To prepare well, first study the NCERT textbook thoroughly, then practise all Exemplar questions regularly, focusing on understanding concepts and revising important numerical and application-based problems.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 2 Solutions
We see different types of solutions in our daily lives like sugar dissolved in water, salt dissolved in water, and air which is a homogeneous mixture of gases. Solution is a homogeneous mixture of two or more compounds. Every mixture is not a solution for example, oil dissolved in water is a mixture but not a solution because it is not a homogeneous mixture. In NCERT Exemplar Solutions Class 12 Chemistry Chapter 2 Solutions students are going to study different types of solutions, their properties, concentration terms, why some substances dissolve and others remain undissolved, and why sugar dissolves easily in hot water as compared to cold water.
CBSE has implemented on-screen marking for evaluating Class 12 board exam answer sheets. However, it has clarified that Class 10 answer scripts will continue to be assessed in the traditional physical mode.
Before the start of the formal evaluation process, the first day will be set aside for reviewing the marking scheme, carrying out mock assessments to minimise discrepancies in scoring, and training evaluators to use the digital platform effectively.
Read More: CBSE explains how two Class 10 board exams 2026, on-screen marking for Class 12 will work
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions chapter 2: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: Long Answer Type
- Class 12 Chemistry NCERT Chapter 2: Higher Order Thinking Skills (HOTS) Questions
- Approaches to Solve Questions of Chapter 2
- Topics Covered in NCERT Exemplar Solutions Class 12 Chemistry Chapter 2
- Formulas for Class 12 Chemistry Chapter 2
- Advantages of Using NCERT Exemplar Solutions Class 12 Chemistry Chapter 2 Solutions
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class 12 Solutions
- NCERT Solution subject-wise
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus

NCERT exemplar solutions class 12 Chemistry, designed by subject experts, provide easy-to-understand and comprehensive explanations of every question. NCERT exemplar solutions help students to develop analytical and problem-solving abilities. Solutions Important Questions are given below, and we have also added higher-order thinking skills questions to enhance students ability to solve tricky questions in the board exam. Scroll down to get detailed answers to the given questions. For more practice and concept clarity, students can also refer to NCERT Solutions.
NCERT Exemplar Class 12 Chemistry Solutions chapter 2: MCQ (Type 1)
The MCQ-type questions from Chapter 2 help boost conceptual understanding. Regular practice of Chapter 2 Solutions NCERT Exemplar Problems will strengthen your clarity and deepen your understanding of key concepts
Question 1 Which of the following units is useful in relating the concentration of a solution to its vapour pressure?
(i) mole fraction
(ii) parts per million
(iii) mass percentage
(iv) molality
Answer:
The answer is option (i).
Vapour pressure and the concentration (mole fraction) of solution are related. Raoult’s law states that the vapour pressure $(p_{A})$ of a component is directly proportional to the mole fraction $(x_{A})$ of the component.
$p_{x}\propto x_{A}$
where, $x_{A}=\frac{n_{A}}{n_{A}+n_{B}}$
Question 2 On dissolving sugar in water at room temperature solution feels cool to the touch. Under which of the following cases dissolution of sugar will be most rapid?
(i) Sugar crystals in cold water.
(ii) Sugar crystals in hot water.
(iii) Powdered sugar in cold water.
(iv) Powdered sugar in hot water.
Answer:
The answer is option (iv).
Dissolution is an endothermic process, which is why the solution is cool to the touch. Also, powdered sugars have a higher surface area than sugar crystals, which further promotes dissolution.
Question 3 At equilibrium, the rate of dissolution of a solid solute in a volatile liquid solvent is __________.
(i) less than the rate of crystallisation
(ii) greater than the rate of crystallisation
(iii) equal to the rate of crystallisation
(iv) zero
Answer:
The answer is option (iii).
The rate of dissolution of a solid solute is equal to the rate of crystallisation at equilibrium.
Question 4 A beaker contains a solution of a substance ‘A’. Precipitation of substance ‘A’ takes place when a small amount of ‘A’ is added to the solution. The solution is _________.
(i) saturated
(ii) supersaturated
(iii) unsaturated
(iv) concentrated
Answer:
The answer is option (ii).
Adding a small amount of solute in an already saturated solution will make the solution supersaturated and will lead to the precipitation of the solute.
Question 5 The maximum amount of a solid solute that can be dissolved in a specified amount of a given liquid solvent does not depend upon ____________.
(i) Temperature
(ii) Nature of solute
(iii) Pressure
(iv) Nature of solvent
Answer:
The answer is option (iii).
Solids don’t compress significantly on application of pressure and thus have no impact to their solubility in solvent.
Question 6 Low concentration of oxygen in the blood and tissues of people living at high altitude is due to ____________.
(i) low temperature
(ii) low atmospheric pressure
(iii) high atmospheric pressure
(iv) both low temperature and high atmospheric pressure
Answer:
The answer is option (ii).
Due to lower atmospheric pressure at high altitude, oxygen concentration is lower in the blood and tissues of people living there.
Question 7 Considering the formation, breaking and strength of hydrogen bond, predict which of the following mixtures will show a positive deviation from Raoult’s law?
(i) Methanol and acetone.
(ii) Chloroform and acetone.
(iii) Nitric acid and water.
(iv) Phenol and aniline
Answer:
The answer is option (i).
Methanol has hydrogen bonds. When acetone is mixed with it, some of the bonds break, weakening the methanol-methanol interaction. This is why they exhibit a positive deviation.
Question 8 Colligative properties depend on ____________.
(i) the nature of the solute particles dissolved in solution.
(ii) the number of solute particles in solution.
(iii) the physical properties of the solute particles dissolved in solution.
(iv) the nature of solvent particles
Answer:
The answer is option (ii).
The number of solute particles determines its colligative properties.
Question 9 Which of the following aqueous solutions should have the highest boiling point?
(i) $1.0 M NaOH$
(ii) $1.0 M Na_2SO_4$
(iii) $1.0 M NH_4NO_3$
(iv) $1.0 M KNO_3$
Answer:
The answer is option (ii).
As $1.0 M Na_2SO_4$ furnishes the maximum number of ions, it should have the highest boiling point.
Question 10 The unit of ebulioscopic constant is _______________.
(i) $K kg mol^{-1} or K (molality)^{-1}$
(ii) $mol kg K^{-1} or K^{-1}(molality)$
(iii) $kg mol^{-1} K^{-1} or K^{-1}(molality)^{-1}$
(iv) $K mol kg^{-1} or K (molality)$
Answer:
The answer is option (i).
$K_{b}=\frac{\Delta T_{b}}{m}=\frac{K}{mol kg^{-1}}$
The unit of ebullioscopy constant is $K kg mol^{-1} or K (molality)^{-1}$
Question 11 In comparison to a 0.01 M solution of glucose, the depression in the freezing point of a 0.01 M $MgCl_2$ solution is _____________.
(i) the same
(ii) about twice
(iii) about three times
(iv) about six times
Answer:
The answer is option (iii).
Colligative properties only depend on the number of solutes. While a 0.01 M solution of glucose does not ionize, 0.01 M $ MgCl_2$ solution ionizes to produce a single $Mg^{2+}$ion and $2 Cl^{-}$ ions.
Question 12 An unripe mango was placed in a concentrated salt solution to prepare pickle shrivels because _____________.
(i) it gains water due to osmosis.
(ii) it loses water due to reverse osmosis.
(iii) it gains water due to reverse osmosis.
(iv) it loses water due to osmosis.
Answer:
The answer is option (iv).
Due to osmosis, Water inside the mango (lower concentration) moves outside in a concentrated salt solution (higher concentration).
Question 13 At a given temperature, the osmotic pressure of a concentrated solution of a substance _____________.
(i) is higher than that at a dilute solution.
(ii) is lower than that of a dilute solution.
(iii) is the same as that of a dilute solution.
(iv) cannot be compared with the osmotic pressure of a dilute solution.
Answer:
The answer is option (i).
At a given temperature, the osmotic pressure of a concentrated solution of a substance is higher than that of a dilute solution.
Question 14 Which of the following statements is false?
(i) Two different solutions of sucrose of the same molality prepared in different solvents will have the same depression at the freezing point.
(ii) The osmotic pressure of a solution is given by the equation $\pi = CRT$ (where C is the molarity of the solution).
(iii) Decreasing order of osmotic pressure for 0.01 M aqueous solutions of barium chloride, potassium chloride, acetic acid and sucrose is $BaCl_2> KCl > CH_3COOH > sucrose.$
(iv) According to Raoult’s law, the vapour pressure exerted by a volatile component of a solution is directly proportional to its mole fraction in the solution.
Answer:
The answer is option (i).
Colligative properties depend on the molarity of the solute and the solvent. Two different solvents (with the same molality of sucrose) will have different freezing points
Question 15 The values of Van’t Hoff factors for KCl, NaCl and $K_2SO_4$, respectively, are
_____________.
(i) 2, 2 and 2
(ii) 2, 2 and 3
(iii) 1, 1 and 2
(iv) 1, 1 and 1
Answer:
The answer is option (ii).
While KCl and NaCl ionise to give 2 ions,$K_2SO_4$ gives 3 ions. So, van’t Hoff factors for KCl, NaCl and $K_2SO_4$ are 2,2 and 3, respectively
Question 16 Which of the following statements is false?
(i) Units of atmospheric pressure and osmotic pressure are the same.
(ii) In reverse osmosis, solvent molecules move through a semipermeable membrane from a region of lower concentration of solute to a region of higher concentration.
(iii) The value of the molal depression constant depends on the nature of the solvent.
(iv) Relative lowering of vapour pressure is a dimensionless quantity.
Answer:
The answer is option (ii).
Solvent molecules move from a solution containing a high concentration of solute to one with a low concentration of solute.
Question 17 Value of Henry’s constant $K_H$. ____________.
(i) increases with an increase in temperature.
(ii) decreases with an increase in temperature.
(iii) remains constant.
(iv) first increases, then decreases.
Answer:
The answer is option (i).
There is an increase in the value of Henry’s constant with an increase in temperature.
Question 18 The value of Henry’s constant $K_H$. is _____________.
(i) greater for gases with higher solubility.
(ii) greater for gases with lower solubility.
(iii) constant for all gases.
(iv) not related to the solubility of gases.
Answer:
The answer is option (ii).
Gases having a lower solubility have a higher $K_H$.
Question 19 Consider the Figure and mark the correct option.
(i) Water will move from the side (A) to side (B) if a pressure lower than the osmotic pressure is applied on piston (B).
(ii) Water will move from the side (B) to the side (A) if a pressure greater than the osmotic pressure is applied on the piston (B).
(iii) Water will move from side (B) to side (A) if a pressure equal to osmotic pressure is applied on piston (B).
(iv) Water will move from side (A) to side (B) if pressure equal to osmotic pressure is applied on the piston (A).
Answer:
The answer is option (ii).
If the piston above B applies a pressure exceeding the osmotic pressure, water will move from the concentrated solution (side B) to the Fresh Water (side A).
Question 20 We have three aqueous solutions of NaCl labelled as ‘A’, ‘B’ and ‘C’ with concentrations 0.1M, 0.01M and 0.001M, respectively. The value of Van’t Hoff factor for these solutions will be in the order______.
(i) $i_A<i_B<i_C$
(ii) $i_A>i_B>i_C$
(iii) $i_A=i_B=i_C$
(iv) $i_A<i_B>i_C$
Answer:
The answer is option (iii).
Due to complete dissociation of NaCl, the van’t Hoff factor will be the same for the three solutions.
Question 21 On the basis of the information given below, mark the correct option.
Information:
(A) In the bromoethane and chloroethane mixture, intermolecular interactions of A–A and B–B type are nearly the same as A–B type interactions.
(B) In ethanol and acetone mixture A–A or B–B type intermolecular interactions are stronger than A–B type interactions.
(C) In a chloroform and acetone mixture A–A or B–B type intermolecular interactions are weaker than A–B type interactions.
(i) Solution (B) and (C) will follow Raoult’s law.
(ii) Solution (A) will follow Raoult’s law.
(iii) Solution (B) will show a negative deviation from Raoult’s law.
(iv) Solution (C) will show a positive deviation from Raoult’s law
Answer:
The answer is the option (ii).
In a Bromoethane (A) and Chloroethane (B) mixture, the interactions A-A, B-B and A-B are nearly equal, so the solution will be nearly ideal and thus follow Raoult’s law.
Question 22 Two beakers of capacity 500 mL were taken. One of these beakers, labelled as “A”, was filled with 400 mL water, whereas the beaker labelled “B” was filled with 400 mL of 2 M solution of NaCl. At the same temperature, both the beakers were placed in closed containers of the same material and the same capacity as shown in the Figure.
At a given temperature, which of the following statements is correct about the vapour pressure of pure water and that of NaCl solution?
(i) The vapour pressure in container (A) is more than that in container (B).
(ii) The vapour pressure in container (A) is less than that in container (B).
(iii) The vapour pressure is equal in both containers.
(iv) The vapour pressure in container (B) is twice the vapour pressure in container (A).
Answer:
The answer is the option (i).
Vapour pressure of container (B) is lower as NaCl, being a non-volatile solute, reduces the vapour pressure.
Question 23 If two liquids A and B form a minimum boiling azeotrope at some specific composition then _______________.
(i) A–B interactions are stronger than those between A–A or B–B.
(ii) The vapour pressure of the solution increases because more molecules of liquids A and B can escape from the solution.
(iii) Vapour pressure of the solution decreases because less number of molecules of only one of the liquids escape from the solution.
(iv) A–B interactions are weaker than those between A–A or B–B.
Answer:
The answer is the option (i).
(i) A–B interactions are stronger than those between A–A or B–B.
Question 24 4L of 0.02 M aqueous solution of NaCl was diluted by adding one litre of water. The molality of the resultant solution is _____________.
(i) 0.004
(ii) 0.008
(iii) 0.012
(iv) 0.016
Answer:
The answer is the option (iv).
$M=\frac{n}{V}$
$0.02=\frac{n}{4}$
$n=0.08$
$M=\frac{n}{(\text { Mass of water in } \mathrm{Kg})}$
$=\frac{0.08}{5}$
$=0.016$
Question 25 On the basis of the information given below, mark the correct option.
Information: On adding acetone to methanol, some of the hydrogen bonds between methanol molecules break.
(i) At a specific composition, the methanol-acetone mixture will form a minimum boiling azeotrope and will show positive deviation from Raoult’s law.
(ii) At a specific composition, the methanol-acetone mixture forms maximum boiling azeotrope and will show positive deviation from Raoult’s law.
(iii) At a specific composition, the methanol-acetone mixture will form a minimum boiling azeotrope and will show negative deviation from Raoult’s law.
(iv) At a specific composition, the methanol-acetone mixture will form a maximum boiling azeotrope and will show negative deviation from Raoult’s law.
Answer:
The answer is option (ii).
At a specific composition methanol-acetone mixture forms maximum boiling azeotrope and will show positive deviation from Raoult’s law.
Question 26 KH value for Ar(g), CO2(g), HCHO (g) and CH4(g) are 40.39, 1.67, $1.83\times 10^{-5}$ and 0.413 respectively.
Arrange these gases in the order of their increasing solubility.
(i) $HCHO < CH_4< CO_2 < Ar$
(ii) $HCHO < CO_2< CH_4< Ar$
(iii) $Ar < CO_2< CH_4< HCHO$
(iv) $Ar < CH_4 < CO_2 < HCHO$
Answer:
The answer is option (iii).
Solubility decreases with increasing value of KH. The increasing order of solubility of the gases is as follows: $Ar < CO_2< CH_4< HCHO$
NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: MCQ (Type 2)
Class 12 Chemistry NCERT Exemplar Chapter 2 MCQs are provided here with clear and simple explanations.
Question 27 Which of the following factor(s) affect the solubility of a gaseous solute in the fixed volume of liquid solvent?
(a) nature of solute (b) temperature (c) pressure
(i) (a) and (c) at constant T
(ii) (a) and (b) at constant P
(iii) (b) and (c) only
(iv) (c) only
Answer:
The answer is the option (i, ii).
solubility of a gaseous solute depends on the nature of the solute, pressure and temperature.
Question 28 Intermolecular forces between two benzene molecules are near to same strength as those between two toluene molecules. For a mixture of benzene and toluene, which of the following is not true?
(i) $\Delta _{mix} H = zero$
(ii) $\Delta _{mix} V = zero$
(iii) These will form a minimum boiling azeotrope.
(iv) These will not form the ideal solution.
Answer:
The answer is option (iii, iv).
As interactions in a Benzene and Toluene solution are nearly identical, they will form an ideal solution.
$\Delta _{mix} H = 0$, $\Delta _{mix} V =0$
Question 29 Relative lowering of vapour pressure is a colligative property because _____________.
(i) It depends on the concentration of a non-electrolyte solute in a solution and does not depend on the nature of the solute molecules.
(ii) It depends on a number of particles of electrolyte solute in a solution and does not depend on the nature of the solute particles.
(iii) It depends on the concentration of a non-electrolyte solute in solution as well as on the nature of the solute molecules.
(iv) It depends on the concentration of an electrolyte or non-electrolyte solute in solution as well as on the nature of solute molecules.
Answer:
The answer is option (i, ii).
Nature of solute particles is not a factor in determining the relative lowering of vapour pressure. It is only affected by the number of solute particles (non-electrolyte and electrolyte solutes)
Question 30 Van’t Hoff factor i is given by the expression _____________.
(i) i = Normal molar mass/Abnormal molar mass
(ii) i = Abnormal molar mass/ Normal molar mass
(iii) i= Observed colligative property/calculated colligative property
(iv) i = Calculated colligative property/ Observed colligative property
Answer:
The answer is option (i,iii).
$i=\frac{\text{Normal molar mass}}{\text{Abnormal molar mass}}\; or\; i=\frac{\text{Observed colligative property}}{\text{Calculated colligative property}}$
Question 31 Isotonic solutions must have the same _____________.
(i) solute
(ii) density
(iii) elevation in boiling point
(iv) depression at the freezing point
Answer:
The answer is option (ii, iii).
Isotonic solutions must have the same
(ii) density
(iii) elevation in boiling point
Question 32 Which of the following binary mixtures will have the same composition in the liquid and vapour phases?
(i) Benzene – Toluene
(ii) Water-Nitric acid
(iii) Water-Ethanol
(iv) n-Hexane – n-Heptane
Answer:
The answer is option (ii, iii).
Water-nitric acid and Water-Ethanol solutions have the same composition in liquid and vapour phases
Question 33 In isotonic solutions ________________.
(i) The solute and the solvent are both the same.
(ii) Osmotic pressure is the same.
(iii) Solute and solvent may or may not be the same.
(iv) A solute is always the same solvent may be different.
Answer:
The answer is option (ii, iii).
Osmotic pressure is the same for isotonic solutions.
Question 34 For a binary ideal liquid solution, the variation in total vapour pressure versus the composition of the solution is given by which of the curves?
Answer:
The answer is option (i, iv).
For the ideal solution.
$p_1 \propto x_1$ and $p_2 \propto y_2$
Question 35 Colligative properties are observed when _____________.
(i) A non-volatile solid is dissolved in a volatile liquid.
(ii) A non-volatile liquid is dissolved in another volatile liquid.
(iii) A gas is dissolved in a non-volatile liquid.
(iv) A volatile liquid is dissolved in another volatile liquid.
Answer:
The answer is option (i, ii).
Colligative properties can be observed on the dissolution of a non-volatile solid or liquid in a volatile liquid
NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: Short Answer Type
Class 12 chemistry chapter 2 questions also include short answer type questions for thorough practice. This chapter covers key concepts from solutions that are frequently asked in examinations
As the composition of both the liquid and vapour phase is same and both components are coming in the distillate, this implies that they have formed an azeotropic mixture.
The boiling point of water increases on the addition of NaCl, as it is a non-volatile solute and, hence, reduces the vapour pressure of water and increases the boiling point. Unlike NaCl, Methyl alcohol is more volatile than water and thus increases the vapour pressure of water and reduces the boiling point.
Question 38 Explain the solubility rule “like dissolves like” in terms of intermolecular forces that exist in solutions.
Answer:
“Like dissolves like” means that a solute dissolves in a solvent if its intermolecular interactions are similar. In other words, we can say that polar solutes can dissolve in polar solvents, and non-polar solutes can dissolve in non-polar solvents.
Question 39 Concentration terms such as mass percentage, ppm, mole fraction and molality are independent of temperature, however, molarity is a function of temperature. Explain.
Answer:
$\text{Molarity}=\frac{\text{number of moles of solute}}{\text{Volume of solution in litres} }$
Since molarity is dependent on the volume of the solution, it changes with temperature. The other concentration terms, like mass percentage, ppm, mole fraction, and morality, involve moles or mass of components, which are independent of temperature.
Question 40 What is the significance of Henry’s Law constant KH?
Answer:
According to Henry’s law:
$p=K_H\times x$
$K_H=\frac{p}{x}$
The solubility of a gas in a liquid decreases with increasing Henry’s law constant.
Question 41 Why are aquatic species more comfortable in cold water in comparison to warm water?
Answer:
For a given pressure, as the temperature decreases, the solubility of oxygen in water increases. This means that cold sea water has a higher concentration of oxygen, and aquatic species thrive more in cold water.
(a) (i). Deep-sea divers require compressed air for breathing underwater, which has both $N_2$ and $O_2$. In atmospheric conditions, $N_2$ isn’t soluble in blood, but at higher pressure (as you go deeper into the ocean, the pressure increases) it starts dissolving. When the diver comes back up, the pressure decreases and $N_2$ comes out of the body, leaving behind bubbles in the bloodstream, which restrict the flow of blood affecting nerve impulses. In worse cases, these bubbles can burst the capillaries and not let $O_2$ reach the tissues. This condition, known as bends, is very painful and can even be life-threatening.
(ii). In lower pressure (at higher altitudes), $O_2$ exhibits a lower partial pressure, resulting in lower oxygen concentration in blood and tissues. This condition is known as anoxia and leads to climbers becoming weak and losing the ability to think clearly.
(b) $CO_2$ isn’t very soluble in soft drinks at atmospheric pressure. So, to dissolve $CO_2$ in soft drinks, soda bottles are sealed under high pressure. However, when the bottle is opened, the pressure decreases suddenly and excess $CO_2$ fizzes out.
Question 43 Why is the vapour pressure of an aqueous solution of glucose lower than that of water?
Answer:
In pure liquid water, molecules of water cover the entire surface. When a non-volatile solute is dissolved in water, it replaces part of the surface water molecules. As a result, the number of solvent molecules which can escape also gets reduced, and thus, the vapour pressure of the solution reduces.
Question 44 How does sprinkling of salt help in clearing snow-covered roads in hilly areas? Explain the phenomenon involved in the process.
Answer:
Salt is spread over snow-covered roads to lower the freezing point enough to melt the ice (snow). This leads to the snow melting away and clearing the road.
Question 45 What is a “semi-permeable membrane”?
Answer:
Semipermeable membranes are membranes which only permit the flow of solvent molecules, but not the solute molecules. Only the solvent molecules move across the semipermeable membrane during osmosis and Reverse osmosis.
Question 46 Give an example of a material used for making a semipermeable membrane for carrying out reverse osmosis.
Answer:
For carrying out reverse osmosis, cellulose acetate, potassium ferrocyanide, etc. are used as semipermeable membranes.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: Matching Type
This section of NCERT Exemplar Class 12 Chemistry Chapter 2 covers matching-type questions designed to test your understanding of key concepts in solutions. Practising Important Questions on Solutions will help reinforce important relationships and definitions.
Question 47 Match the items given in Column I and Column II.
|
Column I |
Column II |
|
(i) Saturated solution |
(a) Solution having the same osmotic pressure at a given temperature as that of the given solution |
|
(ii) Binary solution |
(b) A solution whose osmotic pressure is less than that of another. |
|
(iii) Isotonic solution |
(c) Solution with two components. |
|
(iv)Hypotonic solution |
(d) A solution which contains the maximum amount of solute that can be dissolved in a given amount of solvent at a given temperature. |
|
(v) Solid solution |
(e) A solution whose osmotic pressure is greater than another. |
|
(vi) Hypertonic solution |
(f) A solution in the solid phase |
Answer:
(i $\longrightarrow$ d), (ii $\longrightarrow$ c); (iii$\longrightarrow$ a); (iv $\longrightarrow$ b), (v $\longrightarrow$ f); (vi $\longrightarrow$ e)
i.Saturated solution: It is a solution where no more of the solute can be dissolved at the given temperature and pressure.
ii. Binary solution: It is a solution with two components.
iii. Isotonic solution: It is a solution having the same osmotic pressure at a given temperature as that of a given solution.
iv. Hypotonic solution: It is a solution whose osmotic pressure is less than that of a given solution.
v. Solid solution: It is a solution in a solid phase.
vi. Hypertonic solution: It is a solution whose osmotic pressure is greater than that of a given solution
Question 48 Match the items given in Column I with the type of solutions given in Column II.
|
Column I |
Column II |
|
(i) Soda water |
(a) A solution of the gas in a solid |
|
(ii) Sugar solution |
(b) A solution of the gas in gas |
|
(iii) German silver |
(c) A solution of a solid in a liquid |
|
(iv) Air |
(d) A solution of solid in solid |
|
(v) Hydrogen gas in palladium |
(e) A solution of the gas in liquid |
|
|
(f) A solution of a liquid in a solid |
Answer:
(i → e), (ii → c), (iii → d); (iv → b), (v → a)
i. Soda water: A solution of gas in $(CO_2)$ liquid(soft drink)
ii. Sugar solution: A solution of solid (sugar) in liquid (water)
iii. German silver: It is an alloy of Cu, Zn and Ni and a solid solution of solid in solid.
iv. Air: A solution of gas. Air is a mixture of various gases.
v. Hydrogen gas in palladium: Hydrogen gas in palladium is used as a reducing agent and is an example of a solution of gas in a solid.
Question 49 Match the laws given in Column I with expressions given in Column II.
|
Column I |
Column II |
|
(i) Raoult’s law |
(a)$\Delta T_f= K_fm$ |
|
(ii) Henry’s law |
(b) $\pi = CRT$ |
|
(iii) Elevation of boiling point |
(c) $p = x_{1}P_1^0+ x_{2}P_2^0$ |
|
(iv) Depression in freezing point |
(d) $\Delta T_b= K_bm$ |
|
(v) Osmotic pressure |
(e) $p = K_H.x$ |
Answer:
(i) → (c) (ii) → (e) (iii) → (d) (iv) → (a) (v) → (b)
i.Raoult’s law: $p = x_{1}P_1^0+ x_{2}P_2^0$
ii. Henry’s law: $p = K_H.x$
iii. Elevation of the boiling point: $\Delta T_b= K_bm$
iv. Depression in freezing point: $\Delta T_f= K_fm$
v. Osmotic pressure: $\pi = CRT$
Question 50 Match the terms given in Column I with expressions given in Column II.
|
Column I |
Column II |
|
(i) Mass percentage |
(a) $\frac{Number \;of\; moles\; of\; the\; solute\; component}{Volume\; of \;solution \;in \; litres}$ |
|
(ii) Volume percentage |
(b) |
|
(iii) Mole fraction |
(c) $\frac{Volume\; of\; the\; solute\; component\; in \;solution}{Total\; volume \;of solution\;}\times 100$ |
|
(iv) Molality |
(d) $\frac{Mass\; of\; the\; solute\; component\; in \;solution}{Total \;mass \;of \;the\; solution}\times 100$ |
|
(v) Molarity |
(e) $\frac{Number\; of \;moles \;of\; the\; solute\; components}{Mass\; of \;solvent \;in\; kilograms}\times 100$ |
Answer:
(i → d), (ii → c), (iii →b), (iv → e), (v →a)
NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: Assertion and Reason Type
Assertion and Reason Type questions of Class 12 NCERT Exemplar Solutions Chapter 2 aims to deepen your understanding of core concepts
The answer is option (a).
Molarity depends on volume, which changes with temperature. Hence, molarity also changes with a change in temperature.
The answer is option (d).
Addition of a volatile solute to a volatile solvent increases vapour pressure and decreases boiling point. Methyl alcohol and water interaction are an example of the same.
The answer is option (a).
Addition of a non-volatile solute to a volatile solvent decreases vapour pressure and increases boiling point.
The answer is option (b).
Assertion and reason both are correct statements, but the reason is not the correct explanation for the Assertion.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 2: Long Answer Type
Solutions NCERT Exemplar Problems features long-answer type questions, focusing on essential topics from Solutions. These questions are often asked in exams to assess detailed understanding and explanation skills.
-
$\text{w/w(Mass percentage)}=\frac{\text{Mass of component in the solution}}{\text{Total mass of the solution}}\times 100$
-
$\text{V/V(Volume percentage)}=\frac{\text{Volume of component in the solution}}{\text{Total volume of the solution}}\times 100$
-
$\text{w/V (mass by Volume percentage)}=\frac{\text{Mass of the solute}}{\text{Total volume of the solution}}\times 100$
-
$\text{ppm (parts per million)}=\frac{\text{Number of parts of component}}{\text{Total number of parts of all components}}\times 10^{6}$
-
$\text{x( Mole fraction)}=\frac{\text{Number of moles of component}}{\text{Total number of moles of all components}}$
-
$\text{M (Molarity)}=\frac{\text{Moles of solute}}{\text{Volume of the solution in litre}}$
-
$\text{m (Molality)}=\frac{\text{Moles of solute}}{\text{Mass of solvent in kg}}$
Mass percentage, ppm, mole fraction and molality are only dependent on mass or number of moles making them independent of temperature changes.
$p=p_A^ox_A+p_B^ox_B$
Where, $p_A^o,p_B^o$ are the vapour pressure of pure components (A) and (B)
And $x_{A}\text{and }x_{B}$ are the mole fractions of the components in the solution.
(b) On dissolving in water, the vapour pressure lowers. The vapour pressure of the solution is:
$p=p^ox_A$
Where $x_{A}$=mole fraction of solvent
$p^{0}$=vapour pressure of pure solvent
p=vapour pressure of the solution
Similarly,
$\frac{\Delta p}{p^o}=x_B$
$\Delta p=p^o-p$
$x_{B}$=mole fraction of solute
Question 57 Explain the terms ideal and non-ideal solutions in light of forces of interactions operating between molecules in liquid solutions.
Answer:
Ideal solutions: They obey Raoult’s law irrespective of the concentration. For an ideal solution,
$\Delta _{mix}H=0, \Delta _{mix}V=0$
$\text{A-B interactions}\approx \text{A-A interactions and B-B interactions}$
Non-ideal solutions: They don’t follow Raoult’s law over the entire range of concentration
Positive deviations: Vapour pressure is higher than the calculated values
$\Delta _{mix}H=+ve, \Delta _{mix}V=+ve$
A-B interactions < A-A interactions and B-B interactions
Negative deviations: Vapour pressure is lower than the calculated values
$\Delta _{mix}H=-ve, \Delta _{mix}V=-ve$
A-B interactions>A-A interactions and B-B interactions
Azeotropes are solutions which have the same composition of components in both the liquid and vapour phases and boil at a constant temperature. The components cannot be separated by fractional distillation as they have the same boiling point. There are two types of azeotropes
-
Minimum boiling azeotropes: They show a large positive deviation from Raoult’s law (A-B interactions are weaker than A-A and B-B interactions), e.g., an ethanol-water mixture
-
Maximum boiling azeotropes: They show large negative deviation from Raoult’s law (A-B interactions are stronger than A-A and B-B interactions), e.g., the solution having composition 68% $HNO_{3}$ and 32% water by mass.
Question 59 When kept in water, the raisin swells in size. Name and explain the phenomenon involved with the help of a diagram. Give three applications of the phenomenon.
Answer:
Raisins swell in water due to osmosis. Water moves from a place of lower concentration (Water holder) to a higher concentration (Raisin) through the skin of raisin, which acts as a semipermeable membrane.
Applications of the phenomenon
-
Water moves from the soil to the plant roots partly due to osmosis.
-
Preservation of meat against bacterial action by adding salt.
-
Preservation of fruits against bacterial action by adding sugar. Bacterium in canned fruit loses water through the process of osmosis, shrivel, and die.
-
Reverse osmosis is used for the desalination of water.
Question 60 Discuss the biological and industrial importance of osmosis.
Answer:
Some of the biological and industrial importance of osmosis are as follows-
-
Osmosis is responsible for the circulation of water to all the body parts of animals
-
Water moves from soil to plant roots partly due to osmosis. The concentration of cell sap inside the root hair cells is higher compared to that of water present in the soil.
-
Water circulation inside the plant body from root to treetop is also because of osmosis.
-
Osmosis helps in the growth of plants and the germination of seeds.
-
Due to endosmosis, Red Blood Cells burst when they are placed in water.
-
Osmosis controls various functions of plants, e.g., the stretching of leaves and flowers, the opening, and the closing of flowers.
-
Salt and sugar in pickles prevent the growth of bacteria and fungi by osmosis and thus act as preservatives.
-
Endosmosis is responsible for the swelling of dead bodies under water.
-
Dried fruits and vegetables swell and return to their original form when placed in water. It is also due to the osmosis of water.
-
Edema: Tissues become puffy when a person ingests an excess amount of salt.
This can be achieved as under:
-
Take a mineral acid solution and put an egg inside it, and leave it for 2 hours. Most of the outer shell will dissolve. Remove any remaining parts with your fingers.
-
Take a saturated solution (hypertonic) and place the egg in it for 3 hours. The egg’s size reduces as the egg shrivels due to osmosis.
-
Place the egg in a bottle with a narrow neck and fill water (hypotonic) it. The egg will regain its shape.
Question 62 Why is the molar mass determined by measuring a colligative property in case some solutes are abnormal? Discuss it with the help of the Van’t Hoff factor.
Answer:
Abnormal molecular masses are shown by the compounds which dissociate/associate in the solvent.
-
Association: Colligative properties depend on the number of particles in a solution. Certain compounds like benzoic acid or ethanoic acid dimerise in benzene due to hydrogen bonding, resulting in the reduction of the number of particles and thus, solutes show lower colligative properties.
-
Dissociation: Similarly, certain compounds like electrolytes (NaCl, KCl), etc dissociate into ions, increasing the number of particles, and thus, show a higher value of colligative property.
Van’t Hoff introduced a factor to account for association or dissociation, known as the Van’t Hoff factor.
$i=\frac{\text{Expected molar mass}}{\text{Abnormal molar mass}}$
$=\frac{\text{Observed colligative property}}{\text{Calculated colligative property}}$
$=\frac{\text{Total number of moles of particles after association or disaasociation}}{\text{Total number of moles of particles before association or disaccioation}}$
Class 12 Chemistry NCERT Chapter 2: Higher Order Thinking Skills (HOTS) Questions
Below are some Important Questions on Solutions that will help students tackle complex problems with ease. To understand the concepts better, students can also refer to Class 12 Solutions notes.
Question 1: $\mathrm{HA}(\mathrm{aq}) \rightleftharpoons \mathrm{H}^{+}(\mathrm{aq})+\mathrm{A}^{-}(\mathrm{aq})$
The freezing point depression of a 0.1 m aqueous solution of a monobasic weak acid HA is $0.20^{\circ} \mathrm{C}$. The dissociation constant for the acid is
Given :
$\mathrm{K}_{\mathrm{f}}\left(\mathrm{H}_2 \mathrm{O}\right)=1.8 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$, molality $\equiv$ molarity
(1) $1.38 \times 10^{-3}$
(2) $1.1 \times 10^{-2}$
(3) $1.90 \times 10^{-3}$
(4) $1.89 \times 10^{-1}$
Answer:
$\begin{aligned} & \Delta \mathrm{T}_{\mathrm{f}}=\mathrm{i} \mathrm{K}_{\mathrm{f}} \mathrm{m} \\ & \mathrm{i}=\frac{\Delta \mathrm{T}_f}{\mathrm{~K}_{\mathrm{f}} \cdot \mathrm{m}} \\ & \mathrm{i}=\frac{0.20}{1.8 \times 0.1}=1.11 \\ & \mathrm{i}=1.11 \\ & \alpha=\frac{i-1}{\mathrm{n}-1}(\text { for } \mathrm{HA}, \mathrm{n}=2) \\ & \alpha=\frac{1.11-1}{1}=0.11 \\ & \mathrm{~K}_{\mathrm{a}}=\frac{c \alpha^2}{1-\alpha}=\frac{0.1 \times(0.11)^2}{1-0.11}=1.38 \times 10^{-3}\end{aligned}$
Hence, the correct answer is option (1).
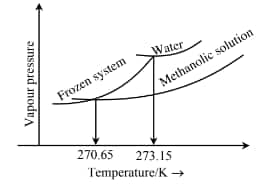
Question 2: When ' $x$ ' $\times 10^{-2} \mathrm{~mL}$ methanol (molar mass $=32 \mathrm{~g}$; density $=0.792 \mathrm{~g} / \mathrm{cm}^3$ ) is added to $100 \mathrm{~mL}$ water (density $=1 \mathrm{~g} / \mathrm{cm}^3$ ), the following diagram is obtained.
$x=\ldots \ldots . . . . . . . . .$. (nearest integer)
[Given: Molal freezing point depression constant of water at $273.15 \mathrm{~K}^2$ is $1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$ ]
i) 543
ii) 559
iii)550
iv)540
Answer:
$\begin{aligned} & \Delta \mathrm{T}_{\mathrm{f}}=273.15-270.65=2.5 \mathrm{~K} \\ & \Delta \mathrm{T}_{\mathrm{f}}=\mathrm{K}_{\mathrm{f}} \mathrm{m} \Rightarrow 2.5=1.86 \times \frac{\mathrm{n}}{0.1} \\ & \Rightarrow \mathrm{n}=0.1344 \text { moles } \\ & \Rightarrow \mathrm{w}=0.1344 \times 32=4.3 \mathrm{~g} \\ & \text { Volume }=\frac{4.3}{0.792}=5.43 \mathrm{ml}=543 \times 10^{-2} \mathrm{ml}\end{aligned}$
Hence, the correct answer is option (1).
Question 3: Given below are two statements :
Statement (I) : Molal depression constant $\mathrm{K}_{\mathrm{f}}$ is given by $\frac{M_1 R T_f}{\Delta S_{f u s}}$, where symbols have their usual meaning.
Statement (II) : $\mathrm{K}_{\mathrm{f}}$ for benzene is less than the $\mathrm{K}_f$ for water.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Statement I is incorrect but Statement II is correct
(2) Both Statement I and Statement II are incorrect
(3) Both Statement I and Statement II are correct
(4) Statement I is correct but Statement II is incorrect
Answer:
Statement-I
Molar depression constant $\mathrm{k}_f=\frac{\mathrm{M}_{\mathrm{i}} \mathrm{RT}_{\mathrm{f}}^2}{\Delta \mathrm{H}_{\text {fius }}}$
$\begin{aligned}
& \mathrm{k}_f=\frac{\mathrm{M}_1 \mathrm{RT}_{\mathrm{f}}}{\left[\frac{\Delta \mathrm{H}_{\text {fis }}}{\mathrm{T}_{\mathrm{f}}}\right]} \\
& \mathrm{k}_f=\frac{\mathrm{M}_1 \mathrm{RT}_f}{\Delta \mathrm{~S}_{\text {fus }}}
\end{aligned}$
Hence statement-I is correct
but $\mathrm{k}_{\mathrm{f}}$ for benzene $=5.12 \frac{{ }^{\circ} \mathrm{C}}{\text { molal }}$
$\mathrm{k}_{\mathrm{f}}$ for water $=1.86 \frac{{ }^{\circ} \mathrm{C}}{\text { molal }}$ Hence statement- II is incorrect
Hence, the correct answer is option (4).
Question 4: The vapour pressure of an aqueous solution is found to be 750 torr at a temperature $T$, and the same solution shows an elevation in boiling point equal to 1.04 K . If $T$ is the boiling point of pure water, then the atmospheric pressure should be ( $K_b$ of water $=0.52 \mathrm{Kkg} / \mathrm{mol}$ )
Answer:
$\Delta \mathrm{T}_{\mathrm{b}}=\mathrm{K}_{\mathrm{b}} \mathrm{~m} \Rightarrow \mathrm{~m}=1.04 \mathrm{~K} / 0.52 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}=2 \mathrm{~mol} \mathrm{~kg}^{-1}$
For $1 \mathrm{~kg} \mathrm{H}_2 \mathrm{O}$ :
$\mathrm{n}_{\text {solvent }}=1000 \mathrm{~g} / 18 \mathrm{~g} \mathrm{~mol}^{-1}=55.56 \mathrm{~mol}$
$\mathrm{n}_{\text {solute }}=2 \mathrm{~mol}$
Mole-fraction of solvent
$X_w=\frac{55.56}{55.56+2}=0.965$
Raoult: $P_{\text {solution }}=X_w P^{\circ}$
Given $P_{\text {solution }}=750$ torr,
$P^{\circ}=\frac{750}{0.965} \approx 7.77 \times 10^2 \text { torr. }$
Atmospheric pressure $=P^{\circ} \approx 777$ torr.
Hence, the answer is 777 torr.
Question 5: Choose the correct observation

(1) Vapour pressure of solution $I$ is lowest
(2) Relative lowering of vapour pressure is maximum in III
(3) Freezing point is maximum for III
(4) Boiling point is minimum for II
Answer:
$\begin{array}{ccc}
\text { (I) } & \text { (II) } & \text { (III) } \\
\text { Urea } & \mathrm{NaCl} & \mathrm{CaCl}_2 \\
c=0.1 M & 0.1 M & 0.1 M \\
i=1 & i=2 & i=3
\end{array}$
1. Vap. pressure $\propto \frac{1}{i}$
$\begin{gathered}
\therefore \quad V \cdot P \quad I>I I>I I I \\
\frac{\Delta p}{p_0} \propto i \\
\text { 2. } \quad \therefore \frac{\Delta p}{p_0} \quad I I I>I I>I \\
\text { 3. } \Delta T_f=i k_f \cdot m . \\
\Delta T_f \propto i \\
T_f^0-T_f \propto i \\
\therefore I I I<I I<I
\end{gathered}$
4.$\begin{aligned}
& \Delta T_b=i K_b \cdot m \\
\end{aligned}$
$T_b-T_b^0 \propto i \quad \therefore I I I>I I>I$
Hence, the correct answer is option (2).
Approaches to Solve Questions of Chapter 2
To solve Solutions question effectively, students should focus on understanding the core concepts, formulas, and their applications. NCERT Exemplar Class 12 Solutions Chapter 2 questions will strengthen problem-solving skills.
1. The solve questions of this chapter first step is to identify the type of solution, whether the solution is solid in liquid, liquid in liquid or gas in liquid.
2. The next step is to understand the basic terms frequently used in almost every question, like solute, solvent and concentration.
3. Concentration terms like molarity, molality, mass per cent, mole per cent, parts per million are often asked in exams, and the questions are interrelated with these concentration terms. So, proper knowledge of these concentration terms is a must.
4. Then we must have the knowledge of topics like:
- Solubility
- Ideal solution
- Factors Affecting Solubility like the identification of the effect of Temperature and Pressure.
5. Students must be aware of the Colligative properties, like:
- Relative lowering of vapour pressure
- Elevation of Boiling Point
- Depression of Freezing point
6. A Proper understanding of basic concepts and practice helps students clear their doubts and solve questions effectively for NCERT Exemplar Class 12 Chemistry Solutions Chapter 2 Solutions. While finalising answer, do not forget to verify whether units are correct and also check for significant figures.
Topics Covered in NCERT Exemplar Solutions Class 12 Chemistry Chapter 2
Students must understand some important topics given below to solve NCERT Exemplar Problems. To understand them better and get more practice, check out the NCERT Class 12 Chemistry Chapter 2 Solutions.
- Types Of Solution
- Expression Of Concentration Of Solution
- Solubility
- Solubility of a Solid in a Liquid
- Solubility of a Gas in a Liquid
- Vapour Pressure of Liquid Solutions
- Vapour Pressure of Liquid-Liquid Solutions
- Raoult’s Law as a special case of Henry’s Law
- Vapour Pressure of Solutions of Solids in Liquids
- Ideal and Non- ideal Solutions
- Ideal Solution
- Non-ideal Solutions
- Colligative Properties and Determination of Molar Mass
- Relative Lowering of Vapour Pressure
- Elevation In Boiling Point
- Depression In Freezing Point
- Osmosis and Osmotic Pressure
- Reverse Osmosis and Water Purification
- Abnormal Molar Masses
Formulas for Class 12 Chemistry Chapter 2
Some important formulas of chapter 2 questions are given below.
1. Mass percentage (w/w)
Mass percent $=\frac{\text { Mass of the component in the solution }}{\text { Total mass of the solution }} \times 100$
2. Volume percentage (V/V):
Volume $\%$ of a component $=\frac{\text { Volume of the component }}{\text { Total volume of solution }} \times 100$
3. Mass by volume percentage (w/V):
Mass by Volume $\%(\mathrm{w} / \mathrm{V})=\left(\frac{\text { Mass of solute }(\mathrm{g})}{\text { Volume of solution }(\mathrm{mL})}\right) \times 100$
4. Parts per million:
$\begin{aligned} & \text { Parts per million }= \\ & \frac{\text { Number of parts of the component }}{\text { Total number of parts of all components of the solution }} \times 10^6\end{aligned}$
5. Mole fraction:
Mole Fraction =$\frac{\text { Number of moles of the component }}{\text { Total number of moles of all the components }}$
For example, in a binary mixture, if the number of moles of $A$ and $B$ are $n_{\mathrm{A}}$ and $n_{\mathrm{B}}$ respectively, the mole fraction of A will be
$x_{\mathrm{A}}=\frac{n_{\mathrm{A}}}{n_{\mathrm{A}}+n_{\mathrm{B}}}$
For a solution containing i number of components, we have:
$
x_i=\frac{n_i}{n_1+n_2+\ldots \ldots+n_i}=\frac{n_i}{\sum n_i}
$
6. Molarity:
Molarity $=\frac{\text { Moles of solute }}{\text { Volume of solution in litre }}$
7. Molality:
Molality $(\mathrm{m})=\frac{\text { Moles of solute }}{\text { Mass of solvent in } \mathrm{kg}}$
8. Raoult's law
$P_{\text {solution }}=($ Mole fraction of solvent $) \times($ Vapour pressure of pure solvent $)$
9. Dalton's law of partial pressures
$P_{\text {total }}=P_1+P_2+P_3+\ldots$
10. Relative lowering of vapour pressure :
By Raoult's law,
$\frac{P^0-P}{P^0}=X_{\text {solute }}$
11. Elevation of boiling point :
$\Delta T_b=K_b \cdot m$
where $m$ is molality of solution and $K_b$ is called boiling point elevation
12. Depression of freezing point :
$\Delta T_f=K_f \cdot m$
$K_f=$ Molal depression constant
13. Osmosis and osmotic pressure :
$\Pi=C R T$
14. van't Hoff factor $(i)$
$i=\frac{\text { Observed colligative property }}{\text { Calculated colligative property }}$
15. Relation between van't Hoff factor and degree of dissociation
$\alpha=\frac{i-1}{n-1}$
16. Relation between van't Hoff factor and degree of association
$\alpha=\frac{1-i}{1-1 / n}$
Advantages of Using NCERT Exemplar Solutions Class 12 Chemistry Chapter 2 Solutions
These NCERT Exemplar Class 12 Chemistry Solutions Chapter 2 Solutions are prepared in an organised way and offer various benefits for students preparing for board exams and competitive exams. The advantages of using these solutions are given below:
- These NCERT Exemplar Solutions for Class 12 summarise important formulas, definitions, and concepts of solutions with the help of solved questions.
- These solutions are written in simple language with proper explanations that make it easier to understand topics like solubility, concentration terms, Raoult’s law, and colligative properties.
- Class 12 chemistry chapter 2 Solutions Question Answers are prepared by subject experts in a very detailed way that eliminates the need to read the entire NCERT book.
- These solutions focus on high-weightage topics and frequently asked questions in board exams.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are prepared strictly according to the latest NCERT syllabus. Below are the NCERT Exemplar chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
These NCERT Solutions for Class 12 Chemistry are prepared as per the latest NCERT curriculum and CBSE guidelines. Below is a list of NCERT chapter-wise solutions:
NCERT Exemplar Class 12 Solutions
Students can refer to the links given below for the NCERT Exemplar subject-wise solutions for Class 12:
NCERT Exemplar Class 12 Solutions
Students can refer to the links given below for the NCERT Exemplar subject-wise solutions for Class 12:
NCERT Solution subject-wise
Students can refer to the links given below for the NCERT subject-wise solutions:
NCERT Notes subject-wise
Students can refer to the links given below for the NCERT subject-wise notes:
NCERT Books and NCERT Syllabus
Students can refer to the links given below for the NCERT Books and Syllabus:
Frequently Asked Questions (FAQs)
This chapter focuses on the concepts of solutions, their properties, types, and methods of expressing concentration. It also covers important topics like colligative properties, Raoult's Law, and the behavior of solutions.
Colligative properties are properties of solutions that depend on the number of solute particles in a given amount of solvent, regardless of the nature of the solute. Key colligative properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure.
A saturated solution contains the maximum amount of solute that can be dissolved in a solvent at a given temperature and pressure. In contrast, an unsaturated solution can still dissolve more solute because it has not reached its saturation point.
Understanding solution concentrations is crucial because it allows chemists to quantitatively describe the amount of solute in a solution relative to the solvent. This knowledge is essential for performing reactions, analyzing substances, and applying principles in various scientific and industrial fields.
Vapor pressure lowering occurs when a non-volatile solute is added to a solvent, which results in a decrease in the vapor pressure of the solvent. This happens because the solute particles disrupt the ability of the solvent molecules to escape into the vapor phase, effectively reducing the number of solvent molecules in the vapor.
NCERT Class 12 Chemistry Chapter 2 is Solutions, which discusses different types of solutions, concentration terms, solubility, Raoult’s law, and colligative properties. The chapter helps students understand how substances dissolve and how solution behaviour is used in chemical calculations.
You can download the Class 12 chemistry chapter 2 Solutions Question Answers for free from trusted educational websites such as NCERT’s official site, Careers360 and other educational platforms.
Matching questions in Solutions NCERT Exemplar Problems require you to pair terms, laws, or concepts in one column with their correct descriptions or applications in another.
Concepts like solubility, concentration, and colligative properties apply to real-life processes such as making beverages, pharmaceutical formulations, and antifreeze use in vehicles.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Hello,
Here is your Final Date Sheet Class 12 CBSE Board 2026 . I am providing you the link. Kindly open and check it out.
https://school.careers360.com/boards/cbse/cbse-class-12-date-sheet-2026
I hope it will help you. For any further query please let me know.
Thank you.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters





