NCERT Solutions for Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes
Do you know why fire extinguishers are so effective, how medicines work so fast, and what makes refrigerants cool? The answer to all these questions lies in Haloalkanes and Haloarenes. The word Haloalkanes and Haloarenes are made up of two words: the first is ‘Halo’, which means halogens, and the second word is ‘alkanes’ or ‘arenes’, which means aliphatic or aromatic hydrocarbons. So, these are organic compounds containing halogens. Due to their unique properties, they are used in industrial applications and biological processes. Many organic compounds containing halogens occur naturally and have clinical significance.
The Central Board of Secondary Education (CBSE) will release the answer key soon for CBSE Class 12 Psychology paper. Students will be able to use it to cross-check their answers and estimate scores before the official results are declared.
This Story also Contains
- NCERT Solutions for Class 12 Chemistry Chapter 6: Download PDF
- NCERT Solutions for Class 12 Chemistry Haloalkanes and Haloarenes (Intext Question from 6.1 to 6.9)
- NCERT Solutions for Class 12 Chemistry Haloalkanes and Haloarenes- (Exercise Questions)
- Class 12 Chemistry NCERT Chapter 6: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 6 Haloalkanes and Haloarenes
- Topics of NCERT Syllabus Class 12 Chemistry Haloalkanes and Haloarenes
- What Extra Should Students Study Beyond the NCERT for JEE/NEET?
- What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes
- Importance of Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes Solutions
- NCERT Solutions Class 12 Chemistry

Haloalkanes and haloarenes play a vital role in the Class 12 Chemistry curriculum. The NCERT Class 12 Chemistry Solutions are prepared by subject experts in a very easy and comprehensive way. This will help to resolve all the problems covered in class 12 chemistry chapter 6 haloalkanes and haloarenes solutions. These NCERT solutions will help you score well in board exams as well as in competitive exams. To enhance your understanding this article also includes Higher order thinking skills questions.
NCERT Solutions for Class 12 Chemistry Chapter 6: Download PDF
Students can download the haloalkanes and haloarenes ncert solutions pdf for free. These solutions are designed to help you understand the fundamental concepts and solve textbook questions with ease.
Also read
NCERT Solutions for Class 12 Chemistry Haloalkanes and Haloarenes (Intext Question from 6.1 to 6.9)
These haloalkanes and haloarenes class 12 question answer provide clear and accurate answers to all the intext questions, helping students build a strong understanding. These solutions of NCERT simplify complex reaction mechanisms and making it easier for students to learn and apply them in exams.
Question 6.1 Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
Answer :
Main chain will be of five carbon atoms. At second position chlorine will be present and at third position, methyl group will be present.
The structure of 2-Chloro-3-methylpentane is given below :-
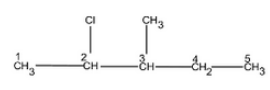
Question 6.1 Write structures of the following compounds:
(ii) 1-Chloro-4-ethylcyclohexane
Answer :
A cyclic structure is present in which at 1st carbon there is chlorine present and at the 4th position ethyl group is present.
The structure of 1-Chloro-4-ethylcyclohexane is given below :-
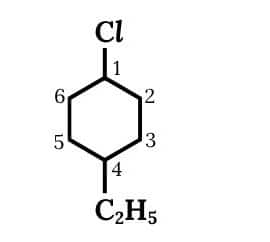
Question 6.1 Write structures of the following compounds:
(iii) 4-tert. Butyl-3-iodoheptane
Answer :
Main chain will be of seven carbon atoms. At 4th position tertiary butyl will be present and at the 3rd position iodine will be present.
The structure of 4-tert. Butyl-3-iodoheptane is given below :-
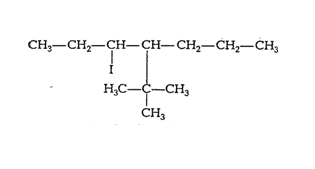
Question 6.1 Write structures of the following compounds:
Answer :
Main chain will be of 4 carbon atoms and it is an alkene. There are two bromine atoms on the terminal carbon atoms.
The structure of 1,4-Dibromobut-2-ene is given below :-
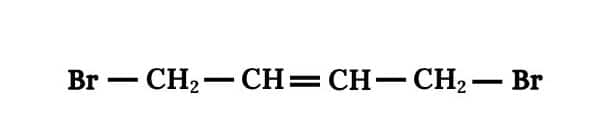
Question 6.1 Write structures of the following compounds:
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Answer :
Main chain will be the benzene. Three substituents are present on benzene
The structure of 1-Bromo-4-sec. butyl-2-methylbenzene is shown below :-
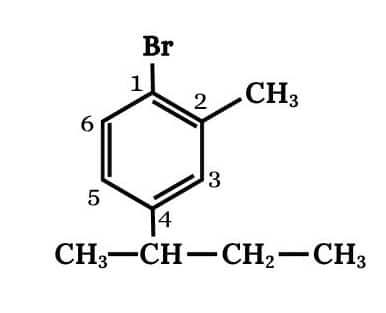
Question 6.2 Why is sulphuric acid not used during the reaction of alcohols with KI?
Answer :
We don't use sulphuric acid because it acts as an oxidising agent and the required alkyl iodide is not produced. KI is expected to give HI on reacting with $\mathrm{H}_2 \mathrm{SO}_4$ which will convert alcohols (R – OH) to alkyl iodides (R – I). However, $\mathrm{H}_2 \mathrm{SO}_4$ is a strong oxidising agent and it oxidises HI formed during the reaction to I2 which does not react with alcohol.
The reactions are given below :-
$2 \mathrm{KI}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow 2 \mathrm{KHSO}_4+2 \mathrm{HI}$
$2 \mathrm{HI}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{I}_2+\mathrm{SO}_2+\mathrm{H}_2 \mathrm{O}$
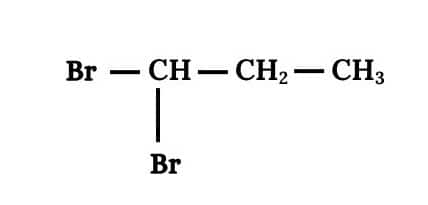
Question 6.3 Write structures of different dihalogen derivatives of propane.
Answer :
We obtain four dihalogen derivatives of propane :-
(i) 1,1 Dibromopropane
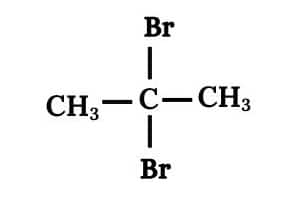
(ii) 2, 2 Dibromopropane
(iii) 1, 2 Dibromopropane
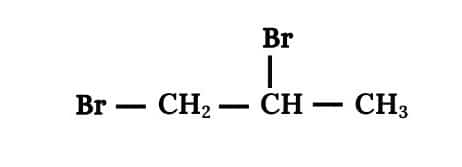
(iv) 1, 3 Dibromopropane
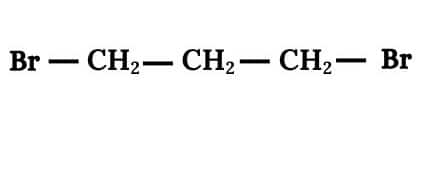
Question 6.4 Among the isomeric alkanes of molecular formula C5H12 , identify the one that on photochemical chlorination yields
Answer :
In this we have to find an isomer in which replacement of any hydrogen atom gives the same compound for all replacements.
So the isomer is Neopentane.
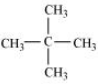
Question 6.4 Among the isomeric alkanes of molecular formula C5H12 , identify the one that on photochemical chlorination yields
(ii) Three isomeric monochlorides.
Answer :
For the given condition we must have three different hydrogens so that we can get three different monochlorides on the replacement.
Thus the isomer is n-pentane.
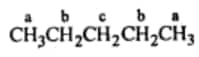
Question 6.4 Among the isomeric alkanes of molecular formula $\mathrm{C}_5 \mathrm{H}_12$ , identify the one that, on photochemical chlorination, yields
(iii) Four isomeric monochlorides .
Answer :
For four monochlorides, we need four different hydrogens which can be replaced by chlorine.
Hence the required isomer is iso pentane:-
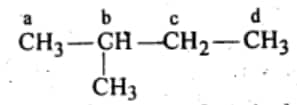
Question 6.5 Draw the structures of major monohalo products in each of the following reactions:
(i) 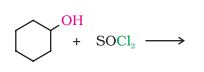
Answer: In this reaction the hydroxyl group will be replaced with the chlorine atom.
The final products are:-
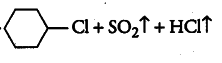
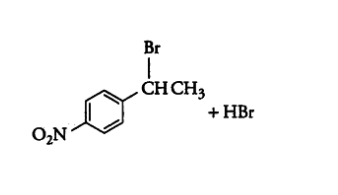
Question 6.5 Draw the structures of major monohalo products in each of the following reactions:
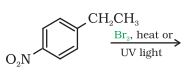
Answer :
In this reaction the amine group will be replaced with methyl bromide.
The reaction is given below:
Question 6.5 Draw the structures of major monohalo products in each of the following reactions:
(iii) 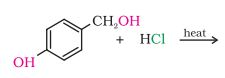
Answer :
In this reaction the hydroxyl group with the methyl group will be replaced with the chlorine atom.
The reaction is given below:
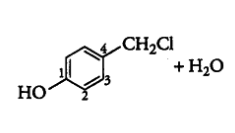
Question 6.5 Draw the structures of major monohalo products in each of the following reactions:
(iv) 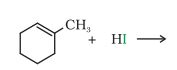
Answer :
In this reaction the iodine from the hydrogen iodide will attach the carbon atom having the double bond as well as methyl group.
The reaction is given below:
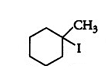
Question 6.5 (V) Draw the structures of major monohalo products in each of the following reactions:
$CH_3CH_2Br+\ NaI\rightarrow$
Answer :
In this reaction the iodine atom will replace the bromine in the haloalkane.
The reaction is given below:
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{I}+\mathrm{NaBr}$
Question 6.5 Draw the structures of major monohalo products in each of the following reactions:
(vi) 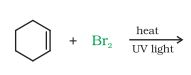
Answer :
In this reaction, the bromine atom will attack the alpha-carbon atom of the double bond.
The reaction is given below:
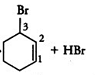
Question 6.6 Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane.
Answer :
It is known that boiling point increases with increase in molecular mass when the alkyl group is the same.
So the order of increasing boiling point is Chloromethane < Bromomethane < Dibromomethane < Bromoform
Question 6.6 Arrange each set of compounds in order of increasing boiling points.
(ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Answer :
In the given compounds the halide groups are same. In these cases, the boiling point depends on the bulkiness of the alkyl group. The boiling point increases with an increase in the chain length. Also, the boiling point decreases with an increase in branching.
So the order is :- 1- Chlorobutane > 1- Chloropropane > Isopropyl Chloride
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Br} or
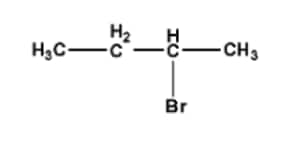
Answer :
In this case, the rate of $S_{N}2$ reaction will depend on the hindrance of the substrate.
Since 1-Bromobutane is a $1^{\circ}$ alkyl halide and 2-Bromobutane is a $2^{\circ}$ alkyl halide, 2-Bromobutane gives more hindrance to the nucleophile.
Hence, 1-bromobutane reacts faster.

Answer :
The rate of $S_{N}2$ reaction decreases with an increase in hindrance to the attack of the nucleophile.
In the first compound the alkyl halide is secondary halide while the second compound is a tertiary halide. So 2-bromobutane will react faster than 2-bromo-2-methylpropane in the nucleophilic attack.
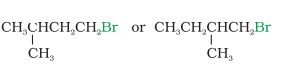
Answer :
Both compounds are secondary halides .In these kinds of cases, we see where is the substituent is attached i.e., how far from the halide group. It can be clearly seen that the methyl group attached in 1-bromo-2-methylbutane is near than that attached in 1-bromo-3-methyl butane.
Hence, the rate of $S_{N}2$ reaction will be faster in the case of 1-bromo-3-methylbutane.
Question 6.8 In the following pairs of halogen compounds, which compound undergoes faster $S_{N}1$ reaction?
(i) 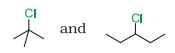
Answer :
In $S_{N}1$ reactions, we see the formation of carbocation and this is the rate determining step for this kind of reactions. So the compound having more stable carbocation will react faster. For $S_N 1$ the order of ractivity is $3^{\circ}>2^{\circ}>1^{\circ}$. In the given case 2- Chloro, 2- Methylpropane we have $3^{\circ}$ carbon whereas in 3- Chloropentane we have $2^{\circ}$ carbon.
Question 6.8 In the following pairs of halogen compounds, which compound undergoes faster $S_{N}1$ reaction?
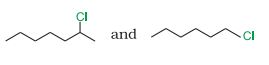
Answer :
In $S_{N}1$ reactions, we see the formation of carbocation and this is the rate determining step for these kinds of reactions.So the compound having more stable carbocation will react faster. The first compound is a secondary compound and the second compound is primary compound. Hence 2-Chloroheptane will react faster than 1- Chlorohexane.
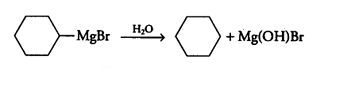
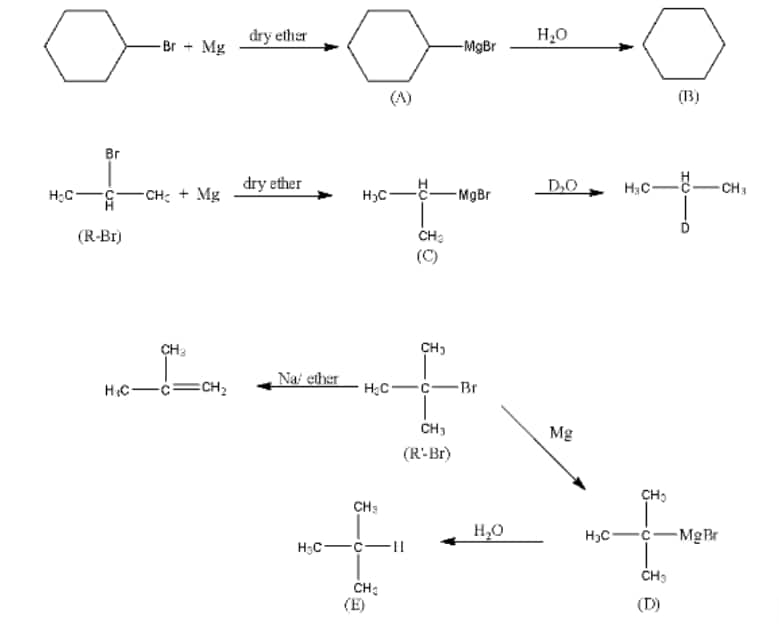
Question 6.9 Identify A, B, C, D, E, R and R1 in the following:
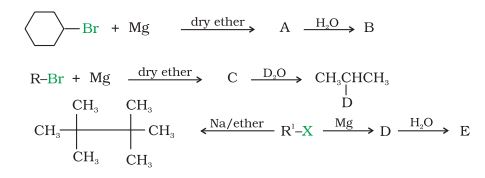
Answer :
1st reaction:-
The compound A in the reaction is cyclohexylmagnesium bromide, compound B is Cyclohexane.
2nd reaction:-
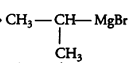
3rd reaction:-
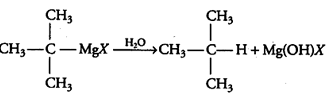
The overall reaction is given below:
NCERT Solutions for Class 12 Chemistry Haloalkanes and Haloarenes- (Exercise Questions)
These class 12 chemistry chapter 6 haloalkanes and haloarenes solutions offer detailed answers to all the exercise questions from the chapter. NCERT solutions for Class 12 help students strengthen their conceptual understanding, practise important reactions, and prepare effectively for board and competitive exams.
(i) $\left ( CH_{3} \right )_{2}CHCH (Cl)CH_{3}$
Answer :
(i) 2-Chloro-3-methylbutane. And it is a secondary alkyl halide.
(ii) $CH_{3}CH_{2}CH(CH_{3})CH(C_{2}H_{5})Cl$
Answer :
3-Chloro-4-methylhexane. And it is a secondary alkyl halide.
(iii) $CH_{3}CH_{2}C(CH_{3})_{2}CH_{2}I$
Answer :
(iii) 1-Iodo-2, 2-dimethylbutane. And it is a primary alkyl halide.
Question 6.1 Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
(iv) $(CH_{3})_{3}CCH_{2}CH(Br)C_{6}H_{5}$
Answer :
(iv) 1-Bromo-3, 3-dimethyl-1-phenylbutane. And it is secondary benzyl halide.
(v) $CH_{3}CH(CH_{3})CH(Br)CH_{3}$
Answer :
(v) 2-Bromo-3-methylbutane. And it is a secondary alkyl halide.
(vi) $CH_{3}C(C_{2}H_{5})_{2}CH_{2}Br$
Answer :
(vi) 1-Bromo-2-ethyl-2-methylbutane. And it is a primary alkyl halide.
Question 6.1 Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
(vii) $CH_{3}C(Cl)(C_{2}H_{5})CH_{2}CH_{3}$
Answer :
(vii) 3-Chloro-3-methylpentane. And it is a tertiary alkyl halide.
(viii) $CH_{3}CH = C(Cl)CH_{2}CH(CH_{3})_{2}$
Answer :
(viii) 3-Chloro-5-methylhex-2-ene. And it is vinyl halide
(ix) $CH_{3}CH = CH C (Br)( CH_{3})_{2}$
Answer :
(ix) 4-Bromo-4-methylpent-2-ene. And it is allyl halide.
(x) $p- Cl C_{6}H_{4}CH_{2}CH(CH_{3})_{2}$
Answer :
(x) 1-Chloro-4-(2-methylpropyl) benzene. And it is an aryl halide.
Question 6.1 Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
(xi) $m- Cl C_{6}H_{4}CH_{2}CH (CH_{3})_{2}$
Answer :
(xi) 1-Chloromethyl-3-(2, 2-dimethylpropyl) benzene. And it is a primary benzyl halide.
(xii) $o- Br C_{6}H_{4}CH (CH_{3})CH_{2}CH_{3}$
Answer :
(xii) 1-Bromo-2-(1-methylpropyl) benzene. And it is an aryl halide.
Question 6.2 Give the IUPAC names of the following compounds:
(i) $CH_{3}CH(Cl)CH (Br)CH_{3}$
Answer :
(i) 2-Bromo-3-chlorobutane
Question 6.2 Give the IUPAC names of the following compounds:
(ii) $CHF_{2}CBrCIF$
Answer :
1-Bromo-1-chloro-1, 2, 2-trifluroethane
Question 6.2 Give the IUPAC names of the following compounds:
(iii) $Cl CH_{2}C \equiv C CH_{2}Br$
Answer :
1-Bromo-4-chlorobut-2-yne
Question 6.2 Give the IUPAC names of the following compounds:
(iv) $(CCl_{3})_{3}CCl$
Answer :
2-(Trichloromethyl)-1, 1, 1, 2, 3, 3, 3-heptachloropropane
Question 6.2 Give the IUPAC names of the following compounds:
(v) $CH_{3}(p- Cl C_{6}H_{4})_{2}CH(Br)CH_{3}$
Answer :
2-Bromo-3, 3-bis(4-Chlorophenyl) butane
Question 6.2 Give the IUPAC names of the following compounds:
(vi) $(CH_{3})_{3}CCH = CCl C_{6}H_{4}I - p$
Answer :
1-Chloro-1-(4-iodophenyl)-3, 3-dimethylbut-1-ene
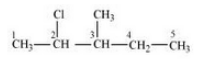
Question 6.3 Write the structures of the following organic halogen compounds.
(i) 2-Chloro-3-methylpentane
Answer :
(i)
Question 6.3 Write the structures of the following organic halogen compounds.
(ii) p-Bromochlorobenzene
Answer :
(ii)
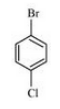
Question 6.3 Write the structures of the following organic halogen compounds.
(iii) 1-Chloro-4-ethylcyclohexane
Answer :
(iii)

Question 6.3 Write the structures of the following organic halogen compounds.
(iv) 2-(2-Chlorophenyl)-1-iodooctane
Answer :
(iv)
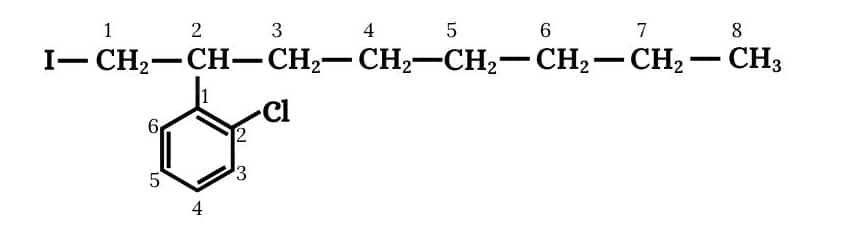
Question 6.3 Write the structures of the following organic halogen compounds.
(v) 2-Bromobutane
Answer :
(v)
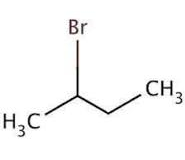
Question 6.3 Write the structures of the following organic halogen compounds.
(vi) 4-tert-Butyl-3-iodoheptane
Answer :
(vi)
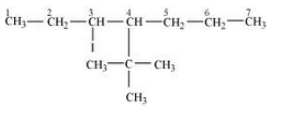
Question 6.3 Write the structures of the following organic halogen compounds.
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
Answer :
(vii)
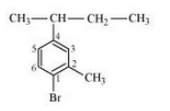
Question 6.3 Write the structures of the following organic halogen compounds.
(viii) 1,4-Dibromobut-2-ene
Answer :
(viii)
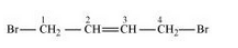
Question 6.4 Which one of the following has the highest dipole moment?
(i)$\mathrm{CH}_2 \mathrm{Cl}_2$ 2 (ii) $\mathrm{CHCl}_3$ (iii) $\mathrm{CCl}_4$
Answer :
Below are the three-dimensional structures of the three compounds, as well as the direction of each bond's dipole moment:
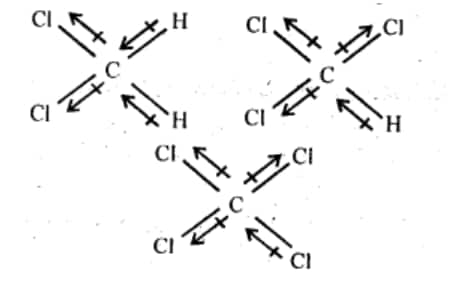
The order of dipole moment will be :- $\mathrm{CH}_2 \mathrm{Cl}_2>\mathrm{CHCl}_3>\mathrm{CCl}_4$.
The reason for the above order is given as $\mathrm{CCl}_4$ is a symmetrical compound so its dipole moment will be zero. In case of $\mathrm{CHCl}_3$ , one of the Cl atoms cancels dipole moment of the opposite Cl atom, so the net dipole moment is just due to one Cl. In the case of $\mathrm{CH}_2 \mathrm{Cl}_2$ , both Cl groups contribute to the dipole moment so it has the highest dipole moment among all.
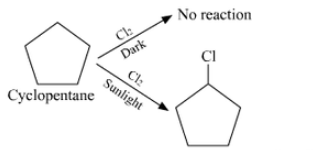
Answer :
We are given the formula $\mathrm{C}_5 \mathrm{H}_{10}$ which can be either of an alkene or of cycloalkane. Since the hydrocarbon doesn't react with chlorine in dark thus it cannot be alkene. So the only option left out is cyclopentane.
Since the cycloalkane interacts with $\mathrm{Cl}_2$ in the presence of strong sunshine to form a single monochloro compound, $\mathrm{C}_5 \mathrm{H}_9 \mathrm{Cl}$, all ten hydrogen atoms in the cycloalkanes must be equivalent. Thus, cyclopentane is the cycloalkane.
Question 6.6 Write the isomers of the compound having formula $C_{4}H_{9}Br$ .
Answer :
The isomers of the compound $C_{4}H_{9}Br$ are :-
(i) 1-Bromobutane
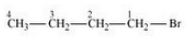
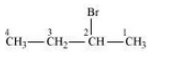
(ii) 2-Bromobutane
(iii) 1-Bromo-2-methylpropane
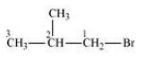
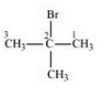
(iv) 2-Bromo-2-methylpropane
Question 6.7 Write the equations for the preparation of 1-iodobutane from:
(i) 1-butanol
Answer :
(i) The procedure given below can be used :-

Question 6.7 Write the equations for the preparation of 1-iodobutane from
Answer :
(ii) The required product can be obtained as shown below :-
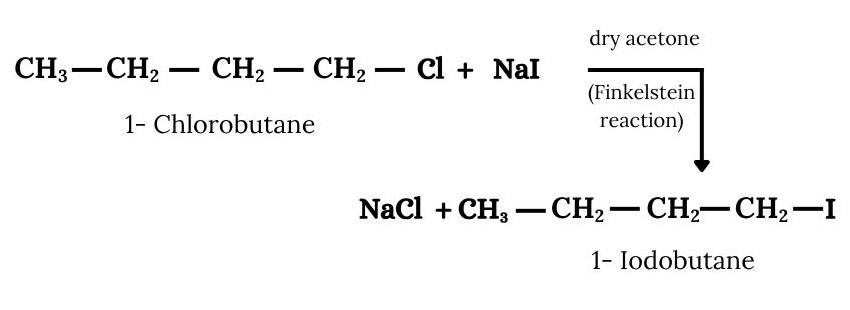
Question 6.7 Write the equations for the preparation of 1-iodobutane from
Answer :
(iii) The required product is obtained by following procedure :-
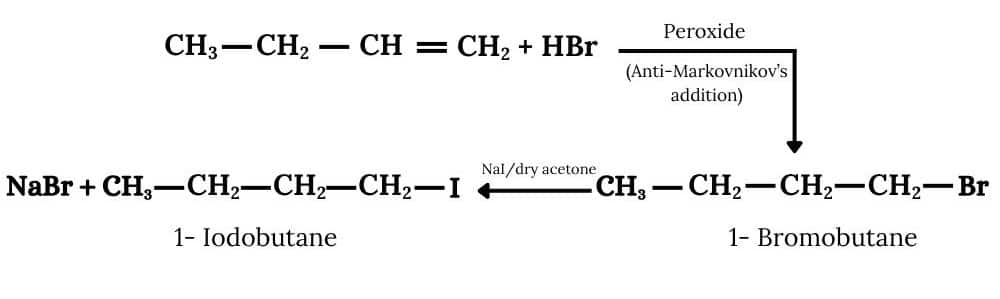
Question 6.8 What are ambident nucleophiles? Explain with an example.
Answer :
Ambident nucleophiles are nucleophiles that can attack at two distinct locations. It is a resonance hybrid of the following two structures (for example, the cyanide ion).Cyanide ion is a resonance hybrid of the following two structures:
$: \bar{C} \equiv N: \longleftrightarrow: C=\bar{N}:$
It may attack carbon to produce cyanide, and nitrogen to form isocyanide, depending on the chemical.
Question 6.9 Which compound in each of the following pairs will react faster in SN2 reaction with –OH?
Answer :
In this case, we have the same alkyl group but different halide ions. For this rate of $\mathrm{S}_{\mathrm{N}} 2$ reaction increases with an increase in atomic mass. So, $\mathrm{CH}_3 \mathrm{I}$ will react faster than $\mathrm{CH}_3 \mathrm{Br}$.
Question 6.9 Which compound in each of the following pairs will react faster in $\mathrm{S}_{\mathrm{N}} 2$ reaction with –OH?
(ii) $(CH_{3})_{3}CCl$ or $CH_{3}Cl$
Answer :
In this case, the hindrance will be a deciding factor for the rate of $\mathrm{S}_{\mathrm{N}} 2$ reaction because hindrance will directly affect the attack of the nucleophile. $(\mathrm{CH})_3 \mathrm{CCl}$ has very high steric hindrance and $\mathrm{CH}_3 \mathrm{Cl}$ has less steric hindrance. So $CH_{3}Cl$ will react faster as compared to $(CH_{3})_{3}CCl$ .
Question 6.10 Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(i) 1-Bromo-1-methylcyclohexane
Answer :
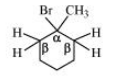
In this compound, it is clear that we have identical $\beta$ hydrogen; therefore, dehalogenation of the given compound gives the same alkene.
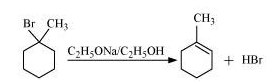
Question 6.10 Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
Answer :
(ii)
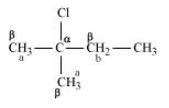
In this compound we have two kinds of $\beta$ hydrogen. So dehalogenation will give two kinds of alkenes, namely 2-Methylbut-2-ene and 2-Methylbut-1-ene.
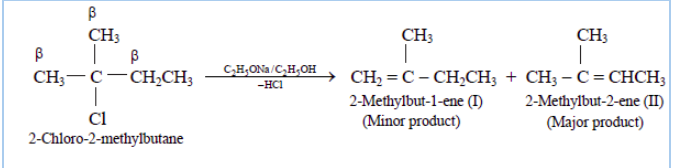
The major product of this reaction will be 2-Methylbut-2-ene as the number of $\ alpha$-hydrogens attached to double-bonded carbon is greater in case of this compound.
Question 6.10 Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(iii) 2,2,3-Trimethyl-3-bromopentane
Answer :
(iii)
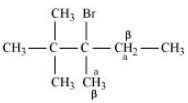
In this compound, we have two types of $\beta$ - hydrogen thus dehalogenation we get two types of products namely 3, 4, 4-Trimethylpent-2-ene and 2-Ethyl-3,3-dimethylbut-2-ene.
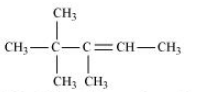 and
and 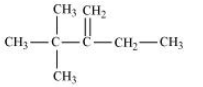
Here, 3, 4, 4-Trimethylpent-2-ene will be major product, since the $\ alpha$-hydrogen attached to the double bond are greater.
Question 6.11 How will you bring about the following conversions?
Answer :
Ethanol will react with SOCl2 and pyridine to Chloroethane. Acetylene will react with NaNH2 to form sodium acetylide. Now Chloroethane and Sodium acetylide will react to form But-1-yne. The reactions are given below:
The conversion will take place by following procedure:-
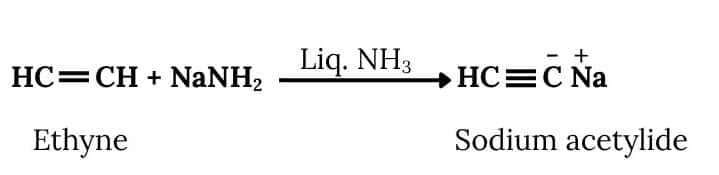
Now,
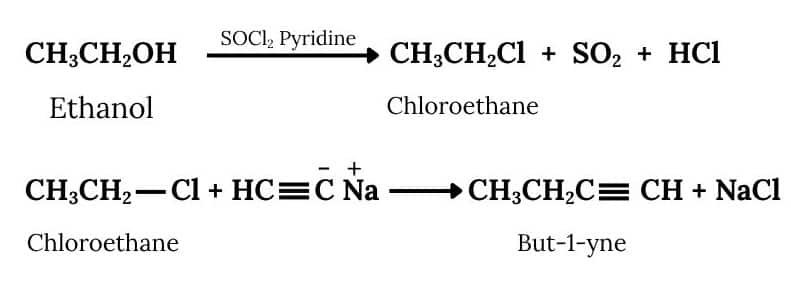
Question 6.11 How will you bring about the following conversions?
Answer :
Ethane will react with bromine to form bromoethane. Now, this bromoethane will react with KOH to form ethene and then there will be the formation of 1, 2- Dibromoethane. Now, 1, 2-Dibromoethane will react with alcoholic KOH to form Bromoethene. The reactions are given below:
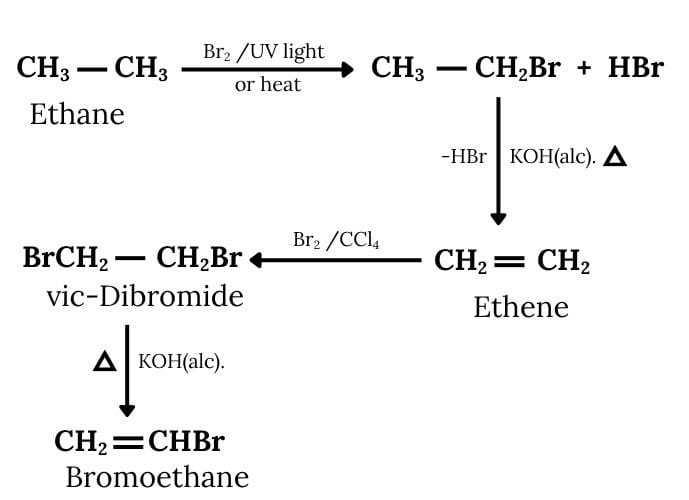
Question 6.11 How will you bring about the following conversions?
(iii) Propene to 1-nitropropane
Answer :
Propene will react with Hydrogen bromide in peroxide effect to form 1- Bromopropane. Now, 1-Bromopropane will react with silver nitrite to given 1- Nitropropane. The reaction is given below:
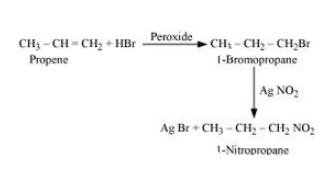
Question 6.11 How will you bring about the following conversions?
(iv) Toluene to benzyl alcohol
Answer :
Toluene will react with chlorine in the presence of light to give benzyl chloride and this benzyl chloride will react with aqueous NaOH to give benzyl alcohol. The reaction is given below:
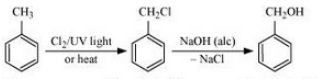
Question 6.11 How will you bring about the following conversions?
Answer :
Propene will react with bromine in the presence of carbon tetrachloride to give 1, 2-Dibromopropane. Now, 1, 2-Dibromopropane will react with NaNH2 and liquid ammonia to give propyne. The reaction is given below:
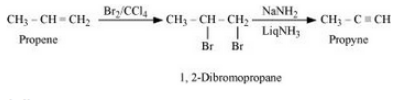
Question 6.11 How will you bring about the following conversions?
(vi) Ethanol to ethyl fluoride
Answer :
Ethanol will react with PCl5 to form ethyl chloride. Now, ethyl chloride will react with AgF to form Ethyl fluoride. The reaction is given below:
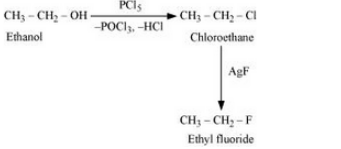
Question 6.11 How will you bring about the following conversions?
(vii) Bromomethane to propanone
Answer :
Bromomethane will react with KCN in the presence of ethanol to form acetonitrile. Acetonitrile will react with CH3 MgBr in the presence of ether and then with water to give Propanone. The reaction is given below:
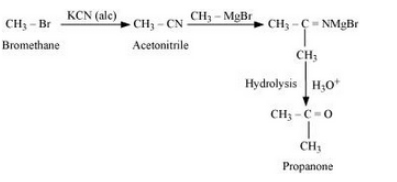
Question 6.11 How will you bring about the following conversions?
Answer :
But-1-ene will react with Hydrogen bromide to form 2-Bromobutane and this will react with alcoholic KOH to form But-2-ene. The reaction is given below:
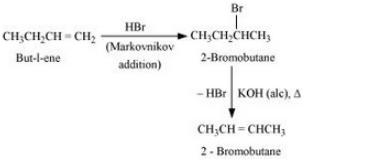
Question 6.11 How will you bring about the following conversions?
(ix) 1-Chlorobutane to n-octane
Answer :
1-Chlorobutane will react with sodium and dry ether to form n-octane. The reaction is given below:
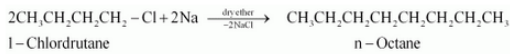
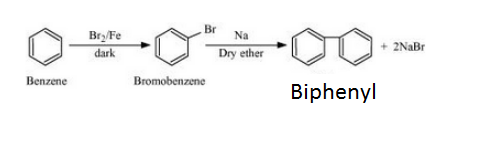
Question 6.11 How will you bring about the following conversions?
Answer :
Benzene will react with Bromine in the presence of Fe/Br2 to form Bromobenzene. Bromobenzene will react with sodium to form Biphenyl. The reaction is given below:
Question 6.12 Explain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
Answer :
We know that the Cl-atom in chlorobenzene is attached to a $\mathrm{sp}^2$ hybridized carbon atom whereas in cyclohexyl chloride, the Cl-atom is attached to a $\mathrm{sp}^3$ hybridized carbon atom. It is known that $\mathrm{sp}^2$ hybridized carbon has more s-character than $\mathrm{sp}^3$ hybridized carbon atom. Thus, chlorobenzene is more electronegative than cyclohexyl chloride.
Apart from this, the - R effect of the benzene ring of chlorobenzene results in decreasing the electron density of the C-Cl bond near the Cl-atom. And the C-Cl bond in chlorobenzene becomes less polar. Due to these reasons, the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Question 6.12 Explain why
(ii) alkyl halides, though polar, are immiscible with water?
Answer :
For being soluble in water, we have a condition that the solute-water force of attraction must be stronger than the solute-solute and water-water forces of attraction. Alkyl halides are held together by dipole-dipole interactions and there are polar molecules. Similarly, the intermolecular force of attraction present between the water molecules is hydrogen bonding. The new force of attraction after we dissolve solute in water, i.e., between the alkyl halides and water molecules, is weaker than the alkyl halide-alkyl halide and water-water forces of attraction. That is why alkyl halides (though polar) are immiscible with water.
Question 6.12 Explain why
(iii) Grignard reagents should be prepared under anhydrous conditions?
Answer :
The reactivity of the Grignard reagent is very high. When they come in contact with water they readily react and form alkanes. The general reaction is given below:
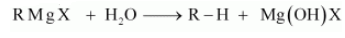
Question 6.13 Give the uses of freon 12, DDT, carbon tetrachloride and iodoform.
Answer :
(i) Freon-12 or dichlorodifluoromethane is generally known as CFC. It is used in refrigerators and air conditioners as a refrigerant. It is also used in body sprays, hair sprays, etc. But it has environmental impacts as it damages the ozone layer.
(ii) DDT or p, p-dichlorodiphenyltrichloroethane is one of the best-known insecticides which was used very widely all over the world. It is very effective against mosquitoes, insects and lice. But it has harmful effects.
(iii) CCl4 :- It is mostly used for manufacturing refrigerants for refrigerators and air conditioners. It is also used as a solvent in the manufacture of pharmaceutical products. In the early years, carbon tetrachloride was widely used as a cleaning fluid and a fire extinguisher.
(iv) Iodoform was used earlier as an antiseptic. And this antiseptic property of iodoform is due to the liberation of free iodine when it comes in contact with the skin.
Question 6.14 Write the structure of the major organic product in each of the following reactions:
(i) $CH_{3}CH_{2}CH_{2}Cl+NaI\xrightarrow[heat]{acetone}$
Answer :
The Chlorine atom from the alkyl halide by iodine. The major product of the reaction is 1-Iodopropane.
The obtained product is:-
$CH_{3}CH_{2}CH_{2}Cl+NaI\xrightarrow[heat]{acetone}$$\underset{\text { 1-Iodopropane }}{\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{I}}+\mathrm{NaCl}$
Question 6.14 Write the structure of the major organic product in each of the following reactions:
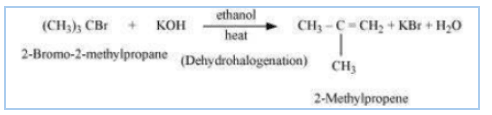
(ii) $(CH_{3})_{3}CBr+KOH \xrightarrow[heat]{ethanol}$
Answer :
There will be Dehydrohalogenation and the major product of the reaction is 2- methylpropene. The reaction is given below:
Question 6.14 Write the structure of the major organic product in each of the following reactions:
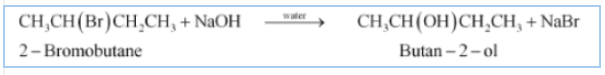
(iii) $CH_{3}CH(Br)CH_{2}CH_{3}+ NaOH \overset{water}{\rightarrow}$
Answer :
The bromine atom will be replaced with the hydroxyl ion. The major product will be Butan-2-ol. The reaction is given below:
Question 6.14 Write the structure of the major organic product in each of the following reactions:
(iv) $CH_{3}CH_{2}Br+KCN \overset{aq. ethanol}{\rightarrow}$
Answer :
The bromide ion will be replaced with the cyanide ion. The major product will be propanenitrile. The reaction is given below

Question 6.14 Write the structure of the major organic product in each of the following reactions:
(v) $C_{6}H_{5}ONa + C_{2}H_{5}Cl \rightarrow$
Answer :
The major product in the above reaction will be Phenetole. The reaction is given below:
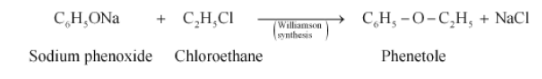
Question 6.14 Write the structure of the major organic product in each of the following reactions:
(vi) $CH_{3}CH_{2}CH_{2}OH+SOCl_{2}\rightarrow$
Answer :
The hydroxyl ion in the alkyl halide will be replaced with chloride ion. The major product of the reaction will be 1-Chloropropane. The reaction is given below:
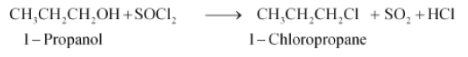
Question 6.14 Write the structure of the major organic product in each of the following reactions:
(vii) $CH_{3}CH_{2}CH = CH _{2}+HBr \overset{peroxide}{\rightarrow}$
Answer :
Since peroxide is used in the reaction, there will be Anti-Markovnikov's addition. The major product in the reaction will be 1-Bromobutane. The reaction is given below:

Question 6.14 Write the structure of the major organic product in each of the following reactions:
(viii) $CH_{3}CH = C(CH_{3})_{2}+ HBr \rightarrow$
Answer :
In this reaction there will be Markovnikoff’s addition. The major product of the reaction will be 2-Bromo-2-methylbutane. The reaction is given below:
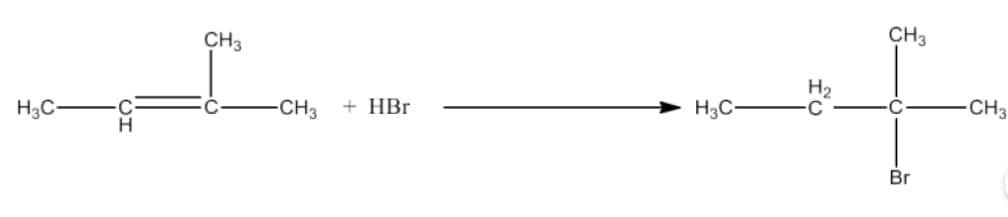
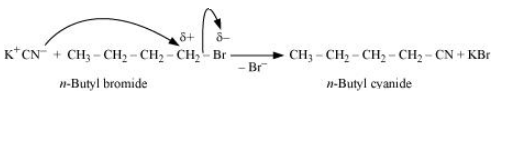
Question 6.15 Write the mechanism of the following reaction:
$nBuBr + KCN \overset{EtOH -H_{2}O}{\rightarrow} nB_{4}CN$
Answer :
The reaction will proceed through SN2 mechanism. The mechanism for the given reaction is shown below:-
Question 6.16 Arrange the compounds of each set in order of reactivity towards S N 2 displacement:
(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
Answer :
(i) Here, the deciding factor the rate of reaction will be a steric hindrance.
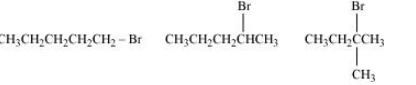
It is clear from the above that the order of hindrance is:-
1-Bromopentane < 2-bromopentane < 2-Bromo-2-methylbutane
So the order of rate of reaction will be:-
2-Bromo-2-methylbutane < 2-Bromopentane < 1-Bromopentane
Question 6.16 Arrange the compounds of each set in order of reactivity towards S N 2 displacement:
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
Answer :
(ii) In this case, also, the order of the rate of reaction will be decided from the steric hindrance factor.
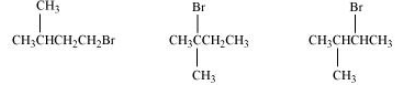
It is clear from the above that the steric hindrance in 2-Bromo-2-methylbutane is highest (note that hindrance of the carbon attached to halide ion is seen). So the order of the rate of reaction is:-
2-Bromo-2-methylbutane < 2-Bromo-3-methylbutane < 1-Bromo-3-methylbutane
Question 6.16 Arrange the compounds of each set in order of reactivity towards SN2 displacement:
(iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane.
Answer :
(iii) The steric hindrance is the deciding factor here.
The order of steric hindrance is :-
1-Bromobutane < 1-Bromo-3-methylbutane < 1-Bromo-2-methylbutane< 1-Bromo-2, 2-dimethylpropane
Thus, the order of the rate of reaction will be : -
1-Bromo-2, 2-dimethylpropane < 1-Bromo-2-methylbutane < 1-Bromo-3- methylbutane < 1-Bromobutane
Question 6.17 Out of $C_{6}H_{5}CH_{2}Cl$ and $C_{6}H_{5}CHCl C_{6}H_{5}$ , which is more easily hydrolysed by aqueous KOH.
Answer :
Hydrolysis by KOH will take place by the formation of a carbocation in its rate-determining step. So the compound having a stable carbocation will hydrolyse faster.
The carbocations of both the compounds are given below:-
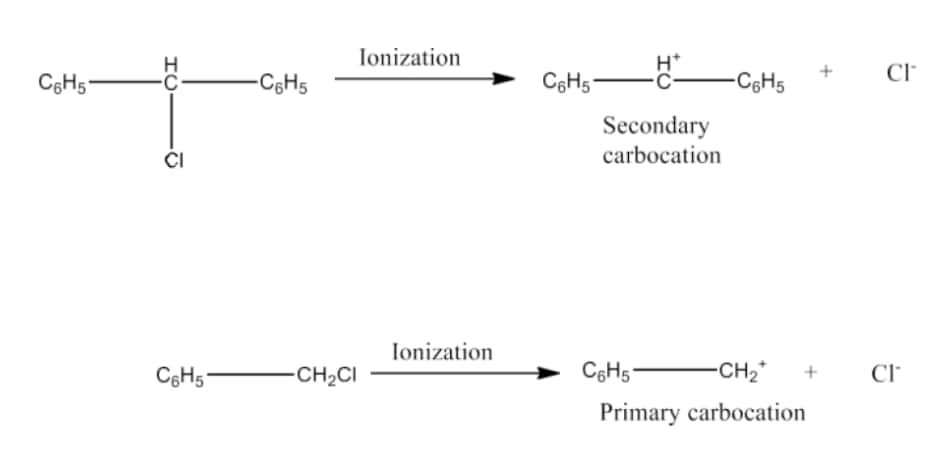
It is clear that carbocation of $C_{6}H_{5}CHCl C_{6}H_{5}$ is more stable.
Hence $C_{6}H_{5}CHCl C_{6}H_{5}$ will hydrolyse faster than $C_{6}H_{5}CH_{2}Cl$ .
Question 6.18 p-Dichlorobenzene has higher m.p. than those of o- and m-isomers. Discuss.
Answer :
The structures of o-Dichlorobenzene, m-Dichlorobenzene and p-Dichlorobenzene are given below.
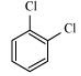
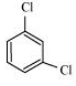
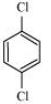
We can see that p-Dichlorobenzene is a very symmetric structure, thus its packing will be maximum. As a result, more and more energy will be required to break bonds (during boiling). Thus boiling point is high for p-Dichlorobenzene.
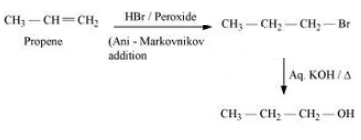
Question 6.19 How can the following conversions be carried out?
Answer :
Propene will react with hydrogen bromide in the presence of peroxide to give 1- Bromopropane. Now, this 1-Bromopropane will react with aqueous KOH to form Propan-1-ol.
The mechanism is given below:-
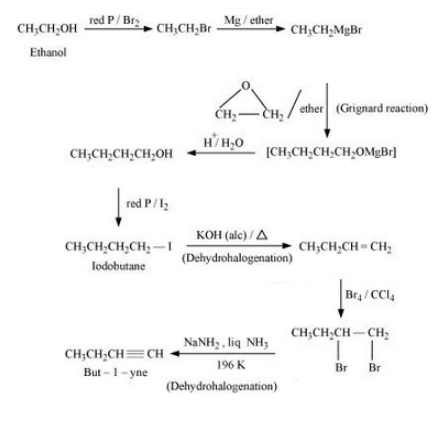
Question 6.19 How the following conversions can be carried out?
Answer :
The reaction mechanism is given below :-
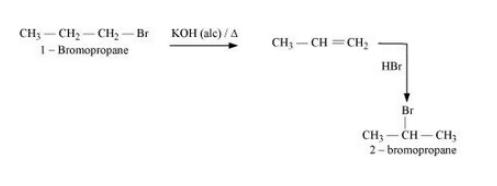
Question 6.19 How the following conversions can be carried out?
(iii) 1-Bromopropane to 2-bromopropane
Answer :
1-Bromopropane will react with alcoholic KOH to form propene. Propene will react with HBr to form 2-Bromopropane.
The mechanism is given below :-
Question 6.19 How the following conversions can be carried out?
(iv) Toluene to benzyl alcohol
Answer :
Toluene will react with chlorine in the presence of light to give benzyl chloride and this benzyl chloride will react with aqueous KOH to give benzyl alcohol.
The mechanism is given below :-
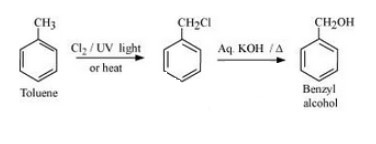
Question 6.19 How the following conversions can be carried out?
(v) Benzene to 4-bromonitrobenzene
Answer :
Benzene will react with Bromine in the presence of FeBr3to form Bromobenzene. Bromobenzen will react with concentrated nitric acid and concentrated sulfuric acid to form 4-Bromonitrobenzene. The reaction is given below:
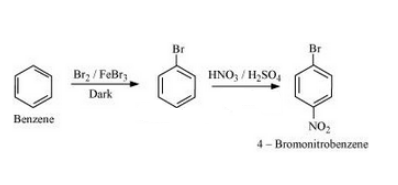
Question 6.19 How the following conversions can be carried out?
(vi) Benzyl alcohol to 2-phenylethanoic acid
Answer :
Benzyl alcohol will react with PCl5 to form Benzyl chloride. Benzyl chloride will react with KCN to form Benzyl cyanide. Now, benzyl cyanide on hydrolysis will give 2-Phenylethanoic acid. The reaction is given below:
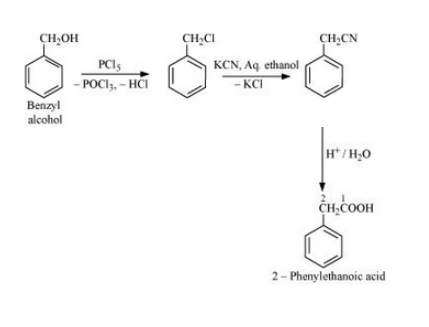
Question 6.19 How can the following conversions be carried out?
(vii) Ethanol to propanenitrile
Answer :
Ethanol will react with bromine in the presence of phosphorus to form Bromoethane. Iodoethane will react with KCN in the presence of aqueous ethanol to give Propanenitrile. The reaction is given below:
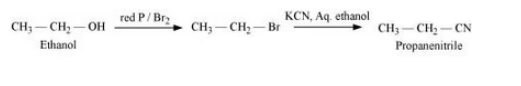
Question 6.19 How can the following conversions be carried out?
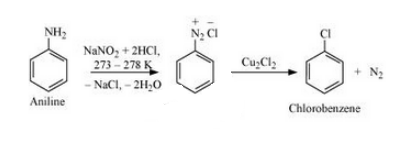
(viii) Aniline to chlorobenzene
Answer :
Aniline will undergo diazotization to form Benzene diazonium chloride. The Benzene diazonium chloride will react with copper chloride in the presence of hydrochloric acid to give Chlorobenzene. The reaction is given below:
Question 6.19 How can the following conversions be carried out?
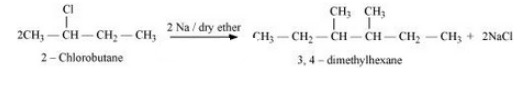
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
Answer :
2-Chlorobutane will react with sodium in the presence of dry ether to form 3, 4- Dimethylhexane. The rection is given below:
Question 6.19 How can the following conversions be carried out?
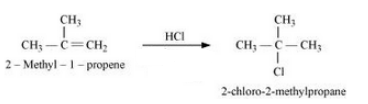
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
Answer :
2-Methyl-1-propene will react with Hydrogen chloride to give 2-Chloro-2- methylpropane. The reaction is given below:
Question 6.19 How can the following conversions be carried out?
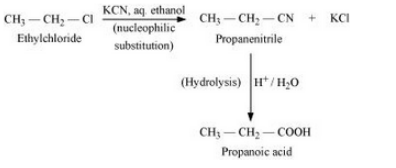
(xi) Ethyl chloride to propanoic acid
Answer :
Ethyl chloride will react with KCN to give propanenitrile. Propanenitrile on hydrolysis will give propanoic acid. The reaction is given below:
Question 6.19 How can the following conversions be carried out?
(xii) But-1-ene to n-butyliodide
Answer :
But-1-ene will react with HBr in the presence of peroxide to form 1- Bromobutane. 1-Bromobutane will react with NaI in the presence of Acetone to give n-butyl iodide. The reaction is given below:
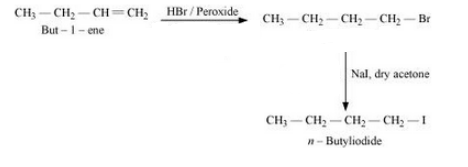
Question 6.19 How can the following conversions be carried out?
(xiii) 2-Chloropropane to 1-propanol
Answer :
2-Chloropropane will undergo dehydrohalogenation to give propene. Propene will react with HBr in the presence of peroxide to give 1-bromopropane. 1- Bromopropane will react with KOH to form 1-Propanol. The reaction is given below:
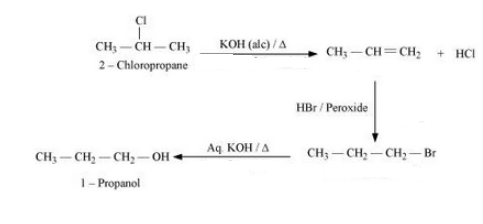
Question 6.19 How can the following conversions be carried out?
(xiv) Isopropyl alcohol to iodoform
Answer :
The proposed mechanism is:-
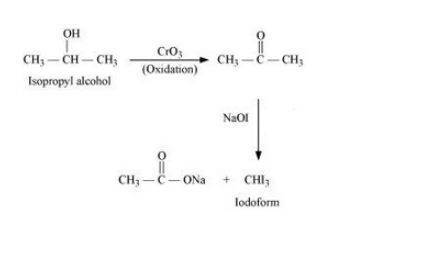
Question 6.19 How can the following conversions be carried out?
(xv) Chlorobenzene to p-nitrophenol
Answer :
Chlorobenzene will react with concentrated nitric acid and concentrated sulfuric to give p-Chloronitrobenzene. p-Chloronitrobenzene will react with 15% sodium hydroxide and hydrochloric acid to give p-nitrophenol. The reaction is given below:
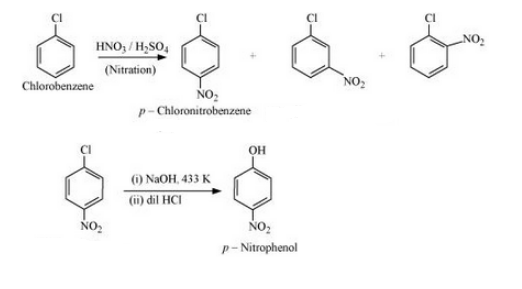 `
`
Question 6.19 How can the following conversions be carried out?
(xvi) 2-Bromopropane to 1-bromopropane
Answer :
2-Bromopropane will react with alcoholic KOH to form Propene. Now, propene will react with HBr in the presence of peroxide to form 1-Bromopropane. The reaction is given below:
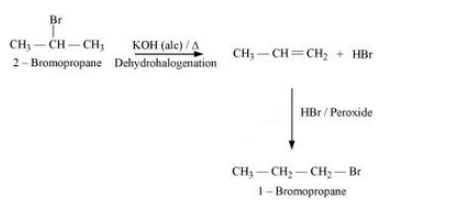
Question 6.19 How can the following conversions be carried out?
Answer :
Chloroethane will react with sodium in the presence of butane. The reaction is given below:
$\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{Cl} \xrightarrow{2 \mathrm{Na} / \text { dry ether }} \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CH}_3+2 \mathrm{NaCl}$
Question 6.19 How can the following conversions be carried out?
Answer :
Benzene will react with Bromine in the presence of FeBr to form Bromobenzene. Bromobenzene will react with sodium to form Biphenyl. The reaction is given below:

Question 6.19 How can the following conversions be carried out?
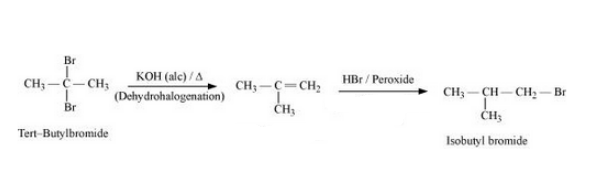
(xix) tert-Butyl bromide to isobutyl bromide
Answer :
Tert-butyl bromide will react with alcoholic KOH to form 2-Methyl-1-propene. 2-Methyl-1-propene will react with HBr in the presence of peroxide to form isobutyl bromide. The reaction is given below:
Question 6.19 How can the following conversions be carried out?
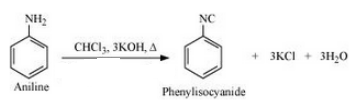
(xx) Aniline to phenylisocyanide
Answer :
Aniline will react with chloroform and alcoholic KOH to form Phenyl isocyanide. The reaction is given below:
Answer :
In an aqueous solution, KOH almost completely dissociates into $\mathrm{OH}^{-}$ ions. We know that $\mathrm{OH}^{-}$ ions are strong nucleophile, which leads the alkyl chloride to undergo a reaction to form alcohol. But an alcoholic solution of KOH contains alkoxide (RO-) ion, which is a strong base. Thus, it can remove hydrogen from the β-carbon of the alkyl chloride and form an alkene. The $\mathrm{OH}^{-}$ ion is a weaker base than RO- ion. The basic character of $\mathrm{OH}^{-}$ ion decreases in aqueous solution. Therefore, it cannot remove hydrogen from the β-carbon.
$\mathrm{R}-\mathrm{Cl}+\mathrm{KOH}_{(a q)} \longrightarrow \mathrm{R}-\mathrm{OH}+\mathrm{KCl}$
$\mathrm{R}-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Cl}+\mathrm{KOH}(\mathrm{alc}) \longrightarrow \mathrm{R}-\mathrm{CH}=\mathrm{CH}_2+\mathrm{KCl}+\mathrm{H}_2 \mathrm{O}$
Answer :
With the given formula we have two alkyl halides They are n - butyl bromide and isobutyl bromide.
For the first set of reaction we get two possibilities:-
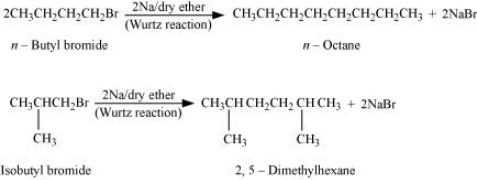
Therefore, compound (d) is 2, 5-dimethylhexane.

Question 6.22 What happens when
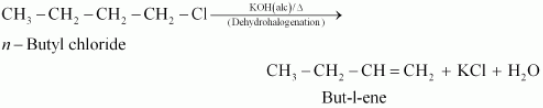
(i) n-butyl chloride is treated with alcoholic KOH,
Answer :
When n−butyl chloride is treated with alcoholic KOH, the formation of but−l−ene takes place. This reaction is a dehydrohalogenation reaction.
Question 6.22 What happens when
(ii) bromobenzene is treated with Mg in the presence of dry ether
Answer :
When bromobenzene is treated with Mg in the presence of dry ether there will be the formation of Phenylmagnesium bromide. The reaction is given below:
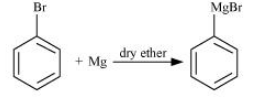
Question 6.22 What happens when
(iii) chlorobenzene is subjected to hydrolysis
Answer :
Chlorobenzene does not undergo hydrolysis under normal conditions. However, it undergoes hydrolysis when heated in an aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atm to form phenol.
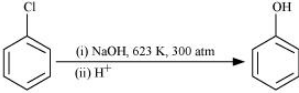
Question 6.22 What happens when
(iv) ethyl chloride is treated with aqueous KOH
Answer :
When ethyl chloride is treated with aqueous KOH, it undergoes hydrolysis to form ethanol.

Question 6.22 What happens when
(v) methyl bromide is treated with sodium in the presence of dry ether
Answer :
When methyl bromide is treated with sodium in the presence of dry ether then Wurtz reaction will take place and the product of the reaction will be Ethane. The reaction is given below:

Question 6.22 What happens when
(vi) methyl chloride is treated with KCN?
Answer :
When methyl chloride is treated with KCN then there will be a Nucleophilic substitution reaction and the product will be Methyl cyanide. The reaction is given below:

Class 12 Chemistry NCERT Chapter 6: Higher Order Thinking Skills (HOTS) Questions
Selected Higher Order Thinking Skills questions of class 12 chemistry chapter 6 haloalkanes and haloarenes question answer are given below. These questions go beyond basic learning and help you understand the concepts well.
Question 1. Given below are two statements:
Statement (I) : Alcohols are formed when alkyl chlorides are treated with aqueous potassium hydroxide by elimination reaction.
Statement (II) : In alcoholic potassium hydroxide, alkyl chlorides form alkenes by abstracting the hydrogen from the $\beta$-carbon.
In light of the above statements, choose the most appropriate answer from the options given below:
1) Both Statement I and Statement II are incorrect
2) Statement I is incorrect, but Statement II is correct
3) Statement I is correct, but Statement II is incorrect
4) Both Statement I and Statement II are correct
Answer:
Statement (I) :
$\mathrm{R}-\mathrm{Cl} \xrightarrow{(\mathrm{aq}, \mathrm{KOH})} \mathrm{R}-\mathrm{OH}\left(\mathrm{~S}_{\mathrm{N}}\right. \text { reaction) }$
Statement (II) :
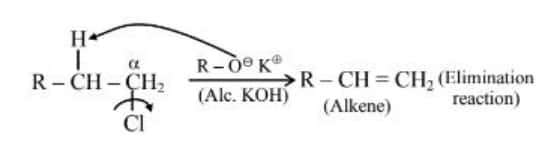
Hence, the correct answer is option (2).
Question 2. The major product of the following reaction is :

1) 6-Phenylhepta-2,4-diene
2) 2-Phenylhepta-2,5-diene
3) 6-Phenylhepta-3,5-diene
4) 2-Phenylhepta-2,4-diene
Answer:

Hence, the correct answer is option (4).
Question 3. Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : Aryl halides connot be prepared by replacement of hydroxyl group of phenol by halogen atom.
Reason R : Phenols react with halogen acids violently.
In the light of the above statements, choose the most appropriate from the options given below :
(1) A is false but R is true
(2) Both A and R are true and R is the correct explanation of A
(3) A is true but R is false
(4) Both A and R are true but R is NOT the correct explanation of A
Answer:
$\rightarrow$ does not give substitution $\mathrm{rx}^{\mathrm{n}}$ due to double bond character in $\mathrm{C}-\mathrm{O}$ bond.
does not give substitution $\mathrm{rx}^{\mathrm{n}}$ due to double bond character in $\mathrm{C}-\mathrm{O}$ bond.
$\rightarrow \quad$ Phenol does not react with HX violently.
Hence, the answer is the option (3)
Question 4: Identify the structure of the final product (D) in the following sequence of the reactions :

Total number of $sp^2$ hybridised carbon atoms in product D is.
Answer:

When vicinal dichloride reacts with NaNH2, a strong base, it undergoes a double dehydrohalogenation reaction, resulting in the formation of an alkyne. This reaction proceeds via two consecutive E2 elimination steps. Later on this alkyne reacts with diborane to give an aldehyde.
Number of $\mathrm{sp}^2$ hybridised carbon $=7$
Hence, the answer is 7.
Question 5: Given below are two statements :
Statement (I) : is more polar than
is more polar than
Statement (II) : Boiling point of  is lower than
is lower than  but it is more polar than
but it is more polar than 
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Statement I is correct but statement II is incorrect
(2) Statement I is incorrect but statement II is correct
(3) Both statement I and statement II are incorrect
(4) Both statement I and statement II are correct
Answer:
 is more polar than
is more polar than  because
because
C – Cl bond is more polar than C – Br bond.
Boiling point of  is less than that of
is less than that of  because of non-polar nature and hence lower intermolecular forces.
because of non-polar nature and hence lower intermolecular forces.  is non-polar.
is non-polar.
Hence, the correct answer is option (1).
Question 6:

The total number of compounds obtained (including stereoisomers) of all monochloroderivative products of (c)?
(1) 10
(2) 11
(3) 12
(4) 13
Answer:

Hence, the correct answer is option 1.
Question 7: Arrange the following in order of ease of formation of white ppt on reaction with $\mathrm{AgNO}_3$.
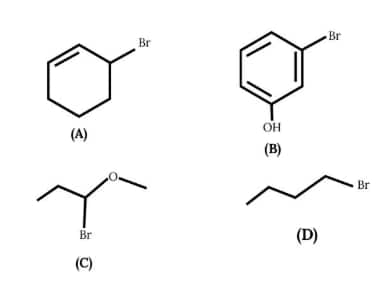
(1) $C>A>D>B$
(2) $A>C>B>D$
(3) $C>A>B>D$
(4) $A>D>C>B$
Answer:
Stabilizing of carbocation after loss of Br-
$C>A>D>B$
Hence, the correct answer is option 1.
NCERT Solutions for Class 12
- NCERT Solutions for Class 12 Biology
- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 12 Physics
Approach to Solve Questions of Chapter 6 Haloalkanes and Haloarenes
Sometimes, problems related to haloalkanes and haloarenes class 12 question answer seem difficult, but once we understand the basic formulas, it becomes very easy to solve all the questions. We can follow the steps given below to solve the questions based on haloalkanes and haloarenes:
1) Before attempting the questions, make sure you have revised all the concepts
- Identify whether the compound is a Haloalkane or Haloarene, which can be determined based on the Carbon chain.
- Then determine the degree of Halogenated carbon
2) Nomenclature plays an important role in organic compounds and questions related to nomenclature are frequently asked in exams. Below are some points to take care while naming compounds.
- Write the IUPAC name
- Check for multiple halogens, and if available, then they are named according to alphabetical order.
3) Reaction mechanisms help us to understand why and how any reaction takes place. To learn more about this you can refer to haloalkanes and haloarenes ncert solutions.
- Identify the type of reaction, whether it is addition, elimination, or substitution.
- Then identify the mechanism type whether it is $\mathrm{S}_{\mathrm{N}} 1, \mathrm{~S}_{\mathrm{N}} 2, \mathrm{E} 1, \mathrm{E} 2$.
4) These are important and are often asked in the exams. You can practice more and more questions of class 12 chemistry haloalkanes and haloarenes question answer to understand the concept.
5) This is one of the most important topics and is often asked in exams
- Learn to determine whether the reaction involves inversion, retention, or racemization.
- Also, learn to check for optical activity.
6). Follow the solved examples, Haloalkanes and Haloarenes intext questions to know the correct way to solve the question. You can also practice the questions provided in the NCERT textbook.
NCERT Exemplar Solutions
Topics of NCERT Syllabus Class 12 Chemistry Haloalkanes and Haloarenes
This section lists all the topics covered in the haloalkanes and haloarenes class 12 question answer. Knowing these topics helps students organise their study, focus on essential concepts, and ensure thorough preparation for exams.
6.1 Classification
6.2 Nomenclature
6.3 Nature of C–X Bond
6.4 Methods of Preparation of Haloalkanes
6.5 Preparation of Haloarenes
6.6 Physical Properties
6.7 Chemical Properties
6.8 Polyhalogen compounds
NCERT Books and NCERT Syllabus
What Extra Should Students Study Beyond the NCERT for JEE/NEET?
The table contains some important topics that will help you prepare the topics according to the exams. This comparison table highlighting what to study beyond the NCERT for JEE. For detailed learning refer the NCERT Solutions for Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes:
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes
These class 12 chemistry chapter 6 haloalkanes and haloarenes question answer help students understand the fundamental concepts, properties, and reactions of haloalkanes and haloarenes in a structured way. Given below some points on key learnings of this solutions:
- Nomenclature of haloalkanes and haloarenes according to IUPAC rules and classification of them based on the number of halogen atoms are explained in these solutions.
- Polarity and bond strength of the carbon halogen bond are explained in these haloalkanes and haloarenes ncert solutions.
- Using these class 12 chemistry chapter 6 haloalkanes and haloarenes solutions students will learn about laboratory and industrial methods of preparing haloalkanes and haloarenes.
- Physical characteristics and different types of chemical reactions are explained well in these solutions.
- Here the mechanism of substitution reactions, elimination reactions are explained well in these solutions.
Importance of Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes Solutions
The haloalkanes and haloarenes class 12 question answer is important for organic chemistry that introduces students to halogen-containing compounds.
- These solutions help students understand substitution and elimination reactions.
- Haloalkanes and haloarenes are used in everyday products such as refrigerants, solvents, and pharmaceuticals.
- The class 12 chemistry haloalkanes and haloarenes question answer are important from the point of view of CBSE exams and competitive exams like JEE and NEET.
- This chapter also raises awareness about the environmental effects of certain halo compounds.
NCERT Solutions Class 12 Chemistry
Apart from the class 12 chemistry chapter 6 haloalkanes and haloarenes question answer, students can also explore the links provided below for solutions to other chapters.
Frequently Asked Questions (FAQs)
Haloalkanes are organic compounds in which halogen atoms have replaced one or more hydrogen atoms in an alkane. Haloarenes, on the other hand, are similar compounds where one or more hydrogen atoms in an aromatic ring are substituted with halogen atoms.
Haloalkanes can be classified based on the number of carbon atoms bonded to the carbon atom attached to the halogen. They are categorized as primary, secondary, and tertiary haloalkanes.
The carbon-halogen bond in haloalkanes is polar because of the difference in electronegativity between carbon and halogens. This polarity influences the reactivity of haloalkanes, making them susceptible to nucleophilic substitution and elimination reactions.
The important topics of Chapter 6 include the nomenclature and classification of haloalkanes and haloarenes, nature of the carbon–halogen bond, methods of preparation, physical and chemical properties, mechanisms of substitution and elimination reactions, reactivity and uses, and the environmental effects of organohalogen compounds.
Haloalkanes and haloarenes are generally insoluble in water. Although they are polar molecules, they cannot form strong hydrogen bonds with water molecules. The energy required to overcome the attractions between water molecules is greater than the energy released when haloalkanes or haloarenes interact with water.
Haloalkanes react mainly by nucleophilic substitution. In SN1, the halide leaves first forming a carbocation, then the nucleophile attacks. In SN2, the nucleophile attacks as the halide leaves in one step, causing inversion.
Primary haloalkanes have the halogen atom attached to a carbon that is bonded to only one other carbon atom. Secondary haloalkanes are attached to a carbon bonded to two other carbons. Tertiary haloalkanes are connected to a carbon that is bonded to three other carbon atoms. This classification affects their chemical reactivity, especially in nucleophilic substitution reactions.
Haloarenes generally exhibit different chemical behaviors compared to haloalkanes due to the resonance stabilization of the aromatic ring. For instance, haloarenes are less reactive in nucleophilic substitution reactions than haloalkanes because the aromatic system is stable, while haloalkanes are more reactive toward nucleophiles due to their sp³ hybridization.
NCERT Solutions provide a structured approach to understanding the concepts covered in the chapter. They help clarify complex topics, offer step-by-step explanations for exercises, and reinforce learning through practice.
To use class 12 chemistry haloalkanes and haloarenes question answer effectively, start by reading the chapter thoroughly to grasp the concepts. After that, solve the exercise problems without looking at the solutions first. Once you attempt each question, refer to the NCERT Solutions for guidance on how to approach the questions and to verify your answers.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
