NCERT Class 12 Chemistry Chapter 4 Notes Chemical Kinetics- Download PDF Notes
The most recent topic in today's world is nuclear power. Have you ever wondered why some reactions, like nuclear explosions, happen within a fraction of second while some reactions take years to happen like rusting of iron? Well, this depends on the rate of reaction, and in class 12 chemistry chapter 3 chemical kinetics notes you will learn everything about it. Similarly, food preservation is something that we see every day. Fresh fruits and vegetables spoil due to interactions between microbes and oxygen in the air. Storing them in the refrigerator slows down these processes, allowing them to last longer. In medicine, how rapidly a drug dissolves in the body influences its effectiveness. All this involves study of rate of reaction, and hence, chemical kinetics.
This Story also Contains
- NCERT Notes for Class 12 Chapter 3 Chemical Kinetics: Download PDF
- NCERT Notes For Class 12 Chapter 3 Chemical Kinetics
- NCERT Chapter 3 Chemical Kinetics Previous Year Question and Answer
- How to Master Class 12 Chemistry Chapter 3 Chemical Kinetics
- Advantages of Class 12 Chemistry Chapter 3 Chemical Kinetics Notes
- CBSE Class 12 Chemistry Chapter-wise Notes
- NCERT Solutions for Class 12 Chemistry
- Subject Wise NCERT Exemplar Solutions
- Subject-Wise NCERT Solutions
Chemical Kinetics is the branch of Chemistry that investigates how quickly certain reactions occur. It investigates what influences the rate of reactions and how compounds transform into new products. NCERT Notes of class 12 helps in scoring well in CBSE board exams by simplifying complex concepts and formulas of Class 12 Chemistry Chemical Kinetics . These NCERT notes align with the updated CBSE syllabus covering all the important topics like Molecularity of reaction, Integrated rate equations, Temperature Dependence of the Rate of a Reaction, etc.
NCERT Notes for Class 12 Chapter 3 Chemical Kinetics: Download PDF
Students can download the ncert class 12 chemistry chapter 3 chemical kinetics notes pdf from the icon given below. Whether reading for the first time or revising for exams, these NCERT notes for Class 12 Chemistry are very helpful.
Also, students can refer,
NCERT Notes For Class 12 Chapter 3 Chemical Kinetics
In chemical kinetics class 12 ncert notes, students will learn about different factors that affect reaction rates, Important formulas,Diagrams and graphs, how to calculate rate of reaction mathematically, and how to interpret experimental data using integrated rate laws. Below is a structured breakdown of each concept from the NCERT to help you master the topic with clarity.
Rate of a Chemical Reaction
The rate of a reaction can be defined as the change (decrease or increase) in the concentration of a reactant or product in unit time. The rate of a reaction can be given as:
Average rate
The average rate of reaction can be defined as the change in the concentration of a reactant or product in a definite time interval (Δt). It is denoted by the symbol $r_{av}$.
Consider a hypothetical reaction:
Reactant (M) $\rightarrow$ Product ( N )
The rate of disappearance of reactant-
$\begin{aligned} & r_{a v}=\frac{\text { Decrease in concentration of } M}{\text { Time taken }} \\ & r_{a v}=\frac{-[M]}{\Delta t}\end{aligned}$
A minus sign indicates a decrease in concentration.
The rate of appearance of the product-
$\begin{aligned} & r_{a v}=\frac{\text { Increase in concentration of } N}{\Delta t} \\ & r_{a v}=\frac{+[N]}{\Delta t}\end{aligned}$
Instantaneous rate
The instantaneous rate of reaction can be defined as the change in the concentration of a reactant or product at a particular instant of time. It is denoted by the symbol $r_{\text {inst }}$..
For an infinitesimally small instant of time $\Delta t \rightarrow 0$,
Therefore,
$r_{i n s t}=\frac{-\mathrm{d}[M]}{\mathrm{d} t}=\frac{\mathrm{d}[P]}{\mathrm{d} t}$
The overall rate of reaction
For an overall rate of reaction, the rate of disappearance of any of the reactants (or the rate of appearance of products) is divided by their corresponding stoichiometric coefficients.
Such that for a reaction,
$\mathrm{aA}+\mathrm{bB} \rightarrow \mathrm{cC}+\mathrm{dD}$
The overall rate is given as:
$\frac{1}{a} \frac{-\mathrm{d}[A]}{\mathrm{d} t}=\frac{1}{b} \frac{-\mathrm{d}[B]}{\mathrm{d} t}=\frac{1}{c} \frac{+\mathrm{d}[C]}{\mathrm{d} t}=\frac{1}{d} \frac{+\mathrm{d}[D]}{\mathrm{d} t}$
Or
$\frac{-[A]}{a \Delta t}=\frac{-[B]}{b \Delta t}=\frac{+[C]}{c \Delta t}=\frac{+[D]}{d \Delta t}$
.png)
Factors Influencing Rate of a Reaction
Major factors that influence the rate of reaction are given below. For better understanding of these topics students can refer Chemical Kinetics Class 12 Chemistry Chapter 3 CBSE Notes.
-
Concentration of reactants
-
Temperature
-
Catalyst
-
Rate Law
Concentration of reactants
When the rate of reaction is represented in terms of the concentration of the reactants is known as rate law. It is also known as the rate equation or rate expression. Refer to chemical kinetics class 12 ncert notes for understanding these concepts better.
Consider the following reaction:
$\mathrm{aA}+\mathrm{bB} \rightarrow \mathrm{cC}+\mathrm{dD}$
The rate law for this reaction is:
Rate $\propto[A]^x[B]^y$
Where x and y represent order w.r.t to A and B and may or may not be equal to a and b.
The differential form of the rate law is:
$\frac{-\mathrm{d}[R]}{\mathrm{d} t}=k[A]^x[B]^y$
Order of a reaction
The order of the chemical reaction is the summation of powers raised to the concentration of the reactants in the rate law.
For a reaction,
$\mathrm{aA}+\mathrm{bB} \rightarrow \mathrm{cC}+\mathrm{dD}$
The rate law is:
Rate $\propto[A]^x[B]^y$
Therefore, the order of the reaction is x+y.
The order of a reaction can be 0,1,2,3 and fraction.
Order can never be a negative number.
Units of rate constant
|
REACTION |
ORDER |
UNITS |
|
Zero-order reaction |
0 |
mol $^{-1}$ time ${ }^{-1}$ |
|
First-order reaction |
1 |
$time^{-1}$ |
|
Second-order reaction |
2 |
$l \mathrm{~mol}^{-1}$ time $^{-1}$ |
|
Third-order reaction |
3 |
$l^2 \mathrm{~mol}^{-2}$ time ${ }^{-1}$ |
Molecularity of a reaction:
- The sum total of species reacting or (molecules, atoms, ions) taking part in a chemical reaction is called the molecularity of a reaction.
- The reaction can be unimolecular when one reacting species yields product(s).
- The reaction can be bimolecular when two reacting species are involved.
- The reaction can be trimolecular when three reacting species are involved.
| Molecularity | No. of reactants | Example Reaction |
|---|---|---|
| 1 (Unimolecular) | One | $\mathrm{O}_3 \rightarrow \mathrm{O}_2+\mathrm{O}$ |
| 2 (Bimolecular) | Two | $\mathrm{NO}+\mathrm{O}_3 \rightarrow \mathrm{NO}_2+\mathrm{O}_2$ |
| 3 (Termolecular) | Three | $2 \mathrm{NO}+\mathrm{O}_2 \rightarrow 2 \mathrm{NO}_2$ |
Integrated rate Equations
-
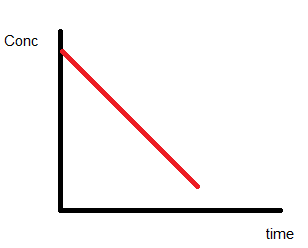
Zero-order reactions
Consider the reaction,
A → P
The rate law of this reaction is given by:
Rate = k[A]0
On integrating the above equation we get,
$[A]_t=[A]_0-k t$
The graph of the above equation can be plotted as:
-
First-order reaction
Consider the reaction,
A → P
The rate law of this reaction is given by:
Rate = k[A]
On integrating the above equation we get,
$\ln \left(\frac{[A]_0}{[A]_t}\right)=k t$
The exponential form of a first-order reaction is given as:
$[A]_t=[A]_0 e^{-k t}$
Let us consider a typical first order gas phase reaction
$
\mathrm{A}(\mathrm{~g}) \rightarrow \mathrm{B}(\mathrm{~g})+\mathrm{C}(\mathrm{~g})
$
Let $p_{\mathrm{i}}$ be the initial pressure of A and $p_{\mathrm{t}}$ the total pressure at time ' $t$ '. Integrated rate equation for such a reaction can be derived as Total pressure $p_{\mathrm{t}}=p_{\mathrm{A}}+p_{\mathrm{B}}+p_{\mathrm{C}}$ (pressure units)
$p_{\mathrm{A}}, p_{\mathrm{B}}$ and $p_{\mathrm{C}}$ are the partial pressures of $\mathrm{A}, \mathrm{B}$ and C , respectively.
If x atm be the decrease in pressure of A at time $t$ and one mole each of B and C is being formed, the increase in pressure of B and C will also be x atm each.
$
\mathrm{A}(\mathrm{~g}) \rightarrow \mathrm{B}(\mathrm{~g})+\mathrm{C}(\mathrm{~g})
$
Initial conditions (at $t=0$ ):
- Partial pressure of $\mathrm{A}=p_i \mathrm{~atm}$
- Partial pressure of $\mathrm{B}=0$ atm
- Partial pressure of $\mathrm{C}=0 \mathrm{~atm}$
At time $t$ :
- Partial pressure of $\mathrm{A}=\left(p_i-x\right)$ atm
- Partial pressure of $\mathrm{B}=\boldsymbol{x}$ atm
- Partial pressure of $\mathrm{C}=\boldsymbol{x}$ atm
$
\begin{gathered}
\qquad \begin{array}{c}
p_{\mathrm{t}}=\left(p_{\mathrm{i}}-\mathrm{x}\right)+\mathrm{x}+\mathrm{x}=p_{\mathrm{i}}+\mathrm{x} \\
\mathrm{x}=\left(p_{\mathrm{t}}-p_{\mathrm{i}}\right)
\end{array} \\
\text { where, } p_{\mathrm{A}}=p_{\mathrm{i}}-\mathrm{x}=p_{\mathrm{i}}-\left(p_{\mathrm{t}}-p_{\mathrm{i}}\right) \\
=2 p_{\mathrm{i}}-p_{\mathrm{t}} \\
k=\left(\frac{2.303}{t}\right)\left(\log \frac{p_{\mathrm{i}}}{p_{\mathrm{A}}}\right) \\
=\frac{2.303}{t} \log \frac{p_{\mathrm{i}}}{\left(2 p_{\mathrm{i}}-p_{\mathrm{t}}\right)}
\end{gathered}
$
The following equation can be used to plot graphs of first-order reactions:
$k=\frac{1}{t} \ln \left(\frac{[A]_0}{[A]_t}\right)$
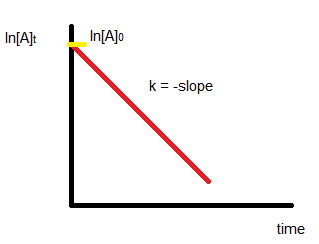
$\log \frac{[A]_0}{[A]_t}=\frac{k t}{2.303}$
The Half-life of a reaction:
The time in which the concentration of a reactant is decreased to one-half of its initial concentration is known as the half-life of a reaction. Half-life is represented as t1/2.
-
For zero-order reactions
$t_{1 / 2}=\frac{[A]_0}{2 k}$
- For first-order reactions
$t_{1 / 2}=\frac{0.693}{k}$
Note that for zero-order reaction $t_{1 / 2} \propto[A]_0$. For the first-order reaction, $t_{1 / 2}$ is independent of $[A]_0$.
Pseudo-first-order reactions:
The reactions in which the actual order is different from the expected rate order predicted using rate law are called pseudo-first-order reactions Students can also download the class 12 chemistry chapter 3 chemical kinetics notes pdf to study offline anytime and anywhere.
$\mathrm{CH}_3 \mathrm{COOC}_2 \mathrm{H}_5+\mathrm{H}_2 \mathrm{O} \xrightarrow{\mathrm{H}^{+}} \mathrm{CH}_3 \mathrm{COOH}+\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$
The rate law of the reaction:
Rate $=k^{\prime}\left[\mathrm{CH}_3 \mathrm{COOC}_2 \mathrm{H}_5\right]\left[\mathrm{H}_2 \mathrm{O}\right]$
Expected order = 2
The concentration of water does not get altered much during the reaction as it is in excess and can be considered constant.
Rate $=k\left[\mathrm{CH}_3 \mathrm{COOC}_2 \mathrm{H}_5\right]$
Actual order = 1
Therefore, the reaction behaves as a first-order reaction, and such reactions are called pseudo-first-order reactions.
Table of Integrated Rate Laws for the Reactions of Zero and First Order
| Order | Reaction Type | Differential Rate Law | Integrated rate law | Striaght line plot | Half-life | Units of k |
| 0 | $\mathrm{R} \rightarrow \mathrm{P}$ | $\mathrm{d}[\mathrm{R}] / \mathrm{d} t=-k$ | $k t=[\mathrm{R}]_0-[\mathrm{R}]$ | $[\mathrm{R}]$ vs $t$ | $[\mathrm{R}]_0 / 2 k$ | $\begin{aligned} & \text { conc time }{ }^{-1} \\ & \text { or } \mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}\end{aligned}$ |
| 1 | $\mathrm{R} \rightarrow \mathrm{P}$ | $\mathrm{d}[\mathrm{R}] / \mathrm{d} t=-k[\mathrm{R}]$ | $\begin{aligned} & {[\mathrm{R}]=[\mathrm{R}]_0 \mathrm{e}^{-k t}} \\ & \text { or } k t= \\ & \ln \left\{[\mathrm{R}]_0 /[\mathrm{R}]\right\}\end{aligned}$ | $\ln [\mathrm{R}]$ vs $t$ | $\ln 2 / k$ | time $^{-1}$ or $\mathrm{s}^{-1}$ |
Temperature Dependence of the Rate of a Reaction
The fact that the rate of a chemical reaction is dependent on temperature can be explained by the Arrhenius equation.
The mathematical form of the Arrhenius equation is given below:
$k=A e^{-E a / R T}$
where A is the Arrhenius factor / the frequency factor / the pre-exponential factor.
According to Arrhenius, a reaction can take place only when two (or more) molecules collide to form an unstable intermediate. This intermediate exists for a very short time and then breaks up to form product(s).
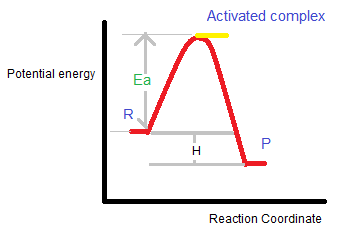
The activation energy ($E_a$) is the mandatory energy required to produce the intermediate activated complex (C). The reaction coordinate depicts the energy change profile when reactants change into products.
The minimum energy which the colliding molecules required to collide effectively is called threshold energy.
Threshold energy = Activation energy + Energy of the reactants
The rate of reaction depends on the temperature as it is found that with the rise in temperature by 10°, the rate constant is nearly doubled.
Temperature coeff icient $=\frac{\left(\text { Rate constant at } T+10^{\circ}\right)}{\left(\text { Rate constant at } T^{\circ}\right)}$
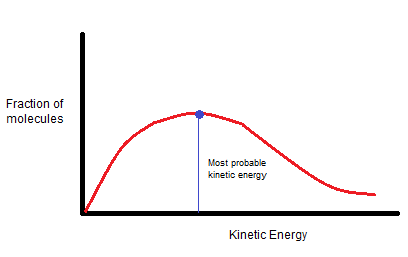
At a particular temperature, the fractions of molecules are plotted against corresponding kinetic energies, a graph given below is obtained.
The peak of the curve represents the kinetic energy possessed by the maximum fraction of molecules and is called the most probable kinetic energy.
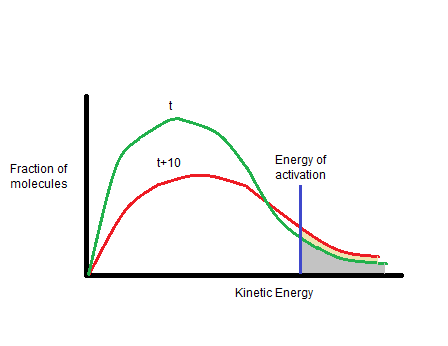
Here, the yellow area shows the fraction of additional molecules which react at t+10 while the grey area shows the fraction of molecules reacting at t.
Taking ln on both sides of the following equation:
$\begin{aligned} & k=A e^{-E a / R T} \\ & \ln (k)=\frac{-E_a}{R T}+\ln (A)\end{aligned}$
Arrhenius equation for two different temperatures can be modified as:
$\log \frac{k_2}{k_1}=\frac{E_a}{R T} \frac{\left[T_2-T_1\right]}{T_1 T_2}$
Effect of Catalyst:
-
A catalyst is a substance that alters or changes the rate of a reaction without undergoing any permanent chemical change.
-
According to intermediate complex theory, a catalyst forms temporary bonds with the reactants, forming an intermediate complex. This is how a catalyst participates in a chemical reaction.
-
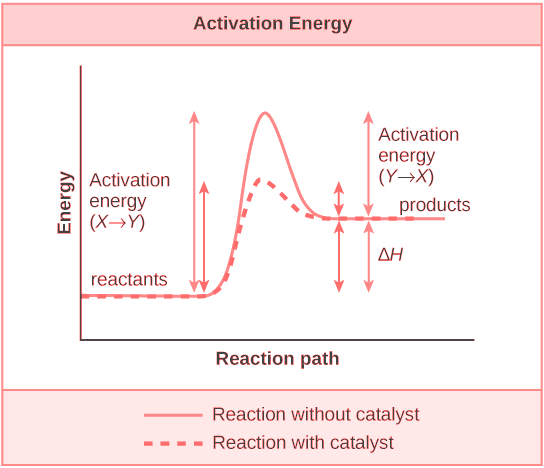
The catalyst reduces the energy difference known as activation energy between products and reactants which in turn lowers the potential energy barrier.
-
A catalyst catalyses to the same extent the forward as well as the backward reactions so that the equilibrium state remains same but equilibrium is reached faster.
Collision Theory of Chemical Reactions
- According to the collision theory of chemical reactions, the reactant molecules are supposed as hard spheres, and the reaction takes place when molecules collide with each other.
$A+B \rightarrow$ Products
- The collision frequency (Z) can be defined as the number of collisions per second per unit volume of the reaction mixture.
- Activation energy also affects the rate of chemical reactions.
- Only effective collisions lead to the formation of products.
- The proper orientation of reactant molecules in space leads to bond formation whereas due to improper orientation no products are formed.
- The factor for proper orientation during effective collisions is called the probability or steric factor (P).
.png)
$k=P Z_{A B} e^{-E_a / R T}
NCERT Chapter 3 Chemical Kinetics Previous Year Question and Answer
Below are the previous year questions and answers from Chemical Kinetics to help you understand the exam pattern and important topics. Practising these will boost your confidence and improve your problem-solving speed. Students can follow NCERT Notes for Class 12 Chemistry Chapter 3 Chemical Kinetics to understand concepts better to solve these questions.
Question 1: In a first order decomposition reaction, the time taken for the decomposition of reactant to one fourth and one eighth of its initial concentration are $t_1$ and $t_2(\mathrm{~s})$, respectively. The ratio $t_1 / t_2$ will :
(1) $\frac{4}{3}$
(2) $\frac{3}{2}$
(3) $\frac{3}{4}$
(4) $\frac{2}{3}$
Answer:
$\begin{aligned} & \mathrm{t}_1=\mathrm{t}_{\frac{1}{4}}=\frac{1}{\mathrm{k}} \ln \frac{\mathrm{A}_0}{\frac{\mathrm{~A}_0}{4}}=\frac{1}{\mathrm{k}} \ln 4 \\ & \mathrm{t}_2=\mathrm{t}_{\frac{1}{8}}=\frac{1}{\mathrm{k}} \ln \frac{\mathrm{A}_0}{\frac{\mathrm{~A}_0}{8}}=\frac{1}{\mathrm{k}} \ln 8 \\ & \frac{\mathrm{t}_1}{\mathrm{t}_2}=\frac{\ln 4}{\ln 8}=\frac{2 \ln 2}{3 \ln 2}=\frac{2}{3}\end{aligned}$
Hence, the correct answer is option (4).
Question 2: Half life of zero order reaction $\mathrm{A} \rightarrow$ product is 1 hour, when initial concentration of reaction is $2.0 \mathrm{~mol} \mathrm{~L}^{-1}$. The time required to decrease concentration of A from 0.50 to $0.25 \mathrm{~mol} \mathrm{~L}^{-1}$ is:
(1) 0.5 hour
(2) 4 hour
(3) 15 min
(4) 60 min
Answer: For zero order reaction
$\begin{aligned}
& \text { Half life }=\frac{\mathrm{A}_0}{2 \mathrm{k}} \\
& 60 \mathrm{~min}=\frac{2}{2 \mathrm{k}} \\
& \mathrm{k}=\frac{1}{60} \mathrm{M} / \mathrm{min}
\end{aligned}$
Now
$\begin{aligned}
& \mathrm{A}_{\mathrm{t}}=\mathrm{A}_{\mathrm{o}}-\mathrm{kt} \\
& \mathrm{t}=\frac{\mathrm{A}_{\mathrm{o}}-\mathrm{A}_1}{\mathrm{k}} \\
&=\frac{0.5-0.25}{1 / 60} \\
& 0.25 \times 60 \\
& \mathrm{t}=15 \mathrm{~min}
\end{aligned}$
Hence, the correct answer is option (3).
Question 3: Which among the following is a false statement?
- Rate of zero order reaction is independent of initial concentration of reactant
- Half-life of a zero order reaction is inversely proportional to the rate constant.
- Molecularity of a reaction may be zero.
- For a first order reaction, $\mathrm{t}_{1 / 2}=0.693 / \mathrm{k}$.
Answer: Statement (1). Rate of zero order reaction is independent of initial concentration of reactant.
This statement is correct because for zero order reaction
Rate $=k$
Means rate is independent of concentration.
Statement 2. Half-life of a zero order reaction is inversely proportional to the rate constant.
This statement is correct for zero order reaction because,
$t_{1 / 2}=\frac{[A]_0}{2 k}$
Statement 3. Molecularity of a reaction may be zero.
This statement is incorrect because Molecularity is the number of molecules involved in the reaction. It is always a positive integer. It can never be zero.
Statement 4: For a first order reaction, $\mathrm{t}_{1 / 2}=0.693 / \mathrm{k}$.
This statement is true because for first-order reaction, the half-life is :
$
t_{1 / 2}=\frac{0.693}{k}
$
Statement (1). Rate of zero order reaction is independent of initial concentration of reactant.
This statement is correct because for zero order reaction
Rate $=k$
Means rate is independent of concentration.
Statement 2. Half-life of a zero order reaction is inversely proportional to the rate constant.
This statement is correct for zero order reaction because,
$t_{1 / 2}=\frac{[A]_0}{2 k}$
Statement 3. Molecularity of a reaction may be zero.
This statement is incorrect because Molecularity is the number of molecules involved in the reaction. It is always a positive integer. It can never be zero.
Statement 4: For a first order reaction, $\mathrm{t}_{1 / 2}=0.693 / \mathrm{k}$.
This statement is true because for first-order reaction, the half-life is :
$
t_{1 / 2}=\frac{0.693}{k}
$
Hence, the correct answer is option (3).
Question 4: $\mathrm{A}(\mathrm{g}) \rightarrow \mathrm{B}(\mathrm{g})+\mathrm{C}(\mathrm{g})$ is a first order reaction.
| Time | T | $\infty$ |
| $\mathbf{P}_{\text {system }}$ | $\mathrm{P}_{\mathrm{t}}$ | $\mathrm{P}_{\infty}$ |
The reaction was started with reactant A only. Which of the following expression is correct for rate constant k ?
(1) $k=\frac{1}{t} \ln \frac{2\left(p_{\infty}-P_t\right)}{P_t}$
(2) $k=\frac{1}{t} \ln \frac{p_{\infty}}{P_t}$
(3) $k=\frac{1}{t} \ln \frac{p_{\infty}}{2\left(p_{\infty}-P_t\right)}$
(4) $k=\frac{1}{t} \ln \frac{p_{\infty}}{\left(p_{\infty}-P_t\right)}$
Answer:
$\mathrm{A}(\mathrm{g}) \rightarrow \mathrm{B}(\mathrm{g})+\mathrm{C}(\mathrm{g})$
$\begin{array}{ccccc} & \\ \mathrm{t}=0 & \mathrm{p}_0 & & 0 & 0 \\ \mathrm{t}=\mathrm{t} & \mathrm{p}_0-\mathrm{p} & & \mathrm{p} & \mathrm{p} \\ \mathrm{t}=\infty & 0 & & p_0 & p_0\end{array}$
$\begin{array}{ll}\text { At } \begin{aligned} t & =t \\ t & =\infty\end{aligned} \quad \begin{array}{l}p_t=p_0+p \\ 2 p_0=p_{\infty}\end{array}\end{array}$
For first order reaction
$\begin{array}{ll}k=\frac{1}{t} \ln \frac{\left(p_0\right)_A}{\left(p_t\right)_A} & p_0-p=\frac{p_{\infty}}{2}-p_t+\frac{p_{\infty}}{2} \\ =\frac{1}{t} \ln \frac{p_0}{p_0-p} & =p_{\infty}-p_t\end{array}$
$=\frac{1}{t} \ln \frac{p_{\infty}}{2\left(p_{\infty}-p_t\right)}$
Hence, the correct answer is option (3).
Question 5: Rate law for a reaction between $A$ and $B$ is given by
$\mathrm{R}=\mathrm{k}[\mathrm{~A}]^{\mathrm{n}}[\mathrm{~B}]^{\mathrm{m}}$
If concentration of A is doubled and concentration of $B$ is halved from their initial value, the ratio of new rate of reaction to the initial rate of reaction $\left(\frac{r_2}{r_1}\right)$ is
(1) $2^{(n-m)}$
(2) $(n-m)$
(3) $(m+n)$
(4) $\frac{1}{2^{m+n}}$
Answer:
$\mathrm{r}_1=\mathrm{k}[\mathrm{A}]^{\mathrm{n}}[\mathrm{B}]^{\mathrm{m}}$
Now A is doubled & B is halved in concentration
$\begin{aligned} & \Rightarrow \mathrm{r}_2=\mathrm{k} 2^{\mathrm{n}}[\mathrm{A}]^{\mathrm{n}} \cdot \frac{[\mathrm{B}]^{\mathrm{m}}}{2^{\mathrm{m}}} \\ & \text { Now } \frac{\mathrm{r}_2}{\mathrm{r}_1}=2^{(\mathrm{n}-\mathrm{m})}\end{aligned}$
Hence, the correct answer is option (1).
Also read
How to Master Class 12 Chemistry Chapter 3 Chemical Kinetics
These Chemical Kinetics Class 12 Chemistry Chapter 3 CBSE Notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 3.
- Firstly students need to understand the concepts like reaction rate, order and molecularity of reactions, and factors affecting reaction rates.
- Concepts like rate laws for different reactions play an important role in this chapter. To understand these concepts better refer class 12 chemistry chapter 3 chemical kinetics notes.
- Students must interpret and draw concentration vs. time and rate vs. concentration graphs.
- They must solve numerical questions and strengthen problem solving skills.
- Understand the concepts like reaction mechanisms and how they relate to overall reaction rates.
- After that students can solve previous year questions from this chapter.
Advantages of Class 12 Chemistry Chapter 3 Chemical Kinetics Notes
NCERT Notes for Class 12 Chemistry Chapter 3 Chemical Kinetics provide a clear explanation of concepts that helps in mastering topics and scoring well in board and competitive exams. The advantages of using these notes are given below:
- These notes cover all the topics from NCERT in a detailed and comprehensive manner.
- Students can refer to these notes anytime, whether revising concepts and reading important formulas for the first time
- The ncert class 12 chemistry chapter 3 chemical kinetics notes helps students clearly differentiate between zero, first, and second order reactions
- These notes are prepared by experts to help students in conceptual understanding for boards and competitive exams.
CBSE Class 12 Chemistry Chapter-wise Notes
NCERT Solutions for Class 12 Chemistry
These solutions serve as a comprehensive guide to help students understand complex chemical concepts with ease. They provide step-by-step answers to all textbook questions, enabling students to build a strong foundation for board exams and competitive tests.
Subject Wise NCERT Exemplar Solutions
The hyperlinks of NCERT Exemplar Solutions of all subjects are given below:
Subject-Wise NCERT Solutions
The hyperlinks of NCERT Solutions of all subjects are given below:
| NCERT Solutions for Class 12 Mathematics |
| NCERT Solutions for Class 12 Chemistry |
| NCERT Solutions for Class 12 Physics |
| NCERT Solutions for Class 12 Biology |
Frequently Asked Questions (FAQs)
Zero-order reactions have a rate that is independent of the concentration of reactants. In first-order reactions, the rate is directly proportional to the concentration of one reactant, while in second-order reactions, the rate depends on the square of the concentration of one reactant or is proportional to the product of concentrations of two reactants.
Activation energy is the minimum energy required for a chemical reaction to occur. It is the threshold that reactants must overcome to transform into products. The higher the activation energy, the slower the reaction tends to be, as fewer reactant molecules will have enough energy to initiate the reaction.
Studying chemical kinetics is crucial because it provides insights into reaction mechanisms, helps predict how long a reaction will take, and is essential for designing chemical processes in industries. It is also important in fields like pharmacology, environmental science, and biochemistry.
The rate law is an equation that relates the rate of a reaction to the concentrations of its reactants raised to a power.
Zero-order reactions have a rate that is independent of the concentration of the reactants (Rate = k)
The rate constant (k) is a proportionality factor in the rate law that provides information about the speed of the reaction at a given temperature.
Chemical kinetics is the branch of chemistry that deals with the rates of chemical reactions and the factors that affect these rates. It helps us understand how fast a reaction occurs and what influences that speed, such as concentration, temperature, and the presence of catalysts.
The rate of a chemical reaction can be determined by measuring the change in concentration of reactants or products over time. For example, if we start with a known concentration of a reactant and measure how much of it reacts in a specific time interval, we can calculate the reaction rate.
Several factors influence the rate of a chemical reaction, including:
1. Concentration of reactants, Higher concentration generally increases the rate.
2. As temperature increases, reactant particles move faster, increasing the rate.
3.A greater surface area of solid reactants leads to more collisions and a higher rate.
4. Substances that speed up reactions without being consumed in the process.
Catalysts are substances that increase the rate of a chemical reaction by lowering the activation energy required for the reaction to proceed. They help in forming transition states more easily, allowing the reaction to occur faster without being consumed in the process.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
