Aldehydes, Ketones and Carboxylic Acids Class 12th Notes- Free NCERT Class 12 Chemistry Chapter 12 Notes - Download PDF
What is the first thing that comes to mind when you smell something delicious or try a new scent? Perhaps it's the aroma of freshly baked bread or the sweet scent of a flower? Did you know that aldehydes, ketones, and carboxylic acids are often responsible for these smells? These are found in many industries, biological systems, and household items. This article provides a brief overview of Chapter 8. These aldehydes, ketones and carboxylic acids ncert notes are useful for CBSE board examinations.
This Story also Contains
- NCERT Notes for Class 12 Chapter 8: Download PDF
- NCERT Notes for Class 12 Chapter 8
- Aldehydes, Ketones and Carboxylic Acids Previous year Question and Answer
- How to Master Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids
- Advantages of Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids Notes
- CBSE Class 12 Chemistry Chapter-wise Notes
- NCERT Solutions for Class 12 Chemistry
- Subject-Wise NCERT Exemplar Solutions
- Subject-Wise NCERT Solutions
NCERT Class 12 Chemistry notes provides a structured approach to understanding the classification, properties, preparation, and reactions of Chapter 8 . These class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids notes discuss the structure of the carbonyl group, methods of preparation, physical properties and some of the important chemical reactions of Aldehydes and Ketones. We are also providing NCERT notes that cover all the topics and concepts presented in the NCERT textbook in a clear and comprehensive manner. These notes are also valuable resources for various competitive exams like JEE Main, NEET, and JEE Advanced. Also, check the NCERT Solutions for all the chapters.
NCERT Notes for Class 12 Chapter 8: Download PDF
Students can download the aldehydes, ketones and carboxylic acids Class 12 Chemistry Chapter 8 CBSE notes from the download icon given below. These concise NCERT notes for Class 12 cover all the key concepts of alcohol, phenol and ethers to help students in quick revision before the exam.
Also, students can refer,
NCERT Notes for Class 12 Chapter 8
These class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids notes provide a concise yet comprehensive summary of key concepts, making it easier for students to revise and prepare effectively for exams. These notes cover important definitions, reactions, and applications from the chapter.
Nomenclature and Structure of Carbonyl Group
Aldehydes, ketones, carboxylic acids, and their derivatives are organic compounds with the carbonyl group (CO) as the functional group. These are referred to as carbonyl compounds together. The carbonyl group's oxygen atom is significantly more electronegative than the carbon atom. As a result, the oxygen atom attracts the electron cloud of the - bond towards itself, resulting in an unsymmetrical electron cloud of >C = O. As a result, carbonyl carbon has a positive charge while carbonyl oxygen has a negative charge. Thus, the carbonyl group is polar. In aldehydes, one hydrogen and an alkyl/aryl group are attached to carbonyl group. But in ketone, two alkyl/aryl groups are attached to the carbonyl group.
(a) Common Names: Aldehydes are often named based on the corresponding carboxylic acids by replacing the suffix –ic acid with –aldehyde. The names usually reflect the Latin or Greek origin of the compound. Substituent positions are indicated using Greek letters: α (alpha) for the carbon next to the aldehyde group, β (beta) for the next, and so on. Ketones are commonly named by listing the two alkyl or aryl groups attached to the carbonyl carbon. Substituent positions are shown as α, α′; β, β′, etc., starting from the carbon atoms next to the carbonyl group. Some ketones have traditional names (e.g., acetone). Alkyl phenyl ketones are often named by adding the acyl group as a prefix to phenone. Use aldehydes, ketones and carboxylic acids ncert notes to understand these concepts better.
(b) IUPAC Names:
- Aldehydes: Replace –e of the alkane with –al. The chain is numbered starting from the aldehyde carbon.
- Ketones: Replace –e with –one. Numbering starts from the end closest to the carbonyl group.
Substituents are named alphabetically with position numbers. In cyclic ketones, the carbonyl carbon is position 1. For ring-attached aldehydes, add the suffix –carbaldehyde to the ring name, starting numbering from the aldehyde carbon. The simplest aromatic aldehyde, benzenecarbaldehyde, is commonly known (and IUPAC accepted) as benzaldehyde. Other aromatic aldehydes are named as substituted benzaldehydes.
| Structure | Common Name | IUPAC Name |
|---|---|---|
| Aldehydes | ||
| HCHO | Formaldehyde | Methanal |
| CH₃CHO | Acetaldehyde | Ethanal |
| (CH₃)₂CHCHO | Isobutyraldehyde | 2-Methylpropanal |
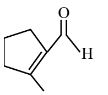
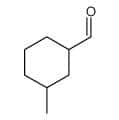 | γ-Methylcyclohexanecarbaldehyde | 3-Methylcyclohexanecarbaldehyde |
| CH₃CH(OCH₃)CHO | α-Methoxypropionaldehyde | 2-Methoxypropanal |
| CH₃CH₂CH₂CH₂CHO | Valeraldehyde | Pentanal |
| CH₂=CHCHO | Acrolein | Prop-2-enal |
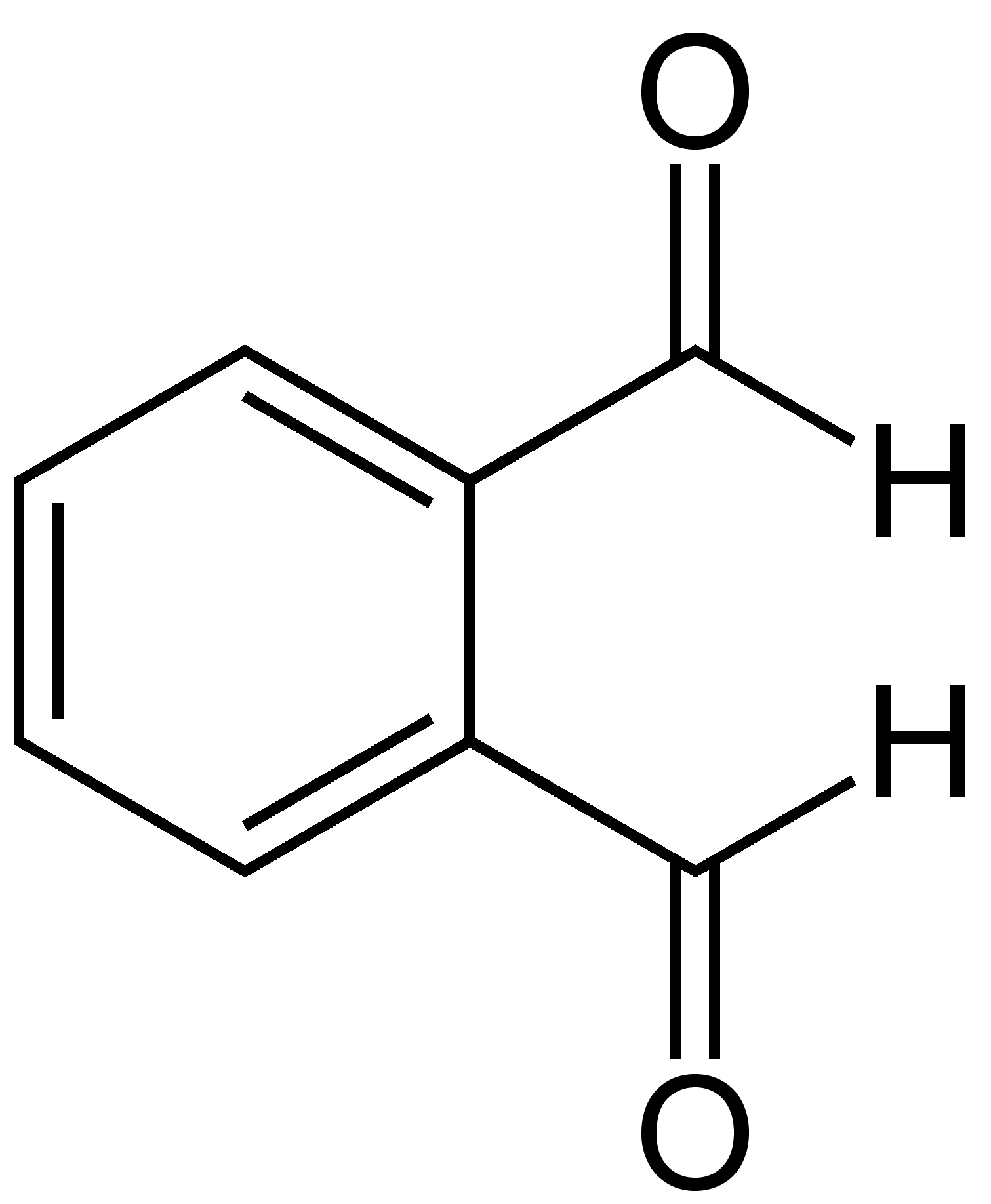 | Phthalaldehyde | Benzene-1,2-dicarbaldehyde |
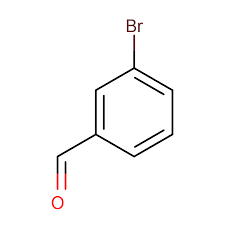 | m-Bromobenzaldehyde | 3-Bromobenzenecarbaldehyde or 3-Bromobenzaldehyde |
| Ketones | ||
| CH₃COCH₂CH₂CH₃ | Methyl n-propyl ketone | Pentan-2-one |
| (CH₃)₂COCH(CH₃)₂ | Diisopropyl ketone | 2,4-Dimethylpentan-3-one |
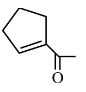
.png) | α-Methylcyclohexanone | 2-Methylcyclohexanone |
| (CH₃)₂C=CHCOCH₃ | Mesityl oxide | 4-Methylpent-3-en-2-one |
Structure of the Carbonyl Group:
The carbonyl carbon is sp²-hybridised, forming three sigma (σ) bonds in a trigonal planar arrangement with bond angles close to 120°. Its unhybridised p-orbital overlaps with the p-orbital of oxygen to form a π-bond, while oxygen also carries two lone pairs. This results in a planar structure with the π-electron cloud above and below the plane., as shown in the figure.
.png)
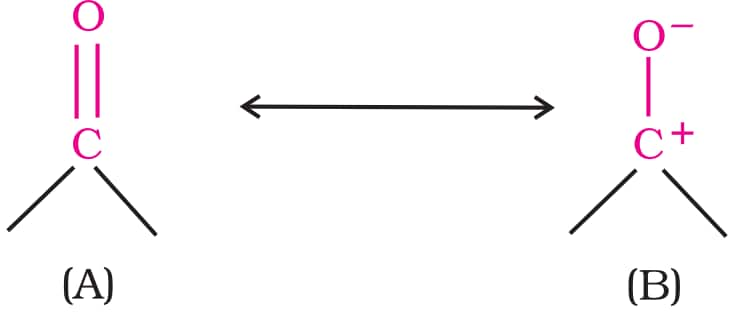
The C=O bond is polar due to oxygen's higher electronegativity, making the carbonyl carbon electrophilic and the oxygen nucleophilic. Carbonyl compounds have significant dipole moments, making them more polar than ethers. The high polarity of the carbonyl group is explained on the basis of resonance involving a neutral (A) and a dipolar (B) structures as shown.
Preparation of Aldehydes and Ketones
a) By oxidation of alcohols
Aldehydes are formed when primary alcohols are oxidized in the presence of an oxidizing agent such as $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7 / \mathrm{H}_2 \mathrm{SO}_4, \mathrm{KMnO}_4$, or $\mathrm{CrO}_3$
b) By the dehydrogenation of alcohols
Aldehyde is formed when primary alcohol vapours pass over heated copper or silver at 573 K.
c) From Hydrocarbons
- By ozonolysis of alkenes: As we know, ozonolysis of alkenes followed by reaction with zinc dust and water gives aldehydes,ketones or a mixture of both depending on the substitution pattern of the alkene.
- By hydration of alkynes: Addition of water to ethyne in the presence of $\mathrm{H}_2 \mathrm{SO}_4$ and $\mathrm{HgSO}_4$ gives acetaldehyde.
Preparation of Aldehydes
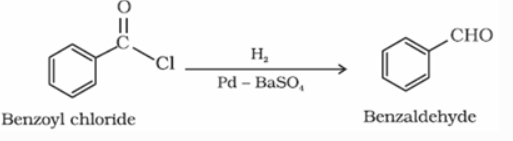
a) From acyl chloride (acid chloride):
Acyl chloride (acid chloride) is hydrogenated over catalyst, palladium on barium sulphate. This reaction is called Rosenmund reduction.
b) From nitriles and esters
When nitriles are reduced in the presence of stannous chloride and HCl, an imine is formed, which when hydrolyzed yields the corresponding aldehyde. This reaction is called Stephen reaction.
$\mathrm{RCN}+\mathrm{SnCl}_2+\mathrm{HCl} \rightarrow \mathrm{RCH}=\mathrm{NH}+\mathrm{H}_3 \mathrm{O}^{+} \rightarrow \mathrm{RCHO}$
Alternatively, DIBAL-H (Diisobutylaluminium Hydride) preferentially reduces nitriles and esters to aldehydes.
Nitriles are reduced to aldehydes using DIBAL-H at low temperatures $\left(-78^{\circ} \mathrm{C}\right)$.
$\mathrm{R}-\mathrm{C} \equiv \mathrm{N} \xrightarrow[-78^{\circ} \mathrm{C}]{\text { DIBAL-H }} \mathrm{R}-\mathrm{CHO}$
Esters can also be selectively reduced to aldehydes using DIBAL-H.
$\mathrm{R}-\mathrm{COOR}$ ' $\xrightarrow[-78^{\circ} \mathrm{C}]{\text { DIBAL-H }} \mathrm{R}-\mathrm{CHO}$
e) From Hydrocarbons: Benzaldehyde and its derivatives are syntehsized from hydrocarbons using the following methods:
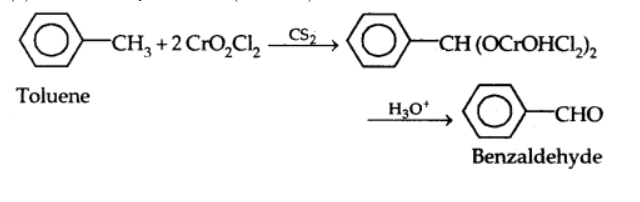
By oxidation of methylbenzene: Strong oxidising agents oxidise toluene and its derivatives to benzoic acids. However, it is possible to stop the oxidation at the aldehyde stage with suitable reagents that convert the methyl group to an intermediate that is difficult to oxidise further. The following methods are used for this purpose.
a) Use of chromyl chloride $\left(\mathrm{CrO}_2 \mathrm{Cl}_2\right.$): Chromyl chloride converts the methyl group to a chromium complex, which when hydrolyzed, yields the benzaldehyde. This is known as Etard Reaction.
b)With chromic oxide: In the presence of chromic oxide, toluene or substituted toluene is converted to benzaldehyde in acetic anhydride.
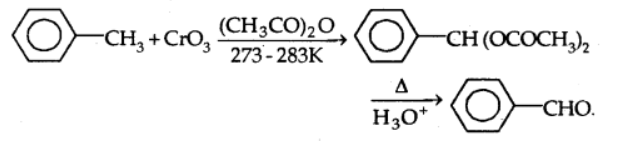
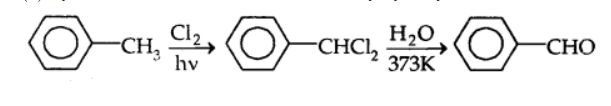
By sidechain chlorination followed by hydrolysis: Toulene is chlorinated which gives benzal chloride, which on further hydrolysis gives benzaldehyde. This is a commercial method of manufacture of benzaldehyde.
By the Gattermann-Koch reaction:
Benzaldehyde or substituted benzaldehydes are produced by treating benzene or its derivatives with carbon monoxide and HCl in the presence of anhydrous aluminium chloride or cuprous chloride (CuCl).
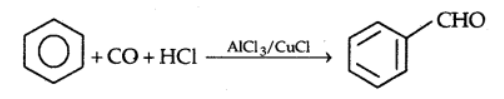
Preparation of Ketones
According to ncert class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids notes the preparation of ketones is given by:
a) From acyl chlorides
Acyl chloride is converted to a ketone when it is treated with dialkyl cadmium (made by reacting cadmium chloride with a Grignard reagent).
$2 \mathrm{R}-\mathrm{Mg}-\mathrm{X}+\mathrm{CdCl}_2 \rightarrow \mathrm{R}_2 \mathrm{Cd}+2 \mathrm{MgXCl}$
2 R'-CO-Cl $+\mathrm{R}_2 \mathrm{Cd} \rightarrow 2$ R'-CO-R $+\mathrm{CdCl}_2$
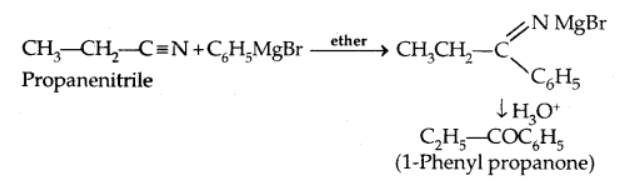
b) From nitriles
Nitriles are converted to ketones after being treated with a Grignard reagent and then hydrolyzed.
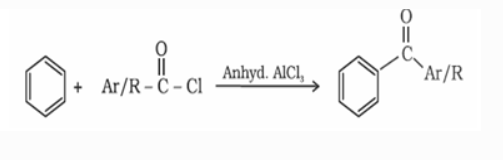
c) From benzene or substituted benzene
Ketone is formed when benzene or substituted benzene is treated with acid chloride in the presence of anhydrous aluminium chloride.
Physical Properties of Aldehydes and Ketones
-
At room temperature, methane is a gas. Ethanol is a very flammable liquid. At room temperature, other aldehydes and ketones are liquid or solid.
-
Boiling points are greater than hydrocarbons and ethers of comparable molecular weights. This is because of weak molecular association in aldehydes and ketones due to the dipole-dipole interactions. Due to the lack of H-bonding, their boiling points are lower than those of alcohols.
-
Because they form H-bonds with water, lower aldehydes and ketones like methanal, ethanal, and propanone are miscible with water in all proportions.
.png)
-
Lower aldehydes have a harsh, pungent odour. The odour gets less strong and more delicate as molecular mass increases.
Chemical Reactions of Aldehydes and Ketones
Aldehydes and ketones undergo a wide variety of chemical reactions due to the presence of the carbonyl group. Their reactivity is mainly influenced by the polar nature of the C=O bond.
1. Nucleophilic addition reactions
The hybridization of C shifts from $s p^2$ to $s p^3$ when a nucleophile attacks the carbonyl carbon. Due to steric and electrical reasons, aldehydes are more sensitive to nucleophilic addition processes than ketones.
a) Addition of hydrogen cyanide
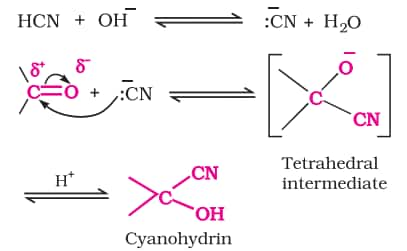
b) Addition of sodium hydrogensulphite
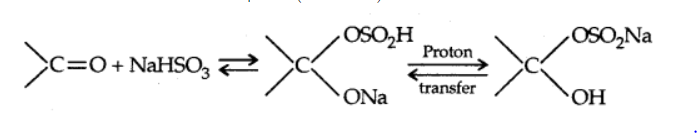
c) Addition of alcohols
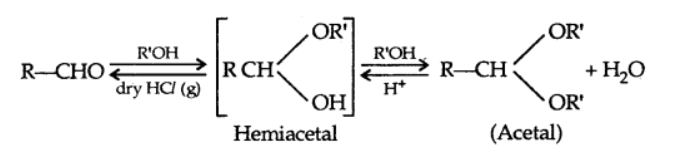
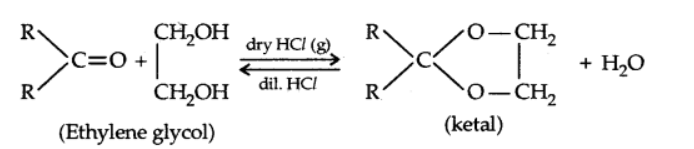
d) Addition of ammonia and its derivatives
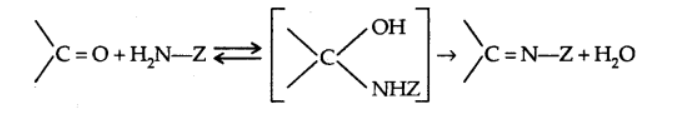
2. Reduction
a) Reduction to alcohols
Sodium borohydride or lithium aluminium hydride, as well as catalytic hydrogenation, reduce aldehydes and ketones to primary and secondary alcohols, respectively.
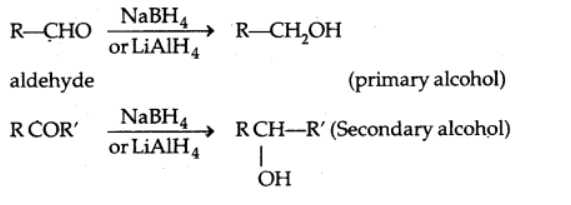
b) Reduction to hydrocarbons
When aldehydes and ketones are treated with zinc amalgam and strong hydrochloric acid, the carbonyl group is reduced to $\mathrm{CH}_2$,(Clemmensen reduction)
On treating carbonyl compound with hydrazine followed by sodium or potassium hydroxide in a high boiling solvent such as ethylene glycol reduces the carbonyl group of aldehydes and ketones to $\mathrm{CH}_2$ (Wolff-Kishner reaction)
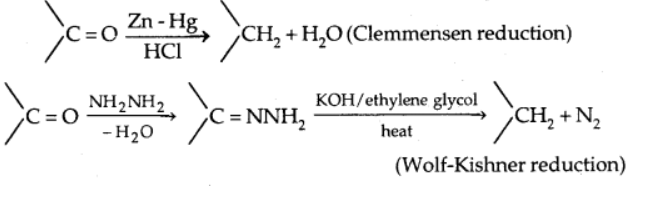
3. Oxidation
Oxidation reactions of aldehyde and ketones are different
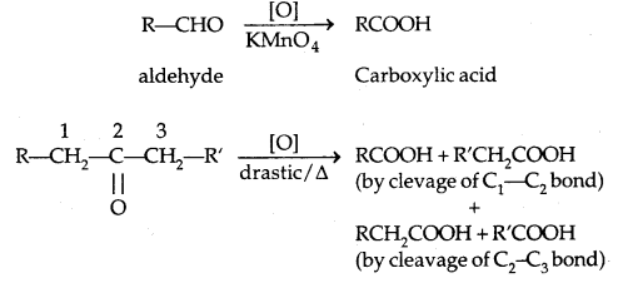
Aldehydes and ketones can be distinguished using the 2 reactions below because aldehydes are easier to oxidize than ketones.
a) Tollen’s test
A bright silver mirror is formed by heating an aldehyde with freshly made ammonical AgNO3 solution (Tollen's reagent).
$\mathrm{RCHO}+2\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)_2\right]^{+}+3 \mathrm{OH}^{-} \rightarrow \mathrm{RCOO}^{-}+2 \mathrm{Ag}+2 \mathrm{H}_2 \mathrm{O}+4 \mathrm{NH}_3$
b) Fehling’s test
A reddish-brown precipitate is formed when an aldehyde is heated with Fehling reagent.
$\mathrm{R}-\mathrm{CHO}+2 \mathrm{Cu}^{2+}+5 \mathrm{OH}^{-} \rightarrow 2 \mathrm{ROO}^{-}+\mathrm{Cu}_2 \mathrm{O}+3 \mathrm{H}_2 \mathrm{O}$
c) Oxidation of methyl ketone by the haloform reaction
This test is positive for all carboxyl compounds with the $\mathrm{COCH}_3$ group.
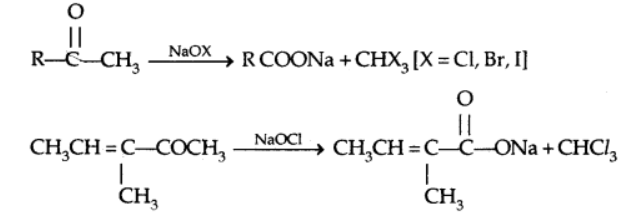
4. Reactions due to α-hydrogen
Because of the strong electron-withdrawing impact of the carbonyl group and the resonance stabilization of the conjugate base, α-hydrogens in aldehydes and ketones are acidic in nature.
a) Aldol condensation
In the presence of dil. alkali, aldehydes and ketones with at least one -hydrogen undergo Aldol Condensation to create β-hydroxy aldehydes (aldol) or β-hydroxy ketones [ketol].
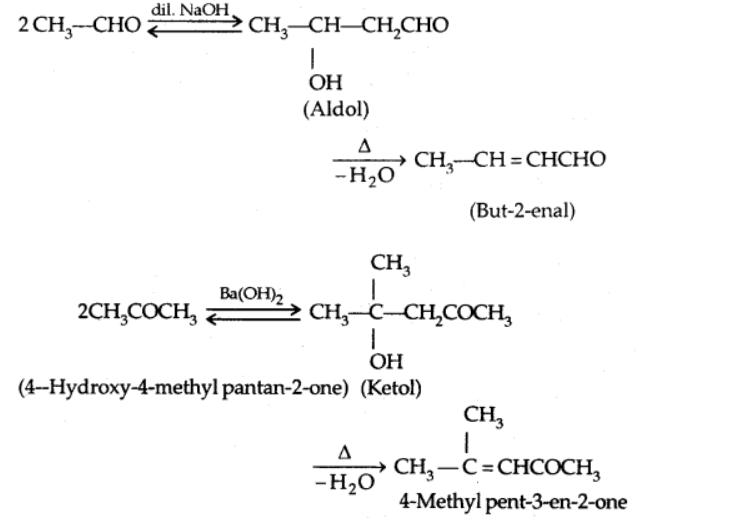
b) Cross Aldol condensation
Cross aldol condensation occurs when aldol condensation occurs between two separate aldehydes and/or ketones.
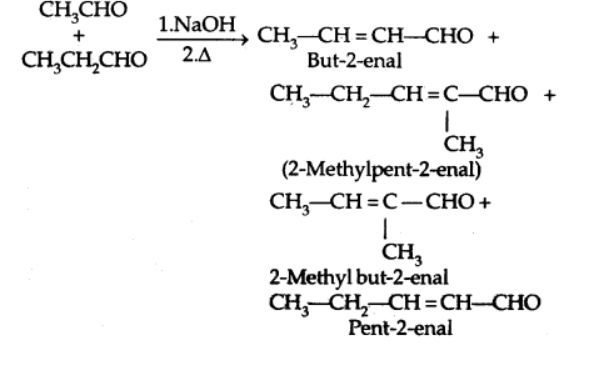
5. Other reactions
a) Cannizzaro reaction
When aldehydes without hydrogen atom are treated with strong alkali, they undergo self-oxidation and reduction reactions, resulting in alcohol and acid salt.
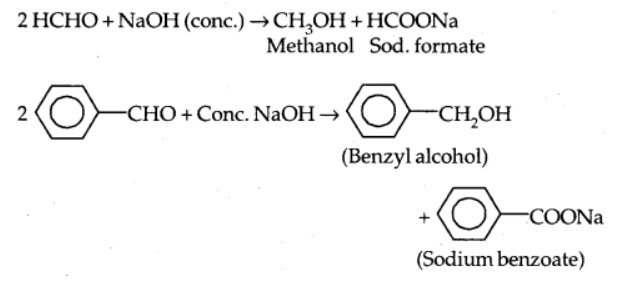
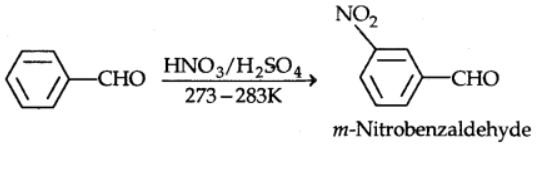
6) Electrophilic substitution reaction
Electrophilic substitution reactions occur at the ring of aromatic aldehydes and ketones, while the carbonyl group serves as a deactivating and meta-directing group.
Uses of Aldehyde and Ketones
Aldehydes and ketones serve as important solvents, reagents, and starting materials in the chemical industry.
- Formaldehyde (as 40% formalin) is used to preserve biological specimens and in the production of bakelite, urea-formaldehyde resins, and other polymers.
- Acetaldehyde is a key raw material for making acetic acid, ethyl acetate, vinyl acetate, polymers, and pharmaceuticals.
- Benzaldehyde finds applications in perfumery and the dye industry.
- Acetone and ethyl methyl ketone are widely used as industrial solvents.
- Several aldehydes and ketones like butyraldehyde, vanillin, acetophenone, and camphor are valued for their distinctive odours and flavours.
Carboxylic Acid
Carboxylic acids are organic compounds containing the –COOH group, which combines a carbonyl and a hydroxyl group. They are classified as aliphatic (RCOOH) or aromatic (ArCOOH) based on the attached group. Many carboxylic acids occur naturally, including fatty acids ($C_{12}-C_{18}$) found in fats as glycerol esters.
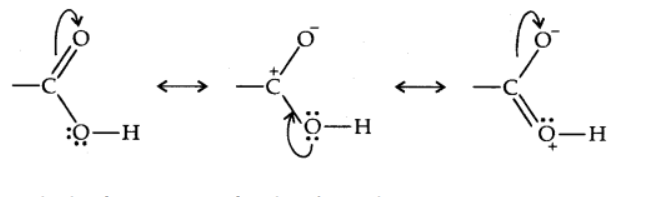
Structure of Carboxyl Group
The carboxylic carbon is less electrophilic due to the potential resonance configuration depicted below. The carbonyl carbon and the carboxylic carbon bonds are then aligned in the plane and separated by 120°.
Methods of Preparation of Carboxylic Acids
a) From primary alcohols and aldehydes
$\mathrm{R}-\mathrm{CH}_2 \mathrm{OH} \xrightarrow[\mathrm{H}_3 \stackrel{+}{\mathrm{O}}]{\text { alkaline } \mathrm{KMnO}_4} \mathrm{RCOOH}$
$\underset{\text { 1-Deanol }}{\mathrm{CH}_3\left(\mathrm{CH}_2\right)_8 \mathrm{CH}_2 \mathrm{OH}} \xrightarrow{\mathrm{CrO}_3-\mathrm{H}_2 \mathrm{SO}_4} \underset{\text { Decanoic acid }}{\mathrm{CH}_3\left(\mathrm{CH}_2\right)_8 \mathrm{COOH}}$
$\mathrm{RCHOH}+2\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)_2\right]^{+}+3 \mathrm{OH}^{-} \rightarrow \mathrm{RCOO}^{-}+2 \mathrm{Ag}+2 \mathrm{H}_2 \mathrm{O}+4 \mathrm{~N}$
$\mathrm{R}-\mathrm{CHO}^{+} 2 \mathrm{Cu}^{2+}+5 \mathrm{OH}^{-} \rightarrow \mathrm{RCOO}^{-}+\mathrm{Cu}_2 \mathrm{O}+3 \mathrm{H}_2 \mathrm{O}$
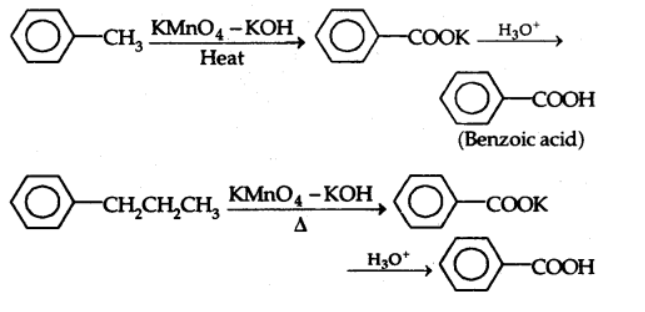
b) From alkylbenzenes
Aromatic carboxylic acids are made by oxidizing alkylbenzenes with chromic acid or acidic or alkaline potassium permanganate at high temperatures.
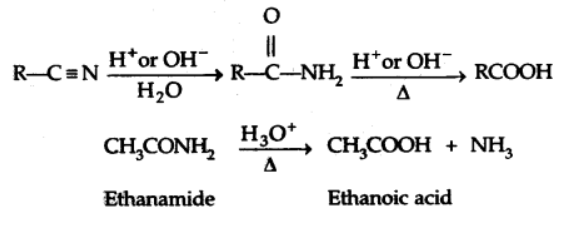
c) From nitriles and amides
When nitriles are hydrolyzed in the presence of dilute acids or bases, an amide is formed, which can then be further hydrolyzed to produce carboxylic acid.
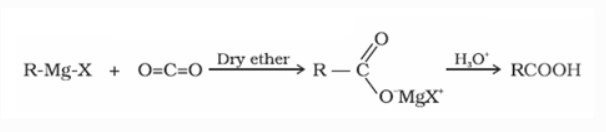
d) From Grignard reagent
Grignard reagents react with carbon dioxide (dry ice) to produce carboxylic acid salts, which are then hydrolyzed to produce carboxylic acids.
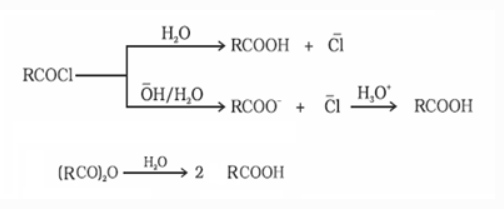
e) From acyl halides and anhydrides
When acid chlorides are hydrolyzed with water, carboxylic acids are formed. Carboxylate ions are generated during basic hydrolysis and are then acidified to form carboxylic acids. When anhydrides are hydrolyzed, the corresponding acid is produced
f) From esters
Basic hydrolysis of esters produces carboxylates, which are acidified to produce corresponding carboxylic acids. Acidic hydrolysis of esters directly produces corresponding carboxylic acids.
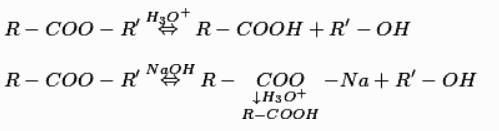
Physical Properties of Carboxylic Acid
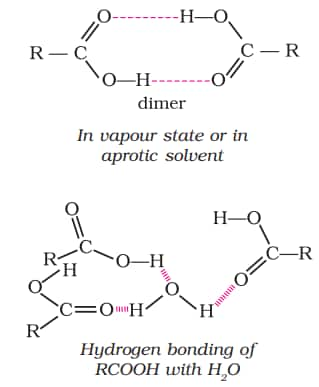
Because the non-polar component of the acid increases as the size of the alkyl group increases, the solubility of carboxylic acid decreases. Carboxylic acids boil at a higher temperature than aldehydes, ketones, or even alcohols with similar molecular weights. This is due to significant intermolecular hydrogen bonding between carboxylic acid molecules. The hydrogen bonds are not broken completely even in the vapour phase. Most carboxylic acids exist as dimer in the vapour phase or in the aprotic solvents. Download aldehydes, ketones and carboxylic acids class 12 ncert notes pdf to access these notes anytime.
Chemical Reactions of Carboxylic Acid
1. Reactions Involving Cleavage of O–H Bond (Acidity)
Reaction with metals
$2 \mathrm{RCOOH}+2 \mathrm{Na} \rightarrow 2 \mathrm{R}^{-} \mathrm{COO}^{-} \mathrm{Na}^{+}+\mathrm{H}_2$
$\mathrm{R}-\mathrm{COOH}+\mathrm{NaOH} \rightarrow 2 \mathrm{R}-\mathrm{COO}^{-} \mathrm{Na}^{+}+\mathrm{H}_2 \mathrm{O}$
$\mathrm{R}-\mathrm{COOH}+\mathrm{NaHCO}_3 \rightarrow 2 \mathrm{R}-\mathrm{COO}^{-} \mathrm{Na}^{+}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
Carboxylic acid dissociates in water to yield the carboxylate anion and the hydronium ion, which are both resonance-stabilized.
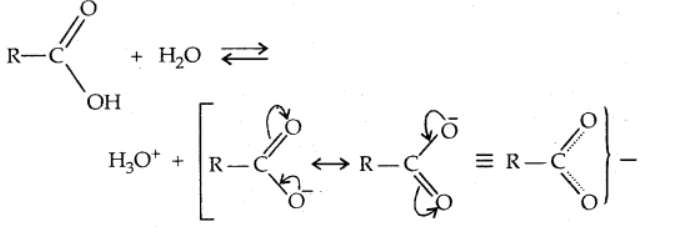
Effects of substituents on carboxylic acid acidity: Electron-withdrawing groups increase carboxylic acid acidity by stabilizing the conjugate base by delocalization of the negative charge via inductive and/or resonance effects. Electron-donating groups, on the other hand, reduce acidity by destabilizing the conjugate base.
2. Reactions involving the cleavage of the C-OH bond
Formation of anhydride
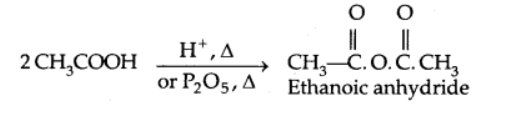
Esterification
In the presence of a mineral acid such as concentrated H2SO4 or HCl gas as a catalyst, carboxylic acids are esterified with alcohols.
$\mathrm{RCOOH}+\mathrm{R}$ 'OH (acidic medium) $\leftrightarrow \mathrm{RCOOR}{ }^{\prime}+\mathrm{H}_2 \mathrm{O}$
Reactions with PCl3, PCl5 and SOCl2
$\mathrm{RCOOH}+\mathrm{PCl}_5 \rightarrow \mathrm{RCOCl}+\mathrm{POCl}_3+\mathrm{HCl}$
$3 \mathrm{RCOOH}+\mathrm{PCl}_3 \rightarrow 3 \mathrm{RCOCl}+\mathrm{H}_3 \mathrm{PO}_3$
$\mathrm{RCOOH}+\mathrm{SOCl}_2 \rightarrow \mathrm{RCOCl}+\mathrm{SO}_2+\mathrm{HCl}$
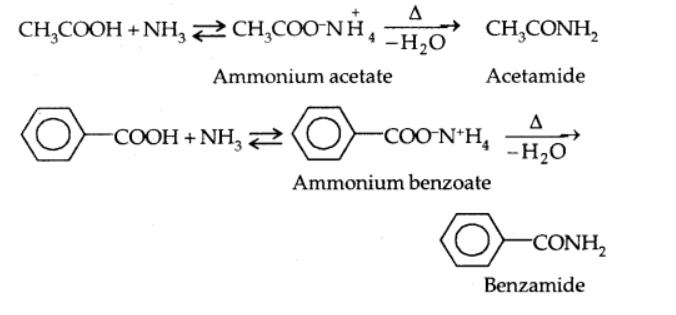
Reaction with ammonia
3. Reactions involving -COOH group
Reduction
In the presence of LiAlH4 or B2H6, carboxylic acids are reduced to alcohols.
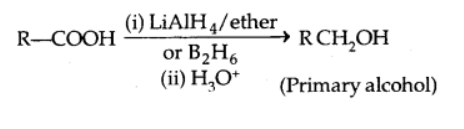
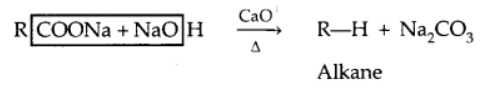
Decarboxylation
Heating sodium or potassium salts of carboxylic acids with soda lime (NaOH + CaO in a 3:1 ratio) produces hydrocarbons with one less carbon than the parent acid.
4. Substitution reactions in the hydrocarbon part
Halogenation (Hell-Volhard-Zelinsky reaction)
Carboxylic acids containing α-hydrogen are halogenated at the α-position with chlorine or bromine in the presence of a small amount of red phosphorus to produce α-halo carboxylic acids).
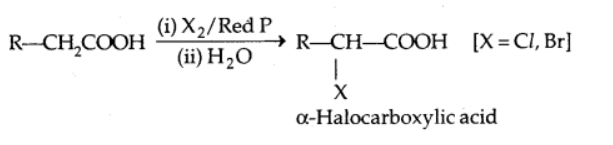
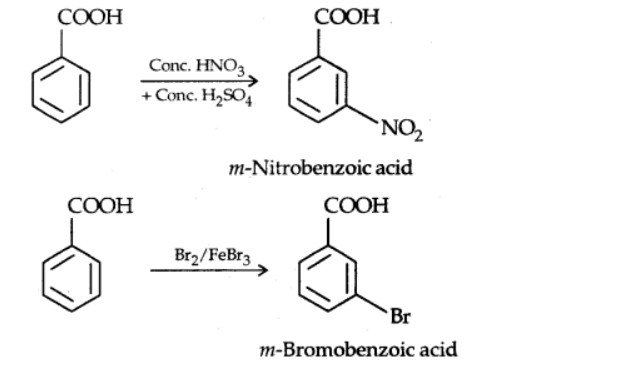
Ring substitution
Electrophilic substitution reactions occur in aromatic carboxylic acids. In benzoic acid, the carboxyl group is an electron-withdrawing and meta-directing group.
Uses of Carboxylic Acid
Methanoic acid is utilised in the rubber, textile, dyeing, leather, and electroplating industries. Ethanoic acid serves as a solvent and is utilized as vinegar in the food sector. Hexanedioic acid is utilized in the production of nylon-6,6. Esters of benzoic acid. Acids are utilized in fragrance. Sodium benzoate serves as a food preservative. Long-chain fatty acids are utilized in the production of soaps and detergents.
Aldehydes, Ketones and Carboxylic Acids Previous year Question and Answer
Selected previous year questions and answers of this chapter are given below they help students practise important questions and understand the application of concepts. The concepts used to solve these questions are explained in aldehydes, ketones and carboxylic acids Class 12 Chemistry Chapter 8 CBSE notes.
Question 1: The number of molecules from below that cannot give an iodoform reaction is :
Ethanol, Isopropyl alcohol, bromoacetone, 2-Butanol, 2-Butanone, Butanal, 2-Pentanone, 3-Pentanone, Pentanal and 3-Pentanol
(1) 5
(2) 4
(3) 3
(4) 2
Answer:
A compound gives a positive iodoform test if it contains either:
- A methyl ketone group $\left(\mathrm{COCH}_3\right)$
- A secondary alcohol that can be oxidized to a methyl ketone
The following will not give an iodoform reaction/test.
- Butanal- Butanal is an aldehyde; butanal does not have a $\mathrm{COCH}_3$ (methyl ketone) group.
- 3-Pentanone - This does have a $-\mathrm{COCH}_3$ group a methyl ketone in its structure.
- Pentanal - Pentanal is also an aldehyde like butanal, but does not have the $\mathrm{CH}_3-\mathrm{CO}-$ group.
- 3-Pentanol- This is a secondary alcohol, but it does not have the structure $-\mathrm{CH}(\mathrm{OH}) \mathrm{CH}_3$, which is required for oxidation to a methyl ketone.
Hence, the correct answer is option (2).
Question 2: Both acetaldehyde and acetone (individually) undergo which of the following reactions?
A. Iodoform Reaction
B. Cannizaro Reaction
C. Aldol condensation
D. Tollen's Test
E. Clemmensen Reduction
Choose the correct answer from the options given below:
(1) A, B and D only
(2) A, C and E only
(3) C and E only
(4) B, C and D only
Answer:
| Sr no |
Name of reaction |
Acetaldehyde
|
Acetone
|
| 1 | Iodoform reaction | $\oplus \mathrm{ve}$ | $\oplus \mathrm{ve}$ |
| 2 | Cannizaro | $\Theta \mathrm{ve}$ | $\Theta \mathrm{ve}$ |
| 3 | Aldol reaction | $\oplus \mathrm{ve}$ | $\oplus \mathrm{ve}$ |
| 4 | Tollen's test | $\oplus \mathrm{ve}$ | $\Theta \mathrm{ve}$ |
| 5 | Clemmensen reduction | $\oplus \mathrm{ve}$ | $\oplus \mathrm{ve}$ |
A, C, and E only
Hence, the correct answer is option (2).
Question 3: Write the structures of $A, B$ and $C$ in the following sequence of reaction:
$\mathrm{CH}_3 \mathrm{COOH} \xrightarrow{\mathrm{SOCl}_2} \mathrm{~A} \xrightarrow{\mathrm{H}_2, \mathrm{Pd}-\mathrm{BaSO}_4} \mathrm{~B} \xrightarrow{\mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2} \mathrm{C}$
Answer:
Given:
$
\mathrm{CH}_3 \mathrm{COOH} \xrightarrow{\mathrm{SOCl}_2} A \xrightarrow{\mathrm{H}_2, \mathrm{Pd} / \mathrm{BaSO}_4} B \xrightarrow{\mathrm{NH}_2 \mathrm{NH}_2} C
$
Acyl chloride formation
$
\mathrm{CH}_3 \mathrm{COOH} \xrightarrow{\mathrm{SOCl}_2} \mathrm{CH}_3 \mathrm{COCl}
$
Structure of A: Acetyl chloride
$
\mathrm{A}=\mathrm{CH}_3 \mathrm{COCl}
$
Rosenmund reduction
Reduction of acid chloride to aldehyde using $\mathrm{H}_2, \mathrm{Pd} / \mathrm{BaSO}_4$ (poisoned catalyst)
$
\mathrm{CH}_3 \mathrm{COCl} \xrightarrow{\mathrm{H}_2, \mathrm{Pd} / \mathrm{BaSO}_4} \mathrm{CH}_3 \mathrm{CHO}
$
Structure of B: Acetaldehyde
$
\mathrm{B}=\mathrm{CH}_3 \mathrm{CHO}
$
Wolff-Kishner Reduction
Aldehyde reacts with hydrazine ($\mathrm{NH}_2 \mathrm{NH}_2$) under basic conditions to give alkane:
$
\mathrm{CH}_3 \mathrm{CHO} \xrightarrow{\mathrm{NH}_2 \mathrm{NH}_2, \text { base }} \mathrm{CH}_3 \mathrm{CH}_3 \quad \text { (C) }
$
Structure of C: Ethane
$
\mathrm{C}=\mathrm{CH}_3 \mathrm{CH}_3
$
Question 4:
When
(1)
(2)
(3)
(4)
Answer:
The first step of the scheme shows aldol condensation in the presence of a base, resulting in the formation of $\beta$-hydroxy ketone with a 5-membered ring. On heating, water is eliminated and the product $\alpha, \beta$-Unsaturated cyclopentanone is obtained.
Hence, the correct answer is option (1).
Question 5: Which of the following compounds will give silver mirror with ammoniacal silver nitrate?
(A) Formic acid
(B) Formaldehyde
(C) Benzaldehyde
(D) Acetone
Choose the correct answer from the options given below :
(1) C and D only
(2) A, B and C only
(3) A only
(4) B and C only
Answer:
Apart from aldehyde, Formic acid 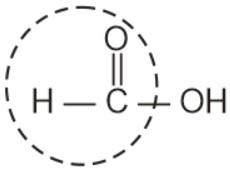
also gives silver mirror test with ammonical silver nitrate.
Hence, the answer is the option (2).
How to Master Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids
These class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 8.
- Firstly understand all fundamental concepts and reaction mechanisms of this chapter.
- Then students must understand the name reactions like Aldol condensation, Cannizzaro reaction, and Kolbe’s reaction.
- Mechanisms of reactions such as nucleophilic addition, oxidation, reduction, and substitution plays an important role in this chapter. Students can understand these concepts with the help of aldehydes, ketones and carboxylic acids ncert notes.
- After that students can solve previous year questions from this chapter.
Advantages of Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids Notes
NCERT notes Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids cover all topics from the NCERT book that helps students to understand complex reactions and mechanisms. The advantages of using these notes are given below:
- Students can use these notes to get clear explanations of the structure, properties, and preparation methods of aldehydes, ketones, and carboxylic acids.
- These notes include detailed reaction mechanisms and important name reactions.
- These ncert class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids notes contain summaries, formulas, and short tips.
- These NCERT Notes are prepared by subject experts in a very clear and comprehensive way that helps students prepare for board and competitive exams.
CBSE Class 12 Chemistry Chapter-wise Notes
Along with the NCERT notes Class 12 Chemistry Chapter 8 Aldehydes, Ketones and Carboxylic Acids students can also explore the hyperlinks provided below for NCERT Class 12 notes of all chapters.
|
NCERT Notes for Class 12 Chemistry Chapter 2 Electrochemistry |
|
NCERT Notes for Class 12 Chemistry Chapter 3 Chemical Kinetics |
|
NCERT Notes for Class 12 Chemistry Chapter 5 Coordination Compounds |
|
NCERT Notes for Class 12 Chemistry Chapter 6 Haloalkanes and haloarenes |
|
NCERT Notes for Class 12 Chemistry Chapter 7 Alcohol phenol and ether |
|
NCERT Notes for Class 12 Chemistry Chapter 8 Aldehyde ketones and carboxylic acids |
NCERT Solutions for Class 12 Chemistry
Along with class 12 chemistry chapter 8 aldehydes, ketones and carboxylic acids notes students can also refer to solutions of NCERT for Class 12 Chemistry chapters.
Subject-Wise NCERT Exemplar Solutions
The hyperlinks of the NCERT exemplar solutions, subject-wise, are given below:
Subject-Wise NCERT Solutions
The hyperlinks of NCERT solutions subject-wise are given below:
Frequently Asked Questions (FAQs)
The IUPAC nomenclature for aldehydes, ketones, and carboxylic acids follows specific rules based on their functional groups.
- Aldehydes: Suffix -al, e.g., Methanal for formaldehyde.
- Ketones: Suffix -one, e.g., Propan-2-one for acetone.
- Carboxylic Acids: Suffix -oic acid, e.g., Ethanoic acid for acetic acid.
Aldehydes can be prepared through several methods, including:
- Oxidation of primary alcohols using oxidizing agents like potassium dichromate.
- Hydroformylation of alkenes in the presence of carbon monoxide and hydrogen.
- Reduction of carboxylic acids from carboxylic acids or esters using lithium aluminum hydride.
Carboxylic acids are organic compounds that contain a carboxyl group. This functional group is responsible for the acidic properties of carboxylic acids, allowing them to donate protons in solution. They have the general formula RCOOH, where R represents a hydrocarbon chain.
Carboxylic acids can be prepared through several methods, including:
- Oxidation of primary alcohols or aldehydes.
- Hydrolysis of nitriles and esters.
- Carboxylation of Grignard reagents, a powerful method to produce carboxylic acids directly.
Aldehydes are generally more reactive than ketones due to having one less alkyl group, which makes the carbonyl carbon more accessible for nucleophilic attack. Carboxylic acids, on the other hand, can undergo further reactions like decarboxylation or esterification, which are not applicable to either aldehydes or ketones.
Aldehydes and ketones undergo nucleophilic addition reactions because the carbonyl carbon is electrophilic due to the polar C=O bond. Nucleophiles can attack this carbon, leading to the formation of an alcohol or hemiacetal, for example.
The aldehydes, ketones and carboxylic acids class 12 ncert notes provide a concise summary of the chapter, covering definitions, nomenclature, physical and chemical properties, preparation methods, and important reactions of these compounds.
Aldehydes, Ketones & Carboxylic Acids Class 12 notes help students by simplifying complex reactions into easy-to-understand steps, highlighting important name reactions, and providing quick revision material before exams.
Carboxylic acids have higher boiling points than aldehydes and ketones because they can form hydrogen bonds due to the presence of the hydroxyl group (-OH), while aldehydes and ketones only have dipole-dipole interactions, which are weaker.
Carboxylic acids are stronger acids than phenols because their conjugate base (carboxylate ion) is stabilized by resonance, while phenols have less effective resonance stabilization in their conjugate base (phenoxide ion).
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters