NCERT Exemplar Class 12 Chemistry Solutions chapter 10 Haloalkanes and Haloarenes
Did you know that replacing just one atom in a molecule can create a whole new class of compounds with completely different properties? This is exactly what is seen in Haloalkanes and Haloarenes, where a single hydrogen atom is replaced by a halogen atom. This small change in configuration leads to the formation of highly reactive and useful compounds found in medicines, refrigerants, and even in pesticides. Haloalkanes and Haloarenes are made up of 2 words, the first is Halo, which means Halogen, and the second word is alkanes or arenes, which means aliphatic or aromatic hydrocarbons.
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: Long Answer Type
- Class 12 Chemistry NCERT Chapter 10: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 10 Haloalkanes and Haloarenes
- Topics Covered in NCERT Exemplar Solutions Class 12 Chemistry Chapter 10
- Important Reactions of Class 12 Chapter 10
- Advantages of Using Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes NCERT Exemplar Solutions
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Exemplar Class 12 Solutions
- NCERT Solution subject-wise
- NCERT Notes Subject-Wise
- NCERT Books and the NCERT Syllabus
In NCERT Exemplar Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes students will learn about types, structures, reactions and real-life examples. The NCERT Exemplar Solutions Class 12 Chemistry are designed in a very comprehensive and systematic way that sharpen your understanding of this chapter, selective Higher Order Thinking Skills (HOTS) questions are also included in this article. These NCERT Exemplar Solutions provide a valuable resource to enhance performance in board exams as well as in competitive exams like NEET, JEE, etc. For better understanding students can also refer to NCERT Solutions.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: MCQ (Type 1)
Below, solutions of MCQ-type questions of Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes are provided which are important for both boards and competitive exams and helps you to improve your conceptual thinking and problem-solving ability.
Question 1 The order of reactivity of the following alcohols with halogen acids is
(A) $CH_{3}CH_{2}-CH_{2}-OH$
(B)
(C)
(i) (A) > (B) > (C)
(ii) (C) > (B) > (A)
(iii) (B) > (A) > (C)
(iv) (A) > (C) > (B)
Answer:
The order of reactivity of alcohols with halogen acids is 3° > 2° > 1°. This is because the stability of carbocations is in the same order.
The reactivity of alcohols with halogen acids is due to the formation of carbocations, which is easier when they are more stable. The rate of reaction is faster when the carbocation is more stable. When alcohols are treated with HCl or HBr, they undergo a nucleophilic substitution reaction to produce an alkyl halide and water.
The order of reactivity of hydrogen halides is HI > HBr > HCl > HF. This order parallels the acidity order.
Hence, the answer is the option (ii) (C) > (B) > (A)
Question 2 Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
Answer:
The tertiary alcohol reacts the most with concentrated HCl, as the tertiary carbocation is the most stable. Therefore, for tertiary alcohol, the room temperature is enough for the reaction. However, for primary and secondary alcohols, there is a need for the presence of a catalyst $(ZnCl_2)$.
Hence, the answer is the option (iv)
Question 3 Identify the compound Y in the following reaction:
Answer:
Dissolved or suspended in cold aqueous mineral acid, a primary aromatic amine turns into a diazonium salt, after treatment with sodium nitrite. The diazonium group is replaced by -Cl as soon as the cuprous chloride is added to the previously prepared diazonium salt. Hence, the ‘Y’ here in the equation is chlorobenzene.
Hence, the answer is the option (i)
Question 4 Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is
(a) electrophilic elimination reaction
(b) electrophilic substitution reaction
(c) free radical addition reaction
(d) nucleophilic substitution reaction
Answer:
Toluene is an aromatic compound, and when it reacts with a halogen in the presence of a Lewis acid catalyst such as FeCl3, the reaction proceeds through an electrophilic substitution mechanism. The methyl group activates the benzene ring and directs the incoming halogen to the ortho and para positions.
This type of reaction is called an electrophilic substitution reaction.
Hence, the answer is the option (b)
Question 5 Which of the following is a halogen exchange reaction?
(i)$RX+NaI\rightarrow RI+NaX$
(ii)
(iii) $R-OH+HX\overset{ZnCl_{2}}{\rightarrow} R-X+H_{2}O$
(iv)
Answer:
(a) Halogen exchange reaction means one halide is replaced by the other. This reaction is also called the Finkelstein reaction.
A halogen exchange reaction is a chemical reaction where one halide ion replaces another halide ion in a compound. The general format for a halogen exchange reaction is:
- X1 and X2: Different halogens
- Na: A sodium ion
- R−X1+NaX2→R−X2+NaX1
The reactivity of the halogen group decreases from top to bottom within the group. Fluorine is the most reactive halogen, while iodine is the least.
This reaction is also called the Finkelstein reaction.
Hence, the answer is the option (i).
Question 6 Which reagent will you use for the following reaction?
$CH_3CH_2CH_2CH_3 \rightarrow CH_3CH_2CH_2CH_2Cl + CH_3CH_2CHClCH_3$
(i) $Cl_{2}$/UV light
(ii) $NaCl + H_2SO_4$
(iii) $Cl_{2}$ gas in dark
(iv) $Cl_{2}$ gas in the presence of iron in dark
Answer:
Here, it is a free radical substitution reaction. That means this reaction happens in the presence of UV light/peroxides/ high temperature. These are all free radical generators.
Hence, the answer is the option (i).
Question 7 Arrange the following compounds in the increasing order of their densities.
(i) (a) < (b) < (c) < (d)
(ii) (a) < (c) < (d) < (b)
(iii) (d) < (c) < (b) < (a)
(iv) (b) < (d) < (c) < (a)
Answer:
(a) Density increases with an increase in molecular mass.
The order of molecular masses is (a) < (b) < (c) < (d)
Hence, the order of densities (a) < (b) < (c) < (d)
Hence, the answer is the option (i).
Question 8 Arrange the following compounds in increasing order of their boiling points
(i)
(ii) $CH_{3}CH_{2}CH_{2}CH_{2}Br$
(iii)
(i) (b) < (a) < (c)
(ii) (a) < (b) < (c)
(iii) (c) < (a) < (b)
(iv) (c) < (b) < (a)
Answer:
Here the given alkanes are isomeric. Therefore, isomeric alkane’s boiling point decreases with increase in branching. When the branching of an alkane increases, the surface area decreases. This causes a decrease in intermolecular force.
Hence, (c) < (a) < (b)
Hence, the answer is the option (iii).
Question 9 In which of the following molecules carbon atom marked with an asterisk (*) is asymmetric?
(i) (a), (b), (c), (d)
(ii) (a), (b), (c)
(iii) (b), (c), (d)
(iv) (a), (c), (d)
Answer:
An Asymmetric carbon atom is linked with four different groups or atoms with all of its four valences. Here, the carbon atoms of (i), (ii), and (iii), all have linked with four different groups of atoms. Hence, those are asymmetric. Whereas, in (iv), the carbon atom is linked with two of its valences with two similar hydrogen atoms. Therefore, it is not an asymmetric carbon atom.
Hence, the answer is the option (ii).
Question 10 Which of the following structures is enantiomeric with the molecule (A) given below:
Answer:
Here the structure (i) is the enantiomer of compound (A).
Here the position two groups, i.e., CH3 and C2H5 in (i) is exactly reversed of the given sample (A) at the chiral carbon.
Hence, the answer is the option (i).
Question 11 Which of the following is an example of vic-dihalide?
(a) Dichloromethane
(b) 1, 2-dichloroethane
(c) Ethylidene chloride
(d) Allyl chloride
Answer:
1, 2-Dichloroethane is an example of a vic-dihalide. This is because ofthe presence of two Cl atoms on vicinal carbon atoms (adjacent).
Hence, the answer is the option (b).
Question 12 The position of -Br in the compound $CH_3CH = CHC(Br)(CH_3)_2$, can be classified as.
(a) allyl
(b) aryl
(c) vinyl
(d) secondary
Answer:
The given sample is an allylic compound. Here the Br atom is attached next to double-bonded carbon.
Hence, the answer is the option (a).
Question 13 Chlorobenzene is formed by the reaction of chlorine with benzene in the presence of $AlCl_3$. Which of the following species attacks the benzene ring in this reaction?
(a) $Cl^{-}$
(b) $Cl^{+}$
(c) $AlCl_3$
(d) $[AlCl_4]^{-}$
Answer:
Here, the $Cl^{+}$ attacks the benzene ring to form chlorobenzene.
In this electrophilic aromatic substitution reaction, chlorine reacts with AlCl3, a Lewis acid, to generate the electrophile Cl+. This Cl+ then attacks the benzene ring, leading to the formation of chlorobenzene.
Hence, the answer is the option (b).
Question 14 Ethylidene chloride is a/an ________.
(a) vic-dihalide
(b) gem-dihalide
(c) allylic halide
(d) vinylic halide
Answer:
Ethylidene chloride is a gem-dihalide. This is because the halogen atoms of $CH_3- CHCl_2$ are connected to the same carbon atom.
Hence, the answer is the option (b).
Question 15 What is A in the following reaction?
Answer:
Here, according to Markownikow's rule, HCl will be attached to the doubly bonded carbons. Therefore, for the carbon atom that has a lesser number of hydrogens, the addition of a negative addendum will take place.
Hence, the answer is the option (c).
Question 16 A primary alkyl halide would prefer to undergo __________ .
(a) $S_N1$ reaction
(b) $S_N2$ reaction
(c) $\alpha$-Elimination
(d) Racemisation
Answer:
$S_N2$ reaction happens through the formation of the transition state. Therefore a primary alkyl halide would prefer to undergo $S_N2$ reaction due to less steric hindrance.
Hence, the answer is the option (b).
Question 17 Which of the following alkyl halides will undergo $S_N1$ reaction most rapidly?
(a) $(CH_3)_3C-F$
(b) $(CH_3)_3C-Cl$
(c) $(CH_3)_3C-Br$
(d)$(CH_3)_3C-I$
Answer:
$(CH_3)_3C-I$ will undergo $S_N1$ reaction most readily. Here, the C-l bond is the weakest because the size difference between carbon and iodine is huge.
Hence, the answer is the option (b).
Question 18 Which is the correct IUPAC name of
(i) 1-Bromo-2-ethylpropane
(ii) 1-Bromo-2-ethyl-2-methylene
(iii) 1-Bromo-2-methylbutane
(iv) 2-Methyl-1-bromobutane
Answer:
The correct IUPAC name of the given compound is 1-Bromo-2-methyl butane.
Hence, the answer is the option (iii).
Question 19 What should be the correct IUPAC name for diethyl bromomethane?
(a) 1-Bromo-1, 1-diethylmethane
(b) 3-Bromopentane
(c) 1-Bromo-1-ethylpropane
(d) 1-Bromopentane
Answer:
The IUPAC name of Diethyl Bromomethane is 3-Bromopentane.
TThe compound has a straight chain of five carbon atoms with a bromine atom attached to the third carbon. Though the common name suggests two ethyl groups and a bromine on a central carbon, it actually forms a continuous pentane chain.
Hence, the answer is the option (b).
Question 20 The reaction of toluene with chlorine in the presence of iron and in the absence of light yields _________ .
Answer:
The reaction of toluene with chlorine in the presence of iron and carried out in absence of light, so the substitution occurs in the benzene ring. The CH3 group of toluene is o and p-directing.
Hence, the answer is the option (d).
Question 21 Chloromethane on treatment with excess of ammonia yields mainly
(i) N,N- Dimethylmethanamine
(ii) N-methylmethanamine $(CH_{3}-NH-CH_{3})$
(iii) Methanamine $(CH_{3}-NH_{2})$
(iv) Mixture containing all of these mixtures in equal proportion
Answer:
Chloromethane on treatment with an excess of ammonia yields mainly methenamine.
$CH_3Cl + NH_3 \rightarrow CH_3NH_2 + HCl$
However, if the two reactants are present in the same amount, the mixture of the primary, secondary, and tertiary amine is obtained.
Hence, the answer is the option (iii).
Question 22 Molecules whose mirror image is non-superimposable over them are known as chiral. Which of the following molecules is chiral in nature?
(a) 2-Bromobutane
(b) 1-Bromobutane
(c) 2-Bromopropane
(d) 2-Bromopropan-2-ol
Answer:
When the mirror images of molecules are identical and superimposable, the molecules are achiral. Achiral molecules should have a plane of symmetry. When the mirror image of achiral structure is rotated, and the structures can be aligned with each other, their mirror images are called achiral carbon atoms.
Chirality often leads to optical activity in a compound. But, it is not a necessary condition for optical activity. Optical activity is the ability of a molecule to rotate plane- polarized light.
Hence, the answer is the option (a).
Question 23 The reaction of $C_6H_5CH_2Br$ with aqueous sodium hydroxide follows _________.
(a)$S_N1$ mechanism
(b)$S_N2$ mechanism
(c) Any of the above two depending upon the temperature of the reaction
(d) Saytzeff rule
Answer:
Reaction of $C_6H_5CH_2Br$ with aqueous sodium hydroxide follows the $S_N1$ mechanism since the carbocation formed $C_6H_5CH_2^{\bigoplus }$ is resonance stabilized cation.
Benzylic halides are highly reactive in nature towards the $S_N1$ reaction.
Hence, the answer is the option (a).
Question 24 Which of the carbon atoms present in the molecule given below are asymmetric?
(i) a, b, c, d
(ii) b, c
(iii) a, d
(iv) a, b, c
Answer:
Carbon has four valences. When all four valencies are attached to four different atoms or groups then the carbon is called asymmetric/ chiral carbon. Here, ‘b’ and ‘c’ are attached to four different atoms/ groups. Hence, these are asymmetric.
Hence, the answer is the option (ii).
Question 25 Which of the following compounds will give a racemic mixture on nucleophilic substitution by $OH^{-}$ ion?
(i) (a)
(ii) (a), (b), (c)
(iii) (b), (c)
(iv) (a), (c)
Answer:
Compounds that undergo nucleophilic substitution by an ion and contain at least one chiral carbon will give a racemic mixture. A chiral carbon is a carbon atom that is attached to four different types of particles or groups of atoms. A racemic mixture is a combination of equal amounts of two enantiomers, which are substances with identical subatomic design. A racemic mixture is also known as a racemate. It has zero optical rotation because the rotation due to one isomer cancels out the rotation due to the other isomer.
As the alkyl halide in it contains asymmetric carbon, therefore, a mixture of an enantiomer is formed during the SN1 reaction.
Hence, the answer is the option (i).
Question 26 In the question arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.
(i) (a) < (b) < (c)
(ii) (c) < (b) < (a)
(iii) (a) < (c) < (b)
(iv) (c) < (a) < (b)
Answer:
$(-NO_2)$ is an electron-withdrawing group and when it is at ortho and para position it causes nucleophilic substitution. Where the effect of this same electron-withdrawing group is much less when it is at the meta position.
Hence, the answer is the option (iii).
Question 27 In the questions, arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.
(i) (a) < (b) < (c)
(ii) (a) < (c) < (b)
(iii) (c) < (b) < (a)
(iv) (b) < (c) < (a)
Answer:
The order of the rate of reaction towards nucleophilic substitution depends on the presence of electron-withdrawing groups and the number of electron-withdrawing groups. The presence of electron-withdrawing groups at the ortho and para positions increases the rate of nucleophilic substitution. The more electron-withdrawing groups there are, the higher the reactivity. The rate of nucleophilic substitution decreases when there is an electron releasing group at ortho or para positions.
Hence, the answer is the option (iv).
Question 28 In the questions, arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.
(i) (c) < (b) < (a)
(ii) (b) < (c) < (a)
(iii) (a) < (c) < (b)
(iv) (a) < (b) < (c)
Answer:
The reactivity of aryl halides increases with the increase in the electron-withdrawing group. Therefore, the more the number of electron-withdrawing groups, the more the rate of nucleophilic substitution.
Hence, the answer is the option (iv).
Question 29 In the questions, arrange the compounds in increasing order of the rate of reaction towards nucleophilic substitution.
(i) (a) < (b) < (c)
(ii) (b) < (a) < (c)
(iii) (c) < (b) < (a)
(iv) (a) < (c) < (b)
Answer:
Electron releasing groups increase the reactivity of aryl halides, less is the number of electron releasing groups, the less is the rate towards nucleophilic substitution.
CH3 is the electron releasing group. More the no. of CH3 groups increases the reactivity of aryl halides.
Hence, the answer is the option (iii).
Question 30 Which is the correct increasing order of boiling points of the following compounds?
1-Iodobutane, 1-Bromobutane, 1-Chlorobutane, Butane
(a) Butane < 1-Chlorobutane < 1-Bromobutane < 1 -Iodobutane
(b) 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane < Butane
(c) Butane < 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane
(d) Butane < 1-Chlorobutane < 1-Iodobutane < 1-Bromobutane
Answer:
The intermolecular force of attraction will increase with the increase in surface area. As a result, the boiling point will also increase. As the boiling point increases with the molecular mass for the similar alkyl halide. The Iodine has the highest atomic mass. Therefore, the boiling point of 1-iodobutane is the highest.
Hence, the answer is the option (a).
Question 31 Which is the correct increasing order of boiling points of the following compounds?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
(a) Bromobenzene < 1-Bromobutane < 1-Bromopropane < 1-Bromoethane
(b) Bromobenzene < 1-Bromoethane < 1 -Bromopropane < 1-Bromobutane
(c) 1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene
(d) 1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Answer:
The boiling point increases with an increase in molecular mass for the same types of alkyl halide.
The correct increasing order of boiling points is 1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene. This order is based on increasing molecular size and intermolecular forces. As the alkyl chain length increases from ethane to butane, van der Waals forces become stronger, raising the boiling point. Bromobenzene, despite having fewer carbons than 1-bromobutane, has a higher boiling point due to the presence of an aromatic ring and stronger intermolecular interactions.
Hence, the answer is the option (d).
NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: MCQ (Type 2)
MCQ-type questions are covered to improve your conceptual thinking and problem-solving ability. These questions from Haloalkanes and Haloarenes NCERT Exemplar Class 12 Chemistry Chapter 10 further enhance exam preparation.
Question 32 Which of the statements are correct about the above reaction?
(i) (a) and (e) both are nucleophiles.
(ii) In (c) carbon atom is sp3 hybridized.
(iii) In (c) carbon atom is sp2 hybridized.
(iv) (a) and (e) both are electrophiles.
Answer:
$OH^{-}$ and $Cl^{-}$are nucleophiles. In (c), the C atom is sp2 hybridized due to the formation of the C – OH bond and the breaking of the C – Cl bond simultaneously.
A nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases.
The movement of an electron pair from the nucleophile to the electrophile is represented by a curved-arrow notation. An example of a nucleophile is negatively charged species such as cyanide ion.
Hence, the answer is the option (i),(iii)
Question 33 Which of the following statements are correct about this reaction?
(i) The given reaction follows $S_{N}2$ mechanism.
(ii) (b) and (d) have opposite configurations.
(iii) (b) and (d) have the same configuration.
(iv) The given reaction follows $S_{N}1$ mechanism.
Answer:
In the given reaction, alkyl halide is primary in nature. Here, a transition state is observed in which one bond is broken and one bond is formed simultaneously i.e., in one step. So, it follows the $S_{N}2$ mechanism.
In this mechanism, the nucleophile attacks the carbon at $180^{\circ}$ to the leaving group. So the reactant and product have opposite configurations.
Hence, the answer is the option (i), (ii)
Question 34 Which of the following statements are correct about the reaction intermediate?
(i) Intermediate (c) is unstable because this carbon is attached to 5 atoms.
(ii) Intermediate (c) is unstable because the carbon atom is $sp^{2}$ hybridized.
(iii) Intermediate (c) is stable because the carbon atom is $sp^{2}$ hybridized.
(iv) Intermediate (c) is less stable than the reactant (b).
Answer:
(i), (iv) are correct options.
Intermediate (c) is unstable because the central carbon is temporarily bonded to five atoms, which violates carbon's tetravalency, making statement (i) correct. Additionally, this intermediate is less stable than the reactant (b) due to its high energy and unusual bonding, validating statement (iv). Statements (ii) and (iii) are incorrect because the carbon is not stably sp2 hybridised in this intermediate.
Hence, the answer is the option (i), (iv)
Question 35 Which of the following statements are correct about the mechanism of this reaction?
(i) A carbocation will be formed as an intermediate in the reaction.
(ii) $OH^{-}$ will attach the substrate (b) from one side and $Cl^{-}$ will leave it simultaneously from the other side.
(iii) An unstable intermediate will be formed in which $OH^{-}$ and $Cl^{-}$ will be attached by weak bonds.
(iv) Reaction proceeds through $S_N1$ mechanism.
Answer:
(i), (iv) are correct.
The given reaction involves a secondary alkyl halide reacting with hydroxide ion, indicating that the reaction proceeds via the SN1 mechanism. In this mechanism, a carbocation is formed as an intermediate after the departure of the chloride ion. This makes statements (i) and (iv) correct. Statement (ii) is incorrect as it describes the SN2 mechanism, where the nucleophile attacks simultaneously as the leaving group departs. Statement (iii) is also incorrect, as no such intermediate with both OH⁻ and Cl⁻ weakly attached is formed.
Hence, the answer is the option (i), (iv)
Question 36 Which of the following statements are correct about the kinetics of this reaction?
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on the concentration of both (a) and (b).
(iii) Molecularity of the reaction is one.
(iv) Molecularity of the reaction is two.
Answer:
The reaction proceeds via the SN1 mechanism, where the rate-determining step involves only the alkyl halide (b). Thus, the rate of reaction depends solely on the concentration of (b), and the molecularity of the reaction is one.
Hence, the answer is the option (i), (iii)
Question 37 Haloalkanes contain halogen atom(s) attached to sp3 hybridized carbon atoms of an alkyl group. Identify haloalkane from the following compounds.
(i) 2-Bromopentane
(ii) Vinyl chloride (chloroethene)
(iii) 2-chloroacetophenone
(iv) Trichloromethane
Answer:
2-Bromopentane and trichloromethane are haloalkanes, as the halogen atoms are attached to sp3 hybridised carbon atoms. Vinyl chloride has the halogen on an sp2 carbon, and 2-chloroacetophenone contains an aryl group, so they are not haloalkanes.
(i), (iv) are the haloalkanes from the above list.
Hence, the answer is the option (i), (iv)
Question 38 Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(i) Both the compounds form the same product on treatment with alcoholic KOH.
(ii) Both the compounds form the same product on treatment with aq.NaOH.
(iii) Both the compounds form the same product on reduction.
(iv) Both the compounds are optically active.
Answer:
Ethylene chloride and ethylidene chloride are structural isomers. Both form the same product, ethene, on treatment with alcoholic KOH due to elimination, and both yield ethane upon reduction. However, they give different products with aqueous NaOH and are optically inactive. Therefore, statements (i) and (iii) are correct.
Hence, the answer is the option (i), (iii)
Question 39 Which of the following compounds are gem-dihalides?
(i) Ethylidene chloride
(ii) Ethylene dichloride
(iii) Methylene chloride
(iv) Benzyl chloride
Answer:
Gem-dihalides are compounds where both halogen atoms are attached to the same carbon. Ethylidene chloride and methylene chloride satisfy this condition, as both have two chlorine atoms on a single carbon atom. Ethylene dichloride is a vicinal dihalide, and benzyl chloride contains only one halogen atom.
Hence, the answer is the option (i), (iii)
Question 40 Which of the following are secondary bromides?
(a) $(CH_3 )_{2}CHBr$
(b) $(CH_3 )_3C CH_2Br$
(c) $CH_3 CH(Br)CH_2CH_3$
(d) $(CH_3 )_2CBrCH_2CH_3$
Answer:
Secondary bromides are compounds in which the bromine atom is attached to a secondary carbon, meaning a carbon bonded to two other carbon atoms. In option (a), the bromine is attached to a carbon connected to two methyl groups, making it a secondary bromide. In option (c), which is 2-bromobutane, the Br is also attached to a secondary carbon. However, in option (b), the bromine is bonded to a primary carbon, and in option (d), the bromine is attached to a tertiary carbon. Therefore, only options (a) and (c) are secondary bromides.
Hence, the answer is the option (i), (iii)
Question 41 Which of the following compounds can be classified as aryl halides?
(i) $p-ClC_6H_4CH_2CH(CH_3)_2$
(ii) $p-CH_3CHCl(C_6H_4)CH_2CH_3$
(iii) $o-BrH_2C-C_6H_4CH(CH_3)CH_2CH_3$
(iv) $C_6H_5Cl$
Answer:
Aryl halides are compounds where a halogen atom is directly bonded to an aromatic ring. In compound (i), the chlorine is directly attached to the benzene ring in the para position, making it an aryl halide. In compound (iv), chlorobenzene the chlorine is directly attached to the benzene ring in the para position However, in compound (ii), the chlorine is bonded to a side chain rather than the aromatic ring, and in compound (iii), bromine is part of a benzyl group.
Hence, the answer is the option (i), (iv)
Question 42 Alkyl halides are prepared from alcohol by treating with
(i) $HCl + ZnCl_2$
(ii) $RedP + Br_2$
(iii) $H_2SO_4 + KI$
(iv) all the above
Answer:
Alkyl halides can be prepared from alcohols using specific reagents depending on the halogen required. HCl with ZnCl2, known as Lucas's reagent, is effective for converting alcohols to alkyl chlorides. Red phosphorus with bromine generates PBr3 in situ, which reacts with alcohols to form alkyl bromides. However, the combination of H2SO4 and KI is not suitable for preparing alkyl iodides
Hence, the answer is the option (i), (ii)
Question 43 Alkyl fluorides are synthesized by heating an alkyl chloride/bromide in
presence of ____ or ____
(i) CaF2
(ii) CoF2
(iii) Hg2F2
(iv) NaF
Answer:
(ii), (iii) are correct options
Alkyl fluorides are prepared by heating alkyl chlorides or bromides in the presence of metal fluorides like cobalt(II) fluoride CoF2 or mercurous fluoride Hg2F2, which facilitate halogen exchange in the Swarts reaction.
Hence, the answer is the option (ii), (iii)
NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: Short Answer Type
Short-answer type questions are covered to improve your problem-solving ability. These Chapter 10 Haloalkanes and Haloarenes important questions help in building a strong foundation for board and competitive exams.
Answer:
Iodination reactions are reversible in nature as because HI is formed in the process. To keep the reaction in forward direction, we need to remove the HI by oxidation process by adding some oxidizing agents like $HIO_{4}.$
Question 45 Out of o- and p-bromobenzene, which one has a higher melting point, and why?
Answer:
p-Dibromobenzene has a higher melting point. Because, in a crystal lattice, the symmetry of p-bromobenzene makes it fit better. As a result, a higher temperature is required to break the bond between the molecules and hence the melting point is higher.
Question 46 Which of the compounds will react faster in $S_N1$ reaction with the $OH^{-}$ ion?
$CH_3 - CH_2 - Cl\; or\; C_6H_5 - CH_2 - Cl$
Answer:
$C_6H_5- CH_2 - Cl$ will react faster in $S_N1$ reaction with the $OH^{-}$ ion. This is because there is enough stability of the carbocation in this compound. Here, due to resonance, the $C_6H_5$ group is already stable and it is attached with $CH_{2}$ and makes the whole structure stable.
Question 47 Why does iodoform have appreciable antiseptic properties?
Answer:
Because there are liberal free iodines, iodoform is an applicable antiseptic.
Question 48 Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
Answer:
This is because there is a resonance stabilization formed in the aryl ring. Where the C -Cl bond acts as a partial double bond because of resonance. Therefore, they are less reactive in nucleophilic substitution.
Question 49 Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Answer:
Aryl bromides and chlorides can be prepared in the presence of Lewis acid catalysts (iron or iron chloride) from the electrophilic substitution of arenas with bromine and chlorine respectively.
Question 50 Which of the following compounds (a) and (b) will not react with a mixture of NaBr and $H_{2}SO_4$. Explain why?
(a) $CH_{3}CH_{2}CH_{2}OH$
(b)
Answer:
If we mix NaBr and $H_2SO_4$, we will get $Br_{2}$ gas. Here, the product (b) will not react with $Br_{2}$ gas. In phenol, the –OH group is directly attached to the aromatic ring and is involved in resonance, making the C–OH bond stronger and less reactive toward substitution.
Question 51 Which of the products will be the major product in the reaction given below? Explain.

Answer:
As per Markovnikov's rule, B will be the major product
This addition reaction is carried out by Markovnikoff's rule, which states that in a double bond, the hydrogen from the hydrogen halide is added to the carbon atom with the most hydrogen atoms attached to it, while the halogen atom is attached to the carbon atom with the fewest hydrogens attached to it. The main product in the combination will be the molecule that follows this guideline. As a result, the molecule (B) will be the reaction's main product.
Question 52 Why is the solubility of haloalkanes in water very low?
Answer:
The solubility of the haloalkanes in water is low. This is because, to make a haloalkane soluble in water, we need energy. Which will help to overcome the attraction between the haloalkane molecules and also help to break the hydrogen bonds between water molecules.
Answer:
The functional group present in this ring is ortho-para directing. This is because the density of electrons at that position is higher.
Question 54 Classify the following compounds as primary, secondary and tertiary halides.
(i) 1-Bromobut-2-ene
(ii) 4-Bromopent-2-ene
(iii) 2-Bromo-2-methylpropane
Answer:
(i) 1-Bromobut-2-ene: It is a primary halide.
(ii) 4-Bromopent-2-ene: Here Bromine is attached to the secondary carbon. Hence, it is a secondary halide.
(iii) 2-Bromo-2-methylpropane: Here Bromine is attached to the tertiary carbon. Hence, it is a tertiary halide.
Answer:
(i) The rate of reaction with aqueous KOH of the compound ‘A’ only depends upon the concentration of ‘A’, therefore, the reaction mechanism is $S_N1$ and ‘A’ is 2-Bromo-2-methylpropane(tertiary bromide).
Whereas, ‘B’ is optically active and is an isomer of ‘A’. Therefore, ‘B’ must be 2-Bromobutane. As the concentration of ‘B’ is responsible for the rate of reaction of ‘B’ with aqueous KOH, therefore, the reaction mechanism is $S_N2$
(ii) Because of the $S_N2$ reaction, compound ‘B’ will have an inversion of configuration and turn out to be an inverted product.
Question 56 Write the structures and names of the compounds formed when compound ‘A’ with the molecular formula, $C_{7}H_8$ is treated with $Cl_2$ in the presence of $FeCl_3$.
Answer:
Toluene$(C_6H_5CH_3)$ is the compound that has a molecular formula $C_{7}H_8$. When toluene is treated with $Cl_2$ in the presence of $FeCl_3$, we get o-chlorotoluene and p-chlorotoluene where the p-isomer predominates. This is because of the $-CH_3$ group is o-, p-directing.
Question 58 Which of the following compounds will have the highest melting point and why?
Answer:
The structure (II) has the symmetry of para-positions.
Because both methyl groups and chlorine atoms are symmetrically positioned at para-positions in compound (II), these molecules fit better in the crystal lattice than other isomers and hence have the greatest melting point.
Question 59 Write down the structure and IUPAC name for neo-pentyl bromide.
Answer:
1-Bromo-2,2-dimethylpropane is the IUPAC name for neo-pentyl bromide.
Question 60 A hydrocarbon of molecular mass 72 g/ mol gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.
Answer:
We can say pentane $(C_5H_{12})$ has a molecular mass of 72 g/ mol. Therefore, the isomer of pentane with a single monochloride derivative should consists of 12 hydrogens equivalent.
Question 61 Name the alkene which will yield 1-chloro-1-methylcyclohexane by its reaction with HCl. Write the reactions involved.
Answer:
Here, two different alkenes such as methylene cyclohexane and 1-methylcyclohex-1-ene can be found.
Question 62 Which of the following haloalkanes reacts with aqueous KOH most easily? Explain by giving a reason.
(i) 1-Bromobutane
(ii) 2-Bromobutane
(iii) 2-Bromo-2-methylpropane
(iv) 2-Chlorobutane
Answer:
(iii) 2-Bromo-2-methylpropane
This complex will easily react with the aqueous KOH. Here, tertiary carbocation will be formed and this will be most stable.
Question 63 Why can aryl halides not be prepared by the reaction of phenol with HCl in the presence of $ZnCl_{2}$ ?
Answer:
In the presence of $ZnCl_{2}$, phenol will not react with HCl. This is because there is a partial double bond characteristic present between the benzene ring and O, which will be produced between the benzene ring and OH group due to resonance. As a result, aryl halide will not be prepared.
Question 64 Which of the following compounds would undergo $S_{N}1$ reaction faster and why?
Answer:
B will undergo $S_{N}1$ reaction faster. This is because when Cl is removed, carbocation is formed and stabilized by resonance.
On the other hand, the carbonation formed during the reaction (A) is not a resonance situation.
Question 65 Allyl chloride is hydrolyzed more readily than n-propyl chloride. Why?
Answer:
Allyl chloride becomes highly reactive in nature due to the formation of carbocation by hydrolysis, which is very much stable due to resonance. In the case of n-propyl chloride, there will be no such stability exists due to carbocation.
Question 66 Why is it necessary to avoid even traces of moisture during the use of a Grignard reagent?
Answer:
It is necessary to avoid even traces of moisture because the Grignard reagents are highly reactive in nature. Those reagents can react with even traces of water and produce corresponding hydrocarbons.
$RMgX + H_2O \rightarrow RH + Mg(OH)X$
Question 67 How do polar solvents help in the first step in $S_{N}1$ mechanism?
Answer:
Through the formation of carbocation, the mechanism of the $S_{N}1$ moves forward. In this process, the breaking of the C-halogen bond takes place. As a result, the solvation of the halide ions is done with the protons of the of the protic solvent. In this way, polar solvents help in ionisation step by stabilizing the ions by solvation.
Question 68 Write a test to detect the presence of a double bond in a molecule.
Answer:
If a molecule contains a double bond, then it can be detected easily by the bromine water test and Baeyer’s test. The bromine water or aqueous $KMnO_{4}$ becomes colorless.
Question 69 Diphenyls are a potential threat to the environment. How are these produced from aryl halides?
Answer:
Diphenyls (ex: p,p ‘-dichlorodiphenyltrichloroethane., i.e DDT) are a serious threat to the environment. This is because those are chemically stable and soluble in fat. The long-term presence of it in the atmosphere is extremely dangerous.
Di-phenyls can be prepared from aryl halides by the following two methods:
Question 70 What are the IUPAC names of the insecticide DDT and benzene hexachloride? Why is their use banned in India and other countries?
Answer:
IUPAC name of DDT is 2, 2-bis (4-chlorophenyl)-1, 1, 1-trichloroethane that of benzene hexachloride is 1, 2, 3, 4, 5, 6-hexachlorocyclohexane.
They are non-biodegradable and toxic at the same time. They are soluble in fat and their concentration keeps increasing in the food chain. This is why their use is banned in India and other countries.
Answer:
Alkyl halides respond in nucleophilic substitution as well as elimination ($\beta$-elimination) reaction.
However, by setting the reaction condition and the right choice of reagent, a particular product can be obtained. Normally, elimination reaction suits the strong and larger bases along with high temperature. Whereas substitution reaction is suitable for the weaker and smaller bases in lower temperatures.
Ex:
$CH_{3}CH_{2}Br\xrightarrow[alc. KOH]{473-573K}CH_{2}=CH_{2}$ (Elimination)
$CH_{3}CH_{2}Br\xrightarrow[aq. KOH]{373K}CH_{3}-CH_{2}OH$ (Substitution)
Nucleophilic Subsitution : Reagents used are nucleophilies like $^{-}{O}H, NH_{3}, ^{-}{C}\equiv N, O=N-O, ^{-}OR$, etc at lower temperature under goes Substitution reaction.
Question 72 How will you obtain mono bromobenzene from aniline?
Answer:
Mono bromobenzene from aniline:
When the nucleophile is attached to the carbon carrying -Cl, due to resonance a stabilised intermediate compound is found. As the $-NO_{2}$ is electron withdrawing in nature, the nucleophile will get attached to the benzene ring easily. When the molecule contains more number of $-NO_{2}$ groups, the nucleophile will be attached more easily. Hence, the order of reactivity is III > II > I.
Question 74 tert-Butylbromide reacts with aq. NaOH by $S_N1$ mechanism while n-butylbromide reacts by $S_N2$ mechanism. Why?
Answer:
In general, the $S_N1$ reaction goes forward by the formation of carbocation. The tert-butyl bromide will form $3^{\circ}$ carbocation by losing the $Br^{-}$ ion, which is actually stable. Therefore, it reacts with aqueous KOH by $S_N1$ mechanism as:
On the other hand, due to its unstable in nature, n-butyl bromide does not form 1° n-butyl carbocation by ionization. Therefore, it follows the mechanism of $S_N2$ mechanism. This happens through a transition state where $OH^{-}$ ion form (nucleophilic attack) in the remote side with simultaneous expulsion of $Br^{-}$ ion from the front side.
Question 75 Predict the major product formed when HCl is added to isobutylene. Explain the mechanism involved.
Answer:
The major product is 2-chloro-2-methylpropane
Question 76 Discuss the nature of the C – X bond in the haloarenes.
Answer:
Resonance effect: The C – X bond holds the nature of a partial double bond character and as a result, the C – X bond is very difficult to break.
(i) The C – X bond in haloarenes is extremely less reactive towards nucleophilic
(ii) In C – X bond, the C atom attached to the halogen is sp2 hybridized. Carbon that is sp2 hybridized with a greater s-character is more electronegative in nature and it can hold the electron pair of C – X bond more tightly than sp3 hybridized carbon in haloalkanes with less s-character.
Question 77 How can you obtain iodoethane from ethanol when no other iodine-containing reagent except Nal is available in the laboratory?
Answer:
Ethanol can be converted into chloroethane with the help of $HCl + ZnCl_2$ and Cl in the chloroethane can be replaced by I with the help of NaI.
$C_{2}H_{5}OH+HCl \overset{ZnCl_2}{\rightarrow}C_{2}H_{5}Cl\overset{NaI}{\rightarrow}C_{2}H_{5}I$
Question 78 Cyanide ion acts as an ambident nucleophile. From which end it act as a stronger nucleophile in the aqueous medium? Give a reason for your answer.
Answer:
A cyanide ion acts as a stronger nucleophile from the carbon end. This is because it will form a C – C bond which is more stable (a bond between two similar atoms) than a C – N bond.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: Matching Type
Matching-type questions are covered to improve conceptual clarity and topic awareness. Such exercises in NCERT Exemplar Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes strengthen understanding.
Question 79 Match the compounds given in Column I with the effects given in Column II.
|
Column I |
Column II |
|
i. Chloramphenicol |
a. Malaria |
|
ii. Thyroxine |
b. Anesthetic |
|
iii. Chloroquine |
c. Typhoid fever |
|
iv. Chloroform |
d. Goiter |
|
|
e.Blood substituent |
Answer:
$(i \rightarrow c); (ii \rightarrow d); (iii \rightarrow a); (iv \rightarrow b)$
(i) Chloramphenicol is a broad-spectrum antibiotic that is used to treat typhoid fever.
(ii) Thyroxine is a hormone secreted by the thyroid gland. When the thyroxine is secreted more, then the patient will notice an enlarged thyroid gland which is called goiter.
(iii) Chloroquine is used to prevent malaria parasite plasmodium vivax in the blood.
(iv) Chloroform is trichloromethane $(CHCl_3)$. It is used as an anesthetic.
Question 80 Match the items of Column I and Column II.
|
column I |
Column II |
|
i. $S_N1$ reaction |
a. vic-dibromides |
|
ii. Chemicals in fire extinguisher |
b. gem - dihalides |
|
iii. Bromination of alkene |
c. Racemization |
|
iv. Alkylidene halides |
d. Saytzeff rule |
|
v. Elimination of HX from alkyl halide |
e. Chlorobromocarbons |
Answer:
$(i \rightarrow c), (ii \rightarrow e), (iii \rightarrow a), (iv \rightarrow b), (v \rightarrow d)$
(i) When any mixture has two enantiomers in equal proportions, then there will be zero optical rotation observed. These kinds of mixtures are called racemic mixtures and this process of conversion of enantiomers into a racemic mixture is known as racemization. When alkyl halide follows SN1 mechanism then racemization is observed.
(ii) Chlorofluorocarbons are used in fire extinguishers.
(iii) Halogen atoms are present on the adjacent carbon atom in vic-dihalides. vic-dihalides are observed from the Bromination of alkanes.
(iv) Alkylidene halides are also known as gem-dihalides. Halogen atoms are present on the same carbon atom in the case of gem-dihalides.
(v) Elimination of HX from alkyl halide follows the Saytzeff rule. This rule states that “in dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms”.
Question 81 Match the reactions given in column I with the types of reactions given in column II.
|
Column I |
Column II |
|
i. |
a. Aryl halide |
|
ii. $CH_{2}=CH-CH_{2}-X$ |
b. Alkyl halide |
|
iii. |
c. Vinyl halide |
|
iv. $CH_{2}=CH-X$ |
d. Allyl halide |
Answer:
$(i \rightarrow b), (ii \rightarrow d), (iii \rightarrow a), (iv \rightarrow c)$
(i) A halogen atom in alkyl halide is bonded to a carbon atom that is sp3 hybridized, where further addition of alkyl group may happen. $CH_3-CH (X) - CH_3$ is alkyl halide.
(ii) A halogen atom in allyl halide is bonded to a carbon atom next to $C=C$ double bond that is $sp^3$ hybridized. $CH_2= CH - CH_2- X$ is allyl halide.
(iii) Halogen atoms in aryl halide are bonded to a carbon atom that is $sp^2$ hybridized aromatic ring. $C_6H_5X$ is aryl halide.
(iv) Halogen atoms in vinyl halide is bonded to a $sp^2$ hybridized carbon atom of a $C=C$ double bond. $CH_2=CH-X$ is vinyl halide.
Question 82 Match the reactions given in column I with the types of reactions given in column II.
Answer:
$(i \rightarrow b), (ii \rightarrow d), (iii \rightarrow e), (iv \rightarrow a), (v \rightarrow c)$
(i) Here, substitution takes place when an electrophile $Cl^+$ attacks on the benzene ring.
(ii) Here, according to Markownifoff’s rule, on the double-bonded carbons the addition of HBr takes place followed by electrophilic addition.
(iii) Here, the reactant is a secondary halide in nature and the hydroxyl ion will substitute the halogen. Here the reaction mechanism is $S_N1$ due to the presence of secondary halide.
(iv) Here the aromatic ring and the halogen atom are attached directly. The halogen of the given compound is substituted by the $OH^-$ group here, due to nucleophilic substitution.
(v) It follows Saytzeff's rule, i.,e elimination reaction.
Question 83 Match the structures given in Column I with the names given in Column II.
Answer:
$(i \rightarrow a), (ii \rightarrow c), (iii \rightarrow b), (iv \rightarrow d)$
(i) A: 4-bromopent-2-ene.
(ii) B: 4-bromo-3-methylpent-2-ene.
(iii) C: 1-bromo-2-methylbut-2-ene.
(iv) D: 1 -bromo-2-methylpent-2-ene.
Question 84 Match the reactions given in Column I with the names given in Column II.

Answer:
$(i\rightarrow b), (ii \rightarrow a), (iii \rightarrow d), (iv \rightarrow c)$
(i) Alkyl arene is produced by the mixture of an alkyl halide and aryl halides when treated with sodium in dry ether. This reaction is called the Wurtz-Fittig reaction.
(ii) Analogous compound is found from the aryl halides when it is treated with sodium in dry ether. Here, two aryl groups are attracted together. This reaction is called the Fittig reaction.
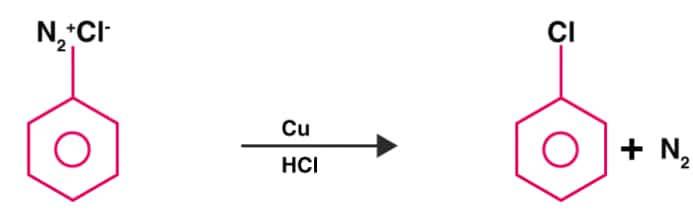
(iii) Chlorobenzene or bromobenzene is formed from the diazonium salt when it is treated with cuprous chloride or cuprous bromide. This reaction is called Sandmeyer’s reaction.
(iv) In dry acetone, alkyl chlorides, and sodium iodide react with each other and form Alkyl iodides. The reaction is called the Finkelstein reaction.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: Assertion and Reason Type
Assertion and Reason type questions are covered to improve your critical thinking and problem-solving solving ability.These are an important part of Chemistry Class 12 NCERT Exemplar Chapter 10 Haloalkanes and Haloarenes, helping students prepare effectively for board and competitive exams.
Question 85 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of assertion.
Assertion (A): Phosphorus chlorides (tri and penta) are preferred over thionyl chloride for the preparation of alkyl chlorides from alcohols.
Reason (R): Phosphorus chlorides give pure alkyl halides.
Answer:
To convert alcohol to alkyl halide, thionyl chloride is the best halogen carrier. Because all the byproducts are in a gaseous state. As a result, a pure form of alkyl halide can be prepared.
Hence, the correct answer is option (ii)
Question 86 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and the reason is the correct explanation of the assertion.
(ii) Both the assertion and the reason both are wrong statements.
(iii) The assertion is correct but the reason is a wrong statement.
(iv) The assertion is wrong, but the reason is the correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of the assertion.
Assertion (A): The boiling points of alkyl halides decrease in the order: $RI > RBr > RCl > RF$
Reason (R): The boiling points of alkyl chlorides, bromides, and iodides are considerably higher than that of the hydrocarbon of comparable molecular mass.
Answer:
The order of boiling point :
$RI > RBr > RCl > RF$
As the halides are polar molecules, therefore, their boiling temperature is always more than the boiling temperature of the hydrocarbons.
Hence, the correct answer is option (v)
Question 87 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and the reason is the correct explanation of the assertion.
(ii) Assertion and reason both are wrong statements.
(iii) The assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of assertion.
Assertion (A): KCN reacts with methyl chloride to give methyl isocyanide
Reason (R):$CN^{-}$ is an ambident nucleophile.
Answer:
$R-Cl + KCN \rightarrow R-CN + KCl$
Alkyl Cyanide
Methyl cyanide is also obtained as $CN^{-}$ group present here is an ambident nucleophile.
Hence, the correct answer is option (iv)
Question 88 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of assertion.
Assertion (A): Tert-butyl bromide undergoes a Wurtz reaction to give 2, 2, 3, 3-tetramethylbutane.
Reason (R): In the Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbons containing double the number of carbon atoms present in the halide.
Answer:
In the Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide.
tert-Butyl bromide undergoes a Wurtz reaction to give 2, 2, 3, 3-tetramethylbutane.
Hence, the correct answer is option (i)
Question 89 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of assertion.
Assertion (A): The presence of a nitro group at the ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution.
Reason (R): The Nitro group, being an electron-withdrawing group decreases the electron density over the benzene ring.
Answer:
The nitro group is used to decrease the electron density in the ring as it is an electron-withdrawing group. As a result, this will cause nucleophilic substitution due to an increase in reactivity of haloarenes.
Hence, the correct answer is option (i)
Question 90 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of assertion.
Assertion (A): In mono haloarenes, further electrophilic substitution occurs at ortho and para positions.
Reason (R): The halogen atom is a ring deactivator.
Answer:
Due to the (+M) or (+R) effect, the halogens are usually ortho-para directing in nature. However, due to high electronegativity, they are also deactivating in nature.
Hence, the correct answer is option (v)
Question 91 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of assertion.
Assertion (A): Aryl iodides can be prepared by a reaction of arenes with iodine in the presence of an oxidizing agent.
Reason (R): Oxidising agent oxidizes $I_{2}$ into $HI$.
Answer:
HI converts into $I_{2}$ by the oxidising agent such as $HIO_3$. Although, HI can convert aryl halides into arenes.
$5HI + HIO_3 \rightarrow 3H_2O + 3I_2$
Hence, the correct answer is option (iii)
Question 92 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not the correct explanation of assertion.
Assertion (A): It is difficult to replace chlorine by -OH in chlorobenzene in comparison to that in chloroethane.
Reason (R): Chlorine-carbon (C – Cl) bond in chlorobenzene has a partial double bond character due to resonance.
Answer:
There is a partial double bond formed due to resonance in between the benzene ring and halogen. As a result, it is difficult for the aryl halide to take part in nucleophilic substitution as compared to the alkyl halide.
Hence, the correct answer is option (i)
Question 93 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of assertion.
Assertion (A): Hydrolysis of (-)-2-bromooctane proceeds with inversion of configuration.
Reason (R): This reaction proceeds through the formation of a carbocation.
Answer:
Here, $S_N2$ mechanism will be seen due to the hydrolysis of 2-bromooctane. As a result, the configuration will be inverted. Here no carbocation will be formed.
Hence, the correct answer is option (iii)
Question 94 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question:
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but the reason is not the correct explanation of assertion.
Assertion (A): Nitration of chlorobenzene leads to the formation of m-nitrochlorobenzene.
Reason (R): $-NO_2$ group is a m-directing group.
Answer:
Nitration of chlorobenzene results in ortho and para nitro chlorobenzene. Although, the nitro groups are meta-directing in nature.
Hence, the correct answer is option (iv).
NCERT Exemplar Class 12 Chemistry Solutions Chapter 10: Long Answer Type
Long-answer type questions are covered to improve your subject knowledge and conceptual thinking. These NCERT Exemplar Class 12 Chemistry Solutions chapter 10 Haloalkanes and Haloarenes enhance analytical skills and strengthen exam preparation.
Primary alkyl halides prefer SN2 mechanism in which a nucleophile attacks at 1800 to the halogen atom. A transition state is formed in which carbon is bonded to two nucleophiles and finally halogen atom is pushed out. Below is the mechanism-
Hence, in SN2 mechanism, substitution takes place. Tertiary alkyl halides follow SN1 mechanism. In this case, there alkyl halides form 3? carbocations. If the reagent used is a weak base then substitution occurs while if it is a strong base then instead of substitution elimination occurs.
The reagent used is aq. KOH. It is a weak base so, substitution takes place.
As alc. KOH is a strong base, so elimination competes over substitution, and alkene is formed.
Uses of halogen-containing compounds are as follows:
Dichloromethane is mainly used as a solvent. Although it can be used as a paint remover, finishing solvent, metal cleaner, propellant in aerosols, solvent in case of drug manufacturing, etc.
Trichloromethane is used as a fat solvent. However, it can be used as a solvent for iodine, alkaloids, and other substances.
Triiodomethane is used as an antiseptic.
But some compounds of this class are responsible for the exposure of flora and fauna to more and more UV light which causes destruction to a great extent. These are as follows
(i) Tetrachloromethane
• CCl3 is released into the air and starts depleting the ozone layer in the atmosphere. As a result, UV rays will come to the earth easily and humans will get affected. This will cause skin cancer, functional disorder, eye diseases, and also the damage in the immune system. These UV rays not only affect the human but also cause damage to plants, and other animals too.
(ii) Freons
• Freon-113, releases and will move to the top of the atmosphere. Here, it generates Cl atoms in order to damage the ozone layer. As a result of this depletion UV rays enter our atmosphere and become responsible for damage to a great extent.
(iii) p – p’ – Dichlorodiphenyltrichloroethane (DDT)
• DDT can not be destroyed and removed from the atmosphere because it is not completely biodegradable. It is soluble in fats and creates a chain. When DDT goes into the body of a human, then it will affect the reproductive system.
In order to get rid of the harmful effects of the following substances, i.e., freons, hydrofluorocarbons, and fluorocarbons, we should lower the use of these substances.
Question 97 Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides? How can we enhance the reactivity of aryl halides?
Answer:
Aryl halides are less reactive towards nucleophilic substitution reactions for the reasons mentioned as follows:
(i) In the case of haloarenes, the benzene ring is attached to the lone pair electron of the halogen in resonance. As a result, the C-Cl bond acts as a partial double bond and increases the strength of the bond. This bond is difficult to substitute by nucleophilic substitution method. Hence, they usually react less in the above-mentioned reaction.
(ii) In the case of haloarenes, the $sp^2$ hybridized carbon atom is attached to a halogen. An $sp^2$ hybridized carbon atom is more electronegative and it holds the C-Cl pair strongly and forces the C-Cl bond shorter than the haloalkanes.
Method to increase the reactivity:
The reactivity of the aryl halides can be increased when there is an electron-withdrawing group$(NO_2)$ at the ortho and para positions. This presence of this electron-withdrawing group at the above-mentioned position withdraws electron density in the benzene ring. As a result, it will be easier for the nucleophile to attack. As a result, carbocation is formed through resonance.
So, in the case of o- and p-chlorobenzenes, the carbon atom bearing $(NO_2)$ group has a negative charge due to the resonating structure. Therefore, the $(NO_2)$ group along with the π-electrons of the benzene ring stabilizes the carbon ions. However, none of the resonating structures carries the negative charge along with the carbon atom bearing $-NO_2$ group, in the case of m-nitrochlorobenzene. Therefore, the negative charge does not stabilize by the nitro group at the meta position. However, the p-electrons of the benzene ring stabilize the carbanion. In other words, the carbanions formed from o-nitrochlorobenzene and p-nitrochlorobenzene are more stable than those formed from m-nitrochlorobenzene.
Thus, the presence of electron-withdrawing groups at o- and p-positions (but not at m-positions) w.r.t. The halogen atom activates the aryl halides towards nucleophilic substitution reaction.
Class 12 Chemistry NCERT Chapter 10: Higher Order Thinking Skills (HOTS) Questions
Class 12 Chemistry NCERT Exemplar Solutions chapter 10 Haloalkanes and Haloarenes also includes HOTS-type questions that are covered to improve your problem-solving ability and conceptual thinking. These advanced questions help in developing higher-level analytical skills for exams.
Question 1: The products A and B in the following reactions, respectively are
$\mathrm{A} \xleftarrow{\mathrm{Ag}-\mathrm{NO}_2}{ } \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Br} \xrightarrow{\mathrm{AgCN}} \mathrm{B}$
(1) $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{ONO}, \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NC}$
(2) $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{ONO}, \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CN}$
(3) $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NO}_2, \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CN}$
(4) $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NO}_2, \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NC}$
Answer:
Reaction with $\mathbf{A g N O}_{\mathbf{2}}$
(A) $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Br}$$\xrightarrow{A g-\mathrm{NO}_2}$$\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NO}_2$
With $\mathrm{AgNO}_2$ :
The nucleophile is $\mathrm{NO}_2{ }^{-}$, which can attach via oxygen or nitrogen. The product is usually an alkyl nitrite ( $\mathrm{R}-\mathrm{ONO}$ ) or a nitroalkane ( $\mathrm{R}-\mathrm{NO}_2$ ).
- In this case, $\mathrm{AgNO}_2$ reacts to give alkyl nitrite (R-ONO) because the ionic character favors Oalkylation (via oxygen).
- So, $\mathrm{A}=\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{ONO}$ (propyl nitrite)
With AgCN:
The cyanide ion $\mathrm{CN}^{-}$is a strong nucleophile and binds via carbon to give a nitrile.
- The reaction results in nucleophilic substitution forming 1-propionitrile ( $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CN}$ ).
- So, B $=\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CN}$ (butyronitrile)
Reaction with AgCN
(B) $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NO}_2 $ $\xrightarrow{\mathrm{AgCN}} \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NC}$
Hence, the correct answer is option (4).
Question 2: Given below are two statements :
Statement I : $\mathrm{CH}_3-\mathrm{O}-\mathrm{CH}_2-\mathrm{Cl}$ will undergo
$\mathrm{S}_{\mathrm{N}} 1$ reaction though it is a primary halide.
Statement II : will not undergo $\mathrm{S}_{\mathrm{N}} 2$ reaction very easily though it is a primary halide.
will not undergo $\mathrm{S}_{\mathrm{N}} 2$ reaction very easily though it is a primary halide.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Statement I is incorrect but Statement II is correct.
(2) Both Statement I and Statement II are incorrect
(3) Statement I is correct but Statement II is incorrect
(4) Both Statement I and Statement II are correct.
Answer:
$\mathrm{CH}_3-\mathrm{O}-\mathrm{CH}_2-\mathrm{Cl}$ will undergo $\mathrm{S}_{\mathrm{N}} 1$ mechanism because $\mathrm{CH}_3-\mathrm{O}-\stackrel{+}{\mathrm{C}} \mathrm{H}_2$ is highly stable.

Hence, the correct answer is option (4).
Question 3: Given below are two statements :
Statement (I) : Alcohols are formed when alkyl chlorides are treated with aqueous potassium hydroxide by elimination reaction.
Statement (II) : In alcoholic potassium hydroxide, alkyl chlorides form alkenes by abstracting the hydrogen from the $\beta$-carbon.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Both Statement I and Statement II are incorrect
(2) Statement I is incorrect but Statement II is correct
(3) Statement I is correct but Statement II is incorrect
(4) Both Statement I and Statement II are correct
Answer:
Statement (I) :
$\mathrm{R}-\mathrm{Cl} \xrightarrow{(\mathrm{aq}, \mathrm{KOH})} \mathrm{R}-\mathrm{OH}$ this reaction is nucleophilic substitution (SN2 or SN1), not elimination.
Statement (II) :
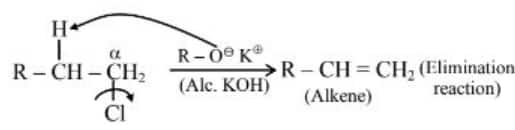
Hence, the correct answer is option (2).
Question 4:

Product (C) is :
(1)

(2)

(3)

(4)

Answer:

Hence, the correct answer is option (2).
Question 5: Identify the structure of the final product (D) in the following sequence of the reactions :

Total number of $sp^2$ hybridised carbon atoms in product D is.
Answer:

When vicinal dichloride reacts with NaNH2, a strong base, it undergoes a double dehydrohalogenation reaction, resulting in the formation of an alkyne. This reaction proceeds via two consecutive E2 elimination steps. Later on this alkyne reacts with diborane to give an aldehyde.
Number of $\mathrm{sp}^2$ hybridised carbon $=7$
Hence, the answer is 7.
Approach to Solve Questions of Chapter 10 Haloalkanes and Haloarenes
To effectively solve the questions of Haloalkanes and Haloarenes, it is important to adopt a structured approach. The following are the ways that can help you attempt the questions with the correct approach:
1) Identify whether the compound is a Haloalkane or Haloarene, which can be determined based on the Carbon chain. Then determine the degree of Halogenated carbon
2) While solving the questions of Chapter 10 NCERT Exemplar Haloalkanes and Haloarenes, it is important to have proper knowledge IUPAC naming rules:
- Identify the longest carbon chain
- Position of the Halogen
- Check for multiple halogens, and if available, then they are named according to alphabetical order
- Presence of functional groups
Also study about nature of C-X bonds, physical and chemical properties of Haloalkanes and Haloarenes.
3) Questions related to reaction mechanisms are asked frequently in exams, like identifying the type of reaction, whether it's addition, elimination, or substitution, then identifying the mechanism type whether it is SN1, SN2, E1, E2.
4) Various important reactions and tests are discussed in this chapter, and questions are asked frequently about these reactions. Hence, a proper knowledge of these reactions is crucial. Some important reactions are given below
- Sandmeyer reaction
- Finkelstein reaction
- Wurtz Fettig reaction
- Test for halogens using $\mathrm{AgNO}_3$
5) Determine whether the reaction involves inversion, retention, or racemization. After that, check for optical activity. Students can also refer to Haloalkanes and Haloarenes notes for conceptual clarity.
6) A Proper understanding of basic concepts and practice helps students clear their doubts and solve questions effectively. Students can refer to the questions provided in the NCERT textbooks and revise them again and again
Topics Covered in NCERT Exemplar Solutions Class 12 Chemistry Chapter 10
Important topics of NCERT Exemplar Class 12 Chemistry Chapter 10 are given below:
- Classification
- Nomenclature
- Nature of C–X Bond
- Methods of Preparation of Haloalkanes
- Preparation of Haloarenes
- Physical Properties
- Chemical Reactions
- Polyhalogen compounds
Important Reactions of Class 12 Chapter 10
Students can refer to the formulas and concepts given below for solving Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes questions:
1) Finkelstein Reaction
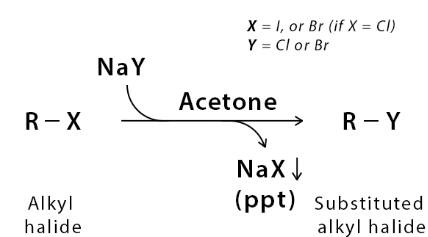
2) Swarts Reaction
$\mathrm{CH}_3 \mathrm{Br}+\mathrm{AgF} \rightarrow \mathrm{CH}_3 \mathrm{~F}+\mathrm{AgBr}$
3) Wurtz Reaction
$2 R-X+2 N a \xrightarrow{\text { dry ether }} R-R+2 N a X$
4) Kolbe's Electrolysis
$2 \mathrm{CH}_3 \mathrm{COONa} \xrightarrow{\text { electrolysis }} \mathrm{C}_2 \mathrm{H}_6+\mathrm{CO}_2+\mathrm{NaOH}+\mathrm{H}_2$
5) Sandmeyer Reaction
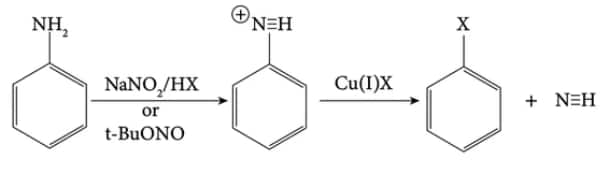
6) Gattermann Reaction
Advantages of Using Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes NCERT Exemplar Solutions
Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes are important for students preparing for boards and competitive exams like JEE and NEET. The advantages of using these solutions are given below:
- Students can cover topics like nomenclature, preparation methods, physical and chemical properties, and mechanisms of substitution, elimination reactions and mechanisms using these solutions.
- NCERT Exemplar Class 12 Solutions help students revise all important topics and reaction mechanisms that are frequently asked in board exams.
- Haloalkanes and Haloarenes NCERT Exemplar Class 12 Chemistry Chapter 10 include diagrams and explanations to make understanding easier.
- These solutions are prepared by subject experts in a very comprehensive manner that helps students build a strong foundation for organic chemistry.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Exemplar Class 12 Solutions
Students can refer to the links given below for the NCERT Exemplar subject-wise solutions for Class 12:
NCERT Solution subject-wise
Refer to the links given below for the NCERT subject-wise solutions:
NCERT Notes Subject-Wise
Do a quick revision by following the NCERT notes. Click on the link given in the table
NCERT Books and the NCERT Syllabus
Follow the links to access the syllabus and books
Frequently Asked Questions (FAQs)
Haloalkanes are organic compounds that contain carbon and halogen atoms in their structure, with halogens replacing one or more hydrogen atoms in an alkane. Haloarenes, on the other hand, are aromatic compounds where a halogen atom replaces a hydrogen atom in an aromatic ring.
Haloalkanes and alcohols differ primarily in their functional groups. Haloalkanes contain a halogen atom attached to a carbon atom, while alcohols contain a hydroxyl group.
Haloalkanes are crucial in organic synthesis as they act as intermediates for producing various other compounds. They can undergo nucleophilic substitution reactions, allowing chemists to synthesize alcohols, amines, and other functional groups by replacing the halogen with other nucleophiles.
Haloalkanes can be prepared through several methods, including:
1. Halogenation of alkanes, where alkanes react with halogens in the presence of UV light.
2. Reaction of alcohols with hydrogen halides, resulting in the substitution of the hydroxyl group with a halogen.
3. Reactions of alkyl halides with metals in the presence of organic solvents
Halogens influence the stability of haloalkanes due to their electronegativity and the strength of the carbon-halogen bond. The greater the bond strength with the halogen, the more stable the haloalkane is against nucleophilic attack.
NCERT Exemplar Solutions Class 12 Chemistry chapter 10 Haloalkanes and Haloarenes provide advanced practice questions with clear solutions to help students understand reactions, mechanisms, and applications of halogen-containing organic compounds.
To prepare Haloalkanes and Haloarenes NCERT Exemplar Class 12 Chemistry Chapter 10 revise core concepts like nomenclature, preparation methods, reaction mechanisms (SN1, SN2), and properties, then practise exemplar questions to strengthen application and problem-solving skills.
NCERT Chemistry Exemplar solutions cover advanced practice questions designed to strengthen concepts and problem-solving skills required for the CBSE Board exams.
Aryl halides are less reactive due to resonance stabilisation and partial double-bond character of the C–X bond.
Haloalkanes are used in solvents, refrigerants, anaesthetics, and as starting materials for synthesis.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters




























































