ acts as an autocatalyst.
Answer:
The answer is option (iv). Acidified KMnO4 oxidises Oxalate ion to CO2 on addition and Mn2+ acts as an auto catalyst. This is why decolorisation is slow in the beginning but becomes instantaneous after some time.
Reduction half
$\left.\mathrm{MnO}_4^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+\mathrm{H}_2 \mathrm{O}\right] \times 2$
Oxidation half
$\left.\mathrm{C}_2 \mathrm{O}_4^{2-} \rightarrow 2 \mathrm{CO}_2+2 \mathrm{e}^{-}\right] \times 5$
Overall equation
$2 \mathrm{MnO}_4^{-}+16 \mathrm{H}^{+}+5 \mathrm{C}_2 \mathrm{O}_4^{2-} \rightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}$
End point of this reaction is colourless to light pink.
Question 10. There are 14 elements in the actinoid series. Which of the following elements does not belong to this series?
(i) U
(ii) Np
(iii) Tm
(iv) Fm
Answer:
The answer is option (iii). Tm (Thulium), which has an atomic number of 69, is a lanthanoid (4f) series.
Question 11. KMnO4 acts as an oxidising agent in acidic medium. The number of moles of KMnO4 that will be needed to react with one mole of sulphide ions in acidic solution is
(i) 25
(ii) 35
(iii) 45
(iv) 15
Answer:
The answer is the option (i).
$2 \mathrm{MnO}_4^{-}+5 \mathrm{~S}^{2-}+16 \mathrm{H}^{+} \rightarrow 2 \mathrm{Mn}^{2+}+5 \mathrm{~S}+8 \mathrm{H}_2 \mathrm{O}$
For 5 moles of S the number of moles of $\mathrm{KMnO}_4$=2
For 1 mole of S the number of moles of $\mathrm{KMnO}_4$=25
Question 12. Which of the following is amphoteric oxide?
Mn2O7, CrO3, Cr2O3,CrO, V2O5,V2O4
(i)$\mathrm{V}_2 \mathrm{O}_5, \mathrm{Cr}_2 \mathrm{O}_3$
(ii) $\mathrm{Mn}_2 \mathrm{O}_7, \mathrm{CrO}_3$
(iii)$\mathrm{CrO}, \mathrm{V}_2 \mathrm{O}_5$
(iv) $\mathrm{V}_2 \mathrm{O}_5, \mathrm{~V}_2 \mathrm{O}_4$
Answer:
The answer is option (i). Since they react with both acids and bases, $\mathrm{V}_2 \mathrm{O}_5$ and $\mathrm{Cr}_2 \mathrm{O}_3$ are amphoteric oxides.
Also, the basic character is predominant in lower oxides, whereas the acidic character is predominant in higher oxides
Question 13. Gadolinium belongs to 4f series. Its atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
(i)$[\mathrm{Xe}] 4 \mathrm{f}^7 5 \mathrm{~d}^1 6 \mathrm{~s}^2$
(ii) $[\mathrm{Xe}] 4 \mathrm{f}^6 5 \mathrm{~d}^2 6 \mathrm{~s}^2$
(iii) $[\mathrm{Xe}] 4 \mathrm{f}^8 6 \mathrm{~d}^2$
(iv) $[\mathrm{Xe}] 44^9 5 \mathrm{~s}^1$
Answer:
The answer is the option (i) ${ }_{64} G d:[\mathrm{Xe}] 4 f^7 5 d^1 6 s^2$ is the correct electronic configuration of gadolinium that belongs to 4f series.
Question 14. Interstitial compounds are formed when small atoms are trapped inside the crystal lattice of metals. Which of the following is not a characteristic property of interstitial compounds?
(i) They have high melting points in comparison to pure metals.
(ii) They are very hard.
(iii) They retain metallic conductivity.
(iv) They are chemically very reactive.
Answer:
The answer is option (iv). Interstitial compounds are not chemically very active. Instead, they are inert in nature.
Question 15. The magnetic moment is associated with its spin angular momentum and orbital angular momentum. Spin only magnetic moment value of Cr3+ ion is___________.
(i) 2.87 B.M.
(ii) 3.87 B.M.
(iii) 3.47 B.M.
(iv) 3.57 B.M
Answer:
The answer is option (ii). The determination of the magnetic moment is done by the number of unpaired electrons. The μ is associated with its spin angular and orbital angular momentum.
Spin only magnetic moment value of Cr3+ ion is 3d3
Hence, magnetic moment (μ)=n(n+2)BM
=3(3+2)
=15=3.87BM
Question 16. KMnO4 acts as an oxidising agent in alkaline medium. When alkaline KMnO4 is treated with KI, iodide ion is oxidised to ____________.
(i) I2
(ii)$\mathrm{IO}^{-}$
(iii) $\mathrm{IO}^{3-}$
(iv)$\mathrm{IO}_4^{-}$
Answer:
The answer is the option (iii). On treating alkaline $\mathrm{KMnO}_4$ with KI , we get
$2 \mathrm{KMnO}_4+\mathrm{KI}+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{KOH}+2 \mathrm{MnO}_2+\mathrm{KIO}_3$
Question 17. Which of the following statements is not correct?
(i) Copper liberates hydrogen from acids.
(ii) In its higher oxidation states, manganese forms stable compounds with oxygen and fluorine.
(iii) Mn3+ andCo3+ are oxidising agents in aqueous solution.
(iv) Ti2+ andCr2+ are reducing agents in aqueous solution.
Answer:
The answer is the option (i). There is no liberation of hydrogen from acids when copper is added.
$\mathrm{Cu}+2 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{CuSO}_4+\mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O}$
$3 \mathrm{Cu}+8 \mathrm{HNO}_3 \rightarrow 3 \mathrm{Cu}\left(\mathrm{NO}_3\right)_2+2 \mathrm{NO}+4 \mathrm{H}_2 \mathrm{O}$
Question 18. When acidified K2Cr2O7 solution is added to Sn2+ salts then Sn2+ changes to
(i) Sn
(ii) Sn3+
(iii) Sn4+
(iv) Sn
Answer:
The answer is the option (iii). On addition of acidified K2Cr2O7(Acidified potassium dichromate) solution to Sn2+ salt, Sn2+ changes to Sn4+.
The reaction is given below
Question 19. Highest oxidation state of manganese in fluoride is +4(MnF4) but highest oxidation state in oxides is +7(Mn2O7) because ____________.
(i) Fluorine is more electronegative than oxygen.
(ii) Fluorine does not possess d-orbitals.
(iii) Fluorine stabilises a lower oxidation state.
(iv) in covalent compounds, fluorine can form a single bond only, while oxygen forms a double bond.
Answer:
The answer is option (iv). This is because in covalent compounds, fluorine can form single bonds only whereas oxygen has the ability to form multiple bonds and therefore it forms a double bond
Question 20. Although Zirconium belongs to the 4d transition series and Hafnium to 5d transition series even then they show similar physical and chemical properties because___________.
(i) both belong to d-block.
(ii) both have the same number of electrons.
(iii) both have a similar atomic radius.
(iv) Both belong to the same group of the periodic table
Answer:
The answer is option (iii). Zirconium and hafnium show similar physical and chemical properties due to the almost identical radii of Zr(160 pm) and Hf(159 pm). A similar atomic radius results from lanthanoid contraction.
Question 21. Why is HCl not used to make the medium acidic in oxidation reactions of KMnO4 in an acidic medium?
(i) Both HCl and KMnO4 act as oxidising agents.
(ii) KMnO4 oxidises HCl into Cl2 which is also an oxidising agent.
(iii) KMnO4 is a weaker oxidising agent than HCl.
(iv) KMnO4 acts as a reducing agent in the presence of HCl.
Answer:
The answer is the option (ii). HCl is oxidised by KMnO4 into Cl2 which is also an oxidising agent. Since hydrochloric acid is oxidised to chlorine, permanganate titrations in their presence are unsatisfactory.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: MCQ (Type 2)
The class 12 chemistry chapter 8 questions are provided here with simple explanations. Learn more through these advanced MCQs
Question 22. Generally, transition elements and their salts are coloured due to the presence of unpaired electrons in metal ions. Which of the following compounds are coloured?
(i) KMnO4
(ii) Ce(SO4)2
(iii) TiCl4
(iv) Cu2Cl2
Answer:
The answer is option (i, ii) Amongst the given options, the compounds KMnO4 and Ce(SO4)2 are coloured due to the presence of unpaired electrons in metal ions.
Question 23. Transition elements show a magnetic moment due to spin and orbital motion of electrons. Which of the following metallic ions have almost the same spin only magnetic moment?
(i) Co2+
(ii) Cr2+
(iii) Mn2+
(iv) Cr3+
Answer:
The answer is the option (i, iv) The metallic ions $\mathrm{Co}^{2+}\left(3 d^7\right)$ and $\mathrm{Cr}^{3+}\left(3 d^3\right)$ have same number of unpaired electrons which is 3. Thus, they have similar spin-only magnetic moments.
Question 24. In the form of dichromate, Cr(VI) is a strong oxidising agent in acidic medium but Mo(VI) in MoO3 and W(VI) in WO3 are not because ___________.
(i) Cr(VI) is more stable than Mo(VI) and W(VI).
(ii) Mo(VI) and W(VI) are more stable than Cr(VI).
(iii) Higher oxidation states of heavier members of group 6 of the transition series are more stable.
(iv) Lower oxidation states of heavier members of group 6 of the transition series are more stable.
Answer:
The answer is option (ii), (iii). In the d-block elements, heavier elements exhibit higher oxidation states in their stable form. Like in group 6, Mo(VI) and W(VI) are more stable than Cr(VI). This is why, in the form of dichromate Cr(VI) is a stronger oxidizing agent in acidic medium whereas Mo(VI) in MoO3 and W(VI) in WO3 are not.
Question 25. Which of the following actinoids show oxidation states up to +7?
(i) Am
(ii) Pu
(iii) U
(iv) Np
Answer:
The answer is the option (ii, iv) oxidation state of Np and Pu is +7 as well.
Question 26. General electronic configuration of actinoids is $(n-2) f^{1-14}(n-1) d^{0-2} n s^2$. Which of the following actinoids have one electron in 6d orbital?
(i) U (Atomic no. 92)
(ii) Np (Atomic no.93)
(iii) Pu (Atomic no. 94)
(iv) Am (Atomic no. 95)
Answer:
The answer is the option (i, ii) U and Np have one electron in 6d orbital.
$\begin{aligned} & 92 \mathrm{U}-5 f^3 6 d^1 7 s^2 \\ & 93 \mathrm{~Np}-5 f^4 6 d^1 7 s^2\end{aligned}$
Question 27. Which of the following lanthanoids show +2 oxidation state besides the characteristic oxidation state +3 of lanthanoids?
(i) Ce
(ii) Eu
(iii) Yb
(iv) Ho
Answer:
The answer is the option (ii, iii)
(a) Cerium (Z=57)
Electronic Configuration =$[X e] 4 f^5 5 d^0 6 s^2$
Oxidation state of Ce=+3,+4
(b) Europium (Z=63)
Electronic configuration = $[X e] 4 f^7 5 d^0 6 s^2$
Oxidation state of Eu=+2,+3
(c) Ytterbium (Z=70)
Electronic Configuration =$[X e] 4 f^{14} 5 d^0 6 s^2$
Oxidation state of Yb=+2,+3
(d) Holmium (Z=67)
Electronic Configuration =$[X e] 4 f^{11} 5 d^0 6 s^2$
The oxidation state of Ho=+3
Question 28. Which of the following ions show higher spin-only magnetic moment value?
(i) Ti3+
(ii) Mn2+
(iii) Fe2+
(iv) Co3+
Answer:
The answer is option (ii, iii).$\mathrm{Mn}^{2+}\left(3 d^5\right)$ and $\mathrm{Fe}^{2+}\left(3 d^6\right)$ will show higher values of spin only magnetic moment.
Question 29. Transition elements form binary compounds with halogens. Which of the following elements will form MF3 type compounds?
(i) Cr
(ii) Co
(iii) Cu
(iv) Ni
Answer:
The answer is option (i, ii). Due to higher lattice energy in CoF3 cobalt can form halides like MF3 type of compounds. On the other hand, chromium can form CrF6 due to higher bond enthalpy.
Question 30. Which of the following will not act as an oxidising agents?
(i) CrO3
(ii) MoO3
(iii) WO3
(iv)$\mathrm{CrO}_4^{2-}$
Answer:
The answer is option (ii, iii). For a species to act as an oxidizing agent, the metal should be in a higher oxidation state, whereas stability is exhibited by its lower oxidation state. Since higher oxidation states of W and Mo are more stable; therefore, they do not act as oxidizing agents.
Question 31. Although +3 is the characteristic oxidation state for lanthanoids but cerium also shows +4 oxidation state because ___________.
(i) it has a variable ionisation enthalpy
(ii) it has a tendency to attain a noble gas configuration
(iii) it has a tendency to attain f0configuration
(iv) it resembles Pb4+
Answer:
The answer is option (ii, iii). The extra stability of empty, half-filled or completely filled f subshell gives rise to this irregularity. The noble gas configuration of Ce(IV) favours its formation.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: Short Answer Type
Some short answer type questions are also given here in the Chemistry NCERT Exemplar Class 12 Solutions Chapter 8 for practice. This section contains surface chemistry important questions that are asked in the exams.
Question 32. Why does copper not replace hydrogen from acids?
Answer:
The standard reduction potential value of copper ($E^o$=+0.34V) is positive. Therefore, obeying the reactivity series of metals, it does not liberate hydrogen from acids.
Question 33. Why E0 values for Mn,Ni and Zn are more negative than expected?
Answer:
Eo values of Mn2+ and Zn2+ are more negative than expected because of the stability they have due to the half-filled d subshell $\left(3 d^5: \mathrm{Mn}^{2+}\right)$ and completely-filled $\left(3 d^{10}: Z n^{2+}\right)$ configuration respectively.
Question 34. Why first ionisation enthalpy of Cr is lower than that of Zn?
Answer:
Given below is the electronic configuration of chromium and zinc
Cr (24)=[Ar]3d54s2
Zn (30)=[Ar]3d104s2
Cr has a half-filled d orbital, which is very stable and results in lower Ionization enthalpy. Similarly, the value for Zn is higher because its electron comes out from completely filled 4s orbital.
Question 35. Transition elements show high melting points. Why?
Answer:
Transition metals have a high melting point because of a higher degree of metallic bonds formed. In addition to the ns electrons, the (n−1) d electrons also contribute to the metallic bonding.
Question 36. When Cu2+ ion is treated with KI, a white precipitate is formed. Explain the reaction with the help of the chemical equation.
Answer:
Iodide ions reduce the Cu2+ ions :
$2 \mathrm{Cu}^{2+}+4 \mathrm{I}^{-} \rightarrow \mathrm{Cu}_2 \mathrm{I}_2$ (White ppt.) $+\mathrm{I}_2$
Question 37. Out of Cu2Cl2 and CuCl2, which is more stable and why?
Answer:
Due to a high hydration enthalpy, CuCl2 exhibits higher stability Cu2Cl2.
Question 38. When a brown compound of manganese (A) is treated with HCl it gives a gas (B). The gas taken in excess reacts with NH3 to give an explosive compound (C). Identify compounds A, B and C.
Answer:
A, B, C are shown below:
$A=\mathrm{MnO}_2 \quad B=\mathrm{Cl}_2 \quad \mathrm{C}=\mathrm{NCl}_3$
The reactions are explained as
$\mathrm{MnO}_2(\mathrm{~A})+4 \mathrm{HCl} \rightarrow \mathrm{MnCl}_2+\mathrm{Cl}_2(\mathrm{~B})+2 \mathrm{H}_2 \mathrm{O}$
$3 \mathrm{Cl}_2($ excess $)+\mathrm{NH}_3 \rightarrow \mathrm{NCl}_3(\mathrm{C})+3 \mathrm{HCl}$
Question 39. Although fluorine is more electronegative than oxygen, the ability of oxygen to stabilise higher oxidation states exceeds that of fluorine. Why?
Answer:
Fluorine $\left(1 s^2 2 s^2 2 p^5\right)$ can form a single bond as it has a single unpaired electron. However, Oxygen $\left(1 s^2 2 s^2 2 p^4\right)$ can form double bonds and can thus stabilize higher oxidation states.
Question 40. Although Cr3+ and Co2+ ions have the same number of unpaired electrons the magnetic moment of Cr3+ is 3.87 B.M. and that of Co2+ is 4.87 B.M. Why?
Answer:
Unlike Cr3+, Co2+ has a symmetrical electronic configuration, due to which the magnetic moment is higher for the Cobalt ion.
Question 41. Ionisation enthalpies of Ce, Pr and Nd are higher than Th, Pa and U. Why?
Answer:
6th period elements like Ce, Pr and Nd have smaller sizes as compared to 7th period elements like Th, Pa and U . As the size of the atom increases, the removal of electrons becomes easier (increased distance between outermost electrons and nucleus).
Question 42. Although Zr belongs to 4d and Hf belongs to 5d transition series but it is quite difficult to separate them. Why?
Answer:
Although Zr belongs to the 4f series and Hf to the 5f series, they have nearly the same size, because of which they exhibit very similar properties.
Question 43. Although +3 oxidation states are the characteristic oxidation state of lanthanoids, cerium also shows a +4 oxidation state. Why?
Answer:
Lanthanoids generally lose 3 electrons from $5 d^1 6 s^2$ to exhibit +3 oxidation state. However, Cerium has a configuration $4 f^1 5 d^1 6 s^2$, and gains additional stability by losing 4f1 electrons as well. It is the case because on losing 4 electrons, it has only completely filled orbitals
Question 44. Explain why the colour of KMnO4 disappears when oxalic acid is added to its solution in an acidic medium.
Answer:
In an acidic medium, KMnO4 acts as an oxidising agent and itself converts to MnSO4, which is colourless.
$\mathrm{KMnO}_4+3 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{~K}_2 \mathrm{SO}_4+2 \mathrm{MnSO}_4+3 \mathrm{H}_2 \mathrm{O}+5[\mathrm{O}]$
$\begin{aligned} & {\left[\mathrm{COOH}+[\mathrm{O}] \rightarrow 2 \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}\right] \times 5} \\ & \mathrm{COOH}\end{aligned}$
$\mathrm{KMnO}_4+3 \mathrm{H}_2 \mathrm{SO}_4+5\binom{C O O H}{C O O H} \rightarrow \mathrm{~K}_2 \mathrm{SO}_4+2 \mathrm{MnSO}_4($ Colourless $)+$10CO2+8H2O
When $\mathrm{Cr}_2 \mathrm{O}_7^{2-}$ is treated with an alkali :
$\mathrm{Cr}_2 \mathrm{O}_7^{2-}$ (orange) $+\mathrm{OH}^{-} \rightarrow 2 \mathrm{CrO}_4^{2-}$ (yellow)
When the yellow solution is treated with an acid, we get back the orange solution
$2 \mathrm{CrO}_4^{2-}+2 \mathrm{H}^{+} \rightarrow \mathrm{Cr}_2 \mathrm{O}_7^{2-}$ (Orange) $+\mathrm{H}_2 \mathrm{O}$
The oxidising nature of KMnO4 depends on the pH of the solution. They change to colourless manganous ions in an acidic medium.
$\mathrm{MnO}_4^{-}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_2 \mathrm{O}$
An alkaline solution will turn green because of the formation of manganate
$\mathrm{MnO}_4^{-}+\mathrm{e}^{-} \rightarrow \mathrm{MnO}_4^{2-}$
In a neutral solution, they will leave a brown precipitate, Manganese Oxide
$\mathrm{MnO}_4^{-}+2 \mathrm{H}_2 \mathrm{O}+3 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_2+4 \mathrm{OH}^{-}$
Question 47. The second and third rows of transition elements resemble each other much more than they resemble the first row. Explain why?
Answer:
The f-orbital contributes extraordinarily little to shielding, because of which the effective nuclear charge increases and the size decreases. Due to this the second and third row transition elements have similar atomic radii and resemble each other much more than the third-row elements.
Question 48. $E^{\Theta}$ of Cu is + 0.34V while that of Zn is – 0.76V. Explain.
Answer:
Cu has to lose an electron from a fully filled 3d10 orbital to become Cu2+. This isn’t balanced by the hydration enthalpy giving Cu a positive $E^{\Theta}$ value. On the other hand, Zn forms a relatively more stable Zn2+ with an electronic configuration of 3d10, which is completely filled, it has a lower ionization enthalpy than Cu2+, but nearly the same hydration energy and a negative $E^{\Theta}$ value.
Question 49. The halides of transition elements become more covalent with increasing oxidation state of the metal. Why?
Answer:
With an increase in the oxidation state, the element’s charge increases and size decreases. Fajan’s rule states that the smaller the ion, the more the bond will exhibit a covalent nature
Question 50. While filling up of electrons in the atomic orbitals, the 4s orbital is filled before the 3d orbital but the reverse happens during the ionisation of the atom. Explain why?
Answer:
While electrons are filled according to n+l rule (which is lower for 4s(4+0=4) than 3d(3+2=5), they leave according to the ionisation enthalpy. 4s electrons, being further from the nucleus, are more loosely held and are removed.
Question 51. Reactivity of transition elements decreases almost regularly from Sc to Cu. Explain.
Answer:
Effective nuclear charge increases as we move along the period from left to right. Due to this, there is a decrease in size as well. Therefore, the electrons will be held more tightly and removing them from the outermost shell will be difficult. Thus, the ionization enthalpy also increases and reactivity decreases. Sc is more reactive than Cu.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: Matching Type
The d and f-block important questions are discussed below. These are generally asked in exams to test your knowledge. The NCERT exemplar Class 12 chemistry solutions chapter 8 d and f-block elements is quite helpful for competitive exams.
Question 52. Match the catalysts given in Column I with the processes given in Column II.
|
Column I (Catalyst) |
Column II (Process) |
|
(i) Ni in the presence of hydrogen |
(a) Ziegler-Natta catalyst |
|
(ii) $\mathrm{Cu}_2 \mathrm{Cl}_2$ |
(b) Contact process |
|
(iii) $\mathrm{V}_2 \mathrm{O}_5$ |
(c) Vegetable oil to ghee |
|
(iv) Finely divided iron |
(d) Sandmeyer reaction |
|
(v) $\mathrm{TiCl}_4+\mathrm{Al}\left(\mathrm{CH}_3\right)_3$ |
(e) Haber's Process |
|
|
(f) Decomposition of KClO3 |
Answer:
(i → c), (ii → d), (iii → b), (iv → e), (v → a)
(i) Vegetable oil (Unsaturated fat) transformed into ghee (saturated fat) in the presence of hydrogen with Ni catalyst.
(ii) In Sandmeyer reaction, $\mathrm{Cu}_2 \mathrm{Cl}_2$ acts as a source of Cu+, which catalyze the replacement of diazonium group with a chlorine atom.
(iii) The Contact Process is used in the industrial production of sulfuric acid. In this process V2O5 (vanadium pentoxide) acts as a solid catalyst to increase the rate of this reaction.
(iv) The Haber Process is the industrial method used to synthesize ammonia from nitrogen and hydrogen gases. In this process, finely divided iron is used as a heterogeneous catalyst, which provides a large surface area for the nitrogen and hydrogen gases to react.
(v) Ziegler-Natta catalyst is used primarily in the polymerization of alkenes. It typically consists of titanium compounds (like TiCl4) supported on magnesium chloride and activated by trialkylaluminum compounds like Al(CH3)3.
Question 53. Match the compounds/elements given in Column I with uses given in Column II.
|
Column I (Compound/element) |
Column II (Use) |
|
(i) Lanthanoid oxide |
(a) Production of iron alloy |
|
(ii) Lanthanoid |
(b) Television screen |
|
(iii)Misch metal |
(c) Petroleum cracking |
|
(iv) Magnesium-based alloy is a constituent of |
(d) Lanthanoid metal+iron |
|
(v) Mixed oxides of lanthanoids are employed |
(e) Bullets |
|
|
(d) In the X-ray screen |
Answer:
(i → b), (ii → a), (iii →d), (iv → e), (v → c)
(i) Uses of Lanthanoid oxide: Colour TV screens, Fluorescent lamps, LEDs, etc.
(ii) Lanthanoids improve the properties of metals, so they are used in making alloys, the production of iron alloys and aluminium alloys.
(iii) Mischmetals are a mix of lanthanides, mainly Ce and La, which are used in high-strength steel and superalloys.
(iv) Magnesium-based alloy is a constituent of bullets.
(v) Mixed oxides of lanthanoids are indeed employed in petroleum cracking processes, especially in catalysis. Lanthanoid oxides have unique redox properties, acid-base characteristics, and thermal stability that make them effective as catalysts.
Question 54. Match the properties given in Column I with the metals given in Column II.
|
Column I (Property) |
Column II (Metal) |
|
(i) An element which can show +8 oxidation state |
(a) Mn |
|
(ii) 3d block element that can show up to +7 oxidation state |
(b) Cr |
|
(iii)3d block element with the highest melting point |
(c) Os |
|
|
(d) Fe |
Answer:
(i → c), (ii → a), (iii → b)
(i) The element Osmium (Os) exhibits multiple oxidation states, ranging from −2 to +8, which depend on the compound it forms.
(ii) Manganese (Mn) is a transition metal known for having the widest range of oxidation states, ranging from −3 to +7, which depends on the compound it forms.
(iii) Chromium (Cr) has the highest melting point due to strong metallic bonding and its electron configuration (five unpaired 3d electrons).
Question 55. Match the statements given in Column I with the oxidation states given in Column II.
|
Column I |
Column II |
|
(i) Oxidation state of Mn inMnO2 is |
(a) +2 |
|
(ii) The most stable oxidation state of Mn is |
(b) +3 |
|
(iii) Most stable oxidation state of Mn in oxides is |
(c) +4 |
|
(iv) The characteristic oxidation state of lanthanoids is |
(d) +5 |
|
|
(e) +7 |
Answer:
(i → c), (ii → a), (iii → e), (iv → b)
(i) Oxidation satate of Mn in MnO2
Let the oxidation state of Mn be x,
x + (-4) = 0
X = +4
(ii) Electronic configuration of Mn: [Ar] 3d5 4s2
In the +2 oxidation state, Mn loses the two 4s electrons. This gives a half-filled 3d subshell (3d5), which is very stable.
(iii) The most unstable oxidation state of Mn in oxides is +7. The +7 oxidation state of manganese occurs in Mn2O7. It is highly oxidizing, volatile, and explosive even in small quantities. It also decomposes easily, releasing oxygen and forming lower oxides (like MnO2).
(iv) Characteristic oxidation state of lanthanoids is +3. It arises from the loss of two 6s and one 4f/5d electron.
Question 56. Match the solutions given in Column I and the colours given in Column II.
|
Column I (Aqueous solution of salt) |
Column II (Colour) |
|
(i) FeSO4.7H2O |
(a) Green |
|
(ii) NiCl2.4H2O |
(b) Light pink |
|
(iii) MnCl2.4H2O |
(c) Blue |
|
(iv) CoCl2.6H2O |
(d) Pale green |
|
(v) Cu2Cl2 |
(e) Pink |
|
|
(f) Colourless |
Answer:
(i → d), (ii → a), (iii → b), (iv→ e), (v →f)
(i) Colour of FeSO4.7H2O: It contains Fe2+ ions. In hydrated form, it appears pale green due to d-d electronic transitions within the 3d orbitals, which are split in the presence of water ligands.
(ii) Colour of NiCl2.4H2O: It contains Ni2+ ions. In the hydrated form, Ni2+ is typically octahedrally coordinated by water and chloride ligands. The green colour appears due to d–d electronic transitions in the 3d8 system under an octahedral crystal field.
(iii) Colour of MnCl2.4H2O: It contains Mn2+ ions. An octahedral field formed by water and chloride ligands and Mn2+ has a high-spin d5 configuration; the d–d transitions in Mn2+ are spin-forbidden, which makes the colour light pink.
(iv) Colour of CoCl2.6H2O: It contains Co2+ ions. In the hydrated form, the Co2+ ions are typically in an octahedral complex with 6 water ligands. In this octahedral environment, it produces a pink colour.
(v) Colour of Cu2Cl2: It contains Cu+ ions. Cu+ has a 3d10 configuration, which means no unpaired electrons, hence it is colourless.
Question 57. Match the property given in Column I with the element given in Column II.
|
Column I (Property) |
Column II (Element) |
|
(i) Lanthanoid, which shows +4 oxidation state |
(a) Pm |
|
(ii) Lanthanoid which can show +2 oxidation state |
(b) Ce |
|
(iii) Radioactive lanthanoid |
(c) Lu |
|
(iv) Lanthanoid which has 4f7 electronic configuration in +3 oxidation state |
(d) Eu |
|
(v) Lanthanoid which has 4f14electronic configuration in +3 oxidation state |
(e) Gd |
|
|
(f) Dy |
Answer:
(i) → (b) (ii) → (d) (iii) → (a) (iv) → (e) (v) → (c)
(i) Cerium has oxidation state +4
$\mathrm{Ce}=[\mathrm{Xe}] 4 \mathrm{~F}^2 5 \mathrm{~d}^0 6 \mathrm{~s}^2 ;$ oxidation state $=+3,+4$
(ii) Europium has an oxidation state of +2
$\mathrm{Eu}=[\mathrm{Xe}] 4 \mathrm{~F}^7 5 \mathrm{~d}^0 6 \mathrm{~s}^2 ;$ oxidation state $=+2,+3$
(iii) Promethium is a man-made radioactive lanthanoid.
(iv) Gadolinium has electronic configuration 4F7 in +3 oxidation state is.
${ }_{64} \mathrm{Gd}=[\mathrm{Xe}] 4 \mathrm{~F}^7 5 \mathrm{~d}^1 6 \mathrm{~s}^2 ;$ oxidation state $=+3$
Question 58. Match the properties given in Column I with the metals given in Column II.
|
Column I (Property) |
Column II (Metal) |
|
(i) Element with the highest second ionisation enthalpy |
(a) Co |
|
(ii) Element with the highest third ionisation enthalpy |
(b) Cr |
|
(iii) M in M(CO)6 is |
(c) Cu |
|
(iv) Element with the highest heat of atomisation |
(d) Zn |
|
|
(e) Ni |
Answer:
(i → c), (ii → d), (iii → b), (iv → a)
i) Cu+ has a configuration 3d10 , making removal of the next electron a challenge
ii) Similar to Cu+ , Zn2+ has a configuration 3d10
iii) M=Cr
iv) Energy of atomization is highest for Nickel
NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: Assertion and Reason Type
This is one of the most important sections covered inthe NCERT exemplar Class 12 chemistry solutions chapter 8 d and f-block elements. These questions will improve your critical thinking.
Question 59. In the following question, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Cu2+ iodide is not known.
Reason: Cu2+ oxidises I– to iodine.
(i) Both assertion and reason are true, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are true, but the reason is not the correct explanation of the assertion.
(iii) The assertion is not true, but the reason is true.
(iv) Both assertion and reason are false.
Answer:
The answer is the option (i). Iodide gets oxidised in the presence of Cu2+ to Iodine.
Question 60. In the following question, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Separation of Zr and Hf is difficult.
Reason: Because Zr and Hf lie in the same group of the periodic table.
(i) Both assertion and reason are true, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are true, but the reason is not the correct explanation of the assertion.
(iii) The assertion is not true, but the reason is true.
(iv) Both assertion and reason are false.
Answer:
The answer is the option (ii). Due to their similar sizes, it is difficult to separate Zr and Hf
Question 61. In the following question, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Actinides form relatively less stable complexes as compared to lanthanoids.
Reason: Actinides can utilise their 5f orbitals along with 6d orbitals in bonding but lanthanoids do not use their 4f orbital for bonding.
(i) Both assertion and reason are true, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are true, but the reason is not the correct explanation of the assertion.
(iii) The assertion is not true, but the reason is true.
(iv) Both assertion and reason are false.
Answer:
The answer is option (iii). Actinoids form more stable complex compounds than Lanthanoids.
Question 62. In the following question, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion : Cu cannot liberate hydrogen from acids.
Reason: Because it has positive electrode potential.
(i) Both assertion and reason are true, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are true, but the reason is not the correct explanation of the assertion.
(iii) The assertion is not true, but the reason is true.
(iv) Both assertion and reason are false.
Answer:
(i) Cu has a higher electrode potential than hydrogen, because of which it can’t liberate Hydrogen in acidic solutions.
Question 63. In the following question, a statement of assertion is followed by a statement of reason. Choose the correct answer out of the following choices.
Assertion: The highest oxidation state of osmium is +8.
Reason: Osmium is a 5d-block element
(i) Both assertion and reason are true, and reason is the correct explanation of the assertion.
(ii) Both assertion and reason are true, but the reason is not the correct explanation of the assertion.
(iii) The assertion is not true, but the reason is true.
(iv) Both assertion and reason are false.
Answer:
The answer is the option (ii). Osmium can use all its 8 electrons to expand its octet and show the oxidation state of +8.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 8: Long Answer Type
The final section contains long-answer type questions. These are d and f-block important questions that are asked in the exams.
Question 64. Identify A to E and also explain the reactions involved.
Answer:
$\mathrm{CuCO}_3 \xrightarrow{\mathrm{Heat}} \mathrm{CuO}+\mathrm{CuO}_2(\mathrm{D})$
$\mathrm{Ca}(\mathrm{OH})_2+\mathrm{CO}_2 \rightarrow \mathrm{CaCO}_3(E)($ Milky $)+\mathrm{H}_2 \mathrm{O}$
$\mathrm{CaCO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}\left(\mathrm{HCO}_3\right)_2($ Clear solution $)$
$2 \mathrm{CuO}+\mathrm{CuS} \xrightarrow{\text { Heat }} 3 \mathrm{Cu}(A)+\mathrm{SO}_2$
$\mathrm{Cu}+4 \mathrm{HNO}_3($ Conc. $) \xrightarrow{\text { Heat }} \mathrm{Cu}\left(\mathrm{NO}_3\right)_2(\mathrm{~B})+2 \mathrm{NO}_2+2 \mathrm{H}_2 \mathrm{O}$
$\mathrm{Cu}\left(\mathrm{NO}_3\right)_2+4 \mathrm{NH}_3 \rightarrow\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]\left(\mathrm{NO}_3\right)_2(\mathrm{C})$ (Blue Solution)
The compounds A, B, C and D are given as under:
$\mathrm{A}=\mathrm{FeCr}_2 \mathrm{O}_4 \quad \mathrm{~B}=\mathrm{Na}_2 \mathrm{CrO}_4 \quad \mathrm{C}=\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7 \cdot 2 \mathrm{H}_2 \mathrm{O} \quad \mathrm{D}=\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$
The following are the reactions :
$4 \mathrm{FeCr}_2 \mathrm{O}_4(\mathrm{~A})+8 \mathrm{NaCO}_3+7 \mathrm{O}_2 \rightarrow 8 \mathrm{Na}_2 \mathrm{CrO}_4(\mathrm{~B})+2 \mathrm{Fe}_2 \mathrm{O}_3+8 \mathrm{CO}_2$
$2 \mathrm{NaCrO}_4+2 \mathrm{H}^{+} \rightarrow \mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{Na}^{+}+\mathrm{H}_2 \mathrm{O}$
$\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7(\mathrm{C})+2 \mathrm{KCl} \rightarrow \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{NaCl}$
$\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{KCl} \rightarrow \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{NaCl}$
The compounds (A), (B), (C) and (D) are given as under:
$A=\mathrm{MnO}_2 \quad B=K_2 \mathrm{MnO}_4 \quad C=K \mathrm{MnO}_4 \quad D=K I O_3$
$2 \mathrm{MnO}_2(A)+4 \mathrm{KOH}+\mathrm{O}_2 \rightarrow 2 \mathrm{~K}_2 \mathrm{MnO}_4(B)+2 \mathrm{H}_2 \mathrm{O}$
$3 \mathrm{MnO}_4^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O}$
$2 \mathrm{MnO}_4^{-}+\mathrm{H}_2 \mathrm{O}+\mathrm{KI} \rightarrow 2 \mathrm{MnO}_2(i)+2 \mathrm{OH}^{-}+\mathrm{KIO}_3(i v)$.
(a). The covalent character of the bond increases as the size of the atom decreases. La is larger than Lu (Lanthanide contraction), hence, La2O3 displays more ionic behaviour, but Lu2O3 displays more covalent behaviour.
(b). Decrease in size of atoms also leads to da ecrease in stability of the oxo-salts.
(c). A decrease in the size of atoms corresponds with the production of lower stable compounds.
(d). 4d and 5d block elements in the same column have nearly equal radii.
(e) There is an increase in the acidic nature of oxides as the size decreases.
(a)
(i)Copper has an electronic configuration $3 d^{10} 4 s^1$ & the second electron, which has to be removed from a fully filled d-orbital, leads to a very high ionization enthalpy.
(ii) Zinc has an electronic configuration of $3 d^{10} 4 s^2$ & the third electron, which has to be removed from a fully filled d-orbital, leads to a very high ionization enthalpy.
(iii) With no unpaired electrons, Zinc has the lowest energy of atomization
(b)
(i) Fe(EANrule)
(ii) Mn (oxidation state of +7)
Interstitial compounds are formed when the crystal lattice of larger transition metals traps smaller atoms like Hydrogen, Carbon and Nitrogen. These compounds have a higher melting point than pure metals, are very hard, conduct electricity and are chemically inert.
(a) The reaction between iodide and persulfate ions is :
$2 \mathrm{I}^{-}+\mathrm{S}_2 \mathrm{O}_8^{2-} \xrightarrow{F e(I I I)} \mathrm{I}_2+2 \mathrm{SO}_4^{2-}$
Role of $\mathrm{Fe}(I I I)$ ions : $2 \mathrm{Fe}^{3+}+2 \mathrm{I}^{-} \rightarrow 2 \mathrm{Fe}^{2+}+I_2$$2 \mathrm{Fe}^{2+}+\mathrm{S}_2 \mathrm{O}_8^{2-} \rightarrow 2 \mathrm{Fe}^{3+}+2 \mathrm{SO}_4^{2-}$
(b) (i)MnO2 acts as a catalyst : $\mathrm{KClO}_3 \rightarrow \mathrm{O}_2$
(ii) Vanadium Oxide acts as a catalyst in the contact process: $\mathrm{SO}_2 \rightarrow \mathrm{SO}_3$
(iii) Finely divided iron acts as a catalyst in Haber's process: $\mathrm{N}_2+\mathrm{H}_2 \rightarrow \mathrm{NH}_3$
The compounds A, B, C and D are given as :
$A=\mathrm{KMnO}_4 \quad B=\mathrm{K}_2 \mathrm{MnO}_4 \quad \mathrm{C}=\mathrm{MnO}_2 \quad D=\mathrm{MnCl}_2$
The reactions are as
$\mathrm{KMnO}_4(A) \xrightarrow{\Delta} \mathrm{K}_2 \mathrm{MnO}_4(B)+\mathrm{MnO}_2(C)+\mathrm{O}_2$
$\mathrm{MnO}_2+\mathrm{KOH}+\mathrm{O}_2 \rightarrow 2 \mathrm{~K}_2 \mathrm{MnO}_4+2 \mathrm{H}_2 \mathrm{O}$
$\mathrm{MnO}_2+4 \mathrm{NaCl}+4 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{MnCl}_2(\mathrm{D})+2 \mathrm{NaHSO}_4+2 \mathrm{H}_2 \mathrm{O}+\mathrm{Cl}_2$
NCERT Exemplar Class 12 Chemistry Chapter 8: Higher Order Thinking Skills (HOTS) Questions
Some tricky Class 12 chemistry chapter 8 questions and answers are given below that will help you tackle complex problems. Students can follow class 12 d and f-block notes to learn the concepts in detail.
Question 1: The correct decreasing order of spin only magnetic moment values $(\mathrm{BM})$ of $\mathrm{Cu}^{+}, \mathrm{Cu}^{2+}, \mathrm{Cr}^{2+}$ and $\mathrm{Cr}^{3+}$ ions is:
1) $\mathrm{Cu}^{+}>\mathrm{Cu}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cr}^{2+}$
2) $\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}>\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}$
3) $\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}$
4) $\mathrm{Cr}^{3+}>\mathrm{Cr}^{2+}>\mathrm{Cu}^{+}>\mathrm{Cu}^{2+}$
Answer:
$\mathrm{Cu}^{+} \Rightarrow 3 \mathrm{~d}^{10} \Rightarrow$ 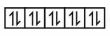
$\mathrm{Cu}^{2+} \Rightarrow 3 \mathrm{~d}^9 \Rightarrow$ 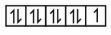
$\mathrm{Cr}^{2+} \Rightarrow 3 \mathrm{~d}^4 \Rightarrow$ 
$\mathrm{Cr}^{3+} \Rightarrow 3 \mathrm{~d}^3 \Rightarrow$ 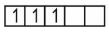
So order :
$\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}$
Hence, the correct answer is option (3).
Question 2:The number of unpaired electrons responsible for the paramagnetic nature of the following complex species are respectively :
$\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-},\left[\mathrm{FeF}_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$
1) $1,5,4,2$
2) $1,5,5,2$
3) $1,1,4,2$
4) $1,4,4,2$
Answer:
$\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-} \Rightarrow \mathrm{Fe}^{3+}, \mathrm{d}^5, \mathrm{t}_{2 \mathrm{~g}}^5 \mathrm{e}_{\mathrm{g}}^0$
$\quad$ $\Rightarrow 1$ unpaired electron
$\left[\mathrm{FeF}_6\right]^{3-} \Rightarrow \mathrm{Fe}^{3+}, \mathrm{d}^5, \mathrm{t}_{2 \mathrm{~g}}^3 \mathrm{e}_{\mathrm{g}}^2 \Rightarrow 5$ unpaired electrons $\left[\mathrm{CoF}_6\right]^{3-} \Rightarrow \mathrm{Co}^{3+}, \mathrm{d}^6, \mathrm{t}_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^2 \Rightarrow 4$ unpaired electrons
$\begin{aligned} {\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-} \Rightarrow } & \mathrm{Mn}^{3+}, \mathrm{d}^4,\end{aligned} \mathrm{t}_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^0 \mathrm{C}$.
$\quad$ $\Rightarrow 2$ unpaired electron
Hence, the correct answer is option (1).
Question 3. The first transition series metal ' M ' has the highest enthalpy of atomisation in its series. One of its aquated ion $\left(\mathrm{M}^{\mathrm{n}}\right)$ exists in green colour. The nature of the oxide formed by the above $\mathrm{M}^{\mathrm{n}-}$ ion is :
(1) neutral
(2) acidic
(3) basic
(4) amphoteric
Answer:
* In 3d series Vanadium has highest enthalpy of atomization and colour of $\mathrm{V}^{+3}$ is green.
* Oxide form by $\mathrm{V}^{+3}$ is $\mathrm{V}_2 \mathrm{O}_3$ (Basic oxide)
Hence, the correct answer is option (3).
Question 4: The incorrect relationship in the following pairs in relation to ionisation enthalpies is :
(1) $\mathrm{Mn}^{+}<\mathrm{Cr}^{+}$
(2) $\mathrm{Mn}^{+}<\mathrm{Mn}^{2+}$
(3) $\mathrm{Fe}^{2+}<\mathrm{Fe}^{3+}$
(4) $\mathrm{Mn}^{2+}<\mathrm{Fe}^{2+}$
Answer:
$\mathrm{Mn}^{2+}:[\mathrm{Ar}] 3 \mathrm{~d}^5$
Half filled stability
Therefore, more I.E. than $\mathrm{Fe}^{2+}$
$\mathrm{Fe}^{2+}:[\mathrm{Ar}] 3 \mathrm{~d}^6$
Hence, the correct answer is option (4).
Question 5: $KMnO_4$ acts as an oxidising agent in acidic medium. ‘X’ is the difference between the oxidation states of Mn in reactant and product. ‘Y’ is the number of ‘d’ electrons present in the brown red precipitate formed at the end of the acetate ion test with neutral ferric chloride. The value of X + Y is _________.
(1) 10
(2) 11
(3) 12
(4) 14
Answer:
In acidic medium, $\mathrm{KMnO}_4$ acts as an oxidizing agent
$
\mathrm{MnO}_4^{-} \rightarrow \mathrm{Mn}^{2+}
$
Oxidation state of Mn in $\mathrm{MnO}_4{ }^{-}=+7$
Oxidation state of Mn in $\mathrm{Mn}^{2+}=+2$
So,
$
X=7-2=5
$
$\begin{aligned} & 6 \mathrm{CH}_3 \mathrm{COO}^{\ominus}+\mathrm{Fe}^{3+}+\mathrm{H}_2 \mathrm{O} \rightarrow\left[\mathrm{Fe}_3\left(\mathrm{OH}_2\right)\left(\mathrm{CH}_3 \mathrm{COO}\right)_6\right]^{\oplus}+2 \mathrm{H}^{\oplus} \\ & {\left[\mathrm{Fe}_3(\mathrm{OH})_2\left(\mathrm{CH}_3 \mathrm{COO}\right)_6\right]^{\oplus}+4 \mathrm{H}_2 \mathrm{O}} \rightarrow \underset{\text { Brown red ppt }}{\left[\mathrm{Fe}(\mathrm{OH})_2\left(\mathrm{CH}_3 \mathrm{COO}\right]+\mathrm{CH}_3 \mathrm{COOH}+\mathrm{H}^{\oplus}\right.}\end{aligned}$
$\mathrm{Fe}^{3+} \Rightarrow 3 \mathrm{~d}^5 4 \mathrm{~s}^0$ contains 5 d electrons
So $\mathrm{Y}=5$
$X+Y=5+5=10$
Hence, the correct answer is 10.
Approach to Solve Questions of Class 12 Chemistry Chapter 8
The approach should be simple and time-saving. The following are the points that can help you build a good question-solving strategy for d and f-block elements Class 12 Chemistry Chapter 8 NCERT Exemplar Solutions:
1. Start by clearly understanding the electronic configuration, oxidation states and general properties of d- and f-block elements. These concepts form the foundation for most questions. The NCERT Exemplar Class 12 Chemistry Solutions Chapter 8 will help you understand these concepts better.
2. Make your focus on periodic trends such as atomic size, ionization enthalpy, melting/boiling points and magnetic properties within the transition and inner transition elements.
3. Try to remember common colored compounds and their oxidation states, especially for transition metals. You can make flowcharts or flashcards to revise it. You can learn these concepts in class 12 chemistry chapter 8 notes to learn more.
4. Give proper attention to the important reactions like the preparation and properties of potassium dichromate and potassium permanganate, and their oxidizing behaviour in acidic, basic, and neutral media.
5. You can refer to the NCERT solved examples and try solving in-text questions for a better understanding of the concept. Also, solve the textbook exercise questions as they are often directly asked in board exams. You can also refer to the NCERT exemplar for better learning. Practice previous year questions and solve mock tests.
Topics and Subtopics Covered in the NCERT Exemplar Class 12 Chemistry Chapter 8
The following are the most important topics covered in the Class 12 NCERT Chemistry book.
- Position in the Periodic Table
- Electronic Configurations of the d-Block Elements
- General Properties of the Transition Elements (d-Block)
- Physical Properties
- Variation in Atomic and Ionic Sizes of Transition Metals
- Ionisation Enthalpies
- Oxidation States
- Trends in the M2+/M Standard Electrode Potentials
- Trends in the M3+/M2+ Standard Electrode Potentials
- Trends in the Stability of Higher Oxidation States
- Chemical Reactivity and Eθ Values
- Magnetic Properties
- Formation of Coloured Ions
- Formation of Complex Compounds
- Catalytic Properties
- Formation of Interstitial Compounds
- Alloy Formation
- Some Important Compounds of Transition Elements
- Oxides and Oxoanions of Metals
- The Lanthanides
- Electronic Configurations
- Atomic and Ionic Sizes
- Oxidation States
- General Characteristics
- The Actinides
- Electronic Configurations
- Ionic Sizes
- Oxidation States
- General Characteristics and Comparison with Lanthanoids
- Some Applications of d- and f-Block Elements
NCERT Exemplar Class 12 Chemistry Chapter 8: Important Formulas and Key Points
Some Important formulas and key points of the d-block and f-block are given below. Practice more at NCERT Exemplar Class 12 Chemistry Solutions Chapter 8 d and f-block elements.
1. General electronic configuration of the d-block:
= $(\mathrm{n}-1) \mathrm{d}^{1-10} \mathrm{~ns}^{0-2}$
2. General electronic configuration of the f-block:
= $(n-2) f^{1-14}(n-1) d^{0-1} n s^2$
3. Magnetic moment μ
=μ=n(n+2)B.M.
Where n = number of unpaired electrons.
4. Most f-block elements are paramagnetic due to unpaired f-electrons.
5. Lanthanides are known for the +3 oxidation state.
6. Actinides show a wider range of oxidation states (+3 to +6 or more).
7. Mostly d-block elements are paramagnetic due to unpaired d-electrons.
8. D-block elements act as good catalysts.
Advantages of Class 12 Chemistry Chapter 8 The d- and f-Block Elements NCERT Exemplar Solutions
The class 12 chemistry ncert exemplar solutions chapter 8 d and f-block elements provide a clear explanation of concepts that helps in mastering topics and scoring well in board and competitive exams. The advantages of using these solutions are given below:
1. The d and f block elements solutions cover all important concepts like electronic configuration, oxidation states, magnetic properties, and complex formation of transition and inner transition elements with the help of solved questions.
2. These NCERT Exemplar Class 12 Solutions are prepared by subject experts and they are well organised that help students quick revision.
3. Some important HOTS questions are also provided in these solutions to help you in scoring good marks.
4. NCERT exemplar Class 12 chemistry solutions chapter 8 d and f-block elements are prepared in a concise and structured manner that are very helpful for last minute revision.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions subject-wise
The NCERT subject-wise solutions will help you broaden your concept and will also help in revision.
NCERT Exemplar Class 12 Solutions subject-wise
Excel your preparation with NCERT exemplar solutions. Click on the links below
NCERT Class 12 subject-wise notes
You can follow the links given in the table below to get access to the Class 12 NCERT notes.
NCERT Books and NCERT Syllabus
The links in the table will give you access to the Class 12 NCERT chemistry book and syllabus for the respective subjects.
Frequently Asked Questions (FAQs)
Transition elements are defined as elements that have incompletely filled d-orbitals in their ground state or in any of their common oxidation states.
The general electronic configuration of d-block elements is (n-1)d1-10 ns1-2, where 'n' represents the outermost shell number.
The colour of transition metal ions arises from d-d transitions. When light is shone on a transition metal ion, electrons in the lower energy d-orbitals can absorb energy and jump to higher energy d-orbitals. The colour observed corresponds to the complementary colour of the light absorbed. The presence of unpaired electrons is essential for d-d transitions to occur.
The transition elements exhibit variable oxidation states because of the small energy difference between the (n-1)d and ns orbitals. Electrons from both orbitals can be involved in bond formation, which leads to multiple possible oxidation states.
The Lanthanide Contraction is the gradual decrease in the atomic and ionic radii of the Lanthanides with increasing atomic number. This is due to the poor shielding of the nuclear charge by the 4f electrons. The inner 4f electrons do not effectively shield the outer electrons from the increasing nuclear charge, leading to a stronger attraction and a smaller size.
Chemistry NCERT Exemplar Class 12 Solutions Chapter 8 provides detailed, high-level practice questions and solved examples on the d- and f-block elements, helping students strengthen concepts and prepare for competitive exams.
Revise each chapter thoroughly from NCERT, then practise exemplar questions regularly to build conceptual clarity and improve problem-solving skills.
NCERT Exemplar Solutions Class 12 Chemistry cover MCQs based on conceptual understanding, numerical applications, reasoning-based questions, and tricky application-based problems from all chapters of the textbook.
Yes, many d-block elements act as good catalysts because they can change oxidation states and provide active sites for reactions due to their partially filled d-orbitals.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Hello,
Here is your Final Date Sheet Class 12 CBSE Board 2026 . I am providing you the link. Kindly open and check it out.
https://school.careers360.com/boards/cbse/cbse-class-12-date-sheet-2026
I hope it will help you. For any further query please let me know.
Thank you.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters


