These alcohols, phenols and ethers Class 12 Chemistry Chapter 7 CBSE notes are concise, organised summaries of all key concepts, preparation methods, properties, reactions, and mechanisms from the NCERT textbook. These notes help students revise quickly, understand concepts clearly, and prepare effectively for exams.
Free NCERT Class 12 Chemistry Chapter 11 Notes Alcohols, Phenols and Ethers - Download PDF
Have you ever wondered where common products such as soft drinks, perfumes, antiseptics, and disinfectants that we use daily come from? These products come from oxygen containing organic compounds. For example ethanol which is most commonly used alcohol is used in making soft drinks, and beverages, Phenols are used in antiseptics and disinfectants, and Ethers are used in perfumes. These alcohols, phenols and ethers ncert solutions discusses three important classes of compounds that feature oxygen containing organic compounds which are widely used in the medical industry and in daily life.
This Story also Contains
- NCERT Notes for Class 12 Chapter 7: Download PDF
- NCERT Notes for Class 12 Chapter 7
- Alcohols ,Phenols and Ethers Previous Year Question and Answer
- How to Master Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers
- Advantages of Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers Notes
- CBSE Class 12 NCERT Chemistry Chapter-Wise Notes
- NCERT Solutions for Class 12 Chemistry
- Subject-Wise NCERT Solutions
- Subject-Wise NCERT Exemplar Solutions
NCERT Class 12 Chemistry notes provide a structured approach to understanding the classification, properties, preparation, and reactions of Chapter 7. These notes are prepared by our subject experts in a comprehensive way that helps students understand the concepts in a very simple and easy way. These alcohols, phenols and ethers ncert notes cover all the topics as prescribed by the latest CBSE syllabus, and selected previous year questions are also added to help enhance the clarity and problem-solving ability of students.
NCERT Notes for Class 12 Chapter 7: Download PDF
These concise class 12 chemistry chapter 7 alcohols, phenols and ethers notes cover all the key concepts to help students in quick revision before the exam. You can download the NCERT Notes from the button given below:
Also Read
NCERT Notes for Class 12 Chapter 7
These ncert class 12 chemistry chapter 7 alcohols, phenols and ethers notes are given below and they covers all concepts of the NCERT textbook and explains them in a concise and easy-to-understand manner. Whether revising or studying for the first time these NCERT notes for Class 12 are a boon for students who aim to score well in their exams.
Classification
The classification of compounds Alcohold, Phenols and Ethers makes their study systematic and hence simpler.
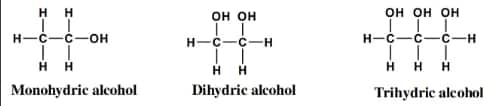
1. Alcohols Mono, Di, Tri or Polyhydric alcohols
Alcohols and phenols are classed as monohydric, dihydric, trihydric, or polyhydric depending on how many hydroxyl groups they have in their molecules.
According to the hybirdisation of Carbon atom to which the hydroxyl group is attached, Monohydric alcohols are further classified:
(i). Compounds containing $\mathrm{C}_{s p^3}-\mathrm{OH}$ group: Alcohols in which the –OH group is attached to an $s p^3$ hybridised carbon atom of an alkyl group.
They are further classified as:
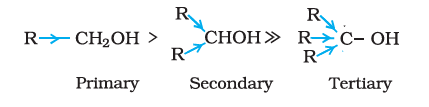
(a). Primary, secondary and tertiary alcohols: The –OH group is attached to primary, secondary and tertiary carbon atom.
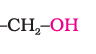
Primary alcohol
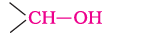
Secondary alcohol
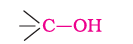
Tertiary alcohol
Primary Secondary Tertiary
(b). Allylic alcohols: The OH group is attached to a $s p^3$ hybridised carbon adjacent to the carbon-carbon double bond, that is to an allylic carbon.
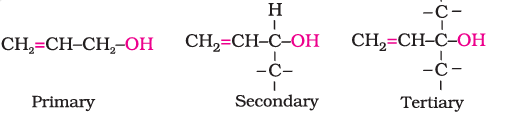
(c). Benzylic alcohols: The OH group is attached to a $s p^3$ hybridised carbon atom next to an aromatic ring.
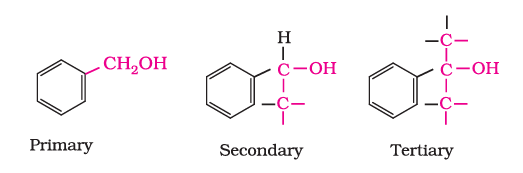
(ii) Compounds containing $\mathrm{C}_{s p^2}-\mathrm{OH}$ bond
These alcohols contain OH group bonded to a carbon-carbon double bond, i.e., to a vinylic carbon or to an aryl carbon. These alcohols are also known as vinylic alcohols.
Vinylic alcohol: $\mathrm{CH}_2=\mathrm{CH}-\mathrm{OH}$
2. Phenols Mono, Di and trihydric phenols
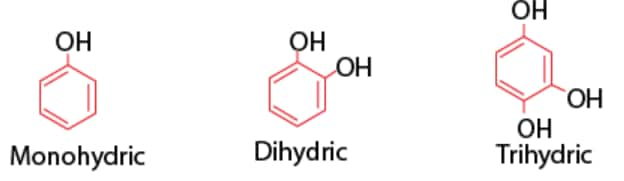
3. Ethers
Ethers are classified as simple or symmetrical, if the alkyl or aryl groups attached to the oxygen atom are the same, and mixed or unsymmetrical, if the two groups are different.
Nomenclature
1. Alcohols
The common name of an alcohol is derived from the common name of the alkyl group and adding the word alcohol to it. For example, $\mathrm{CH}_3 \mathrm{OH}$ is methyl alcohol.
IUPAC Nomenclature:
- Select the longest chain containing the OH group.
-
Number the carbon so that the OH group gets the lowest possible position
-
Remove e from the alkane name and add ol.
-
Mention the position of the OH group
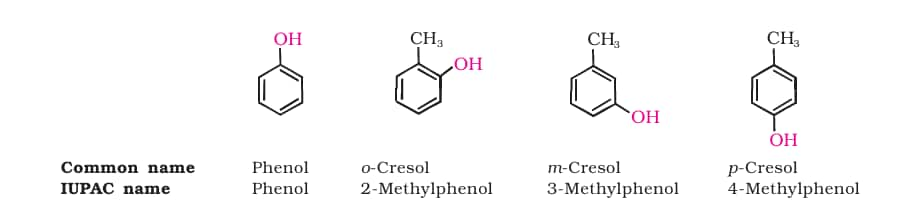
2. Phenols
The simplest hydroxy derivative of benzene is phenol. It is its common name and also an accepted IUPAC name. Students can understand these concepts better with the help of alcohols, phenols and ethers Class 12 Chemistry Chapter 7 CBSE notes.
IUPAC Nomenclature
- The parent compound is phenol.
- Number the ring in such a way so that the substituents gets the lowest possible position.
- Name and prefix substituents with their positions.
3. Ethers
Common names of ethers are derived from the names of alkyl/ aryl groups written as separate words in alphabetical order and adding the word ‘ether’ at the end.
IUPAC Nomenclature
- Name the longerst alkyl group as the base.
- Name the shorter alkyl group as an alkoxy group.
- Combine them as alkoxyalkane.
Structure of Functional Groups
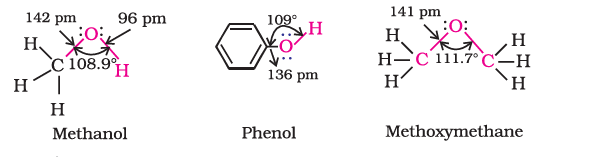
Alcohols have $s p^3$ hybridized oxygen atoms and hybrid atomic orbitals in a tetrahedral configuration. The R group determines the value of the ROH bond angle. Due to lone pair repulsion, this angle for methyl alcohol is (C – O – H) 108.9°.The -OH group in phenols is connected to $s p^2$ hybridized carbon, giving the C – O bond a partial double bond nature.
Alcohols and Phenols
Preparation of Alcohols
Alcohols are prepared by the following methods:
1. From alkenes
(i). By acid catalysed hydration
This reaction is in accordance with Markonikov's rule,
$\mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CH}_3-\mathrm{CH}(\mathrm{OH})-\mathrm{CH}_3$
Mechanism
It involves 3 steps:
Step1. Protonation of alkene to form carbocation by electrophilic attack of $\mathrm{H}_3 \mathrm{O}^{+}$
$\mathrm{H}_2 \mathrm{O}+\mathrm{H}^{+} \rightarrow \mathrm{H}_3 \mathrm{O}^{+}$
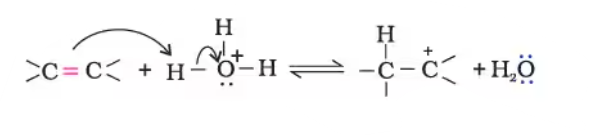
Step 2. Nucleophilic attack of water on carbocation
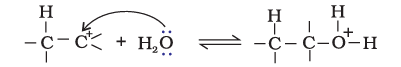
Step 3: Deprotonation to form an alcohol
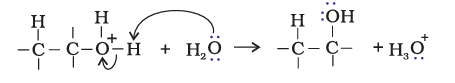
(ii). By hydroboration-oxidation
By hydroboration-oxidation of alkenes, alcohols can be prepared, where BH3 adds across the double bond followed by oxidation with $\mathrm{H}_2 \mathrm{O}_2 / \mathrm{NaOH}$ to give alcohols with anti-Markovnikov orientation.
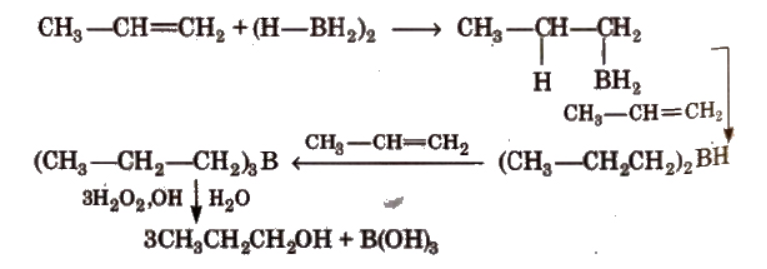
2. From carbonyl compounds
(i). By reduction of aldehydes and ketones
RCHO + H2 → RCH2OH
$\mathrm{RCOR} \xrightarrow{\mathrm{NaBH} 4} \mathrm{R}-\mathrm{CH}(\mathrm{OH})-\mathrm{R}$
(ii). By reduction of carboxylic acids
$\mathrm{RCOOH} \xrightarrow{\text { LiAl }_4, \mathrm{H}_2 \mathrm{O}} \mathrm{ROH}$
3. From Grignard reagents
All three types of monohydric alcohols can be prepared by the use of Grignard reagents. Grignard reagents form addition compounds by nucleophile attack with aldehydes and ketones which on hydrolysis with dilute acid yields alcohol.
$\mathrm{HCHO}+\mathrm{RMgX} \xrightarrow{\mathrm{H}_2 \mathrm{O}} \mathrm{RCH}_2 \mathrm{OH}+\mathrm{Mg}(\mathrm{OH}) \mathrm{X}$
$\mathrm{RCHO}+\mathrm{RMgX} \xrightarrow{\mathrm{H}_2 \mathrm{O}} \mathrm{R}-\mathrm{CH}(\mathrm{R}) \mathrm{OH}+\mathrm{Mg}(\mathrm{OH}) \mathrm{X}$
$\mathrm{RCOR}+\mathrm{RMgX} \xrightarrow{\mathrm{H}_2 \mathrm{O}} \mathrm{R}-\mathrm{C}(\mathrm{R})(\mathrm{R}) \mathrm{OH}+\mathrm{Mg}(\mathrm{OH}) \mathrm{X}$
Preparation of Phenols
Phenol, also known as carbolic acid, and is commercially produced synthetically. In the laboratory, phenols are prepared from benzene derivatives by any of the following methods:
1). From haloarenes
Chlorobenzene is fused with NaOH at 623K and 320 atmospheric pressure. Phenol is obtained by acidification of sodium phenoxide so produced. The reaction occurs as follows.
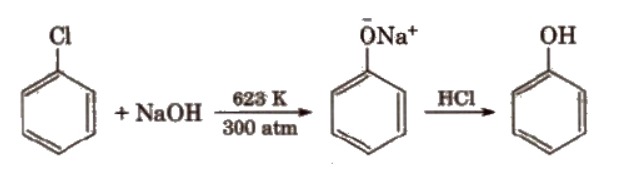
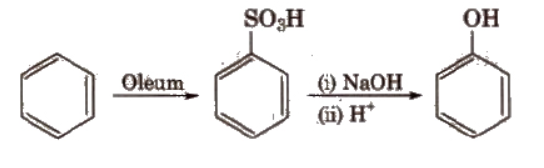
2). From benzenesulfonic acid
Benzene is sulphonated with oleum and benzene sulphonic acid so formed is converted to sodium phenoxide on heating with molten sodium hydroxide. Acidification of the sodium salt gives phenol. The reaction occurs as follows:
3). From diazonium salts
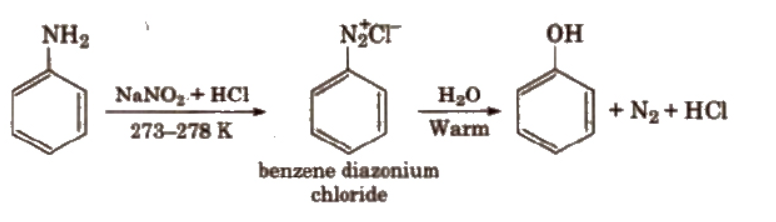
A diazonium salt is formed by treating an aromatic primary amine with nitrous acid (NaNO2 + HCl) at 273-278 K. Diazonium salts are hydrolysed to phenols by warming with water or by treating with dilute acids. The reaction occurs as follows.
4). From Cumenes
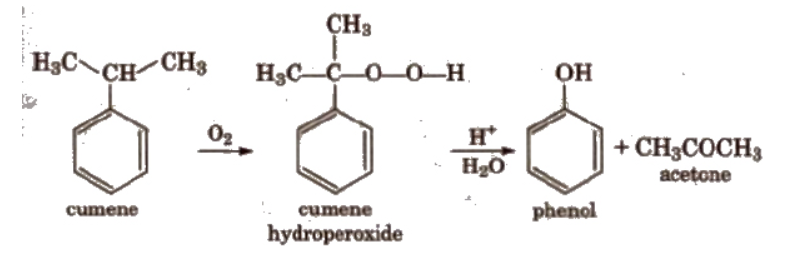
Phenol is manufactured from the hydrocarbon, cumene. Cumene (isopropylbenzene) is oxidised in the presence of air to cumene hydroperoxide. It is converted to phenol and acetone by treating it with dilute acid. Acetone, a by-product of this reaction, is also obtained in large quantities by this method. The reaction occurs as follows:
Physical Properties
Alcohols and phenols consist of two parts, an alkyl/aryl group and a hydroxyl group. The properties of alcohols and phenols are chiefly due to the hydroxyl group. The nature of alkyl and aryl groups simply modify these properties. Students can also follow NCERT notes Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers to understand these concepts with the help of questions.
(i). Boiling points of alcohols and phenols rise with increasing carbon atoms due to stronger van der Waals forces, but decrease with branching due to reduced surface area. They have higher boiling points than hydrocarbons, ethers, haloalkanes, and haloarenes of similar masses, mainly because of intermolecular hydrogen bonding. For example, despite similar molecular masses, ethanol boils much higher than propane, while methoxymethane shows an intermediate boiling point, reflecting the absence of hydrogen bonding in ethers.
(ii). Lower alcohols are colourless liquids, C5–C11 alcohols are oily liquids, and C12 and higher alcohols are waxy solids. Alcohols are miscible with water because their hydroxyl groups can form H-bonds with water. With increasing molecular mass, solubility decreases. Because polar molecules have intermolecular hydrogen bonding, the boiling points of alkanes are greater than expected.
(iii). These are colourless liquids or crystalline solids that turn coloured over time due to gradual oxidation in the presence of air. Carboxylic acid is another name for phenol. Phenols establish intermolecular H-bonds with other phenol molecules and with water due to the presence of a polar -OH bond.
(iv). Alcohols and phenols are soluble in water due to hydrogen bonding with water molecules. However, solubility decreases as the size of the hydrophobic alkyl or aryl group increases. Lower molecular mass alcohols are completely miscible with water.
Chemical Reactions
Alcohols react both as nucleophiles and electrophiles. The bond between O–H is broken when alcohols react as nucleophiles. They are versatile compounds.
Alcohols as Nucleophiles
.png)
Alcohols as electrophiles
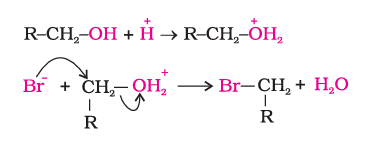
1). Reactions involving cleavage of O–H bond
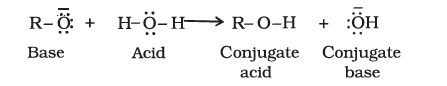
Reactions involving cleavage of the O–H bond in alcohols and phenols typically involve the release of a proton, showcasing their acidic nature. These reactions often form alkoxides or phenoxides when treated with active metals or strong bases.
i). Acidity of alcohols and phenols
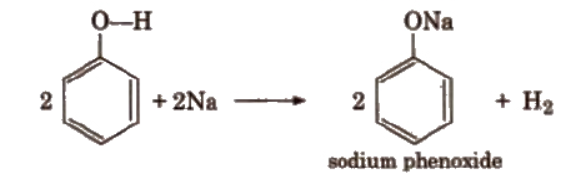
a). Reaction with metals
$2 \mathrm{R}-\mathrm{OH}+2 \mathrm{Na} \xrightarrow{\text { yields }} 2 \mathrm{R}-\mathrm{O}-\mathrm{Na}+\mathrm{H}_2$
b). Acidity of alcohols
The acidic character of alcohols arises from the polar O–H bond. Electron-releasing groups increase electron density on the oxygen atom, reducing the O–H bond polarity and thus decreasing the acidity.
Alcohols are weaker acids than water.
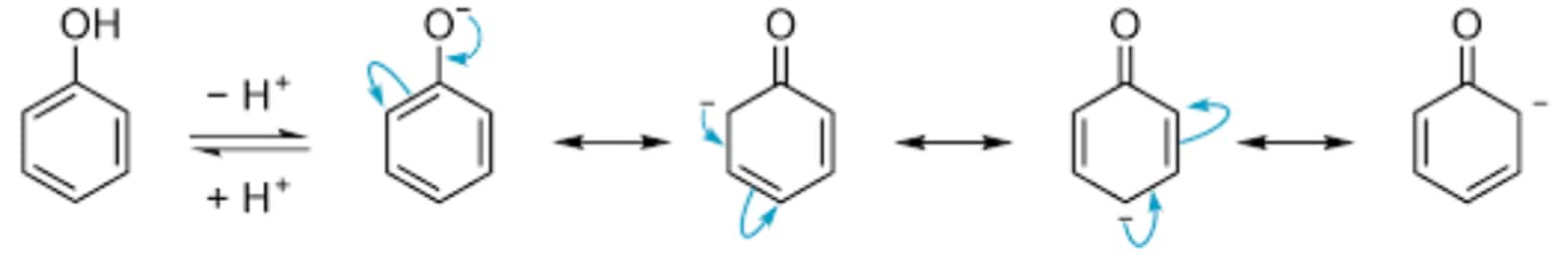
b). Acidity of phenols
Because the phenoxide ion is stabilized through resonance, phenol is more acidic than alcohols. The presence of an electron withdrawing group raises phenol's acidity by stabilizing the phenoxide ion, whereas the presence of an electron releasing group lowers phenol's acidity by destabilizing the phenoxide ion. $s p^2$-hybridised carbon of the benzene ring directly bonded to the hydroxyl group is more electronegative than the oxygen itself, withdrawing electron density from the –OH group. This increases the polarity of the O–H bond, promoting ionisation and formation of the phenoxide ion.
(ii). Esterification
Reaction of Alcohols and phenols with carboxylic acids, acid chlorides and acid anhydrides form esters.
$\mathrm{Ar} / \mathrm{R}-\mathrm{OH}+\mathrm{R}-\mathrm{COOH} \xrightarrow{\mathrm{H}^{+}} \mathrm{Ar} / \mathrm{ROCOR}+\mathrm{H}_2 \mathrm{O}$
$\mathrm{Ar} / \mathrm{R}-\mathrm{OH}+(\mathrm{RCO})_2 \mathrm{O}+\mathrm{H}^{+} \leftrightarrow \mathrm{Ar} / \mathrm{ROCOR}+\mathrm{RCOOH}$
$\mathrm{R} / \mathrm{ArOH}+\mathrm{RCOCl}$ Pyridine $\rightarrow \mathrm{R} / \mathrm{ArOCOR}+\mathrm{HCl}$
2). Reactions involving cleavage of carbon oxygen (C–O) bond in alcohols
Reactions involving cleavage of the C–O bond in alcohols typically occur during substitution or elimination, where the hydroxyl group is replaced or removed forming alkyl halides or alkenes.Download the alcohols, phenols and ethers class 12 ncert notes pdf to study and revise anytime, anywhere.
i). Reaction with hydrogen halides
$\mathrm{ROH}+\mathrm{HX}$ yields $\rightarrow \mathrm{RX}+\mathrm{H}_2 \mathrm{O}$
ii). Reaction with phosphorus trihalides:
$3 \mathrm{R}-\mathrm{OH}+\mathrm{PBr}_3 \rightarrow 3 \mathrm{R}-\mathrm{Br}+\mathrm{H}_3 \mathrm{PO}_3$
iii). Dehydration
Alcohols undergo dehydration means removal of a molecule of water to form alkenes on treating with a protic acid.
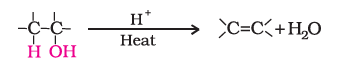
Order of reaction
Tertiary > Secondary > Primary
Mechanism of dehydration
Step 1: Formation of protonated alcohol
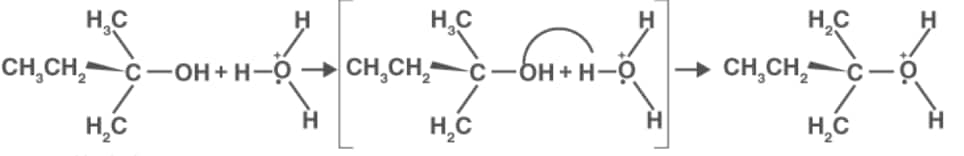
Step 2: Formation of carbocation
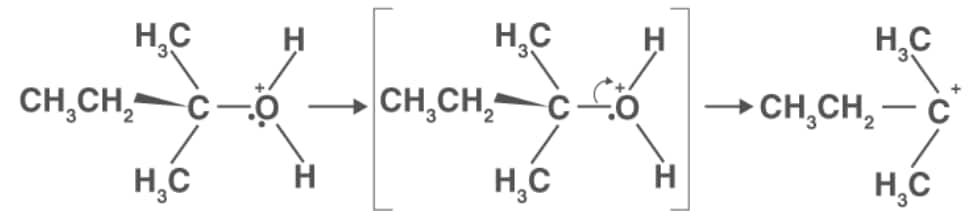
Step 3: Formation of ethene by elimination of a proton.
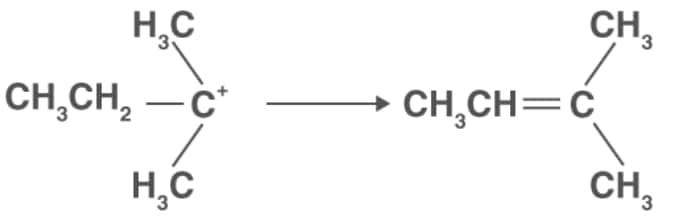
iv). Oxidation
Formation of a carbon oxygen double bond with cleavage of an O-H and C-H bonds is involved in oxidation of alcohol.
Alcohol, Acidified KMnO4→ Carboxylic acid
RCH2OH Oxidation→ RCHO yields→ RCOOH
3). Reactions of phenol
Phenol undergoes characteristic reactions. Its aromatic ring is highly reactive due to the activating effect of the –OH group.
i). Electrophilic aromatic substitution
Electrophilic substitution reactions take place on aromatic ring in phenols. The OH group attached to the benzene ring activates it towards electrophilic substitution. Also, it directs the incoming group to ortho and para positions in the ring as these positions become electron rich due to the resonance effect caused by OH group.
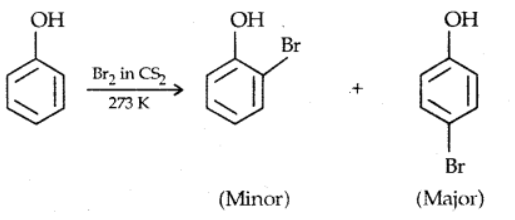
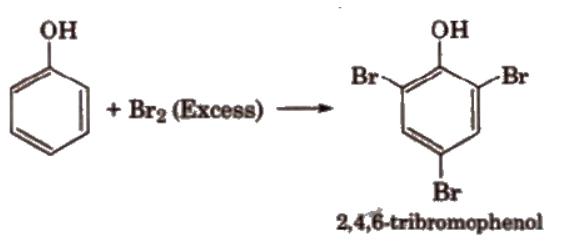
a). Halogenation
Anisole reacts with an alkyl halide and acyl halide introducing alkyl and acyl groups in ortho and para positions.
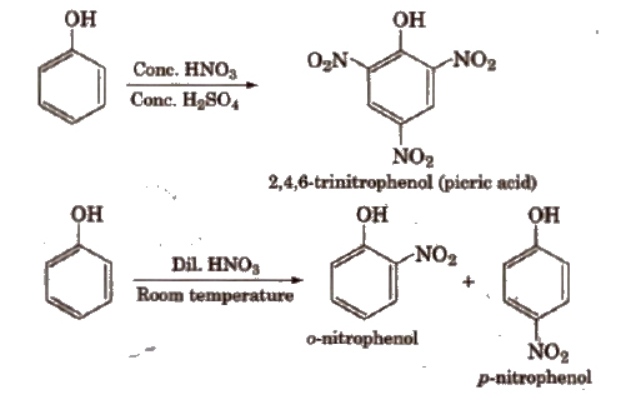
b). Nitration
With dilute nitric acid at low temperature (298 K), phenol yields a mixture of ortho and para nitrophenols. The reaction occurs as follows.
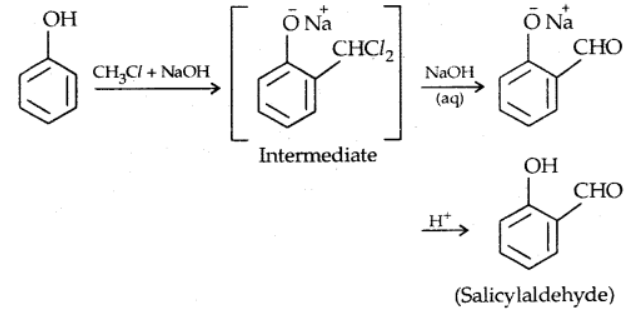
ii). Reimer-Tiemann reaction
Phenols for aromatic compounds containing EDG when refluxed with CHCl3 and alkali yield o- and p- hydroxybenzaldehyde. The ortho product is the predominant product. It is an electrophilic substitution on PhO- ion. The electrophile is dichlorocarbene (:CCl2) which contains a C with only six electrons.
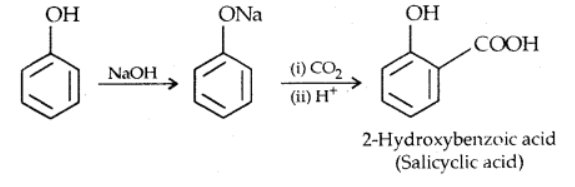
iii). Kolbe’s reaction
Phenol when heated at(390-410K) under pressure with CO2 and alkali gives salicylic acid after acidification in addition to some amount of p-isomer.
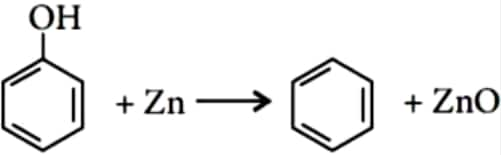
iv). Reaction of phenol with zinc dust
When phenol is distilled with zinc dust, benzene is obtained. The reaction occurs as follows:
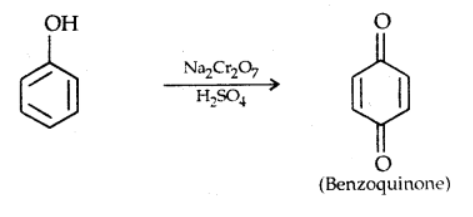
v). Oxidation
Some Commercially Important Alcohols
Two commercially important alchols are Methanol and ethanol.
Methanol (CH3OH)
Wood-spirit is another name for it. It is a clear liquid with no discernible colour. It reaches a temperature of 337 degrees Fahrenheit when it boils. It is extremely poisonous. Even little doses can cause blindness, and excessive doses can be very fatal.

Paints, varnishes, and other products use it as a solvent.It can be used to make formaldehyde.
Ethanol ($\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$)
It is known as denatured spirit when combined with CuSO4 and pyridine.It is a colourless liquid having boiling point 351 K.
$\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}+\mathrm{H}_2 \mathrm{O} \xrightarrow{\text { Invertase }} \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6$
$\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6 \xrightarrow{\text { Zymase }} 2 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+2 \mathrm{CO}_2$
It is a good solvent, Sterilization of surgical tools in laboratories and hospitals.
Ethers
Ethers are organic compounds in which two alkyl or aryl groups are bonded to the same oxygen atom.
Preparation of Ether
1). By dehydration of alcohols
Alcohols lose water in the presence of protic acids like $\mathrm{H}_2 \mathrm{SO}_4$ or $\mathrm{H}_2 \mathrm{PO}_4$. The product formed alkene or ether depends on temperature. For instance, ethanol gives ethene at 443 K, while at 413 K, it mainly forms ethoxyethane.
CH3CH2OH$\xrightarrow[443 \mathrm{~K}]{\mathrm{H}_2 \mathrm{SO}_4} \mathrm{CH}_2=\mathrm{CH}_2$
CH3CH2OH $\xrightarrow[413 \mathrm{~K}]{\mathrm{H}_2 \mathrm{SO}_4} \mathrm{C}_2 \mathrm{H}_5 \mathrm{OC}_2 \mathrm{H}_5$
2). Williamson synthesis
It is the best method to prepare all type of ethers, that is, simple, mixed or aromatic ethers. Here, alkyl halides are treated with sodium alkoxide in presence of magnesium to give ethers. It involves SN2 mechanism during the attack of R-O- on R-X, that is, backside attack occurs here. The reaction occurs as follows:

Some examples include:


Physical Properties of Ethers
Since ethers' C-O bonds are polar, they have a net dipole moment. Their boiling points are equivalent to alkanes with similar molecular weights, although they are lower than alcohols. It's because ethers don't have H-bonding. Ether miscibility with water is similar to that of alcohols of same molar mass. It's because ethers, like alcohols, can make H-bonds with water.
Chemical Reactions
Ethers are generally unreactive but undergo cleavage with strong acids like HI or HBr, forming alcohols and alkyl halides.
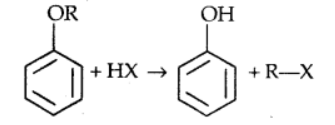
1). Cleavage of C–O bond in ethers
The cleavage of C-O bond in ethers takes place under drastic conditions with excess of hydrogen halides. The reaction of dialkyl ether gives two alkyl halide molecules
$\mathrm{R}-\mathrm{O}-\mathrm{R}+\mathrm{HX} \rightarrow \mathrm{RX}+\mathrm{R}-\mathrm{OH}$
$\mathrm{R}-\mathrm{OH}+\mathrm{HX} \rightarrow \mathrm{R}-\mathrm{X}+\mathrm{H}_2 \mathrm{O}$
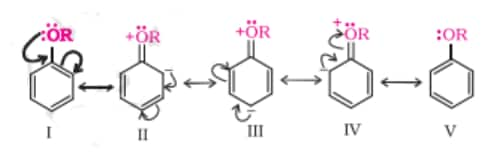
2). Electrophilic substitution reactions
The alkoxy group is ortho, para directing and activates the aromatic ring towards electrophilic substitution in the same way as in phenol.
i). Halogenation
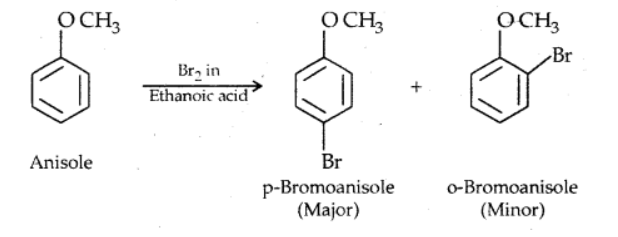
ii). Friedel craft’s reaction
Anisole reacts with an alkyl halide and acyl halide introducing alkyl and acyl groups in ortho and para positions.
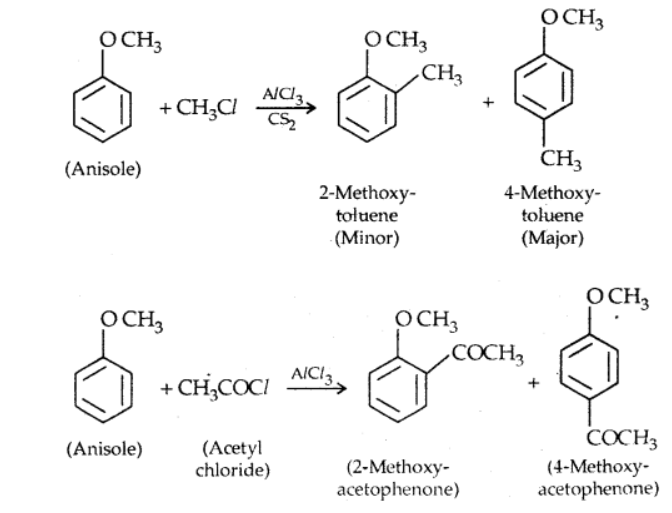
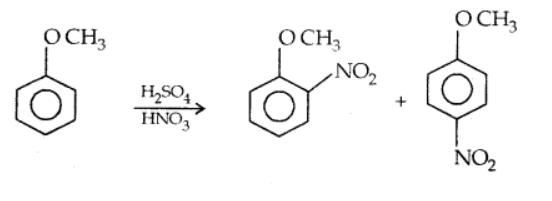
iii). Nitration
Anisole reacts with a mixture of concentrated sulphuric and nitric acids to yield a mixture of ortho and para nitroanisole. The reaction occurs as follows.
Alcohols ,Phenols and Ethers Previous Year Question and Answer
Given below selected previous year questions with answers to help students understand the exam pattern and revise the key concepts effectively. The concepts used to solve these questions are explained in ncert class 12 chemistry chapter 7 alcohols, phenols and ethers notes.
Question 1: Which of the following are benzylic alcohols?
(1) (i) and (ii)
(2) (iii) and (iv)
(3) (ii) and (iii)
(4) (ii) and (iv)
Answer:
The answer is the option (ii) and (iii).
The most prominent way to identify benzylic alcohol is to check if an -OH group is attached to a $s p^3$ hybridised carbon atom on an aromatic ring.
As in the case of option (ii) and (iii), the $s p^3$ hybridised carbon is attached to a benzene ring these are benzylic alcohols but in option (i) and (iv), the $s p^3$ hybridised carbon is not attached to a benzene ring.
Hence, the answer is the option (3).
Question 2: Phenol can be distinguished from ethanol by the reactions with _________.
(i) $B r_2 /$ water
(ii) $N a$
(iii) Neutral $\mathrm{FeCl}_3$
(iv) All the above
(1) (i) and (iii)
(2) (iii) and (iv)
(3) (ii) and (iii)
(4) None of above
Answer:
The answer is the option (i) and (iii).
Phenol can easily be distinguished from ethanol by adding bromine water as it results in a white ppt of tri-bromo phenol or Neutral FeCl3 can also be used for this purpose. But ethanol being aliphatic alcohol will not react with either of the compounds.
Hence, the answer is the option (1). Question:
Question 3: Which of the following reactions will yield phenol?
(1) (A), (B) and (C)
(2) (A), (B) and (D)
(3) (A), (C) and (D)
(4) (B), (C) and (D)
Answer:
The answer is the option (A), (B), (C). To carry out these reactions, the conditions required should be easy to maintain. The reaction occurring in (D) is a nucleophilic substitution of chlorobenzene which is not feasible as it requires drastic temperature and pressure conditions.
The reaction given in (A) is the Dow process, (B) is aniline diazotisation and (C) option contains a reaction from benzene sulphonic acid. All these reactions are feasible due to manageable reaction conditions.
Hence, the answer is the option (1).
Question 4: Match List-I with List-II
| List-I Conversion | List-II Reagents, Conditions used | ||
| (A) | 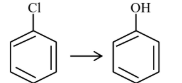 | (I) | Warm, $\mathrm{H}_2 \mathrm{O}$ |
| (B) | 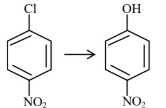 | (II) | (a) $\mathrm{NaOH}, 368 \mathrm{~K}$; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
| (C) | 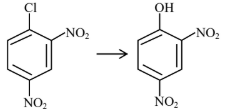 | (III) | (a) $\mathrm{NaOH}, 443 \mathrm{~K}$; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
| (D) | 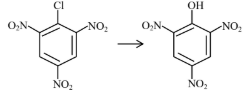 | (IV) | (a) $\mathrm{NaOH}, 623 \mathrm{~K}$, 300 atm ; (b) $\mathrm{H}_3 \mathrm{O}^{+}$ |
Choose the correct answer from the options given below :
(1) (A)-(II), (B)-(III), (C)-(I), (D)-(IV)
(2) (A)-(III), (B)-(IV), (C)-(II), (D)-(I)
(3) (A)-(IV), (B)-(III), (C)-(II), (D)-(I)
(4) (A)-(IV), (B)-(III), (C)-(I), (D)-(II)
Answer:
Presence of EWG at ortho and para-position of chlorobenzene will increase rate of aromatic nucleophilic substitution due to stable intermediate.
A-IV, B-III, C-II, D-I
Hence, the correct answer is option (3).
Question 5: Which one of the following, with HBr will give a phenol?
(1)
(2)
(3)
(4)
Answer:

The phenol product is formed only in the case of option (2) compound.
Hence, the correct answer is option (2).
How to Master Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers
These ncert class 12 chemistry chapter 7 alcohols, phenols and ethers notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 7.
- Firstly understand how alcohols, phenols, and ethers are classified and named according to IUPAC rules.
- Then learn about preparation reactions for each class from alkenes, carbonyl compounds, haloalkanes, phenols, Williamson synthesis for ethers, etc along with reagents and conditions.
- Understand physical and chemical properties and trends in boiling points, solubility, and acidity. Students can follow alcohols, phenols and ethers ncert notes for learning these concepts better.
- Reaction mechanisms like dehydration of alcohols, oxidation of alcohols, electrophilic substitution in phenols, and cleavage of ethers plays an important role to master this chapter.
- Understand why phenols are more reactive than alcohols, and why ethers are relatively inert.
- After that students can solve previous year questions from this chapter.
Advantages of Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers Notes
NCERT notes Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers cover all the important topics from the NCERT book. The advantages of using these notes are given below:
- By using these notes students can get a clear explanation of the physical and chemical properties of alcohols, phenols, and ethers.
- These notes are written in a clear and comprehensive manner that includes structured reaction mechanisms and conversions.
- These alcohols, phenols and ethers class 12 ncert notes are very helpful in revision before exams.
- They contain key points and formulas for quick revision.
CBSE Class 12 NCERT Chemistry Chapter-Wise Notes
Apart from class 12 chemistry chapter 7 alcohols, phenols and ethers notes, you can also check the links below for notes of other Class 12 chapters.
NCERT Solutions for Class 12 Chemistry
Along with alcohols, phenols and ethers Class 12 Chemistry Chapter 7 CBSE notes students can also refer to solutions of NCERT for Class 12 Chemistry chapters.
Subject-Wise NCERT Solutions
Subject-wise links of ncert solutions are given below:
Subject-Wise NCERT Exemplar Solutions
Subject-wise links of ncert exemplar solutions are given below:
Frequently Asked Questions (FAQs)
These alcohols, phenols and ethers ncert notes simplify complex concepts, highlight important reactions and mechanisms, and save time during revision. They help students grasp topics quickly, improve retention, and boost their performance in exams.
Alcohols have diverse applications, including as solvents, in the production of pharmaceuticals, and as fuels. Ethanol is used in alcoholic beverages, as an industrial solvent, and as a biofuel.
Alcohols can undergo several key reactions, including dehydration to form alkenes, oxidation to produce aldehydes or ketones, and esterification where alcohol reacts with acids to form esters.
Ethers are generally less reactive due to the stable nature of the C-O-C bond. They do not readily participate in reactions like alcohols and phenols because they lack a hydroxyl group, which is reactive and can donate protons.
Alcohols have unique physical and chemical properties. They are polar compounds due to the presence of the hydroxyl group, which can form hydrogen bonds, giving them higher boiling points compared to alkanes. Alcohols are also soluble in water, especially shorter-chain alcohols, and they typically have a pleasant odor.
Phenols are a specific type of alcohol where the hydroxyl (-OH) group is directly attached to an aromatic hydrocarbon ring. This structure gives phenols distinct properties compared to alcohols. For instance, phenols are generally more acidic than alcohols due to the resonance stabilization of the phenoxide ion formed when they lose a proton.
Alcohol oxidation is the process where alcohols lose electrons, typically involving the addition of oxygen or the removal of hydrogen. Primary alcohols are oxidized to aldehydes, which can further oxidize to carboxylic acids. Secondary alcohols can be oxidized to ketones, while tertiary alcohols resist oxidation due to the absence of a hydrogen atom on the carbon with the -OH group.
Ethers can react with strong acids through a process called cleavage, where the ether bond is broken, resulting in the formation of alcohols. This reaction typically occurs under acidic conditions and can lead to the formation of alkyl halides if the reactive acid is a haloacid.
The reactivity of alcohols varies significantly based on their classification. Primary alcohols are more easily oxidized than secondary and tertiary alcohols due to the availability of hydrogen atoms on the carbon with the hydroxyl group. Secondary alcohols can oxidize to ketones, while tertiary alcohols do not easily oxidize and are instead more likely to undergo dehydration reactions.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters







