NCERT Exemplar Class 12 Chemistry Solutions chapter 11 Alcohols, Phenols and Ethers
Chemistry has become even more exciting when we study compounds that are essential to our day-to-day life. The chapter Alcohols, Phenols, and Ethers features oxygen-containing organic compounds that are widely used in the medical industry and daily life. Students will learn the uses of alcohol in medicines in various forms, such as antiseptic disinfectants, antidotes, etc. Alcohol is also used in mouthwash, cosmetics, syrups, and so on. Ethers are used in perfumes, resins, dyes, insecticides, etc. Phenols are used as surgical antiseptics and disinfectants in household cleaners.
Before the exam began, candidates were given a 15-minute reading period to review the Mathematics question paper and understand the structure of the exam.
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: Long Answer Type
- Class 12 Chemistry NCERT Chapter 11: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 11 Alcohols, Phenols, and Ethers
- Advantages of Using Alcohols, Phenols, and Ethers NCERT Exemplar Solutions Class 12 Chemistry Chapter 11
- Topics of NCERT Exemplar Class 12 Chemistry Solutions Alcohols, Phenols, and Ethers
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Exemplar Solutions Class 12
- NCERT Solution subject-wise
- NCERT Notes subject-wise

NCERT Exemplar Solutions are designed by subject experts to offer a systematic and structured approach to these important concepts. These solutions provide a valuable resource to enhance performance in board exams as well as in the competitive exams like JEE Advanced, NEET, JEE Mains, etc.The chapter also covers essential reaction mechanisms like dehydration of alcohol, electrophilic substitution in phenols, and Williamson’s synthesis of ethers. In this article, we have added some Higher Order Thinking Skills (HOTS) questions. Students can refer to NCERT Exemplar Class 12 Chemistry Solutions to strengthen their concept and problem-solving ability. Every chapter is fairly and accurately explained in these NCERT solutions.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: MCQ (Type 1)
Below, solutions of MCQ-type questions of Class 12 Chemistry chapter 11 Alcohols, Phenols, and Ethers are provided which are important for both boards and competitive exams and helps you to improve your conceptual thinking and problem-solving ability.
Question 1: Monochlorination of toluene in sunlight followed by hydrolysis with aq. $\text {NaOH}$ yields.
(i) o-Cresol
(ii) m-Cresol
(iii) 2, 4-Dihydroxytoluene
(iv) Benzyl alcohol
Answer:
The answer is the option (iv). The monochlorination of toluene in sunlight results in halogenation that follows a free radical pathway and the product will react with the alkyl group forming haloalkyl.
And in the alkaline medium, the substitution reaction will take place resulting in the deduction of a hydrogen atom from the haloalkyl group. The end product formed will be alcohol; therefore, the monochlorination of toluene in light in the presence of aq. NaOH forms benzyl alcohol. So, option (iv) is the correct answer.
Question 2: How many alcohols with molecular formula $\text {C}_{4}\text {H}_{10}\text {O}$ are chiral?
(i) 1
(ii) 2
(iii) 3
(iv) 4
Answer:
The answer is the option (i). To identify the chirality of any compound in chemistry, the carbon present should be surrounded by four different groups and should not have any plane of symmetry.
The possible alcohols with the molecular formula $\text {C}_{4}\text {H}_{10}\text {O}$ are –
- $\text {1-Butanol}$
- $\text {2-Butanol}$
- $\text { 2-methyl-1-propanol}$
- $\text { 2-methyl-1-propanol}$
If all the alcohol's chemical structure is drawn of the possible compounds, then only one of them contains carbon with four different groups that are $\text {2-Butanol}$ , so the right answer is the option (i) 1.
Question 3: What is the correct order of reactivity of alcohols in the following reaction?
$\text {R-OH+HCl} \overset{ZnCl_2}{\rightarrow} \text {R-Cl}+\text {H}_{2}\text {O}$
(i) $1^{o}>2^{o}>3^{o}$
(ii) $1^{o}<2^{o}>3^{o}$
(iii) $3^{o}>2^{o}>1^{o}$
(iv) $3^{o}>1^{o}>2^{o}$
Answer:
The answer is option (iii). When dry anhydrous $\text {ZnCl}_{2}$ is mixed with concentrated $\text {HCl}$, it forms Lucas reagent and the reaction involved is knows as the Lucas test. Formation of an intermediate carbocation takes place during the reaction, this reaction being a slow step of the mechanism, this carbocation dictates the rates of reaction.
According to the $\text {S}_N{1}$ mechanism, the alcohol reactivity order is $3^{o}>2^{o}$. But tertiary alcohol reacts very fast with Lucas reagent, so the reactivity order of alcohols in this particular reaction is –
$\text {R-OH+HCl} \overset{ZnCl_2}{\rightarrow} \text {R-Cl}+\text {H}_{2}\text {O}$
$3^{o}>2^{o}>1^{o}$
So the correct option is (iii) $3^{o}>2^{o}>1^{o}$
Question 4: $\text {CH}_{3}\text {CH}_{2}\text {OH}$ can be converted into $\text {CH}_{3}\text {CHO}$ by ______________.
(i) catalytic hydrogenation
(ii) treatment with $\text {LiAIH}_{4}$
(iii) treatment with pyridinium chlorochromate
(iv) treatment with $\text {KMnO}_{4}$
Answer:
The answer is the option (iii). The compound $\text {CH}_{3}\text {CH}_{2}\text {OH}$ oxidises and forms $\text {CH}_{3}\text {CHO}$ as the end product. In the oxidation reaction process, the addition of pyridinium chlorochromate, a strong oxidising agent, breaks the cycle by forming aldehydes and preventing complete oxidation of $\text {CH}_{3}\text {CH}_{2}\text {OH}$. As the reaction is halted at the aldehyde stage, the end product formed is $\text {CH}_{3}\text {CHO}$.
So $\text {CH}_{3}\text {CH}_{2}\text {OH}$ can be converted into $\text {CH}_{3}\text {CHO}$ by option (iii) treatment with pyridinium chlorochromate
Question 5: The process of converting alkyl halides into alcohols involves_____________.
(i) addition reaction
(ii) substitution reaction
(iii) dehydrohalogenation reaction
(iv) rearrangement reaction
Answer:
The answer is the option (ii). To covert alkyl halides into alcohols, the reaction involved is –
$\text {R-X}\rightarrow \text {R-OH}$
In order to get the required result, the replacement of $\text {-X}$ into $\text {-OH}$ is necessary. And as replacing one of the groups is required, the type of reaction involved is known as substitution reaction. Thus, the correct option is (ii) substitution reaction.
Question 6: Which of the following compounds is aromatic alcohol?
(i) A, B, C, D
(ii) A, D
(iii) B, C
(iv) A
Answer:
The answer is the option (iii). The characteristics of an aromatic compound are that they have an $\text {-OH}$ group indirectly attached to the benzene ring. So, Option (iii) B, C is the right answer.
Question 7: Give IUPAC name of the compound given below.
(i) $\text {2-Chloro-5-hydroxyhexane}$
(ii)$\text { 2-Hydroxy-5-chlorohexane}$
(iii) $\text { 5-Chlorohexan-2-ol}$
(iv) $\text { 2-Chlorohexan-5-ol}$
Answer:
The answer is the option (iii). To determine the IUPAC name of any chemical compound, the steps of nomenclature included are –
- Identifying the functional group and their correct order of priority. -OH having a higher priority, the suffix used will be '-ol'.
- Recognise the parental chain, which is the longest carbon chain containing the functional group. In this care, the
the chain consists of 6 carbons hence 'hexyl'.
- Numbering of the chain by giving the smallest number to the functional group and other side chains; thus, '2-ol'.
- Organise the side chain atoms/molecules and give, so they are chloro and 5-chloro in the mentioned compound.
- The resulting IUPAC name is 5-Chlorohexan-2-ol.
The conclusion of the correct option is (iii) 5-chlorohexan-2-ol
Question 8: IUPAC name of m-cresol is ___________.
(i) 3-methyl phenol
(ii) 3-chlorophenol
(iii) 3-methoxy phenol
(iv) benzene-1,3-diol
Answer:
The answer is the option (i).
.png)
The structure of m-cresol consists of a benzene ring with $\text {-OH}$ functional and $\text {-CH}_{3}$ substituent attached. m-cresol is the common name assigned to the compound, and according to the rules of nomenclature, the IUPAC name is of m-cresol is 3-methyl phenol. So, Option (i) 3-methyl phenol is the right answer.
Question 9: IUPAC name of the compound is ______________.
(i) $\text {1-methoxy-1-methylethane}$
(ii) $\text {2-methoxy-2-methylethane}$
(iii) $\text {2-methoxypropane}$
(iv) $\text {isopropylmethyl ether}$
Answer:
The answer is the option (iii). To name the compounds containing carbon as their substituent group, steeps of nomenclature involved are –
1. Identify the longest carbon chain, also known as the parent chain. Calculate the number of carbons present and assign its root name.
2. Numbering of the parent chain by assigning the smallest number to the first substituent.
3. Recognise the position and name of all substituent carbon groups.
4. In the presence of identical groups, use the group prefix such as di, tri, tetra, etc.
5. Alphabetically arrange the substituent position and names in front of the root name. While making the order, prefix like di, tri, tetra should not be accounted for except iso and cyclo.
By following these rules, the IUPAC name of the given compound is $\text {2-methoxypropane}$. So the correct option is (iii) $\text {2-methoxypropane}$
Question 10: Which of the following species can act as the strongest base?
Answer:
The answer is the option (ii). The strength of a base is determined by the corresponding conjugate acid. If the acid is weak, the base is strong.
$\text {OH+H}\rightarrow \text {H}_{2}\text {O}\left ( \text {Water} \right )$
$\text {OH+H}\rightarrow \text {ROH}\left ( \text {alcohol} \right )$
$\text {OC}_{6}\text {H}_{5}+\text {H}\rightarrow \text {C}_{6}\text {H}_{5}\text {OH}\left ( \text {phenol} \right )$
$\text {OC}_{6}\text {H}_{5}\left ( \text {m-NO}_{2} \right )+\text {H}\rightarrow \text {HOC}_{6}\text {H}_{5}\left ( \text {m-NO}_{2} \right )\left ( \text {m-nitrophenol} \right )$
In the mentioned options, the phenol having a benzene ring has higher acidity than aliphatic alcohols. Since the acidity of water is more than alcohol, it makes $\text {ROH}$ the least acidic product. Since, according to the above-mentioned point, the weaker acid makes a strong base. $\text {RO}$ will be the strongest base in all four options. This makes option (ii) as is the right answer.
Question 11: Which of the following compounds will react with sodium hydroxide solution in water?
(i) $C_{6}H_{5}OH$
(ii) $C_{6}H_{5}CH_{2}OH$
(iii) $\left ( CH_{3} \right )_{3}COH$
(iv) $C_{2}H_{5}OH$
Answer:
The answer is the option(i). Sodium hydroxide is a strong base and to be able to make the reaction feasible; the other acidic reactant should be strong. Option (ii), (iii), and (iv) are alcohols and are weak in nature due to less affinity towards water thus they are able to dissociate into ions only to a certain extent. Whereas option (i) phenol is a strong acid due to its higher affinity towards water. This makes option (i) as the right answer
Question 12: Phenol is less acidic than ______________.
(i) ethanol
(ii) o-nitrophenol
(iii) o-methyl phenol
(iv) o-methoxy phenol
Answer:
The answer is the option (ii). The acidity of phenol is determined by its electron-withdrawing or donating ability. The $(NO_{2})$ present in phenol is an electron-withdrawing group, so it increases the acidity whereas groups like $(-CH_{3}, -OCH_{3})$ being electron-donating groups, they decrease the acidity of phenol is less acidic compared to o-nitrophenol from the given options. Thus, the correct option is (ii) o-nitrophenol
Question 13: Which of the following is most acidic?
(i) Benzyl alcohol
(ii) Cyclohexanol
(iii) Phenol
(iv) m-Chlorophenol
Answer:
The answer is the option (iv). The addition of electron-withdrawing groups in phenol increases its acidity, whereas the addition of electron-donating groups decreases the acidity. And as a known fact, that the (+R) effect duet to donating is dominant over the (+I) effect. But this is only feasible in the case of substituents at ortho and para positions.
Keeping the above-mentioned point in mind, the m-Chlorophenol compound is the most acidic of all four options. So, option (iv) is the answer.
Question 14: Mark the correct order of decreasing acid strength of the following compounds.
$\\(i) e > d > b > a > c\\$
$(ii) b > d > a > c > e\\$
$(iii) d > e > c > b > a\\$
$(iv) e > d > c > b > a$
Answer:
The answer is the option (ii). According to the (+R) effect in the cyclic compound, the presence of an electron-withdrawing group at para position to $-OH$ makes the acidity level maximum of the compound whereas having an electron-donating group at the para position to $-OH$ makes the acidity level minimum. So, according to this concept, the correct decreasing order of acidity of the given compounds is Option (ii) b > d > a > c > e
Question 15: Mark the correct increasing order of reactivity of the following compounds with $HBr/HCl$.
(i) a < b < c
(ii) b < a < c
(iii) b < c < a
(iv) c < b < a
Answer:
The answer is the option (iii). The type of reaction taking place in this process is the nucleophilic substitution reaction. The mechanism followed is $S_{N}1$, so the first slow step of the mechanism involves the formation of carbocation that determines the rate of reaction and then the addition of a nucleophile. The carbocation formed also controls the reactivity of this product formed. But in case of the presence of a highly electron-withdrawing group $(-NO_{2})$, the carbocation will be destabilized, and the reaction will not be favored.
Whereas the high electron-withdrawing nature of the $(-Cl)$ group due to lone pair presence, its tendency of electron-withdrawing is slightly low than $(-NO_{2})$, it will also manage to destabilize the carbocation and not favor the reaction.
In the case of the first compound, $C_{6}H_{5}CH_{2}OH$, the absence of any destabilizing factors makes the reaction occurrence favorable. Thus, the concluded increasing order of reactivity will be Option (iii) b < c < a
Question 16: Arrange the following compounds in increasing order of boiling point.
$Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol$
$\\(i) Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol \\$
$(ii) Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol \\$
$(iii) Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol \\$
$(iv) Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol$
Answer:
The answer is the option (i). To find out the order of boiling point in alcohols, the important factor is molecular mass. With an increasing molecular mass of the compound, the boiling point also increases. So, the compound with a maximum boiling point will be pentan-1-ol, and the compound with the least boiling point is Propan-1-ol.
The correct order would be Option (i) $\text {Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol}$
NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: MCQ (Type 2)
MCQ-type questions are covered to improve your conceptual thinking and problem-solving ability. These questions from Alcohols, Phenols, and Ethers NCERT Exemplar Class 12 Chemistry Chapter 11 further enhance exam preparation.
Question 17: Which of the following are used to convert $RCHO$ into $RCH_{2}OH$ ?
(i) ${H_{2}}/{Pd}$
(ii) $LiAlH_{4}$
(iii) $NaBH_{4}$
(iv) Reaction with $RMgX$ followed by hydrolysis
Answer:
The answer is the option (i), (ii), and (iii). The above-mentioned reactant is an aldehyde that is converted into alcohol. This type of reaction is called a reduction reaction. The reduction reaction is carried out in the presence of hydrogen metal catalysts to increase the rate of the reaction. The metal catalyst used is ${H_{2}}/{Pd}$. If it is replaced by $NaBH_{4}$ or $LiAlH_{4}$, the reaction will still take place to get the exact result. This makes options (i), (ii), and (iii) as the correct answers.
Question 18: Which of the following reactions will yield phenol?
Answer:
The answer is the option (A), (B), (C). To carry out these reactions, the conditions required should be easy to maintain. The reaction occurring in (D) is a nucleophilic substitution of chlorobenzene which is not feasible as it requires drastic temperature and pressure conditions.
The reaction given in (A) is the Dow process, (B) is aniline diazotization and (C) option contains a reaction from benzene sulphonic acid. All these reactions are feasible due to manageable reaction conditions. The correct options are Options (A), (B), and (C)
The answer is the option (i), (iii), and (iv). The complete oxidation of primary alcohols results in the formation of carboxylic acid unless a mild oxidizing agent is added to terminate the reaction at the aldehyde formation step. The most common mild oxidizing agent used is $PCC$ (Pyridinium chlorochromate). Some other compounds used for this purpose are $CrO_{3}$ in an anhydrous medium and Heat in the presence of $Cu$ at 573K. So, the correct answers are an option (i), (iii), and (iv)
Question 20: Phenol can be distinguished from ethanol by the reactions with _________.
(i) ${Br_{2}}/{water}$
(ii) $Na$
(iii) Neutral $FeCl_{3}$
(iv) All the above
Answer:
The answer is the option (i) and (iii). Phenol can easily be distinguished from ethanol by adding bromine water as it results in a white ppt of tri-bromo phenol or Neutral $FeCl_{3}$ can also be used for this purpose. But ethanol being aliphatic alcohol will not react with either of the compounds. So, the right answer is options (i) and (iii)
Question 21: Which of the following are benzylic alcohols?
Answer:
The answer is the option (ii) and (iii). The most prominent way to identify benzylic alcohol is to check if an $-OH$ group is attached to a $sp^{3}$ hybridized carbon atom on an aromatic ring. As in the case of option (ii) and (iii), the $sp^{3}$ hybridized carbon is attached to a benzene ring these are benzylic alcohols but in option (i) and (iv), the $sp^{3}$ hybridised carbon is not attached to a benzene ring. So, option (ii) and (iii) are the answers.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: Short Answer Type
Short-answer type questions are covered to improve your problem-solving ability. These Chapter 11 Alcohols, Phenols, and Ethers important questions help in building a strong foundation for board and competitive exams.
Question 22: What is the structure and IUPAC name of glycerol?
Answer:
Glycerol comes under the category of trihydric alcohol, and the IUPAC name according to the nomenclature rules is $\text {Propane-1,2,3-triol}$
Question 23: Write the IUPAC name of the following compounds.
Answer:
The systematic structure of naming of organic compounds in chemistry is given International Union of Pure and Applied Chemistry (IUPAC).
The IUPAC name of the mentioned compounds are
(A) $\text {3-Ethyl-5-methyl hexane-2,4-diol}$.
(B) $\text {1-Methoxy-3-nitrocyclohexane.}$
Question 24: Write the IUPAC name of the compound given below.
Answer:
After following the laws of nomenclature designed by IUPAC, the name of the compound is 3-Methylpent-2-ene-1,2-diol.
Question 25: Name the factors responsible for the solubility of alcohols in water.
Answer:
The high affinity of alcohols with water comes due to the following listed reasons –
I) Hydrogen bonds – with a greater number of H-bonds, the solubility of the compound increases.
II) Size of the alkyl or aryl groups- the size of alkyl or aryl groups attached determines the solubility as larger size contributes to a smaller number of H-bonds that decrease the solubility factor.
III) The molecular mass of the Alcohols – the larger molecular mass makes the compound less soluble due to difficulty in dissociation.
Question 26: What is denatured alcohol?
Answer:
To prevent alcohol stealing during the manufacturing process, it is mixed with additional components like copper sulfate and pyridine to make it unfit for drinking purposes. These substances change the colour and make the odour foul.
Question 27: Suggest a reagent for the following conversion.
Answer:
The above-given reaction is the formation of ketone from secondary alcohol through the process of oxidation. The most common oxidizing agents used in this reaction are Pyridinium chlorochromate $(PCC)$, chromic anhydride $(CrO_{3})$, etc.
Question 28: Out of 2-chloroethanol and ethanol, which is more acidic and why?
Answer:
The acidity of the compound depends on the type of group attached to the compound, due to $Cl$ being an electron-withdrawing group, the acidity of 2-chloroethanol is more. This leads to a decrease in electron density in the $-O-H$ bond as a (-I) ( negative inductive effect) effect is created, This makes it very easy for 2-chloroethanol to release a proton
Question 29: Suggest a reagent for conversion of ethanol to ethanal.
Answer:
The reaction involved is the conversion of primary alcohol to an aldehyde by oxidation. The most feasible reagent for the above reaction is Pyridinium chlorochromate $(PCC)$
Question 30: Suggest a reagent for conversion of ethanol to ethanoic acid.
Answer:
To covert primary alcohol to a carboxylic acid through an oxidation reaction, the most efficient reagent is Acidified $\text {KMnO}_{4}$.
Question 31: Out of o-nitrophenol and p-nitrophenol, which is more volatile? Explain.
Answer:
The bonds present in o-nitrophenol are intramolecular H-bonds, but in the case of p-nitrophenol, the bonds formed are intermolecular H-bonds. When these compounds are heated, the boiling temperature of p-nitrophenol gets elevated due to strong intermolecular H-bonds, but the same does not happen with o-nitrophenol. This makes the o-nitrophenol more volatile.
Question 32: Out of $o-nitrophenol \; and\; o-cresol$, which is more acidic?
Answer:
The presence of an electron-withdrawing group at para and ortho position increases the acidity of the cyclic compounds. As $NO_{2}$ is present in o-nitrophenol, it makes it more acidic, but in o-cresol, the electron releasing group decreases its acidity.
Question 33: When phenol is treated with bromine water, white precipitate is obtained. Give the structure and the name of the compound formed.
Answer:
Treating phenol with bromine water makes a white precipitate as a by-product. The compound formed is named as $2,4,6-tribromophenol$, and the structure of this compound is given below -
Question 34: Arrange the following compounds in increasing order of acidity and give a suitable explanation.
$Phenol, o-nitrophenol, o-cresol$
Answer:
The acidity factor of these compounds depends on the type of groups attached. If the group is electron-withdrawing, they increase the acidity of the compound due to (-I) and (-R) effect, which is exactly the case in o-nitrophenol, making it most acidic. Also, due to the (+I) effect depicted by o-cresol, it is weaker than phenol.
So, the correct Increasing order of the acidity of the given compounds: $o-cresol < Phenol < o-nitrophenol.$
All the alkyl groups exhibit the (+I) effect and with an increasing number of alkyl groups from Primary, secondary to tertiary alcohols, the -OH bond strength of increases due to elevation in the electron density. There is a low reactivity of sodium with tertiary alcohol due to steric hindrance and difference in nature. So, the correct decreasing order sodium metal reactivity towards alcohol is Primary alcohols > secondary alcohols > tertiary alcohols.
Question 36: What happens when benzene diazonium chloride is heated with water?
Answer:
During the treatment of benzene diazonium chloride in water, multiple by-products Nitrogen gas and Hydrochloric acid are formed. The major product formed is phenol.
Question 37: Arrange the following compounds in decreasing order of acidity.
$H_{2}O, ROH, HC \equiv CH$
Answer:
To determine the acidity of these given compounds, they are treated with water. So, when $RONa$ is treated with water, it gets replaced by a stronger acid, and a similar happens with sodium ethynide on treatment with water and alcohol. The result is acetylene; this indicates that the strongest acids are water and alcohol. So the decreasing order of acidity of these compounds is- $H_{2}O > ROH > HC \equiv CH$
Question 38: Name the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
Answer:
The enzymes used in the fermentation process of converting ethanol from sucrose are invertase and zymase.
The enzyme invertase processes sucrose into glucose and fructose and later in the presence of zymase, glucose and fructose are converted into ethanol. The reaction involved in the process is given below:
Question 39: How can propane-2-one be converted into tert- butyl alcohol?
Answer:
When ketones are treated with dry ether (Grignard's Reagent), they get converted into tertiary alcohols after hydrolysis. Thus to convert Propane-2-one to tert- butyl alcohol in the presence of Grignard's Reagent, you need to add $CH_{3}MgBr$ and also perform hydrolysis to get the desired result.
Question 40: Write the structures of the isomers of alcohols with molecular formula $C_{4}H_{10}O$. Which of these exhibits optical activity?
Answer:
To become optically active, compounds are required to be chiral. To check the chirality of any compound, they must have a carbon atom attached with four different groups and have no plane of symmetry. From the above-mentioned compounds, only Butan-2-ol is optically active as the rest are achiral compounds.
Question 41: Explain why is $OH$ group in phenols is more strongly held as compared to $OH$ group in alcohols.
Answer:
Even though both alcohol and phenol groups contain $-OH$ group, the bond strength is much higher in phenols as compared to alcohols due to the direct attachment of $-OH$ group to an $sp^{2}$ hybridized carbon in phenol. The bond size is relatively smaller, and because of resonance & charge distribution, it acquires a partial double bond character in phenolic compounds.
Question 42: Explain why nucleophilic substitution reactions are not very common in phenols.
Answer:
For the feasibility of a nucleophilic substitution reaction, the compound present should be able to uptake the nucleophile involved, but in the case of phenols, the resonance effect on $-OH$ bond gives its electron to the benzene ring making the electron density very high. Due to this high electron density, nucleophiles are repelled and are not able to substitute any atom from the phenol compound.
Answer:
To prepare alcohols from alkene in the presence of sulphuric acid through the process of hydration of alkenes. The reactions take place under the influence of Markownikoff's rule, and there are major 3 steps involved in the mechanism.
Step (1) Protonation of alkene and formation of a carbocation
Step (2) Nucleophilic attack of water
Step (3) Deprotonation occurs, and alcohol is formed. $H_{3}O^{+}$ is released.
Question 44: Explain why is $O=C=O$ non-polar while $R-O-R$ is polar.
Answer:
The dipole movement of both compounds decides whether they are polar or non-polar. As $O=C=O$ is a linear molecule, it is non-polar in nature because of equal distribution of its diploe movement in both directions. This makes the net dipole movement zero. But in the case of ethers, the dipole movement might be in opposite directions, but the net movement is not zero, which makes it a non-polar molecule.
Question 45: Why is the reactivity of all the three classes of alcohols with cone. $\text {HCl}$ and $\text {ZnCl}_{2}$ (Lucas reagent) different?
Answer:
The reactivity factor of all alcohols depends on the carbocation formed. Since the reaction mechanism followed by these compounds is $S_{N}1$, the higher stability of intermediate carbocation formed in the first slow step of reaction makes the compound more reactive. The tertiary carbocation formed are most stable, making these compound most reactive. But in case of secondary alcohols, they do not show any turbidity at room temperature, but on heating, they exhibit turbidity, so the carbocation formed is slightly more stable than the primary alcohols.
Question 46: Write steps to carry out the conversion of phenol to aspirin.
Answer:
The most common method of preparing aspirin is reacting salicylic acid with acetic anhydride. Reacting phenol with $CO_{2}$ and $NaOH$ yields salicylic acid. The name of the reaction is Kolbe's reaction. Later in the reaction, the acetylation of $-OH$ group takes place by replacing hydrogen with an acetyl group.
The hydroxyl group present in phenol makes more nitrate. And because of the resonance of $-OH$ bond, it makes the benzene ring rich in electron density which further makes it feasible for electrophilic substitution reaction. This indicates that the nitration, also known as electrophilic substitution, takes place in compounds with high electron density.
Question 48: In Kolbe's reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
Answer:
The phenoxide ion used in the reaction is more reactive as compared to phenol due to the ability to donate its lone pair. This increases the compounds' affinity towards the electrophilic substitution reaction. These abilities make the compound a stronger nucleophile compared to phenol, and it is able to react easily with $CO_{2}$
Question 49: The dipole moment of phenol is smaller than that of methanol. Why?
Answer:
The dipole movement is dependent on the polarity of the compound. They are directly proportional, and in the case of phenol, the carbon atom is $sp^{2}$ hybridised, creating an electron-withdrawing effect on the benzene ring. But in methanol, the hybridisation of the carbon is $sp^{3}$, which results in an electron releasing effect. Due to this scenario, the bond polarity of the $C-O$ bond is more in phenol as compared to methanol. This directly results in smaller dipole movement in phenol.
Question 50: Ethers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether cannot be prepared by this method. Explain.
Answer:
Alkaloids being strong base as well as nucleophiles can react with tertiary alkyl halides through elimination reaction. During the reaction of tert-butyl-bromide with tert-butoxide rather than the expected substitution reaction, it undergoes the elimination reaction. Due to this mishappening, the product formed is Iso butylene instead of Di-tert-butyl ether.
Question 51: Why is the $C-O-H$ bond angle in alcohols slightly less than the tetrahedral angle, whereas the $C-O-C$ bond angle in ether is slightly greater?
Answer:
Bond angle of $C-O-H$ being slightly less in alcohol compared to the tetrahedral bond angle. This phenomenon occurs because-
The C-O-H bond angle in alcohols is slightly less than the tetrahedral angle because of the repulsion between the two lone pairs of electrons on oxygen atoms as these pairs pushes the C-O bonds closer to each other.
In case of ethers, the oxygen atom is attached to bulky alkyl groups, hence repulsion between these R groups pushes the two C-O bonds away from each other and hence the bond angle is slightly greater than the tetrahedral angle.
Question 52: Explain why low molecular mass alcohols are soluble in water.
Answer:
The occurrence of intermolecular hydrogen bonds due to the H-bonds in alcohol compounds plays a major role. With the increasing number of alkyl groups, the molecular mass increases, due to which the polar nature of these compounds gets suppressed. Hence, the solubility factor of alcohol is indirectly proportional to the molecular mass.
Question 53 Explain why p-nitrophenol is more acidic than phenol.
Answer:
The presence of the electron-withdrawing group in cyclic compounds is responsible for providing the compound its acidic strength, and in para-phenol, a strong electron-withdrawing group $(NO_{2})$ is present at par position. There is no group present in phenol. This presence increases the acidic strength as well as stabilises the phenoxide ion.
Question 54: Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Answer:
Due to the presence of intermolecular H-bonds in alcohol, the energy required to break H-bonds is more than the energy required to break simple dipole-dipole bonds present in ethers. Thus, the boiling point of alcohol is higher than the boiling point of ethers.
Question 55: The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Answer:
The higher bond strength in the compound is due to the resonance effect in the molecule that mimics double bond characters in $C-O$ bonding. Therefore, the size of this bond is decreased in phenol. Also, the oxygen atom in phenol is directly attached to the $sp^{3}$ hybridised carbon atom by single bond. Hence the carbon-oxygen bond in phenol is slightly stronger than that in methanol.
Question 56: Arrange water, ethanol and phenol in increasing order of acidity and give a reason for your answer.
Answer:
When all the above molecules are treated with water, the phenol molecule easily forms phenoxide ion and also gets stabilised duet to resonance. These features increase its acidity, but in the case of ethanol, the ion formed is ethoxide ion which is very unstable; thus, the acidity of the compound is very less. But water being a good proton donor is slightly more acidic than ethanol. The increasing order of acidity is: Ethanol < water < phenol
NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: Matching Type
Matching-type questions are covered to improve conceptual clarity and topic awareness. Such exercises in NCERT Exemplar Class 12 Chemistry chapter 11 Alcohols, Phenols, and Ethers strengthen understanding.
Question 57: Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
|
Column I |
Column II | ||
|
i) |
a) |
Hydroquinone | |
|
ii) |
b) |
Phenetole | |
|
iii) |
c) |
Catechol | |
|
iv) |
d) |
o-Cresol | |
|
v) |
e) |
Quinone | |
|
vi) |
f) |
Resorcinol | |
|
|
|
g) |
Anisole |
Answer:
The correct match sequence of structure with their names is –
(i $\rightarrow$ d), (ii $\rightarrow$ c), (iii $\rightarrow$ f), (iv $\rightarrow$ a), (v $\rightarrow$ g), (vi $\rightarrow$ b)
(i) Cresols are methyl phenols that exist in three types namely: w-cresol, o-cresol, and p-cresol.
(ii) The common name of Catechol is pyrocatechol. It is the main constituent of many pesticides and perfumes. The IUPAC name is 1, 2-dihydrobenzene.
(iii). The compound Resorcinol is very commonly used to aid acne, and skin disorders. Its IUPAC name is 1, 3-dihydroxybenzene.
(iv). The IUPAC name of Hydroquinone is 1, 4-dihydroxybenzene, and it is commonly known as quinol. The compound is a good reducing agent and looks like a white granular solid.
(v) It is a colourless liquid also known as methoxy benzene, and the odour is similar to anise seed
(vi) Phenetole is commonly called ethyl phenyl ether; it is an organic compound. With a volatile nature, its vapours have explosive nature.
|
Column I |
Column II | ||
|
(i) |
$\text {CH}_{3}-\text {O}-\text {CH}_{3}$ |
(a) | |
|
(ii) |
(b) | ||
|
(iii) |
(c) | ||
|
(iv) |
(d) |
$\text {CH}_{3}-\text {OH+CH}_{3}-\text {I}$ | |
|
|
|
(e) | |
|
|
|
(f) | |
|
|
|
(g) | |
Answer:
When the given compounds are reacted with HI, their correct product formation is –(i $\rightarrow$ d), (ii $\rightarrow$ e), (iii $\rightarrow$ b), (iv $\rightarrow$ a)
(i) Due to the symmetrical structure of $\text {CH}_{3}-\text {O}-\text {CH}_{3}$, the end products of the reaction are $\text {CH}_{3}\text {I}$ and $\text {CH}_{2}\text {OH}$.
(ii) The compound being asymmetrical follows the $SN^{2}$ mechanism; therefore it forms the following product
(iii) The alkyl group attached to the compound are primary and tertiary; thus, it follows the $SN^{1}$ mechanism. In the process, the halide group attacks the tertiary and forms $\left ( \text {CH}_{3} \right )_{3}\text {C-I}$ and $\text {CH}_{3}\text {OH}$
(iv) The structure mentioned is alkyl aryl ether which is an asymmetrical ether in nature. And having the partial bond structure, the products formed are $\text {C}_{6}\text {H}_{5}-\text {OH}$ and $\text {CH}_{3}\text {I}$.
Question 59: Match the items of Column I with items of Column II.
|
Column I |
Column II | ||
|
(i) |
Antifreeze used in car engine |
(a) |
Neutral ferric chloride |
|
(ii) |
Solvent used in perfumes |
(b) |
Glycerol |
|
(iii) |
Starting material for picric acid |
(c) |
Methanol |
|
(iv) |
Wood spirit |
(d) |
Phenol |
|
(v) |
Reagent used for detection of phenolic group |
(e) |
Ethylene glycol |
|
(vi) |
By product of soap industry used in cosmetics |
(f) |
Ethanol |
Answer:
The correct matching pattern of the given compound and their properties is mentioned below – (i $\rightarrow$e), (ii $\rightarrow$ f), (iii $\rightarrow$ d), (iv $\rightarrow$ c), (v $\rightarrow$ a), (vi $\rightarrow$ b)
(i) Mainly used in the manufacturing of polyester fibres industry as a raw material its IUPAC name is ethane-1, 2-diol.
(ii) The key properties of ethanol include being a good solvent for fatty substances. Being safe for skin use, it is a good constitute of perfumes.
(iii)When phenol reacts with cone. $HNO_{3}$ it gets converted into picric acid (2, 4, 6-trinitro-phenol)
(iv) The widely known 'wood spirit' or Methanol, $CH_{3}OH$ is made by destructive distillation of wood.
(v) Neutral ferric chloride is a well-known reagent used for phenolic group detection, and it imparts purple/red color when mixed with phenols.
(vi). The standard soap preparation is done by reacting $NaOH$ with fatty acids.
Question 60: Match the items of Column I with items of Column II.
|
Column I |
Column II | ||
|
(i) |
Methanol |
(a) |
Conversion of phenol to o-hydroxysalicylic acid |
|
(ii) |
Kolbe's reaction |
(d) |
Ethyl alcohol |
|
(iii) |
Williamson's synthesis |
(c) |
Conversion of phenol to salicylaldehyde |
|
(iv) |
Conversion of 2o alcohol to ketone |
(d) |
wood spirit |
|
(v) |
Reimer-Tiemann reaction |
(e) |
Heated copper at 573 K |
|
(vi) |
Fermentation |
(f) |
Reaction of alkyl halide with sodium alkoxide |
Answer:
The right match options for column 1 are -
(i $\rightarrow$ d), (ii $\rightarrow$ a), (iii $\rightarrow$ f), (iv $\rightarrow$ e), (v $\rightarrow$ c), (vi $\rightarrow$ b)
(i) The destructive distillation method is used to produce methanol, commonly known as 'wood spirit.'
(ii) When phenol reacts with $CO_{2}$ gas, the reaction results in 2-hydroxy benzoic acid (salicylic acid). The name of the process is Kolbe's reaction.
(iii). Another important method of ether preparation of ether is Williamson's Synthesis. In this preparation method, an alkyl halide is made to react with sodium alkoxide.
$R-X+R-ONa\rightarrow ROR+NaX$
(iv) In this method, secondary alcohol passes over highly heated copper up to 573K; this results in dehydrogenation and the ṁṁṁṁformation of a ketone.
(v) An aldehyde group is introduced at the ortho position of the benzene ring when phenol is treated with chloroform in the presence of $NaOH$.
(vi) Preparation of ethanol is done via fermentation of sugars.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: Assertion and Reason Type
Assertion and Reason type questions are covered to improve your critical thinking and problem-solving solving ability.These are an important part of Chemistry Class 12 NCERT Exemplar Chapter 11 Alcohols, Phenols, and Ethers, helping students prepare effectively for board and competitive exams.
Question 61: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Addition reaction of water to but-1-ene in acidic medium yields-butan-1-ol.
Reason (R): Addition of water in acidic medium proceeds through the formation of a primary carbocation.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but the reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(ii) When water is added to but-l-ene in an acidic medium it forms butan-2-ol. The addition of water in the process crosses secondary carbocation formation.
Question 62: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): p-Nitrophenol is more acidic than phenol.
Reason (R): Nitro group helps in the stabilisation of the phenoxide ion by dispersal of negative charge due to resonance.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(i) p-Nitrophenol has a higher acidic nature compared to phenol; this is due to the stabilising of phenoxide ion by the nitro group by negative charge dispersal.
Question 63: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): IUPAC name of the compound
Reason (R): In IUPAC nomenclature, ether is regarded as hydrocarbon derivative in which a hydrogen atom is replaced by $-OR$ or $-OAr$ group [where R = alkyl group and Ar = aryl group]
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(iv) The IUPAC name of the given compound according to the nomenclature rules is 2-propoxypropane.
Question 64: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Bond angle in ethers is slightly less than the tetrahedral angle.
Reason (R): There is a repulsion between the two bulky (-R) groups.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(iv) Because there is repulsion in between two bulky alkyl groups, tetrahedral angle in ethers is slightly less than bond angle.
Question 65: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Boiling points of alcohols and ethers are high.
Reason (R): They can form intermolecular hydrogen bonding.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(ii) Ethers have lower boiling points than alcohols. In hydrogen bonding, ethers cannot form intermolecular whereas alcohols can.
Question 66: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Like bromination of benzene, bromination of phenol is also carried out in the presence of Lewis acid.
Reason (R): Lewis acid polarises the bromine molecule.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(iv) Lewis acid is not useful for the bromination of phenol as its absence won't do anything.
Question 67: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): o-Nitrophenol is less soluble in water than the m- and p-isomers.
Reason (R): m-Nitrophenol and p-Nitrophenol exists as associated molecules.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(v) Intramolecular H-bonding is present in o-nitrophenol.
Thus, m-nitrophenol and p-nitrophenol form H-bonds with H2O but o-nitrophenol does not. Also, m-nitrophenol and p-nitrophenol exist as associated molecules because of the presence of intermolecular H-bonding.
Question 68: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Ethanol is a weaker acid than phenol.
Reason (R): Sodium ethoxide may be prepared by the reaction of ethanol with aqueous $NaOH$
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(iii) Because ethoxide ion cannot be stabilised by resonance, as compared to phenoxide ion; that's why ethanol is a weaker acid than phenol. The reaction between ethanol with sodium is done to obtain Sodium ethoxide.
Question 69: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Phenol forms 2,4, 6-tribromophenol on treatment with $Br_{2}$ in carbon disulphide at 273 K.
Reason (R): Bromine polarises in carbon disulphide.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(ii) When bromine is treated with phenol in water, it forms 2, 4, 6-tribromophenol. Even when the Lewis acid is absent, the polarisation of bromine can still take place in phenols.
Question 70: In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Phenols give o-nitrophenol and p-nitrophenol on nitration with cone. $HNO_{3}$ and $H_{2}SO_{4}$ mixture.
Reason (R):$-OH$ group in phenol is o-,p-directing.
(i) Assertion and reason both are correct and reason is the correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Assertion is wrong statement but reason is correct statement.
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion..
Answer:
(iv) When nitration of Phenols is done with dil. $HNO_{3}$, they give o, p-nitrophenol and 2,4, 6-trinitrophenol is formed with cone. $HNO_{3}$.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 11: Long Answer Type
Long-answer type questions are covered to improve your subject knowledge and conceptual thinking. These NCERT Exemplar Class 12 Chemistry Solutions chapter 11 Alcohols, Phenols, and Ethers enhance analytical skills and strengthen exam preparation.
Question 71: Write the mechanism of the reaction of HI with methoxybenzene.
Answer:
Phenol and an alkyl halide are always the products of alkyl aryl ethers because $C_{6}H_{5}-O$ bond has a partial double bond character when resonance takes place.
Mechanism :
Step 1 – The protonation of ether molecules forms a methylphenyl oxonium ion
The above-mentioned ion has a weaker $O-CH_{3}$ bond as compared to the $O-C_{3}H_{5}$ as it has a partial double bond character. This double bond character is exhibited due to the resonance between the lone pair of electrons present on O-atom and the carbon atom of the phenyl group, which is $sp^{2}$ hybridised.
Step 2 – The protonated ether gives methyl iodide and phenol after interacting with I ion in $SN^{2}$ mechanism.
Solution: (a) The main initiating material used in the industrial preparation of phenol is Cumene (Isopropyl benzene)
(c) In the normal case scenario, Lewis acid is necessary to carry out the halogenation reaction, and $FeBr_{3}$ is responsible for the polarisation of the halogen molecule. But when phenol is taken for halogenation reaction, due to its $-OH$ group no activation agent is required as it is a high activation group present on the benzene ring.
The mechanism involved is that phenoxide ion is given by phenol after ionisation in aqueous solution. And the presence of a negative charge on the oxygen atom dictates it to give its electron to the benzene ring. This makes the ring trisubstituted product due to its high activation. But in non-polar solvents, phenol does not ionise to a greater extent. As a consequence, electrons are donated to a small extent by the $-OH$ group. The ring is activated slightly only contributes to mono-substitution.
Question 73: How can phenol be converted to aspirin?
Answer:
The chemical name of aspirin is salicylic acid. The mechanism of converting phenol into aspirin involves carbon dioxide being absorbed by sodium phenoxide and maintaining the temp at 400 K and 4-7 atm pressure. The first intermediate generated form of sodium salicylate by protonation. The acidification of sodium salicylate results in –
The acetylation of salicylic acid and acetic anhydride in the presence of conc. Sulphuric acid results in the formation of aspirin.
Question 74: Explain a process in which a biocatalyst is used in the industrial preparation of a compound known to you.
Answer:
Biocatalysts, as the name suggests, are the organic compounds that play the role of catalysts by reducing its activation energy and speeding up the process. These are a series of enzymes found in living organisms that catalyse their biochemical reactions. The most famous use of biocatalyst is in ethanol production from sugar solution (molasses):
When sugar solution is crystallised, the left-out solution or the mother liquor is termed as molasses which is the non-crystallised part. Its sugar concentration is 50%. It is kept for about 2-3 days after diluting it with 10% solution and yeast. Yeast has a role in providing enzymes invertase and zymase. The invertase gets broken down into sucrose to glucose and fructose after hydrolyses. Whereas the zymase gets helps in converting fructose and glucose to ethanol.
The winemaking process is very different; the source of sugar and yeast are grapes, not molasses. With time the sugar content increases due to the ripening of the grapes. After crushing of grapes, fermentation take its toll and occurs under anaerobic conditions and releases $CO_{2}$.
When the concentration of alcohol reaches up to 14%, the fermentation stops. If in case there is the presence of air in the fermented mixture, ethanol gets oxidised to ethanoic acid, which degrades the taste and quality of the alcohol
Apart from being informative, the NCERT exemplar solutions for class 12 Chemistry chapter 11 are also fun and interesting for all those chemistry lovers who love to find different reactions and compounds.
Class 12 Chemistry NCERT Chapter 11: Higher Order Thinking Skills (HOTS) Questions
Class 12 Chemistry NCERT Exemplar Solutions chapter 11 Alcohols, Phenols, and Ethers also includes HOTS-type questions that are covered to improve your problem-solving ability and conceptual thinking. These advanced questions help in developing higher-level analytical skills for exams.
Question 1: An organic compound $(\mathrm{X})$ with molecular formula $\mathrm{C}_3 \mathrm{H}_6 \mathrm{O}$ is not readily oxidised. On reduction, it gives $\left(\mathrm{C}_3 \mathrm{H}_8 \mathrm{O}(\mathrm{Y})\right.$ which reacts with HBr to give a bromide ( Z ) which is converted to Grignard reagent. This Grinard reagent on reaction with (X) followed by hydrolysis give 2,3-dimethylbutan-2-ol. Compounds $(\mathrm{X}),(\mathrm{Y})$ and $(\mathrm{Z})$ respectively are :
1) $\mathrm{CH}_3 \mathrm{COCH}_3, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}, \mathrm{CH}_3 \mathrm{CH}(\mathrm{Br}) \mathrm{CH}_3$
2) $\mathrm{CH}_3 \mathrm{COCH}_3, \mathrm{CH}_3 \mathrm{CH}(\mathrm{OH}) \mathrm{CH}_3, \mathrm{CH}_3 \mathrm{CH}(\mathrm{Br}) \mathrm{CH}_3$
3) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Br}$
4) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}, \mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}_2, \mathrm{CH}_3 \mathrm{CH}(\mathrm{Br}) \mathrm{CH}_3$
Answer:

Hence, the correct answer is option (2).
Question 2: Consider the following sequence of reactions
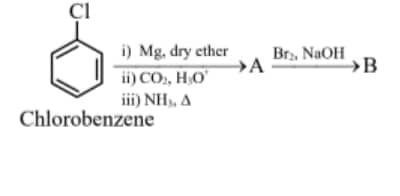
11.25 mg of chlorobenzene will produce $\qquad$ __________$\times 10^{-1} \mathrm{mg}$ of product B .
(Consider the reactions result in complete conversion.)
[Given molar mass of $\mathrm{C}, \mathrm{H}, \mathrm{O}, \mathrm{N}$ and Cl as 12,1 , 16,14 and $35.5 \mathrm{~g} \mathrm{~mol}^{-1}$ respectively]
Answer:


$\begin{aligned} & \frac{11.25 \times 10^3}{112.5}=\frac{x \times 10^1 \times 10^3}{93} \\ & x \times 10^1=93 \times 0.1 \\ & x=93 \mathrm{mg}\end{aligned}$
Hence, the answer is 93.
Question 3: Given below are two statements:
Statement (I): The boiling points of alcohols and phenols increase with increase in the number of C -atoms.
Statement (II): The boiling points of alcohols and phenols are higher in comparison to other class of compounds such as ethers, haloalkanes.
In the light of the above statements, choose the correct answer from the options given below:
(1) Both Statement I and Statement II are false
(2) Statement I is false but Statement II is true
(3) Statement I is true but Statement II is false
(4) Both Statement I and Statement II are true
Answer:
B.P. $\propto$ M.W.
Therefore, the boiling points of alcohols and phenols increase with the increase in the number of C-atoms.
B.P. $\propto$ Intermolecular hydrogen bonding
Alcohol \& Phenol have intermolecular H-bonding
Therefore, the boiling points of alcohols and phenols are higher in comparison to other classes of compounds, such as ethers and haloalkanes.
Hence, the correct answer is option (4).
Question 4: What will the major product of the following reaction?

(1)

(2)

(3)

(4)

Answer:

Hence, the correct answer is option (3).
Question 5: Match List-I with List-II
| List-I Conversion | List-II Reagents, Conditions used | ||
| (A) |  | (I) | Warm, $\mathrm{H}_2 \mathrm{O}$ |
| (B) |  | (II) | (a) $\mathrm{NaOH}, 368 \mathrm{~K}$; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
| (C) |  | (III) | (a) $\mathrm{NaOH}, 443 \mathrm{~K}$; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
| (D) |  | (IV) | (a) $\mathrm{NaOH}, 623 \mathrm{~K}$, 300 atm ; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
Choose the correct answer from the options given below :
(1) (A)-(II), (B)-(III), (C)-(I), (D)-(IV)
(2) (A)-(III), (B)-(IV), (C)-(II), (D)-(I)
(3) (A)-(IV), (B)-(III), (C)-(II), (D)-(I)
(4) (A)-(IV), (B)-(III), (C)-(I), (D)-(II)
Answer:
Presence of EWG at ortho and para-position of chlorobenzene will increase rate of aromatic nucleophilic substitution due to stable intermediate. A-IV, B-III, C-II, D-I
Hence, the correct answer is option (3).
Approach to Solve Questions of Chapter 11 Alcohols, Phenols, and Ethers
To effectively solve the questions of Alcohols, Phenols, and Ethers, it is important to adopt a structured approach. The following are the ways that can help you attempt the questions with the correct approach. The approach to solve the questions must be simple yet effective.
1. Start by mastering key concepts like
- Structure and classification of alcohols, phenols, and ethers.
- Nomenclature
- Methods of preparation from alkenes, carbonyl compounds, haloalkanes.
- Physical properties like boiling point, solubility, etc.
2. Thoroughly revise all key chemical reactions, mainly,
- Reactions of alcohols- oxidation, dehydration, esterification, Lucas test.
- Reactions of phenols- electrophilic substitution , Kolbe’s reaction, Reimer -Tiemann reaction.
- Reactions of ethers- cleavage by acids
3. Many questions are based on mechanisms so practice them thoroughly so that you can solve all the questions related to that reaction. While solving the questions of Chapter 11 NCERT Exemplar Alcohols, Phenols, and Ethers, it is important to have proper knowledge of mechanisms listed below:
- Dehydration of alcohols (E1/E2).
- Electrophilic substitution in phenols.
- Acidic cleavage of ethers.
4. Solve all in-text questions while reading the NCERT Chapter 11 Alcohols, Phenols, and Ethers . Attempt the exercise questions at the end thoroughly as many board exam questions are directly based on these.
5. You can also prepare quick revision charts or flashcards for remembering the concepts like reagents and their effects, name reactions etc.
6. A Proper understanding of basic concepts and practice helps students clear their doubts and solve questions effectively. Students can refer to the questions provided in the NCERT textbooks and revise them again and again.
Advantages of Using Alcohols, Phenols, and Ethers NCERT Exemplar Solutions Class 12 Chemistry Chapter 11
NCERT Exemplar Class 12 Chemistry Solutions chapter 11 Alcohols, Phenols, and Ethers cover all the important topics from the NCERT book. The advantages of using these solutions are given below:
- By using these solutions students can get a clear explanation of the physical and chemical properties of alcohols, phenols, and ethers.
- These NCERT Exemplar Class 12 Solutions are written in a clear and comprehensive manner that includes structured reaction mechanisms and conversions.
- These Chemistry Class 12 NCERT Exemplar Chapter 11 Alcohols, Phenols, and Ethers solutions are very helpful in revision before exams.
- They contain key points and formulas for quick revision.
Topics of NCERT Exemplar Class 12 Chemistry Solutions Alcohols, Phenols, and Ethers
Important topics of NCERT Exemplar Class 12 Chemistry Chapter 11 are given below:
- Alcohols, Phenols and Ethers
- Classification
- Nomenclature
- Structures of Functional Groups
- Alcohols and Phenols
- Some Commercially Important Alcohols
- Ethers
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Exemplar Solutions Class 12
Here is a list of NCERT Exemplar Solution, Subject-wise :
NCERT Solution subject-wise
The hyperlinks of NCERT exemplar of class 12 are given below:
NCERT Notes subject-wise
The hyperlinks of the NCERT solution of class 12 are given below:
NCERT Books and NCERT Syllabus
Students can refer to the links given below for the NCERT books and Syllabus:
Frequently Asked Questions (FAQs)
Alcohols exhibit intermolecular hydrogen bonding due to the presence of the -OH group. Ethers, on the other hand, have only weak dipole-dipole interactions (unless they contain bulky alkyl groups that hinder close packing). Hydrogen bonds are much stronger than dipole-dipole interactions, requiring more energy to overcome, hence the higher boiling point of alcohols.
The -OH group in alcohols can form hydrogen bonds with water molecules, which helps them dissolve in water. However, as the size of the alkyl group (R) increases, the hydrophobic (water-repelling) nature of the alkyl group dominates, decreasing the solubility of the alcohol in water. Smaller alcohols are generally more soluble than larger alcohols.
The general formula for alcohols is R-OH, where R is an alkyl group.
Cleavage of C-O bond- Ethers are relatively unreactive. However, they can be cleaved by strong acids like HI or HBr. The reaction proceeds via SN1 or SN2 mechanisms depending on the nature of the ether. Aromatic ethers undergo electrophilic aromatic substitution due to the +R effect of the oxygen atom.
Denatured alcohol is ethanol that has been made unfit for drinking by adding substances like methanol, copper sulfate, or pyridine. This is done to prevent the misuse of ethanol meant for industrial purposes as an alcoholic beverage, which would avoid excise taxes.
Phenol is a type of organic compound that consists of a hydroxyl (-OH) group attached to a benzene ring. Unlike alcohols, phenols are acidic in nature due to the resonance stabilization of the phenoxide ion.
Alcohols, Phenols, and Ethers NCERT Exemplar Class 12 Chemistry Chapter 11 covers preparation, properties, reactions, and uses of alcohols, phenols, and ethers with focus on mechanism-based questions.
They help you practise higher-order and application-based questions essential for CBSE board and competitive exams.
Lucas test, Kolbe reaction, Reimer–Tiemann reaction, dehydration of alcohols, and electrophilic substitution in phenols.
You get MCQs, reasoning questions, mechanism questions, conversions, and numerical-type problems.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
















































