These haloalkanes and haloarenes class 12 ncert notes are brief, structured summaries of key concepts, reactions, mechanisms, properties, and applications from the NCERT textbook. These notes help students revise quickly and understand the chapter effectively for exams.
NCERT Class 12 Chemistry Chapter 10 Notes Haloalkanes and Haloarenes - Download PDF
Do you know how refrigerant cools, why halogen-containing compounds are widely used in medicines and what makes pesticides so effective? The answer to all these questions lies in haloalkanes and haloarenes class 12 ncert notes. In these compounds, Hydrogen atoms are replaced by Halogens. The word Haloalkanes or Haloarenes is made up of 2 words, the first is Halo, which means Halogen, and the second word is alkanes or arenes, which means aliphatic or aromatic hydrocarbons.
This Story also Contains
- NCERT Notes for Class 12 Chapter 6 : Download PDF
- NCERT Notes for Class 12 Chapter 6
- Haloalkanes and Haloarenes Previous Year Questions and Answers
- How to Master Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes
- Advantages of Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes Notes
- CBSE Class 12 Chemistry Chapter-Wise Notes
- NCERT Solutions for Class 12 Chemistry
- Subject-Wise NCERT Solutions
- Subject-Wise NCERT Exemplar Solutions
NCERT Class 12 Chemistry Notes helps students to understand topics like classification, nomenclature, preparation, physical properties and chemical reactions of chapter 6 haloalkanes and haloarenes. Whether you are revising for an exam or learning the topic for the first time, NCERT notes are an essential resource. These notes include important concepts, definitions, diagrams, and selective previous year solved questions, which helps students in scoring well in the CBSE board as well as competitive exams by simplifying complex concepts.
NCERT Notes for Class 12 Chapter 6 : Download PDF
Students can download the class 12 chemistry chapter 6 haloalkanes and haloarenes notes pdf from the icon given below. These notes help students revise quickly and strengthen their exam preparation.
Also Read
NCERT Notes for Class 12 Chapter 6
These haloalkanes and haloarenes class 12 ncert notes explores Chemistry of organic compounds containing halogens. These NCERT notes for Class 12 cover topics like preparation of Haloalkanes and Haloarenes, their properties, types of reactions, and other important concepts.
Classification
Haloalkanes and haloarenes may be classified as follows
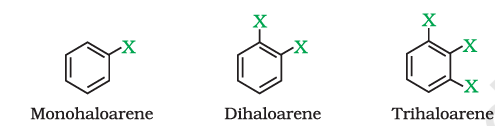
1. On the Basis of Number of Halogen Atoms
Haloalkanes and Haloarenes are classified as mono, di, or polyhalogen compounds depending on whether they contain one, two or more halogen atoms in their structures.
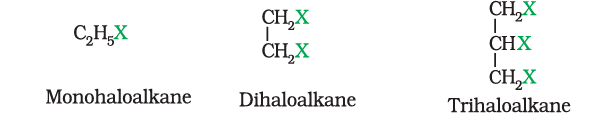
2. Compounds Containing sp3 C—X Bond (X= F, Cl, Br, I)
In these compounds, a halogen atom (F, Cl, Br, or I) is bonded to a carbon atom that is sp3 hybridised, forming a single sigma bond.
These class includes:
(i). Alkyl halides or haloalkanes (R—X)
In alkyl halides, a halogen atom is bonded to an alkyl group (R), forming a homologous series with the formula $\mathrm{C}_n \mathrm{H}_{2 n+1} \mathrm{X}$. They are classified as primary, secondary, or tertiary based on whether the halogen is attached to a primary, secondary, or tertiary carbon atom.
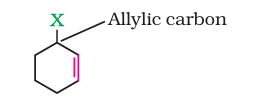
(ii) Allylic halides
These are the compounds in which the halogen atom is bonded to an sp3-hybridised carbon atom adjacent to carbon-carbon double bond (C=C) i.e. to an allylic carbon.
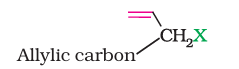
(iii) Benzylic halides
Compounds in which the halogen atom is bonded to an sp3-hybridised carbon atom attached to an aromatic ring.
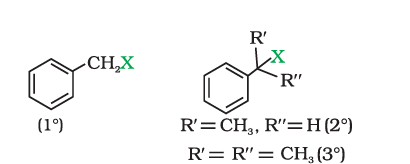
3. Compounds Containing sp2 C—X Bond
These compounds shows halogen atom bonded to an sp2 hybridised carbon. These include haloarenes and vinylic halides, which show distinct reactivity due to the partial double bond character of the C—X bond.
(i). Vinylic halides
These are the compounds in which the halogen atom is bonded to a sp2-hybridised carbon atom of a carbon-carbon double bond (C = C).

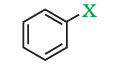
(ii). Aryl halides
These are the compounds in which the halogen atom is directly bonded to the sp2-hybridised carbon atom of an aromatic ring.
Nomenclature
Haloalkanes and haloarenes can be named using either common nomenclature or the IUPAC system. Students can learn these concepts more easily with the help of ncert class 12 chemistry chapter 6 haloalkanes and haloarenes notes.
a) For haloalkanes-
Common name
In the common system, the alkyl group is named first followed by the appropriate word chloride, bromide, etc. The common name of an alkyl halide is always written as two separate words.
IUPAC Name
In the IUPAC system of nomenclature, alkyl halides are named as halosubstituted hydrocarbons.
- Select the longest chain of carbon atoms containing the halogen atom.
- Number the chain to give the minimum number to the carbon-carrying halogen atom.
- If multiple bonds are present, then it is given the preference in numbering the carbon chain.
- The IUPAC name of any halogen derivative is always written as one word.
b) For haloarenes-
Aryl halides are named by prefixing “halo” to the name of the parent aromatic hydrocarbon. If there is more than one substituent on the ring then the relative positions of the substituents are indicated by mathematical numerals. In the common system, the relative position of two groups is shown by prefixes ortho, meta or para.
Halo+ name of aromatic hydrocarbon
Example- chlorobenzene, Iodobenzene, 3-chlorotoluene.
Nature of C-X Bond
Halogen atoms are more electronegative than carbon, therefore, carbon-halogen bond of alkyl halide is polarised; the carbon atom bears a partial positive charge whereas the halogen atom bears a partial negative charge. As we go down the group in the periodic table, the size of halogen atom increases. Fluorine atom is the smallest and iodine atom is the largest. Consequently, the carbon-halogen bond length also increases from$\mathrm{C}-\mathrm{F}$ to $\mathrm{C}-\mathrm{I}$.
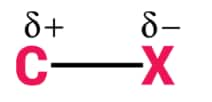
Preparation of Haloalkanes
Haloalkanes can be synthesised through several ways:
1). From Alcohols
Alcohols are converted to haloalkanes by replacing the OH group with halogen using reagents such as concentrated HX , $\mathrm{PCl}_5, \mathrm{PBr}_3$, or $\mathrm{SOCl}_2$. Alkyl bromides form by boiling with 48% HBr, and alkyl iodides by heating with NaI in 95% $\mathrm{H}_3 \mathrm{PO}_4$.
In all cases, tertiary alcohols react most rapidly, followed by secondary and then primary.
$\mathrm{R}-\mathrm{OH}+\mathrm{HCl} \xrightarrow{\mathrm{ZnCl}_2} \mathrm{R}-\mathrm{Cl}+\mathrm{H}_2 \mathrm{O}$
$\mathrm{R}-\mathrm{OH}+\mathrm{NaBr}+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{R}-\mathrm{Br}+\mathrm{NaHSO}_4+\mathrm{H}_2 \mathrm{O}$
$3 \mathrm{R}-\mathrm{OH}+\mathrm{PX}_3 \longrightarrow 3 \mathrm{R}-\mathrm{X}+\mathrm{H}_3 \mathrm{PO}_3 \quad(\mathrm{X}=\mathrm{Cl}, \mathrm{Br})$
$\mathrm{R}-\mathrm{OH}+\mathrm{PCl}_5 \longrightarrow \mathrm{R}-\mathrm{Cl}+\mathrm{POCl}_3+\mathrm{HCl}$
$\mathrm{R}-\mathrm{OH}+\mathrm{SOCl}_2 \longrightarrow \mathrm{R}-\mathrm{Cl}+\mathrm{SO}_2+\mathrm{HCl}$
2). From Hydrocarbons
(i) From alkanes by free radical halogenation
Free radical chlorination or bromination of alkanes gives a complex mixture of isomeric mono- and polyhaloalkanes.
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_3 \xrightarrow[\text { or heat }]{\mathrm{Cl}_2 / \mathrm{UV} \text { light }} \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Cl}+\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHClCH}_3$
(ii) From alkenes
(a). Addition of hydrogen halides:
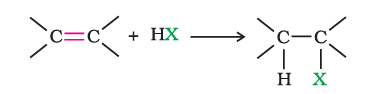
(b). Addition of halogens:
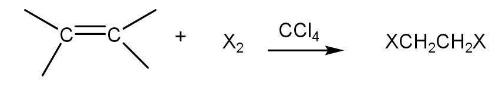
Addition of bromine in $\mathrm{CCl}_4$ to an alkene resulting in discharge of reddish brown colour of bromine constitutes an important method for the detection of double bond in a molecule.
3). Halogen Exchange
This method is also known as Finkelstein's reaction. It is a method of preparation of alkyl iodides from alkyl chlorides or alkyl bromides. In this reaction, alkyl chlorides or bromides are treated with NaI in the presence of acetone to form alkyl iodides. The reaction occurs as follows:
$\mathrm{R}-\mathrm{X}+\mathrm{NaI} \rightarrow \mathrm{R}-\mathrm{I}+\mathrm{NaX}$
We use NaI because it is soluble in acetone as it is covalent in nature. All other sodium halides are ionic in nature and thus not soluble.
For example:
$\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}_2-\mathrm{Cl} \xrightarrow{\mathrm{NaI} / \text { Acetone }} \mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}_2-\mathrm{I}$
Preparation for Haloarenes
Haloarenes can be synthesised through several ways. Download Haloalkanes and Haloarenes Class 12 Chemistry Chapter 6 pdf to access these notes anytime and anywhere.
1). From hydrocarbons by electrophilic substitution
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts like iron or iron(III) chloride.
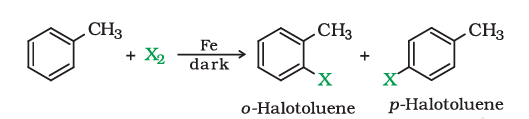
The ortho and para isomers can be easily separated due to large difference in their melting points. Reactions with iodine are reversible in nature and require the presence of an oxidising agent ($\left(\mathrm{HNO}_3, \mathrm{HIO}_4\right)$) to oxidise the HI formed during iodination. Fluoro compounds are not prepared by this method due to high reactivity of fluorine.
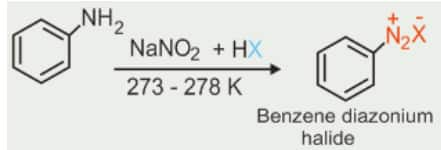
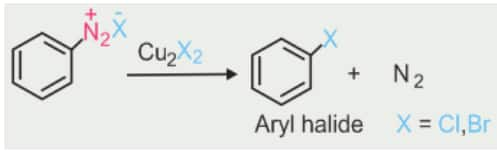
2). From amines by Sandmeyer’s reaction
When a primary aromatic amine, dissolved or suspended in cold aqueous mineral acid, is treated with sodium nitrite, a diazonium salt is formed. Mixing the solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide results in the replacement of the diazonium group by –Cl or –Br.
Physical Properties
Physical properties of Haloalkanes
-
Haloalkanes are colourless and their a sweet-smelling taste.
-
Since haloalkanes cannot break the hydrogen bonds of water, they are slightly soluble in water.
-
As the size of the alkyl group increases, density increases.
-
The boiling as well as melting points of haloalkanes are higher than their parent hydrocarbon.
-
These are not as flammable as hydrocarbons.
-
Dipole moment decreases on going from methyl chloride, methyl bromide, and methyl iodide. However, the dipole moment of methyl fluoride is lower than compared of methyl chloride.
Physical properties of Haloarenes
-
Haloarenes are colourless in nature and heavier than water.
-
Mostly soluble in organic solvents and insoluble in inorganic solvents.
-
As the size of the halogen attached to benzene increases, the boiling point also increases.
-
Out of the three isomers, the boiling point of the ortho isomer is the highest.
-
The dipole moment of the ortho isomer is larger than the meta isomer. Para isomer has zero dipole moment.
Chemical Reactions of Haloalkanes
The reactions of haloalkanes may be divided into the following categories:
1. Nucleophilic substitution
2. Elimination reactions
3. Reaction with metals
1. Nucleophilic substitution reaction
Nucleophiles are electron rich species, they attack at that part of the substrate molecule which is electron deficient. The reaction in which a nucleophile replaces already existing nucleophile in a molecule is called nucleophilic substitution reaction.

Mechanism
Nucleophilic substitution reactions are of two types
i) SN1
ii) SN2
i). Substitution nucleophilic bimolecular (SN2)
The general reaction occurs as follows:
$\mathrm{R}-\mathrm{CH}_2-\mathrm{Cl}+\mathrm{OH}^{-} \rightarrow \mathrm{R}-\mathrm{CH}_2-\mathrm{OH}$
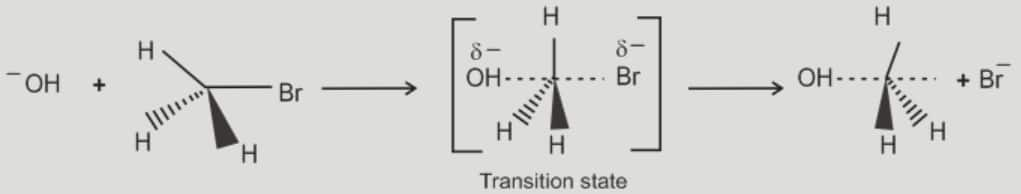
- It is bimolecular nucleophilic substitution (SN2) reaction.
- Rate of reaction follows second order kinetics and depends upon the concentration of both the nucleophile as well as the substrate.
Rate $\propto[\mathrm{R}-\mathrm{X}]\left[\mathrm{Nu}^{-}\right]$
- Rate determinig step depends on how fast the transition state is formed and also the stability of the transition state
- Stronger nucleophile is required as it has to attack and make the leaving group leave
- Polar aprotic solvents favour SN2 reaction as they do not facilitate formation of ions
- The reaction occurs in concerted mechanism and inversion of configuration (Walden Inversion) takes place if the leaving group and the nucleophile have the same priority
- Steric hinderance in the substrate decreases the reactivity of the subtrate towards SN2 reaction
ii). Substitution nucleophilic unimolecular (SN1)
The general reaction occurs as follows:
$\mathrm{R}-\mathrm{CH}_2-\mathrm{Cl}+\mathrm{OH}^{-} \rightarrow \mathrm{R}-\mathrm{CH}_2-\mathrm{OH}$
- The mechanism occurs as follows:
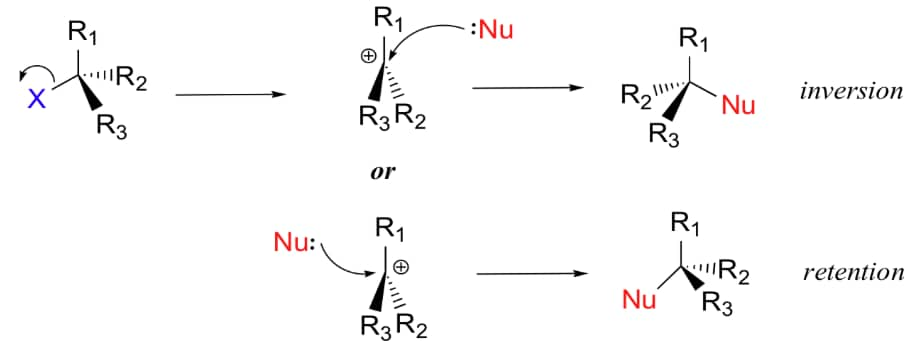
- SN1 reactions are nucleophilic substitution reactions, involving a nucleophile replacing a leaving group.
- SN1 reactions are unimolecular. The rate of this reaction depends only on the concentration of one reactant and does not depend upon the strength of the nucleophile
Rate $\propto[\mathrm{R}-\mathrm{X}]$
- The rate determining step depends on the stability of the intermediate carbocation which is obtained during the course of the reaction
Rate $\propto$ stability of carbocation
- Since the mechanism involves the attack of nucleophiles on an already-formed carbocation, the strength of nucleophiles is unimportant for the rate of the reaction
- The rate of formation of intermediate is independent of concentration of nucleophile and depends only on the concentration of reactants.
- Good ionising solvents (polar protic solvents) are required to carry out SN1 reaction as there has to bea formation of ions
- Configuration of the product may be same or inverted and in cases where the leaving group departs from a chiral centre, racemisation occurs.
- If Nu- attacks on the same side from where X- leaves, then it is called 'Retention'.
- If Nu- attacks from the opposite side from where X- leaves, then it is called 'Inversion'.
- Racemic mixture is obtained when equal amount of retention and inversion products are formed in the reaction.
- Generally, partial racemisation is seen in the reactions as it both SN1 and SN2 are both competing
Stereochemical aspects of nucleophilic substitution reactions
To understand these reactions, it is essential to first learn some fundamental stereochemical concepts and notations such as optical activity, chirality, retention, inversion, and racemisation.
(i) Optical activity:
Plane of plane polarised light produced by passing ordinary light through Nicol prism is rotated when it is passed through the solutions of certain compounds. Such compounds are called optically active compounds. The angle by which the plane polarised light is rotated is measured by an instrument called polarimeter.
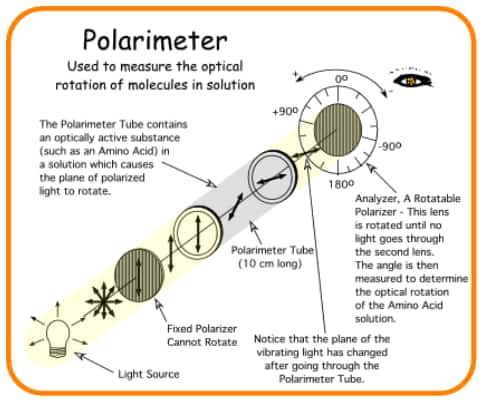
- If the compound rotates the plane of plane polarised light to the clockwise direction, it is called dextrorotatory.
- If the light is rotated towards anticlockwise direction, the compound is said to be laevo-rotatory.
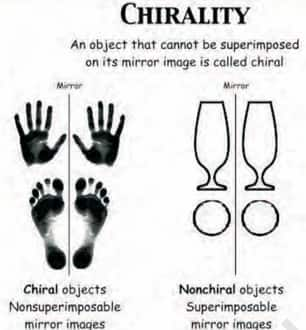
(ii) Molecular asymmetry, chirality and enantiomers:
Molecular asymmetry is basically the absence of symmetry in a molecule, often due to the presence of a carbon atom bonded to four different groups. This leads to chirality a property where a molecule and its mirror image are non-superimposable much like left and right hands. Such mirror-image molecules are called enantiomers.
Enantiomers have identical physical and chemical properties except for the direction in which they rotate plane-polarised light and their behaviour in chiral environments.
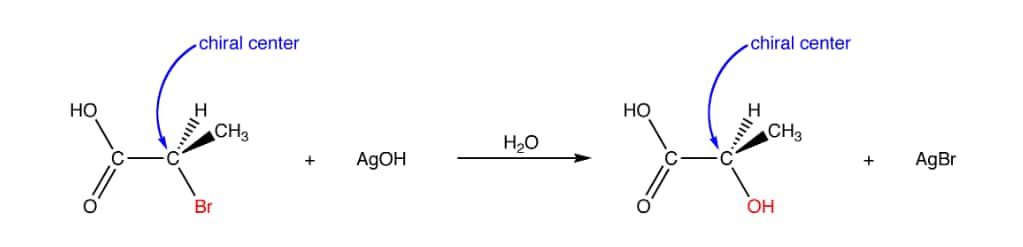
(iii) Retention:
Retention of configuration is the preservation of the spatial arrangement of bonds to an asymmetric centre during a chemical reaction or transformation. In general, if during a reaction, no bond to the stereocentre is broken, the product will have the same general configuration of groups around the stereocentre as that of reactant.
(iv) Inversion, retention and racemisation:
Retention of configuration: A process in which the relative configuration of an atom is retained. If the atom in question is a stereocenter, retention of configuration usually changes R absolute configuration into R, and S into S.
Inversion of configuration: A process in which the relative configuration of an atom is changed. If the atom in question is a stereocenter, inversion of configuration usually changes R absolute configuration into S, and S into R.
Racemization refers to the converting of an enantiomerically pure mixture (one where only one enantiomer is present) into a mixture where more than one of the enantiomers are present. If the racemization results in a mixture where the enantiomers are present in equal quantities.
2. Elimination reactions
When a haloalkane with $\beta$-hydrogen atom is heated with alcoholic solution of potassium hydroxide, there is elimination of hydrogen atom from $\beta$-carbon and a halogen atom from the $\alpha$-carbon atom.
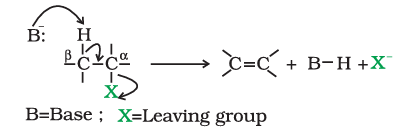
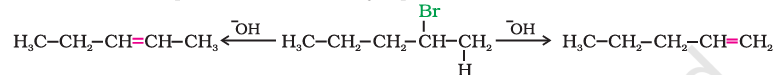
3.Reaction with metals
Haloalkanes react with metals such as Magnesium and sodium.
$R-X + Mg \overset{dry ether}{\longrightarrow} R-MgX$
Wurtz reaction
Alkyl halides react with sodium in dry ether to give hydrocarbons containing double the number of carbon atoms present in the halide. This reaction is known as Wurtz reaction.
$2R-X + 2Na \overset{dry ether}{\longrightarrow} R-R + 2NaX$
Reactions of Haloarenes
1. Nucleophilic substitution
Aryl halides are extremely less reactive towards nucleophilic substitution reactions due to the following reasons:
- Resonance effect: In haloarenes, the electron pairs on halogen atom are in conjugation with π-electrons of the ring and the following resonating structures are possible.
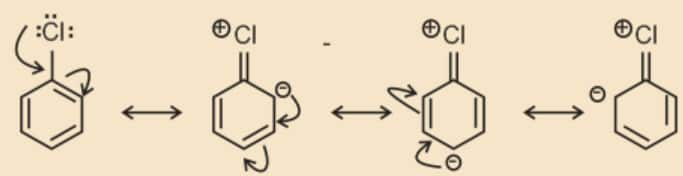
C—Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarene is difficult than haloalkane and therefore, they are less reactive towards nucleophilic substitution reaction.
- Difference in hybridisation of carbon atom in C—X bond: In haloalkane, the carbon atom attached to halogen is sp3 hybridised while in case of haloarene, the carbon atom attached to halogen is sp2-hybridised. The sp2 hybridised carbon with a greater s-character is more electronegative and can hold the electron pair of C—X bond more tightly than sp3 -hybridised carbon in haloalkane with less s-chararcter. Thus, C—Cl bond length in haloalkane is 177pm while in haloarene is 169 pm. Since it is difficult to break a shorter bond than a longer bond, therefore, haloarenes are less reactive than haloalkanes towards nucleophilic substitution reaction.
- Instability of phenyl cation: In case of haloarenes, the phenyl cation formed as a result of self-ionisation will not be stabilised by resonance and therefore, SN1 mechanism is ruled out.
- Because of the possible repulsion, it is less likely for the electron rich nucleophile to approach electron rich arenes.
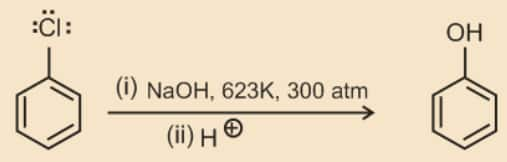
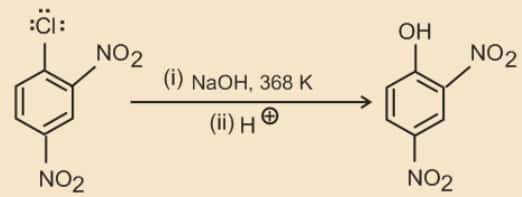
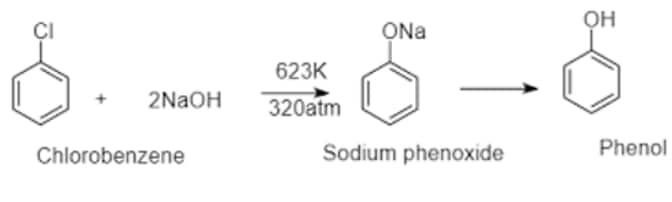
Chlorobenzene can be converted into phenol by heating in aqueous sodium hydroxide solution at a temperature of 623K and a pressure of 300 atmospheres.
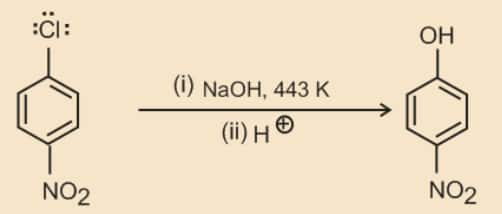
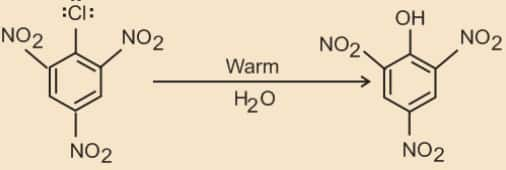
The presence of an electron withdrawing group (-NO2 ) at ortho- and para-positions increases the reactivity of haloarenes.
2. Electrophilic substitution reactions
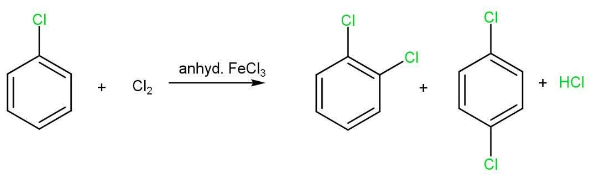
Haloarenes undergo the usual electrophilic reactions of the benzene ring such as halogenation, nitration, sulphonation and Friedel-Crafts reactions. Halogen atom besides being slightly deactivating is o, p directing. Therefore, further substitution occurs at ortho- and para positions with respect to the halogen atom.
(i) Halogenation
(ii) Nitration
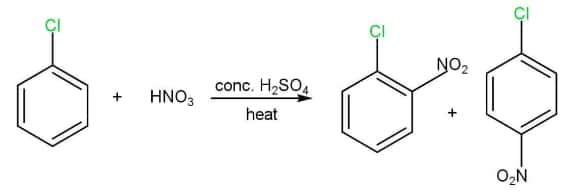
(iii) Sulphonation
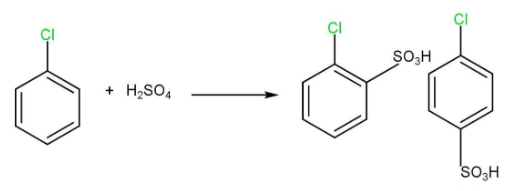
(iv) Friedel-Crafts reaction
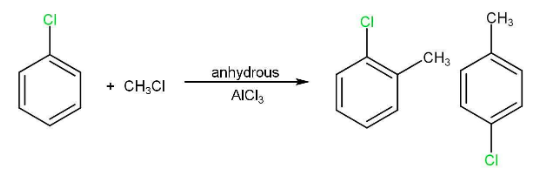
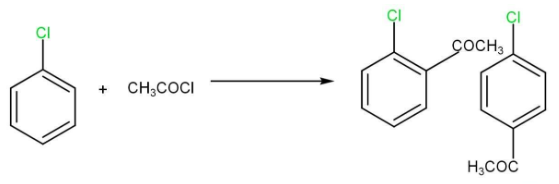
3. Reaction with metals
Wurtz-Fittig reaction
A mixture of an alkyl halide and aryl halide gives an alkylarene when treated with sodium in dry ether and is called Wurtz-Fittig reaction.

Fittig reaction
Aryl halides also give analogous compounds when treated with sodium in dry ether, in which two aryl groups are joined together. It is called Fittig reaction.
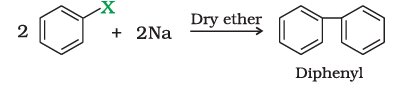
Polyhalogen Compounds
Carbon compounds containing more than one halogen atom are usually referred to as polyhalogen compounds. Many of these compounds are useful in industry and agriculture.
1. Dichloromethane (Methylene chloride)
It is widely used as a solvent in paint removers, aerosols, drug manufacturing, and metal cleaning. However, it is harmful to the central nervous system. Low-level exposure may impair hearing and vision, while higher levels can cause dizziness, nausea, and numbness. Direct skin contact leads to burning and redness, and eye contact can damage the cornea.
2. Trichloro methane (Chloroform)
It is mainly used as a solvent and in making refrigerant R-22. Once used as an anaesthetic, it is now avoided due to its toxicity. Inhalation can cause dizziness and fatigue; long-term exposure may harm the liver and kidneys.
$2 \mathrm{CHCl}_3+\mathrm{O}_2 \xrightarrow{\text { light }} 2 \mathrm{COCl}_2+2 \mathrm{HCl}$
3. Triiodomethane (Iodoform)
It was used earlier as an antiseptic but the antiseptic properties are due to the liberation of free iodine and not due to iodoform itself. Due to its objectionable smell, it has been replaced by other formulations containing iodine
4. Tetrachloromethane (Carbon tetrachloride)
It is used in making refrigerants, solvents, and chemicals. Once common as a cleaner, it is now restricted due to its toxicity. Exposure can cause nausea, nerve damage, heart issues, and liver cancer. It also depletes the ozone layer, increasing the risk of skin cancer and other health problems.
5. Freons
They are chlorofluorocarbon compounds of methane and ethane, widely used as aerosol propellants and in refrigeration and air conditioning. Freon-12 made from tetrachloromethane via the Swarts reaction, is the most common. These stable, non-toxic gases eventually reach the stratosphere, where they trigger radical reactions that deplete the ozone layer.
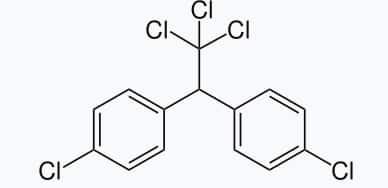
6. p,p’-Dichlorodiphenyltrichloroethane(DDT)
DDT, the first chlorinated organic insecticide. The use of DDT increased enormously on a worldwide basis after World War II, primarily because of its effectiveness against the mosquito that spreads malaria and the lice that carry typhus. However, problems related to the extensive use of DDT began to appear in the late 1940s. Many species of insects developed resistance to DDT, and it was also discovered to have a high toxicity towards fish. The chemical stability of DDT and its fat solubility compounded the problem. DDT is not metabolised very rapidly by animals; instead, it is deposited and stored in the fatty tissues.
Haloalkanes and Haloarenes Previous Year Questions and Answers
Given below selected previous year questions with answers of Haloalkanes and Haloarenes to help students understand the exam pattern and revise the key concepts effectively. The concepts used to solve these questions are explained in haloalkanes and haloarenes class 12 ncert notes.
Question 1: Give reasons for the following observation
Main product obtained when chloroethane reacts with KCN is propane nitrile while with AgCN it is ethyl isocyanide.
Answer:
$C N^{-}$is an ambident nucleophile. In KCN, C acts as the nucleophile because of the ionic nature of $\mathrm{K}-\mathrm{C}$ bond. $\operatorname{In} \mathrm{AgCN}, \mathrm{N}$ acts as the nucleophile because of covalent nature of $\mathrm{Ag}-\mathrm{C}$ bond. Hence, chloroethane reacts with KCN to form ethane nitrile as the main product, while with AgCN, ethyl isocyanide is the chief product.
With KCN (forms ethyl cyanide):
$
\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}+\mathrm{KCN} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{CN}+\mathrm{KCl}
$
With AgCN (forms ethyl isocyanide):
$
\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}+\mathrm{AgCN} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{NC}+\mathrm{AgCl}
$
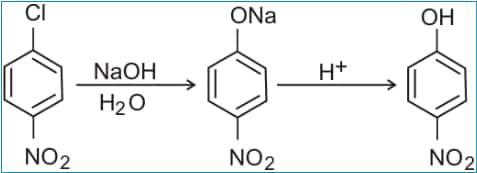
Question 2: Give reasons for the following observation :
p -Chloronitrobenzene reacts with (aq) NaOH at 443 K to give p-nitrophenol whereas chlorobenzene reacts with the same reagent at 623 K and 300 atm.
Answer:
1. p-Chloronitrobenzene reacts with (aq) NaOH at 443 K to give p-nitrophenol
p-chloronitrobenzene undergoes nucleophilic substitution faster than chlorobenzene because p-chloronitrobenzene has a nitro group which is an electron-withdrawing group and chlorine itself is an electronegative group by which chlorine generates electrons on its surface and attract as well as .it also generate partial positive charge therefore now it become an electrophile and it require electron for that positive charge than the nitro group withdraw its electron and increase the partial positive charge over carbon and chloro group and weaken their bond it goes easily on nucleophilic substitution reaction than chlorobenzene.
2. Chlorobenzene reacts with aqueous NaOH at 623 K and 300 atm
Because chlorobenzene lacks strong electron-withdrawing groups that stabilize the intermediate, that's why the reaction requires harsh conditions.
Question 3: Write chemical equations of the following reaction:
Sodium t-butoxide is treated with $\mathrm{CH}_3 \mathrm{Cl}$.
Answer:
When sodium t-butoxide (NaO-t-Bu) is treated with methyl chloride (CH₃Cl), a nucleophilic substitution reaction occurs. The t-butoxide ion acts as a strong nucleophile and attacks the electrophilic carbon in methyl chloride, leading to the formation of t-butyl methyl ether and sodium chloride as a byproduct.
$\left(\mathrm{CH}_3\right)_3 \mathrm{CO}^{-}+\mathrm{CH}_3 \mathrm{Cl} \rightarrow\left(\mathrm{CH}_3\right)_3 \mathrm{COCH}_3+\mathrm{Cl}^{-}$
This is called Williamson ether synthesis.
Question 4: Given below are two statements :
Statement (I) : Alcohols are formed when alkyl chlorides are treated with aqueous potassium hydroxide by elimination reaction.
Statement (II) : In alcoholic potassium hydroxide, alkyl chlorides form alkenes by abstracting the hydrogen from the $\beta$-carbon.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Both Statement I and Statement II are incorrect
(2) Statement I is incorrect but Statement II is correct
(3) Statement I is correct but Statement II is incorrect
(4) Both Statement I and Statement II are correct
Answer:
Statement (I) :
$\mathrm{R}-\mathrm{Cl} \xrightarrow{(\mathrm{aq}, \mathrm{KOH})} \mathrm{R}-\mathrm{OH}$ this reaction is nucleophilic substitution (SN2 or SN1), not elimination.
Statement (II) :
Hence, the correct answer is option (2).
Question 5: The major product of the following reaction is :

(1) 6-Phenylhepta-2,4-diene
(2) 2-Phenylhepta-2,5-diene
(3) 6-Phenylhepta-3,5-diene
(4) 2-Phenylhepta-2,4-diene
Answer:

Hence, the correct answer is option (4).
How to Master Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes
These haloalkanes and haloarenes Class 12 Chemistry Chapter 6 CBSE notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 6.
- Firstly understand the basic concepts like nomenclature, classification, and nature of carbon halogen bonds and IUPAC naming rules.
- Then learn about preparation methods of haloalkanes and haloarenes from alcohols, hydrocarbons, halogen exchange reactions, Sandmeyer reactions, etc.
- Then learn about physical & chemical properties such as boiling points, solubility, and densities.
- Practice reaction mechanisms like nucleophilic substitution and elimination reactions. Students can also refer to haloalkanes and haloarenes class 12 ncert notes.
- Students must learn why haloarenes are less reactive towards nucleophilic substitution, and electrophilic substitution reactions such as nitration, sulphonation, Friedel Crafts reactions.
- Uses of organohalogen compounds and their environmental effects play an important role in this chapter.
- After that students can solve previous year questions from this chapter.
Advantages of Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes Notes
The haloalkanes and haloarenes class 12 ncert notes are important for students preparing for boards and competitive exams like JEE and NEET. The advantages of using these notes are given below:
- Students can cover topics like nomenclature, preparation methods, physical and chemical properties, and mechanisms of substitution, elimination reactions and mechanisms.
- They help students revise all important topics and reaction mechanisms that are frequently asked in board exams.
- NCERT notes Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes include diagrams and explanations to make understanding easier.
- These notes are prepared by subject experts in a very comprehensive manner that helps students build a strong foundation for organic chemistry.
CBSE Class 12 Chemistry Chapter-Wise Notes
Along with class 12 chemistry chapter 6 haloalkanes and haloarenes notes follow these hyperlinks of NCERT notes for other Class 12 chapters:
NCERT Solutions for Class 12 Chemistry
Along with ncert class 12 chemistry chapter 6 haloalkanes and haloarenes notes students can also refer to solutions of NCERT for Class 12 Chemistry chapters.
Subject-Wise NCERT Solutions
The hyperlinks of ncert solutions subject-wise are given below:
Subject-Wise NCERT Exemplar Solutions
The hyperlinks of the NCERT exemplar solutions, subject-wise, are given below:
Frequently Asked Questions (FAQs)
Nucleophilic substitution is a key reaction for haloalkanes where the halogen is replaced by a nucleophile. This reaction is significant for synthesizing various organic compounds, including alcohols and amines.
Haloalkanes can be synthesized through several methods, including:
1. Halogenation of Alkanes: Reacting alkanes with halogens in the presence of UV light.
2. Substitution Reactions: Treating alcohols with hydrogen halides.
3. Nucleophilic Substitution: Reaction of alkyl halides with various nucleophiles
The stability of haloalkanes can differ based on the type of haloalkane. Tertiary haloalkanes are generally more stable than primary haloalkanes due to steric hindrance and hyperconjugation effects, making them more reactive in substitution reactions.
Haloalkanes can have detrimental environmental effects. Some of them are persistent pollutants and can accumulate in the environment, leading to toxic effects on wildlife and even humans.
Haloalkanes generally have higher boiling and melting points compared to their corresponding alkanes due to the presence of polar C-X bonds, which can lead to dipole-dipole interactions. They are usually non-polar and less soluble in water, but soluble in organic solvents.
Haloalkanes are compounds in which one or more hydrogen atoms of an alkane are replaced by halogen atoms (F, Cl, Br, I). Haloarenes are compounds in which one or more hydrogen atoms of an aromatic ring like benzene are replaced by halogen atoms.
Haloalkanes are more reactive than haloarenes due to the different bonding structures. In haloalkanes, the carbon-halogen bond is polarized and can undergo nucleophilic substitution and elimination reactions more readily. Haloarenes, with their stable aromatic rings, are less reactive due to resonance stabilization, making them less susceptible to substitution reactions under normal conditions.
Haloarenes are used in various applications, including as solvents, intermediates in the production of dyes, and in the synthesis of pharmaceuticals.
Haloalkanes typically have higher boiling points than their corresponding alkanes. This is largely due to the stronger polarizability and dipole-dipole interactions associated with the carbon-halogen bond.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters

