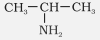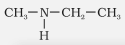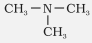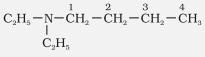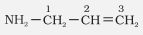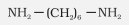Along with the class 12 chemistry chapter 9 amines notes, you can check the hyperlinks given below for notes of all other chapters in Class 12.
Amines Class 12th Notes- Free NCERT Class 12 Chemistry Chapter 13 Notes- Download PDF
What is the first thing that comes to your mind when you are bored or sleepy? An energy drink or maybe a soda? Do you know that coffee contains caffeine, an alkaloid with amine groups? They block sleep-inducing receptors in the brain. This is how active amines are in our daily lives. Amines are vital organic compounds found in many substances, from neurotransmitters and hormones to medicines and industrial chemicals. They are widely found in Proteins, Vitamins, and hormones, and are derivatives of ammonia, where one or more hydrogen atoms are replaced by alkyl or aryl groups.
This Story also Contains
- NCERT Notes for Class 12 Chapter 9 Amines: Download PDF
- NCERT Notes for Class 12 Chapter 9 Amines
- Amines Previous Year Questions and Answers
- How to Master Class 12 Chemistry Chapter 9 Amines
- Advantages of Using Class 12 Chemistry Chapter 9 Amines Notes
- CBSE Class 12 Chemistry Chapter-wise Notes
- NCERT Solutions for Class 12 Chemistry
- Subject-Wise NCERT Exemplar Solutions
NCERT Class 12 Chemistry Notes cover a brief idea of the chapter on Amines. The primary topics discussed in amines class 12 ncert notes are Nomenclature, classification, preparation, physical properties, structure, and chemical reactions of Amines. These notes also comprise a brief introduction to diazonium salts, their Physical Properties, methods of Preparation, chemical reactions, and their Importance in the Synthesis of Aromatic Compounds. Students can also refer to the NCERT Notes for assistance with other chapters. These Notes are an indispensable resource, whether you are preparing for an examination or studying the subject for the first time.
NCERT Notes for Class 12 Chapter 9 Amines: Download PDF
Students can download the ncert class 12 chemistry chapter 9 amines notes pdf from the icon given below to access a clear explanation, important reactions of this chapter. These NCERT notes for Class 12 cover all the key concepts of the Amines.
Also, students can refer,
NCERT Notes for Class 12 Chapter 9 Amines
NCERT notes Class 12 Chemistry Chapter 8 Amines offer a clear and concise explanation of important concepts, reactions, and properties. These notes help students strengthen their understanding and prepare efficiently for board and competitive exams. Scroll down to access these notes.
Amines
When hydrogens in ammonia are replaced by one or more alkyl or aryl groups, amines are formed. They are derivatives of ammonia.
Structure of amines
- Hybridization: sp3
- Geometry: pyramidal
- No. of lone pairs: 1
- Bond angle: less than 109.5°
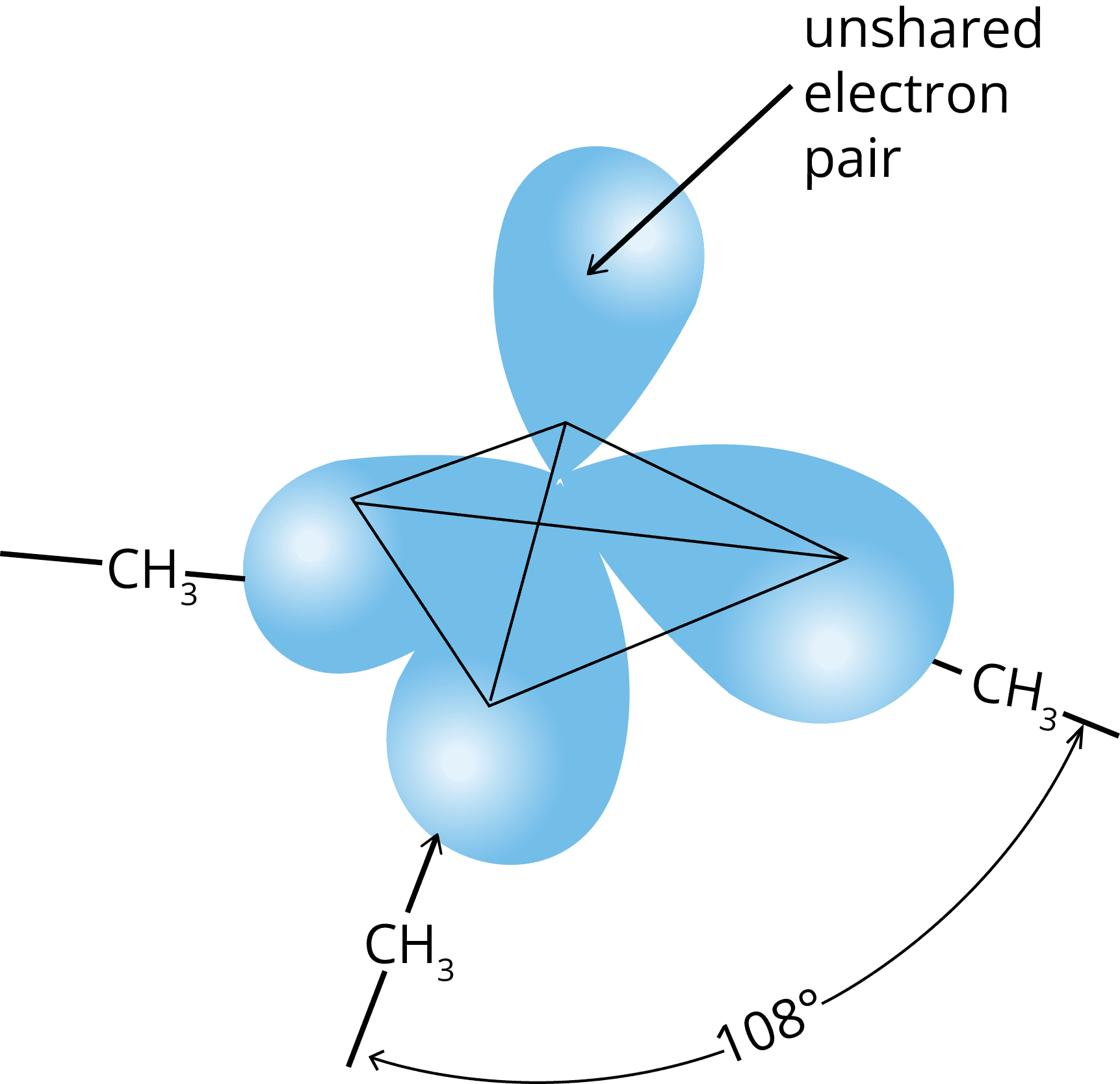
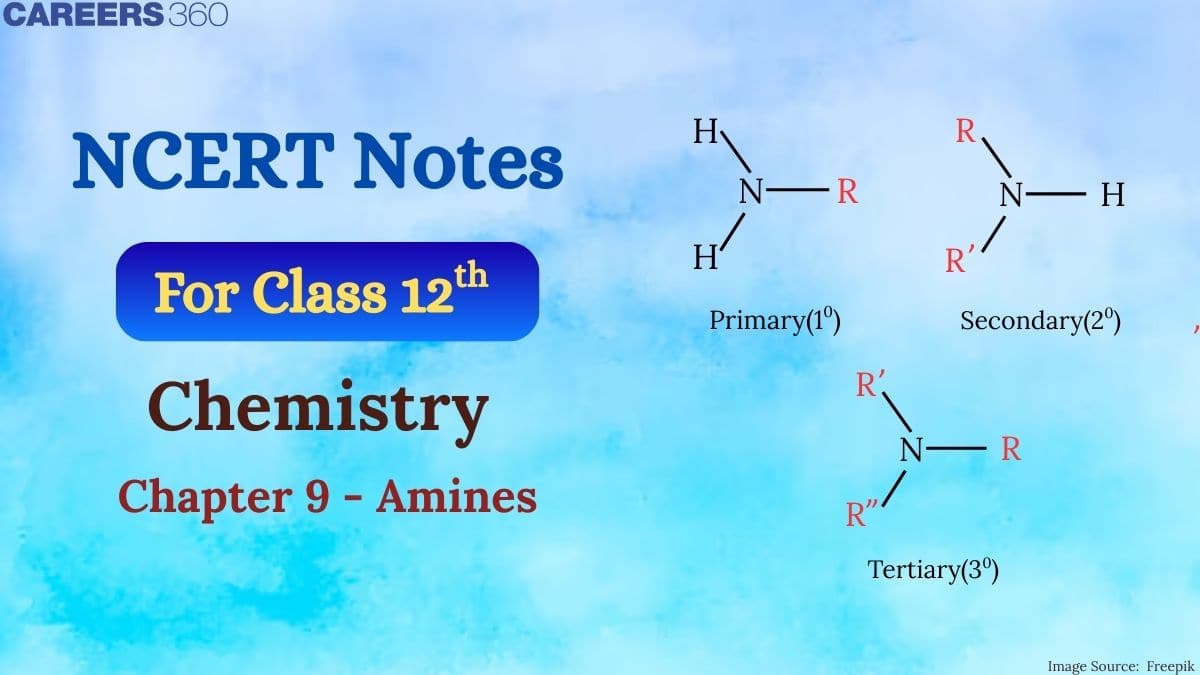
Classification of amines
On the basis of the number of hydrogen atoms replaced by alkyl or aryl groups in the ammonia molecule amines can be of 3 types:
- Primary
- Secondary
- Tertiary
Nomenclature
Amines are named alkanamines in the IUPAC system. Di, tri, etc prefixes are used depending on the no. of $\mathrm{NH}_2$ group attached to the carbon chain.
Examples are given in the tabular form:
|
Amine |
Common name |
IUPAC name |
|
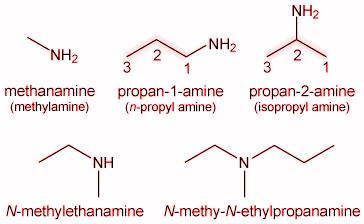
$\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{NH}_2$ |
Ethylamine |
Ethanamine |
|
$\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NH}_2$ |
n-Propylamine |
Propan-1-amine |
|
|
Isopropylamine |
Propan-2-amine |
|
|
Ethylmethylamine |
N-Methylethanamine |
|
|
Trimethylamine |
N, N-Dimethylmethanamine |
|
|
N,N-Diethylbutylamine |
N,N-Diethylbutan-1-amine |
|
|
Allylamine |
Prop-2-en-1-amine |
|
|
Hexamethylenediamine |
Hexane-1,6-diamine |
|
|
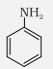
Aniline |
Aniline or Benzenamine |
|
|
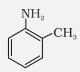
o-Toluidine |
2-Methylaniline |
|
|
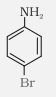
p-Bromoaniline |
4-Bromobenzenamine or 4-Bromoaniline |
|
|
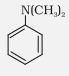
N, N-Dimethylaniline |
N,N-Dimethylbenzenamine |
Preparation of Amine
Preparation involves various methods to synthesise amines from different starting materials. This topic covers important reactions, techniques, and examples essential for preparation of amines. Students can refer to class 12 chemistry chapter 9 amines notes to understand these concepts better.
Given below the various methods
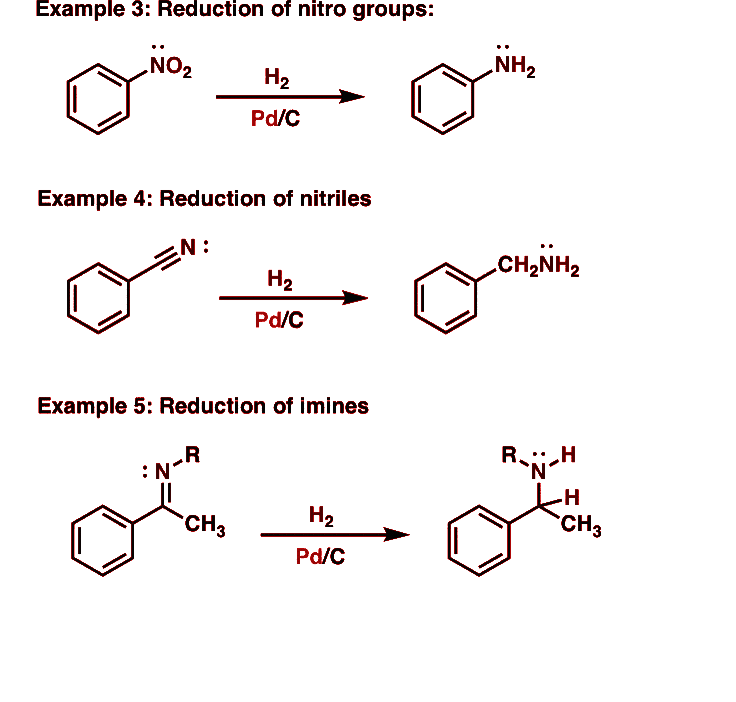
1. Reduction of nitro compounds
Nitro compounds are reduced to amines in the presence of finely divided Ni, Pd, or Pt & by passing hydrogen gas. Amines can also be prepared by reduction with metals in an acidic medium.
2. Ammonolysis of alkyl halides
Ammonia undergoes a nucleophilic substitution reaction in which a halogen atom is replaced by an amino group. The process by which ammonia molecules cleave the C-X bond is called ammonolysis.
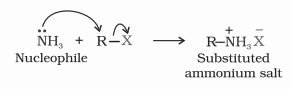
The reactivity order of halides with amines: RI > RBr >RCl
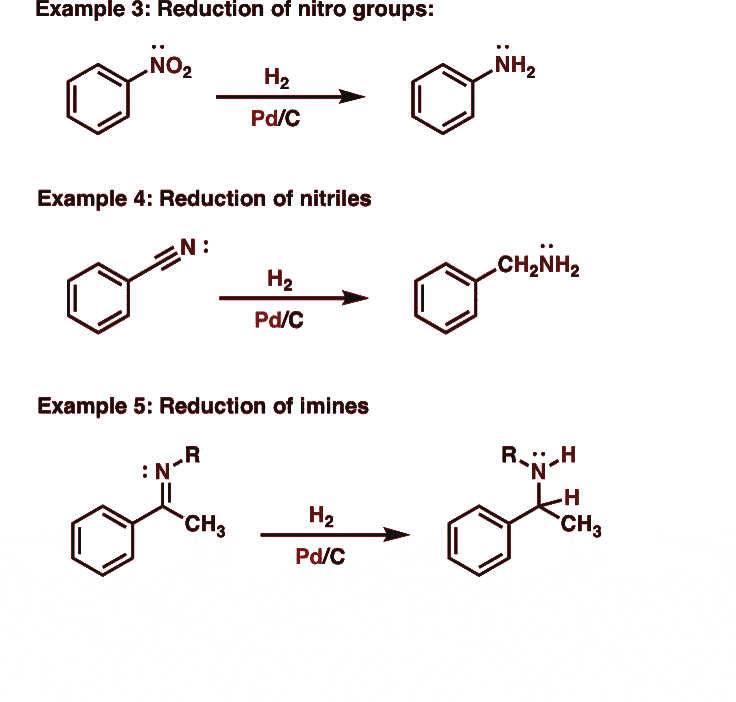
3. Reduction of nitriles
Nitriles on catalytic hydrogenation or reduction with lithium aluminum hydride produce primary amines.
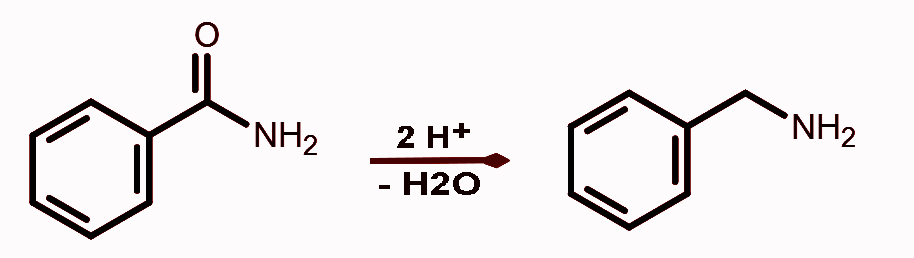
4. Reduction of amides
Amides undergo reduction to produce amines.
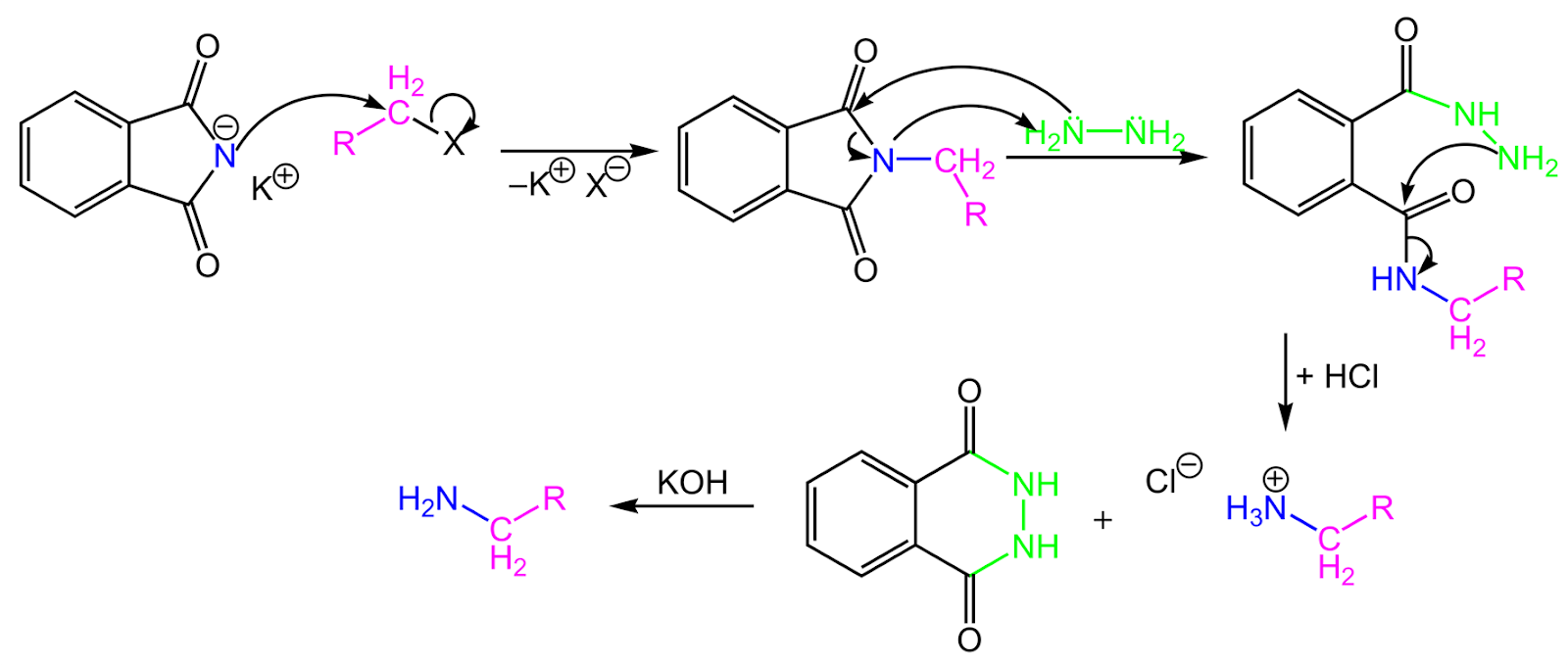
5. Gabriel phthalimide synthesis
Gabriel phthalimide synthesis is a reaction that involves the conversion of primary alkyl halides into primary amines using alkyl halides.
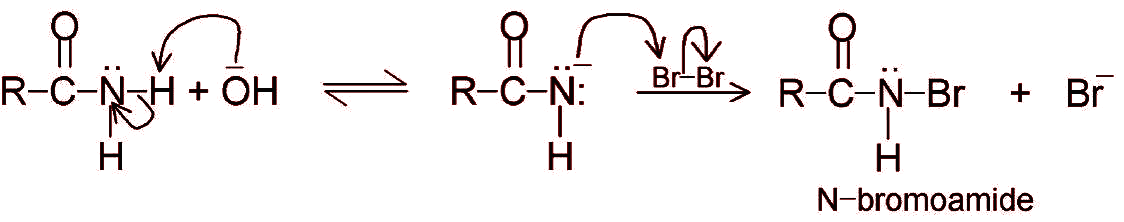
6. Hoffmann bromamide degradation reaction
Physical Properties of Amines:
- Primary amines having 3 or more C are liquid & heavier ones are solid.
- Aryl amines are generally colorless but on atmospheric oxidation gain color.
- Amines are polar molecules due to the presence of lone pairs which induce large dipole moments.
- The primary & secondary amines can undergo H bonding. The tertiary amine cannot undergo H bonding but can accept H bonds from N-H or O-H bonds.
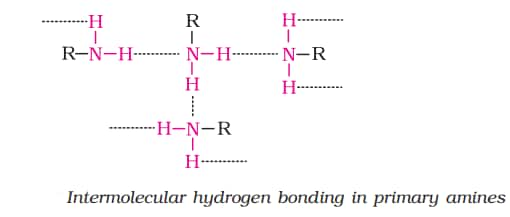
- In amines solubility decrease as the molar mass of amines increases. This is because the hydrophobic alkyl part increases in size. Amines with high molecular mass are insoluble in water.
- Boiling point : Ethers (similar molecular weights) < Primary & secondary amine < alcohols ; Primary ≈ Secondary > Tertiary amines
Chemical Reaction of Amines
Amines are reactive due to the electronegativity difference between N and H, as well as the presence of lone pairs. You can download amines Class 12 Chemistry Chapter 9 CBSE notes for offline access, making it easier to study.
1. Basic character of amines
Amines react with acid to form salts.
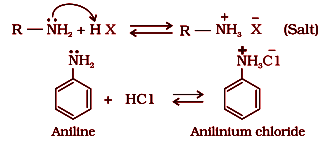
Structure-basicity relationship of amines
(a) Alkanamines versus ammonia
The basicity order of amines in the gaseous phase: 3° amine > 2° amine > 1° amine > ammonia
The basicity order of aliphatic amines: 1° > 2° > 3°
The order of basic strength in case of substituted amines:
$\begin{aligned} & \left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{NH}>\left(\mathrm{C}_2 \mathrm{H}_5\right)_3 \mathrm{~N}>\mathrm{C}_2 \mathrm{H}_5 \mathrm{NH}_2>\mathrm{NH}_3 \\ & \left(\mathrm{CH}_3\right)_2 \mathrm{NH}>\mathrm{CH}_3 \mathrm{NH}_2>\left(\mathrm{CH}_3\right)_3 \mathrm{~N}>\mathrm{NH}_3\end{aligned}$
In the aqueous phase, the substituted ammonium cations get stabilised not only by electron releasing effect of the alkyl group (+I) but also by solvation with water molecules. The greater the size of the ion, lesser will be the solvation and the less stabilised is the ion. The order of stability of ions are as follows:
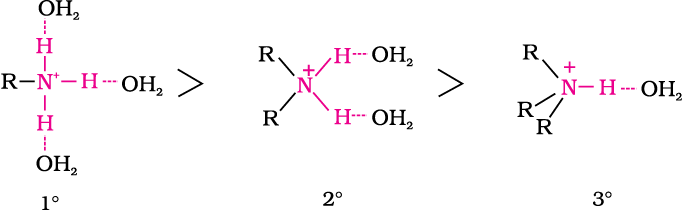
(b) Arylamines vs ammonia
In the case of substituted arylamines, electron-releasing groups like –OCH3, and –CH3 increase basic strength whereas electron-withdrawing groups like –NO2, –SO3H, –COOH, and –X decrease basic strength.
2. Alkylation
3. Acylation
Aliphatic & aromatic primary & secondary amines undergo nucleophilic substitution reactions with acid chlorides, anhydrides & esters.
.png)
4. Carbylamine reaction
$\mathrm{R}-\mathrm{NH}_2+\mathrm{CHCl}_3+3 \mathrm{KOH} \xrightarrow{\text { Heat }} \mathrm{R}-\mathrm{NC}+3 \mathrm{KCl}+3 \mathrm{H}_2 \mathrm{O}$
5. Reaction with nitrous acid
(a) Primary aliphatic amines from aliphatic diazonium salts on reaction with nitrous acid.
$\mathrm{R}-\mathrm{NH}_2+\mathrm{HNO}_2 \xrightarrow{\mathrm{NaNO}_2+\mathrm{HCl}}\left[\mathrm{R}-\stackrel{+}{\mathrm{N}_2} \mathrm{Cl}\right] \xrightarrow{\mathrm{H}_2 \mathrm{O}} \mathrm{ROH}+\mathrm{N}_2+\mathrm{HCl}$
(b) Aromatic amines form diazonium salts on reaction with nitrous acid at low temperatures.
$\mathrm{C}_6 \mathrm{H}_5-\mathrm{NH}_2 \xrightarrow[273-278 \mathrm{~K}]{\mathrm{NaNO}_2+2 \mathrm{HCl}} \mathrm{C}_6 \mathrm{H}_5-\stackrel{+}{\mathrm{N}_2} \stackrel{-}{\mathrm{Cl}}+\mathrm{NaCl}+2 \mathrm{H}_2 \mathrm{O}$
Secondary and tertiary amines react with nitrous acid in a different manner.
6. Reaction with aryl sulphonyl chloride
Benzenesulphonyl chloride $\left(\mathrm{C}_6 \mathrm{H}_5 \mathrm{SO}_2 \mathrm{Cl}\right)$, which is also known as Hinsberg’s reagent, reacts with primary and secondary amines to form sulphonamides.
(a) The reaction of benzene sulphonyl chloride yields N-ethylbenzene sulphonyl amide on reaction with a primary amine.
.jpg)
(b) N, N-diethyl benzene sulphonamide is formed in the reaction with a secondary amine with benzene sulphonyl chloride.
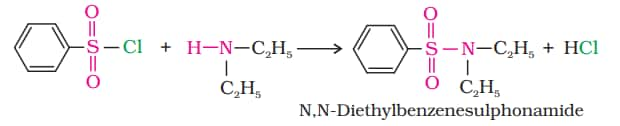
(c) Tertiary amines & benzene sulphonyl chloride do not react.
7. Electrophilic substitution
(a) Bromination:
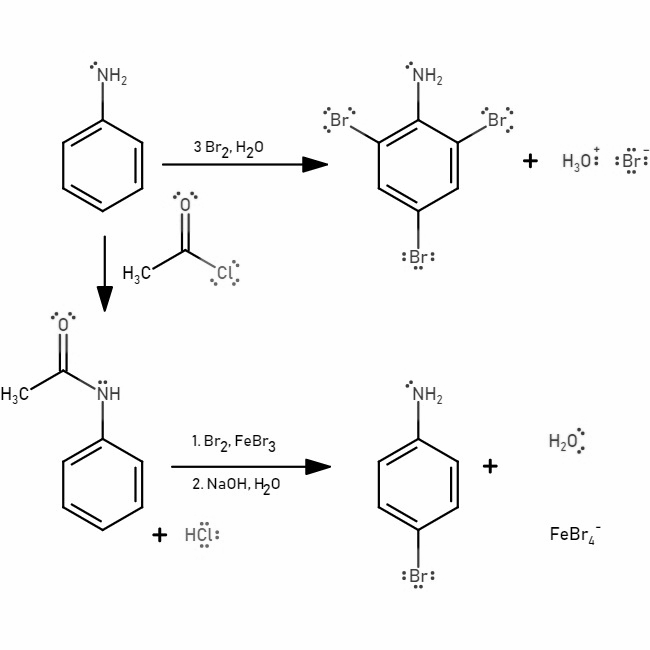
(b) Nitration:
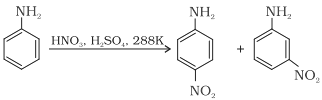
(c) Sulphonation:
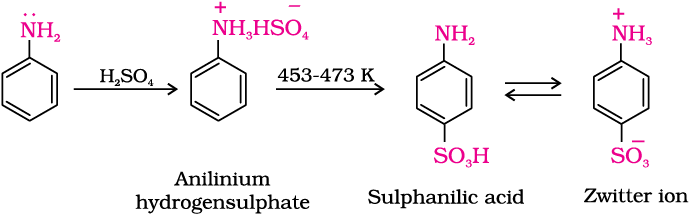
Diazonium Salt
The diazonium salts have the general formula – RN2 + X- where R stands for an aryl group & X- ion may be Cl-, Br-, HSO4-, BF4-, etc.
Nomenclature: The name of parent hydrocarbon is added as prefix & name of anion after diazonium. Example: benzenediazonium chloride etc.
1° aliphatic amines form highly unstable alkyl diazonium salts & 1° aromatic amines form arene diazonium salts (stable at low temperature for short time). The stability of arenediazonium ion is explained on the basis of resonance.
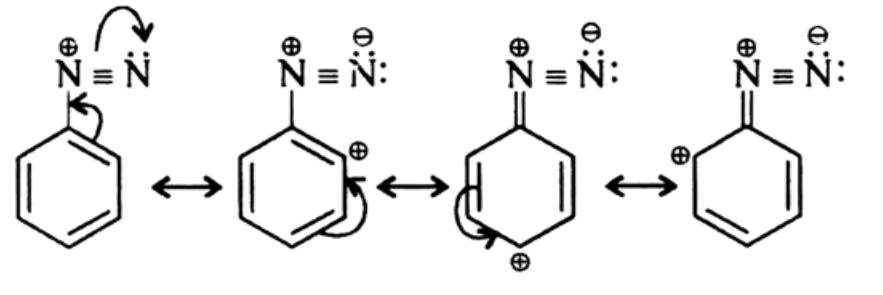
Methods of Preparation of Diazonium Salts
The reaction of aniline with nitrous acid produces Benzene diazonium chloride. Diazotization is the term coined for the conversion of primary aromatic amines into diazonium salts.
$\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2+\mathrm{NaNO}_2+2 \mathrm{HCl} \xrightarrow{273-278 \mathrm{~K}} \mathrm{C}_6 \mathrm{H}_5 \mathrm{~N}_2^{+} \mathrm{Cl}+\mathrm{NaCl}+2 \mathrm{H}_2 \mathrm{O}$
Physical Properties
- Colorless crystalline solid
- Soluble in water (cold water-stable; warm water - react)
- Decomposes easily in the dry state
- Some diazonium salts like Benzenediazonium fluoroborate are water-insoluble & stable at room temperature.
Chemical Reactions
(A) Reactions Involving Displacement of Nitrogen
1. Replacement by halide or cyanide ion:
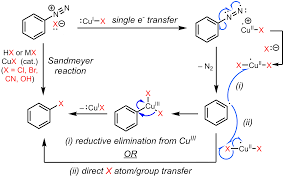
Also known as the Sandmeyer reaction; involves the nucleophilic substitution of diazonium salt to give aryl halide in presence of Cu(I) ion.
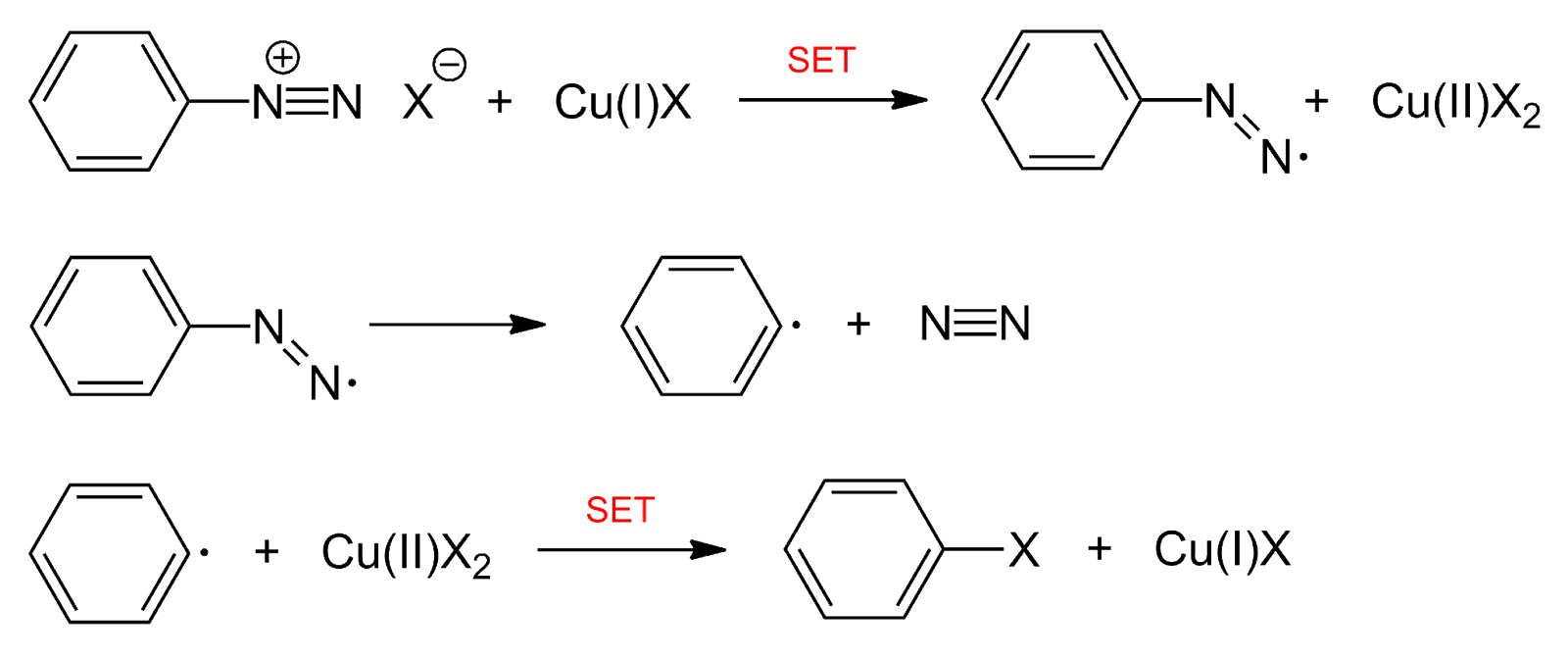
Also known as the Gatterman reaction; involves the nucleophilic substitution of diazonium salt to give aryl halide in the presence of copper powder. Sandmeyer’s reaction is better than Gattermann’s reaction.
2. Replacement by iodide ion:
Iodobenzene is formed when a diazonium salt solution is treated with potassium iodide.
$\mathrm{Ar}_2^{+} \stackrel{-}{\mathrm{N}}_2+\mathrm{KI} \longrightarrow \mathrm{ArI}+\mathrm{KCl}+\mathrm{N}_2$
3. Replacement by fluoride ion:
Arene diazonium fluoroborate is produced on treating arene diazonium chloride with fluoroboric acid which on heating decomposes to yield aryl fluoride.
$\mathrm{ArN}_2^{+} \stackrel{-}{\mathrm{Cl}}+\mathrm{HBF}_4 \longrightarrow \mathrm{Ar}-\stackrel{+}{\mathrm{N}}_2 \mathrm{BF}_4 \xrightarrow{\Delta} \mathrm{Ar}-\mathrm{F}+\mathrm{BF}_3+\mathrm{N}_2$
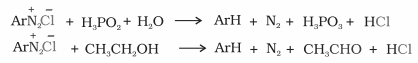
4. Replacement by H:
Diazonium salts are reduced to arenes when treated with ethanol or hypophosphorous acid.
5. Replacement by hydroxyl group:
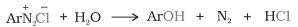
6. Replacement by –NO2 group:
The Diazonium group is replaced by the –NO2 group when the diazonium fluoroborate is heated with aqueous NaNO2 in the presence of Cu.
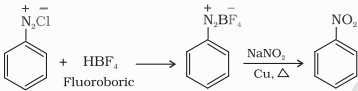
B. Reactions involving retention of diazo group
Coupling reactions:
A reaction between Benzene diazonium chloride & phenol results in coupling between two molecules at the para position of phenol via -N=N- bond. P-hydroxyazobenzene is formed as a product.
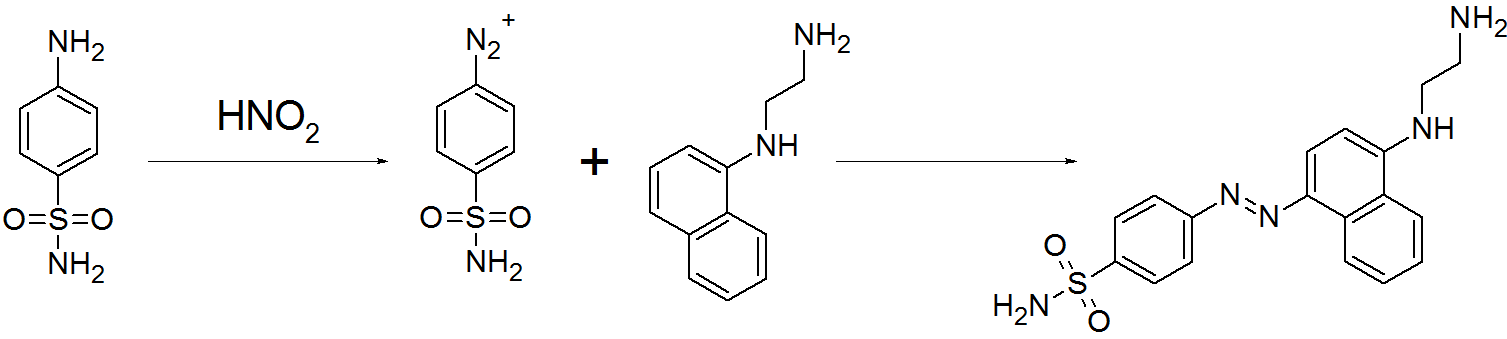
Azo coupling of sulfanilamide acid & N-(1-Naphthyl) ethylene di amine
Importance of Diazonium Salts in the Synthesis of Aromatic Compounds
Diazonium salts are a very good intermediate therefore they are helpful in a reaction where direct substitution is not possible. For example, aryl fluorides, aryl iodides, cyanobenzene, & chlorobenzene can not be prepared by direct substitution but are easily prepared when the diazo group is introduced.
Amines Class 12 notes will be helpful to revise the chapter & to get an idea about the main topics covered in the chapter. Also, amines ncert notes are useful to cover the main topics of the Class 12 CBSE Chemistry Syllabus & also for competitive exams like VITEEE, BITSAT, JEE Core, NEET, etc.
Amines Previous Year Questions and Answers
Selected Previous Year Questions and Answers from this chapter. The concepts used to solve these questions are explained in ncert class 12 chemistry chapter 9 amines notes. They cover basic concepts, properties, reactions, and common uses of amines in a simple way.
Question 1: The correct order of basic nature on aqueous solution for the bases $\mathrm{NH}_3, \mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2$, $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$ and $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}$ is :
(1) $\mathrm{NH}_3<\mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2<\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2<$ $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$
(2) $\mathrm{NH}_3<\mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2<\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}<$ $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}$
(3) $\mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2<\mathrm{NH}_3<\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2<$ $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$
(4) $\mathrm{NH}_2-\mathrm{NH}_2<\mathrm{NH}_3<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2<\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}<$ $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$
Answer:
Basic strength of amine depends on hydrogen bonding and electronic inductive effect.
$\mathrm{NH}(\mathrm{Et})_2>\mathrm{N}(\mathrm{Et})_3>\mathrm{NH}_2 \mathrm{Et}>\ddot{\mathrm{N}}_3>\mathrm{NH}_2-\mathrm{NH}_2$
Hence, the correct answer is option (4).
Question 2: When a concentrated solution of sulphanilic acid and 1-naphthylamine is treated with nitrous acid (273 K) and acidified with acetic acid, the mass (g) of 0.1 mole of product formed is : (Given molar mass in g mol–1 H : 1, C : 12, N : 14,O : 16, S : 32)
(1) 343
(2) 330
(3) 33
(4) 66
Answer:


Molar mass of product formed
$\begin{aligned}
& =(16 \times 12)+(14 \times 3)+(16 \times 3)+32+13 \\
& =327 \mathrm{~g}
\end{aligned}$
Mass of 0.1 mol product
$32.7 \mathrm{~g} \approx 33 \mathrm{~g}$
Hence, the correct answer is option (3).
Question 3: Amines have a lone pair of electrons on nitrogen atom due to which they behave as Lewis base. Greater the value of $K_b$ or smaller the value of $\mathrm{pK}_{\mathrm{b}}$, stronger is the base. Amines are more basic than alcohols, ethers, esters, etc. The basic character of aliphatic amines should increase with the increase of alkyl substitution. But it does not occur in a regular manner as a secondary aliphatic amine is unexpectedly more basic than a tertiary amine in aqueous solutions. Aromatic amines are weaker bases than ammonia and aliphatic amines. Electron releasing groups such as $-\mathrm{CH}_3$, $-\mathrm{OCH}_3,-\mathrm{NH}_2$, etc., increase the basicity while electron-withdrawing substituents such as $-\mathrm{NO}_2,-\mathrm{CN}$, halogens etc., decrease the basicity of amines. The effect of these substitute is more at $\mathrm{p}^{-}$than at $\mathrm{m}^{-}$position.
Why $\mathrm{pK}_{\mathrm{b}}$ of aniline is more than that of methylamine?
Answer: The pKb of aniline is higher than that of methylamine because aniline is a weaker base. The basicity of a compound is related to its ability to accept a proton. Aniline undergoes resonance, and as a result, the lone pair on the N-atom is partially delocalized over the benzene ring. Therefore, the electrons on the N-atom are less available to donate.

On the other hand, in the case of methylamine, due to the +I effect of the methyl group, the electron density on the N-atom is increased, which makes the lone pair more available for protonation.

As a result, aniline is less basic than methylamine. Thus, the pKb of aniline is more than that of methylamine.
Question 4: The descending order of basicity of following amines is :
(A)

(D) $\mathrm{CH}_3 \mathrm{NH}_2$
(E) $\left(\mathrm{CH}_3\right)_2 \mathrm{NH}$
Choose the correct answer from the options given below :
(1) B $>$ E $>$ D $>$ A $>$ C
(2) E $>$ D $>$ B $>$ A $>$ C
(3) E $>$ D $>$ A $>$ B $>$ C
(4) E $>$ A $>$ D $>$ C $>$ B
Answer:
Aliphatic amines are more basic than aromatic amines, as the lone pair on nitrogen is localized.

E > D > B > A > C
Hence, the correct answer is option (2).
Question 5: Which of the following amine (s) show (s) positive carbylamines test ?

Choose the correct answer from the options given below :
(1) A and E Only
(2) C Only
(3) A and C Only
(4) B, C and D Only
Answer:
Only $1^{\circ}$ or primary amines gives positive carbylamines test.

Option (A) and (C) are primary amine and will give positive carbyl amine test
Hence, the correct answer is option (3).
How to Master Class 12 Chemistry Chapter 9 Amines
These ncert class 12 chemistry chapter 9 amines notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 9.
- Firstly students need to understand the basic concepts, nomenclature, and classification of amines.
- Then students need to learn about methods of preparation, physical properties, chemical properties, and uses of amines.
- Important name reactions like Hoffmann bromamide degradation, Carbylamine reaction, Diazotisation, and Sandmeyer reaction are often asked in exams. Students can refer to amines Class 12 Chemistry Chapter 9 CBSE notes for understanding these concepts better.
- Basicity of amines and the factors affecting it plays an important role in this chapter.
- After that students can solve previous year questions from this chapter.
Advantages of Using Class 12 Chemistry Chapter 9 Amines Notes
NCERT notes Class 12 Chemistry Chapter 8 Amines cover all important concepts from the NCERT book in a simple and organised manner. The advantages of using these notes are given below:
- Students can get a clear understanding of the structure, classification, and preparation methods of amines.
- These notes include mechanisms of important reactions like diazotisation and Hoffmann bromamide reaction.
- The amines class 12 ncert notes contain key points, tables, and formulas for easy learning.
- They are created by subject experts in a very clear and comprehensive way that are helpful in both board and competitive exams.
CBSE Class 12 Chemistry Chapter-wise Notes
NCERT Solutions for Class 12 Chemistry
Along with NCERT notes Class 12 Chemistry Chapter 8 Amines, students can also refer to solutions of NCERT for Class 12 Chemistry chapters.
Subject-Wise NCERT Exemplar Solutions
The hyperlinks of the NCERT exemplar solutions, subject-wise, are given below:
Frequently Asked Questions (FAQs)
The amines class 12 ncert notes play a crucial role in the pharmaceutical industry as many drugs contain amine functional groups, which influence their biological activity. For example, many analgesics, antidepressants, and antibiotics contain amine groups that are essential for their therapeutic effects.
When amines react with acids, they form ammonium salts. The basic amine donates a lone pair of electrons to the proton from an acid, resulting in the formation of a positively charged ammonium ion.
Amines generally have higher boiling points than hydrocarbons of comparable molecular weight due to the presence of hydrogen bonding. They are often soluble in water if the amine has a low molecular weight. As you increase the size of the carbon chain, solubility tends to decrease.
Amines are considered basic because they can accept protons due to the lone pair of electrons present on the nitrogen atom. The basicity varies among primary, secondary, and tertiary amines, generally decreasing from primary to tertiary amines due to steric hindrance.
Class 12 Chemistry notes help students by summarising the vast syllabus into concise, easy-to-understand points. They make revision faster, clarify complex concepts, highlight important reactions and mechanisms, and provide a ready reference for practising numerical problems and previous year questions, ensuring effective exam preparation.
Students should care about Class 12 Chemistry Amines notes because they simplify complex reactions, clearly explain mechanisms, and highlight important name reactions and properties. These notes save time during revision, improve understanding, and help in solving exam questions accurately and efficiently.
Amines notes in Class 12 Chemistry are concise study materials that cover the classification, nomenclature, methods of preparation, physical and chemical properties, and important reactions of amines. These notes highlight key mechanisms, name reactions, and differences between primary, secondary, and tertiary amines, making revision easier and helping students prepare effectively for exams.
Amines have a nitrogen atom bonded to alkyl or aryl groups (R-NH₂), making them basic and reactive. Amides have a nitrogen bonded to a carbonyl group (R-CO-NH₂), which reduces the nitrogen's basicity and reactivity due to resonance with the carbonyl group.
Aliphatic amines are more basic than aromatic amines because the lone pair of electrons on nitrogen in aliphatic amines is more accessible for protonation. In aromatic amines, the lone pair on nitrogen is partially delocalized into the aromatic ring, reducing its availability to accept a proton, thus making aromatic amines less basic.
Amines are used in various industries, including: Pharmaceuticals, Dyes and Pigments, Agriculture (Pesticides & Fertilizers), Water Treatment, Personal Care and Cosmetics, Rubber Industry, Flavors and Fragrances,Textiles.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters