Students should take d and f-Block elements Class 12 Chemistry Chapter 4 CBSE notes because this chapter involves many trends, properties, and exceptions that are easier to understand and remember through concise notes.
NCERT Class 12 Chemistry Chapter 8 Notes The d and f-Block Elements - Download PDF
What do the fiery red glow of a ruby, the shimmer of a jet engine, and the flash of a camera have in common? They all owe their magic to the transition and inner transition elements. That glowing red in a ruby is due to $\mathrm{Cr}^{3+}$ ions replacing aluminum in its crystal, creating beauty through chemistry. These d- and f-block, elements are also known as transition metals and the inner transition metals. The d-block elements are elements of groups 3 to 12 of the periodic table in which the 4f and 5f orbitals are filled.
This Story also Contains
- NCERT Notes for Class 12 Chapter 4 : Download PDF
- NCERT Notes for Class 12 Chemistry Chapter 4
- The d- and f- block Elements Previous Year Question and Answer
- How to Master Class 12 Chemistry Chapter 4 The d- and f-Block Elements
- Advantages of Class 12 Chemistry Chapter 4 The d- and f-Block Elements Notes
- CBSE Class 12 Chemistry Chapter-wise Notes
- NCERT Solutions for Class 12 Chemistry
- Subject-Wise NCERT Exemplar Solutions
- Subject-Wise NCERT Solutions
These NCERT Notes of class 12 contains all the important topics of the Chapter 4 Chemistry d and f block elements that are being asked in the Class 12 board examinations and competitive exams. It explains the variable oxidation states of the transition metals, along with magnetic properties, chemical reactivity, electronic configuration, and the formation of colored compounds. These elements are crucial in fields ranging from metallurgy and catalysis to materials science and medicine. These NCERT notes are based on the latest CBSE Syllabus for Class 12 Chemistry and are beneficial for board examinations. The main topics covered in the d and f-block elements class 12 notes are the physical properties of d-block elements, some important compounds of transition elements, lanthanoids, actinoids, lanthanide contraction, etc.
NCERT Notes for Class 12 Chapter 4 : Download PDF
Given below the class 12 chemistry chapter 4 d and f block elements notes pdf cover all important concepts in a simple and exam-friendly format. Students can easily download the PDF of NCERT Class 12 Chemistry Notes of this chapter by clicking the button given below.
Also, students can refer,
NCERT Notes for Class 12 Chemistry Chapter 4
The d- and f-block elements are known as transition and inner-transition metals, respectively. These elements display a wide range of oxidation states, form complex compounds, and play vital roles in catalysis and metallurgy. Below is a structured, topic-wise breakdown of all the concepts covered in the ncert class 12 chemistry chapter 4 d and f block elements notes. These notes are useful for last minute revision as well as exam preparation.
Position of d block in the Periodic Table
In the periodic table, the d-block elements are found in the intermediate region of the s- and p-block elements. Because of its location between s- and p-block components, it was given the term 'transition.'
Electronic Configuration of d Block Elements
$(n-1) d^{1-10} n s^{1-2}$ is the electronic configuration of d-block elements. They have two outer shells that aren't complete.
Where (n–1) denotes inner d orbitals with electrons ranging from 1 to 10, and ns denotes the outermost orbital, which may have one or two electrons (n-1) The electronic configurations of Zn, Cd, and Hg are represented by $\mathrm{d}^{10} \mathrm{~s}^2$.
D-block elements have a variable valency that varies by one unit.
Table : Electronic configuration of 4th period transition elements
| Element | Symbol | Atomic Number (Z) | Electronic Configuration (Outer Orbitals) |
|---|---|---|---|
| Scandium | Sc | 21 | [Ar] $3 d^1 4 s^2$ |
| Titanium | Ti | 22 | [Ar] $3 d^2 4 s^2$ |
| Vanadium | V | 23 | [Ar] $3 d^3 4 s^2$ |
| Chromium | Cr | 24 | [Ar] $3 d^5 4 s^1$(exception) |
| Manganese | Mn | 25 | [Ar] $3 d^5 4 s^2$ |
| Iron | Fe | 26 | [Ar] $3 d^6 4 s^2$ |
| Cobalt | Co | 27 | [Ar] $3 d^7 4 s^2$ |
| Nickel | Ni | 28 | [Ar] $3 d^8 4 s^2$ |
| Copper | Cu | 29 | [Ar] 3$3 d^10 4 s^1$ (exception) |
| Zinc | Zn | 30 | [Ar] $3 d^10 4 s^2$ |
Table : Electronic configuration of 5th period transition elements
| Element | Symbol | Atomic Number (Z) | Electronic Configuration (Outer Orbitals) |
|---|---|---|---|
| Yttrium | Y | 39 | [Kr] $4 d^1 5 s^2$ |
| Zirconium | Zr | 40 | [Kr] $4 d^2 5 s^2$ |
| Niobium | Nb | 41 | [Kr] $4 d^4 5 s^1$ (exception) |
| Molybdenum | Mo | 42 | [Kr] $4 d^5 5 s^1$(exception) |
| Technetium | Tc | 43 | [Kr] $4 d^5 5 s^2$ |
| Ruthenium | Ru | 44 | [Kr] $4 d^7 5 s^1$ (exception) |
| Rhodium | Rh | 45 | [Kr] $4 d^8 5 s^1$ (exception) |
| Palladium | Pd | 46 | [Kr] $4 d^1$ (exception – no 5s electron) |
| Silver | Ag | 47 | [Kr] $4 d^10 5 s^1$ |
| Cadmium | Cd | 48 | [Kr] $4 d^10 5 s^2$ |
Table : Electronic configuration of 6th period transition elements
| Element | Symbol | Atomic Number (Z) | Electronic Configuration (Outer Orbitals) |
|---|---|---|---|
| Hafnium | Hf | 72 | [Xe] $4 f^{14} 5 d^2 6 s^2$ |
| Tantalum | Ta | 73 | [Xe]$4 f^{14} 5 d^3 6 s^2$ |
| Tungsten | W | 74 | [Xe] $4 f^{14} 5 d^4 6 s^2$ |
| Rhenium | Re | 75 | [Xe] $4 f^{14} 5 d^5 6 s^2$ |
| Osmium | Os | 76 | [Xe] $4 f^{14} 5 d^6 6 s^2$ |
| Iridium | Ir | 77 | [Xe] $4 f^{14} 5 d^7 6 s^2$ |
| Platinum | Pt | 78 | [Xe] $4 f^{14} 5 d^9 6 s^1$(exception) |
| Gold | Au | 79 | [Xe] $4 f^{14} 5 d^{10} 6 s^1$(exception) |
| Mercury | Hg | 80 | [Xe] $4 f^{14} 5 d^{10} 6 s^2$ |
General Properties of the Transition Elements (d-Block)
Physical Properties
1)Metallic characteristics :
All transition metals have a metallic character. They are excellent heat and electricity conductors. They are abrasive and persistent. They are malleable, ductile, and resonant since they are metal. They combine with other metals to form alloys. They can be found in three different structures: face-centred cubic (fcc), hexagonal close-packed (hcp), and body-centred cubic (bcc). Within the atoms of transition elements, there is both covalent and metallic bonding. For better understanding of these concepts refer these d and f-Block elements Class 12 Chemistry Chapter 4 CBSE notes till end.
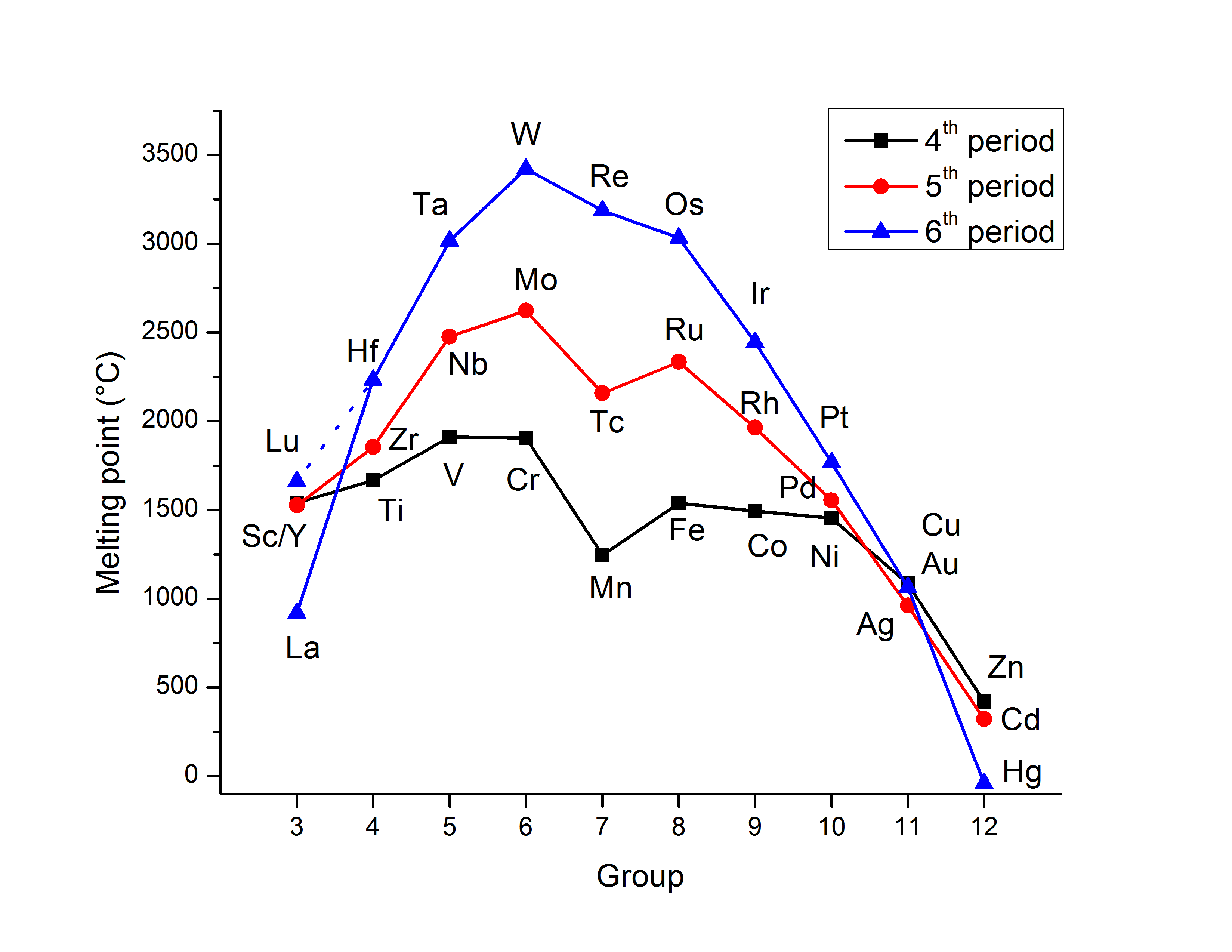
Figure shows the trends in melting points of transition elements.
2)Atomic size:
As the atomic number increases, the atomic radii of the elements in the 3d series decrease. Because of lanthanoid contraction, the atomic radii rise from 3d to 4d, and the atomic radii of the 4d and 5d transition series are quite close. Zirconium and Hafnium, are examples. The rise in the density of elements is caused by a decrease in the metallic radius due to an increase in the atomic mass. As a result, the density of titanium to copper increases.
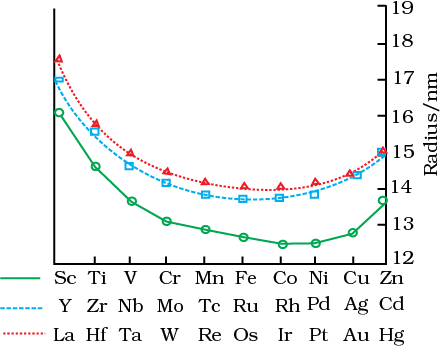
Figure shows the trends in atomic radii of transition elements.
3)Ionisation enthalpy:
Because transition elements are tiny, they have a high ionisation energy. Since their ionisation potentials lie between S and P-block elements, they are less electropositive than s-block elements. Transition elements form covalent compounds. The ionisation potentials of the d-block elements increase from left to right due to the screening effect of the additional electrons added to the (n-1) d subshell. Because of the stable electronic configuration, the second ionisation energy increases with the increase in atomic number in the first transition series. Ionization energy reduces as moves through the group whereas the amount of ionisation energy increases across the period.
4)Oxidation state:
The small energy difference between the ns and (n -1) d orbitals cause transition elements to have different oxidation states. (n -1) d electrons participate in bonding with ns electrons. Scandium, on the other hand, does not have different oxidation states due to the limited number of electrons available for bonding. Zinc has fewer orbitals accessible for bonding due to the existence of more d electrons, and so does not have a variable oxidation state. The elements in the 8th group have the highest oxidation state among the d-block elements. Manganese, which belongs to the 7th group, has the highest oxidation state among the 3d –series elements. Ruthenium, which belongs to the 8th group, has the highest oxidation state among the 4d-Series elements. Osmium, which belongs to the 8th group, has the highest oxidation state of all the elements in the 5d series. Students can also follow NCERT solutions for Class 12 Chemistry Chapter 4 d and f block elements to better understand these concepts through solved questions.
The table shows Oxidation States of the first row Transition Metal (the most common ones are in bold types)
| Element | Symbol | Common Oxidation States |
|---|---|---|
| Scandium | Sc | +3 |
| Titanium | Ti | +2, +3, +4 |
| Vanadium | V | +2, +3, +4, +5 |
| Chromium | Cr | +2, +3, +6 |
| Manganese | Mn | +2, +3, +4, +6, +7 |
| Iron | Fe | +2, +3 |
| Cobalt | Co | +2, +3 |
| Nickel | Ni | +2, +3 |
| Copper | Cu | +1, +2 |
| Zinc | Zn | +2 |
5) Trends in the M2+/M Standard Electrode Potentials:
The stability of $M^{2+}$ ions in aqueous medium depends on three factors:
(i)Enthalpy of atomisation
(ii) Summation of first and second ionization enthalpies
(iii) Hydration enthalpy
An elements in $\mathrm{Mn}^{2+}$ state in aqueous medium is more stabler if the electrode potential $\left(M^{2+} / M\right)$ value of more negative. Across the period, the tendency to form $M^{2+}$ ion decreases. Except copper, all elements of first transition series show negative values of electrode potentials. The exceptional behaviour of the copper due to low enthalpy of atomisation and very high summation of first and second ionization enthalpies which is not compensated by its hydration enthalpy ( $\mathrm{Cu}^{2+}$. Because of positive electrode potential, copper doesnot liberate hydrogen gas from dilute acids and reacts only with oxidizing acids such as nitric acid and hot concentrated sulphuric acid.
The electrode potentials of $\mathrm{Mn}, \mathrm{Ni}$ and Zn are more negative than expected. However, the electrode potential values of Mn and Zn are lowered because of low second ionization enthalpies while Ni has exceptionally more negative electrode potential due to its high hydration enthalpy.
Across the period $M^{2+} / M$ value decreases because of increase in first and second ionization enthalpies.
Table showing thermochemical data (kJ mol-1) for the first row Transition Elements and the Standard Electrode Potentials for the Reduction of MII to M.
| Element | ΔₐH⁰ (M) Atomization Enthalpy | ΔᵢH₁⁰ 1st Ionization Enthalpy | ΔᵢH₂⁰ 2nd Ionization Enthalpy | ΔhydH⁰ (M²⁺) Hydration Enthalpy | E⁰/V (M²⁺/M) |
|---|---|---|---|---|---|
| Ti | 469 | 656 | 1309 | –1866 | –1.63 |
| V | 515 | 650 | 1414 | –1895 | –1.18 |
| Cr | 398 | 653 | 1592 | –1925 | –0.90 |
| Mn | 279 | 717 | 1509 | –1862 | –1.18 |
| Fe | 418 | 762 | 1561 | –1998 | –0.44 |
| Co | 427 | 758 | 1644 | –2079 | –0.28 |
| Ni | 431 | 736 | 1752 | –2121 | –0.25 |
| Cu | 339 | 745 | 1958 | –2121 | +0.34 |
| Zn | 130 | 906 | 1734 | –2059 | –0.76 |
.png)
Figure showing observed and calculated values for the standard electrode potentials (M2+/M°) of the elements Ti to Zn.
6)Trends in the M3+/M2+ Standard Electrode Potentials:
Table showing values of M3+/M2+ Standard Electrode Potentials for First-Row Transition Metals.
| Element | Ion Pair (M³⁺/M²⁺) | $E^\circ(\text{M}^{3+}/\text{M}^{2+})$ (V) |
|---|---|---|
| Sc | $\mathrm{Sc}^{3^{+}} / \mathrm{Sc}^{2^{+}}$ | –2.03 |
| Ti | $\mathrm{Ti}^{3^{+}} / \mathrm{Ti}^{2^{+}}$ | –0.37 |
| V | $V^{3^{+}} / V^{2^{+}}$ | –0.26 |
| Cr | $\mathrm{Cr}^{3^{+}} / \mathrm{Cr}^{2^{+}}$ | –0.41 |
| Mn | $\mathrm{Mn}^{3^{+}} / \mathrm{Mn}^{2^{+}}$ | +1.51 |
| Fe | $\mathrm{Fe}^{3^{+}} / \mathrm{Fe}^{2^{+}}$ | +0.77 |
| Co | $\mathrm{Co}^{3^{+}} / \mathrm{Co}^{2^{+}}$ | +1.82 |
| Ni | $\mathrm{Ni}^{3^{+}} / \mathrm{Ni}^{2^{+}}$ | +1.60 |
| Cu | $\mathrm{Cu}^{3^{+}} / \mathrm{Cu}^{2^{+}}$ | +0.15 (less common) |
| Zn | $\mathrm{Zn}^{3^{+}} / \mathrm{Zn}^{2^{+}}$ | Not commonly observed ($\mathrm{Zn}^{3+}$ is unstable) |
The low value for scandium indicates the exceptional stability of $\mathrm{Sc}^{3+}$, which possesses a noble gas configuration. On the other hand, zinc shows the highest value, as removing an electron from $\mathrm{Zn}^{2^{+}}$ will destabilize its stable $d^{10}$ configuration. The relatively high value for manganese suggests the exceptional stability of $\mathrm{Mn}^{2^{+}}\left(\mathrm{d}^5\right)$, while the lower value for iron reflects the additional stability of $\mathrm{Fe}^{3^{+}}\left(\right.$also $\left.d^5\right)$. Similarly, the low value for vanadium is linked to the stability of $\mathrm{V}^{2+}$, which has a half-filled $\mathrm{t}_2 \mathrm{~g}$ orbital configuration.
7)Trends in Stability of Higher Oxidation States:
Table below shows the stable halides of 3d transition metals. Highest oxidation states are seen in $\mathrm{TiX}_4, \mathrm{VF}_5$, and $\mathrm{CrF}_6 \mathrm{Mn}^{7+}$ does not form simple halides but exists as $\mathrm{MnO}_3 \mathrm{~F}$, and beyond Mn , only $\mathrm{FeX}_3$ and $\mathrm{CoF}_3$ are common trihalides.
Fluorine stabilizes high oxidation states due to strong lattice energies or bond enthalpies (e.g., $\mathrm{VF}_5, \mathrm{CrF}_6$ ). Lower halides like $\mathbf{V X}_{\mathbf{2}}$ and $\mathbf{C u X}$ are unstable. $\mathbf{C u}^{\mathbf{2 +}}$ does not form $\mathbf{C u I}_{\mathbf{2}}$ because it oxidizes $\mathbf{I}^{-}$to $\mathbf{I}_{\mathbf{2}}$.
$
2 \mathrm{Cu}^{2+}+4 \mathrm{I}^{-} \rightarrow \mathrm{Cu}_2 \mathrm{I}_2(\mathrm{~s})+\mathrm{I}_2
$
However, many copper (I) compounds are unstable in aqueous solution, due to lower hydration energy compared to $\mathrm{Cu}^{2+}$ and undergo disproportionation.
$
2 \mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}
$
In oxides, oxygen stabilizes higher oxidation states better than fluorine. The highest oxidation number matches the group number from $\mathrm{Sc}_2 \mathrm{O}_3$ to $\mathrm{Mn}_2 \mathrm{O}_7$. Beyond this, stable higher oxides are rare. Oxocations like $\mathrm{VO}_2{ }^{+}, \mathrm{VO}^{2+}$, and $\mathrm{TiO}^{2+}$ are common. Oxygen's ability to form multiple bonds explains its effectiveness in stabilizing high oxidation states, as seen in tetrahedral oxoanions like $\left[\mathrm{MO}_4\right]^{\mathrm{n}^{-}}$for $\mathrm{V}_{,} \mathrm{Cr}$, and Mn .
| Oxidation Number | +6 | +5 | +4 | +3 | +2 | +1 |
|---|---|---|---|---|---|---|
| $\mathrm{CrF}_6$ | $\mathrm{VF}_5, \mathrm{CrF}_5$ | $\begin{aligned} & \mathrm{TiX}_4, \mathrm{VX}_4, \mathrm{CrX}_4, \\ & \mathrm{MnF}_4\end{aligned}$ | $\mathrm{TiX}_3, \mathrm{VX}_3, \mathrm{CrX}_3$, $\mathrm{MnF}_3, \mathrm{FeX}_3{ }^1$, $\mathrm{CoF}_3$ | $\mathrm{TiX}_2, \mathrm{VX}_2, \mathrm{CrX}_2$, $\mathrm{MnX}_2, \mathrm{FeX}_2$ | CuX |
8) Chemical Reactivity and Eo Values:
Transition metals show a wide range of chemical reactivity. Many are reactive enough to dissolve in mineral acids, though some, known as noble metals, resist attack by simple acids. In the first transition series, all metals except copper are generally more reactive and can be oxidized by $\mathbf{1}$ M $\mathbf{H}^{+}$, though some like titanium and vanadium are passive in dilute non-oxidising acids at room temperature due to slow reaction rates.
The standard electrode potentials, $E^{\circ}\left(\mathrm{M}^{2+} / \mathrm{M}\right)$, show a decreasing tendency to form $\mathbf{M}^{\mathbf{2 +}}$ ions across the series. This trend toward less negative $E^{\circ}$ values correlates with increasing first and second ionisation enthalpies. However, $\mathrm{Mn}, \mathrm{Ni}$, and Zn deviate from this trend:
- $\mathbf{M n}^{2+}\left(\mathbf{d}^5\right)$ and $\mathbf{Z n}{ }^{2+}\left(\mathbf{d}^{10}\right)$ are stabilized by half-filled and fully filled $\mathbf{d}$-subshells, respectively.
- Ni's $ E^{\circ}$ is influenced by its highly exothermic hydration enthalpy.
For the redox couple $\mathrm{M}^{3+} / \mathrm{M}^{2+}$, ions like $\mathrm{Mn}^{3+}$ and $\mathrm{Co}^{3+}$ act as strong oxidising agents in aqueous solutions, while $\mathrm{Ti}^{2+}, \mathrm{V}^{2+}$, and $\mathrm{Cr}^{2+}$ serve as strong reducing agents, capable of liberating hydrogen from dilute acids.
For example:
$
2 \mathrm{Cr}^{2+}(\mathrm{aq})+2 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})
$
9)Magnetic properties:
When a magnetic field is applied, substances exhibit mainly two types of behavior: diamagnetism (repelled by the field) and paramagnetism (attracted by the field). Materials that are very strongly attracted are classified as ferromagnetic, an extreme form of paramagnetism. In paramagnetic substances, the magnetic behavior arises from unpaired electrons; each unpaired electron contributes a magnetic moment (about 1.73 BM) primarily due to its spin, as the orbital contribution is largely quenched in first-row transition metal compounds. Transition metals and most of their compounds are paramagnetic in nature. The spin only formula is used to compute the magnetic moment of these elements.
μ =n(n-1)
where n is the number of unpaired electrons
Table shows the observed and calculated magnetic moment.
|
Ion |
Configuration |
Unpaired Electrons |
Calculated Magnetic Moment (BM) |
Observed Magnetic Moment (BM) |
|---|---|---|---|---|
| Sc³⁺ | 3d⁰ | 0 | 0.00 | 0.00 |
| Ti³⁺ | 3d¹ | 1 | 1.73 | 1.75 |
| Ti²⁺ | 3d² | 2 | 2.84 | 2.76 |
| V²⁺ | 3d³ | 3 | 3.87 | 3.86 |
| Cr²⁺ | 3d⁴ | 4 | 4.90 | 4.80 |
| Mn²⁺ | 3d⁵ | 5 | 5.92 | 5.96 |
| Fe²⁺ | 3d⁶ | 4 | 4.90 | 5.3 – 5.5 |
| Co²⁺ | 3d⁷ | 3 | 3.87 | 4.4 – 5.2 |
| Ni²⁺ | 3d⁸ | 2 | 2.84 | 2.9 – 3.4 |
| Cu²⁺ | 3d⁹ | 1 | 1.73 | 1.8 – 2.2 |
| Zn²⁺ | 3d¹⁰ | 0 | 0.00 | 0.00 |
10)Formation of coloured compounds
There is an excitation of an electron from a lower to a higher level, and the energy of excitation corresponds to the energy difference between the levels. This frequency is usually in the visible range. The existence of unpaired or incomplete d-orbitals gives the transition metal ions their colour. The transition metal cations' absorption of visible light and hence their colourful character is due to the transition of one or more unpaired d-electrons from a lower to a higher level within the same d-subshell. This transition requires a small amount of visible light energy. $\mathrm{Sc}^{3+}, \mathrm{Cu}^{+}, \mathrm{Zn}^{2+}$ and$\mathrm{Ti}^{4+}$ have either a completely empty or completely filled 3d-orbital, i.e. no unpaired d-electron, and hence appear colourless. Download these d and f block elements class 12 ncert notes pdf. It will help you revise important topics quickly.
The table shows the colors of some of the First Row (aquated) Transition Metal Ions
| Ion | Electronic Configuration | Colour (in aqueous solution) |
|---|---|---|
| Ti³⁺ (aq) | 3d¹ | Purple |
| V²⁺ (aq) | 3d³ | Violet |
| V³⁺ (aq) | 3d² | Green |
| Cr³⁺ (aq) | 3d³ | Violet |
| Mn²⁺ (aq) | 3d⁵ | Pale pink |
| Fe²⁺ (aq) | 3d⁶ | Light green |
| Fe³⁺ (aq) | 3d⁵ | Yellow to brown |
| Co²⁺ (aq) | 3d⁷ | Pink |
| Ni²⁺ (aq) | 3d⁸ | Green |
| Cu²⁺ (aq) | 3d⁹ | Blue |
| Zn²⁺ (aq) | 3d¹⁰ | Colourless |
11)Formation of complex compounds
Transition metal cations have a strong tendency to form complexes with several molecules or ions known as ligands. Such complexes are named coordinate complexes because the links involved in their formation are coordinated. The structure of these complex ions might be linear, square, planar, tetrahedral, or octahedral, depending on the nature of the metal ion hybridization. Weak ligands like CO and NO form complexes only when transition metals are in a zero oxidation state. This is because these ligands contain empty orbitals in the donor atom in addition to the lone pair of electrons. Highly electronegative and basic ligands like F-, Cl- can form complexes with transition metals despite being in high oxidation states due to the presence of small, highly charged or neutral ligands with lone pairs of electrons that can form strong sigma bonds by donating a lone pair of electrons. The stability of complexes increases as the atomic number increases in a transition series. The transition metal atom has several oxidation states; the higher the valence, the more stable the complex.
12)Catalytic Properties
Transition metals and their compounds are well-known catalysts, primarily due to their ability to exhibit variable oxidation states and form complexes. Examples include $\mathbf{V}_2 \mathbf{O}_5$ in the Contact Process, finely divided Fe in the Haber Process, and Ni in catalytic hydrogenation. Catalysis on a solid surface typically involves the adsorption of reactant molecules onto the catalyst's surface. First-row transition metals utilize their 3d and 4s electrons to form bonds with reactants, which helps to increase their concentration at the surface and weaken internal bonds, thereby lowering the activation energy.
Their variable oxidation states make transition metal ions particularly effective in redox reactions. For instance, $\mathrm{Fe}^{3+}$ catalyzes the reaction between iodide and persulphate ions as follows:
$
2 \mathrm{I}^{-}+\mathrm{S}_2 \mathrm{O}_8^{2-} \rightarrow \mathrm{I}_2+2 \mathrm{SO}_4^{2-}
$
The catalytic mechanism involves these redox steps:
$
\begin{aligned}
2 \mathrm{Fe}^{3+}+2 \mathrm{I}^{-} & \rightarrow 2 \mathrm{Fe}^{2+}+\mathrm{I}_2 \\
2 \mathrm{Fe}^{2+}+\mathrm{S}_2 \mathrm{O}_8^{2-} & \rightarrow 2 \mathrm{Fe}^{3+}+2 \mathrm{SO}_4^{2-}
\end{aligned}
$
13)Formation of interstitial compounds
Interstitial compounds with non-stoichiometric composition are formed when transition elements combine with tiny atoms such as H, B, C, N, and others. The resulting interstitial compounds are chemically inert and have higher melting points than pure metals. These compounds are strong and durable, and they maintain metallic conductivity.
eg : TiH1.3,VH0.54
14)Alloy formation
Alloys are homogenous mixes of more than one metal. Transition metals are generally smaller in size. So they can displace another metal from the crystal lattices and there by forming alloys. Alloys are hard and have high melting points. Ferrous alloys include chromium, vanadium, tungsten, manganese, and molybdenum, for example. Brass (alloy of copper + zinc), stainless steel, bronze (alloy of copper + tin), and so on are some additional examples.
Some Important Compounds of Transition Metals
Oxides and Oxoanions of Metals
1. Potassium dichromate ($\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$)
Preparation
Initially, chromite ore was fused with sodium or potassium carbonate in the presence of air.
$4 \mathrm{FeCr}_2 \mathrm{O}_4+8 \mathrm{Na}_2 \mathrm{CO}_3+7 \mathrm{O}_2 \rightarrow 8 \mathrm{Na}_2 \mathrm{CrO}_4+2 \mathrm{FeO}_3+8 \mathrm{CO}_2$
A sodium chromate solution is filtered and then acidified with a sulfuric acid solution, yielding an orange sodium dichromate solution that can be crystallized.
$2 \mathrm{Na}_2 \mathrm{CrO}_4+2 \mathrm{H}^{+}->\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{Na}^{+}+\mathrm{H}_2 \mathrm{O}$
Because sodium dichromate is more soluble than potassium dichromate, it is fused with KCl, resulting in the formation of orange potassium dichromate crystals.
$\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{KCl} \rightarrow \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{NaCl}$
The dichromates and chromates exist in equilibrium at pH 4 and can be interconnected.
$2 \mathrm{CrO}_4{ }^{2-}+2 \mathrm{H}^{2+}\rightarrow \mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{H}_2 \mathrm{O}$
$\mathrm{Cr}_2 \mathrm{O}_7{ }^{2-}+2 \mathrm{OH}\rightarrow 2 \mathrm{CrO}_4^{2-}+\mathrm{H}_2 \mathrm{O}$
In the presence of an acidic medium, the yellow colour of chromate changes to an orange-colored dichromate, whereas in the presence of a basic medium, the dichromate transforms back to chromate.
$2 \mathrm{CrO}_4^{2-}+2 \mathrm{H}^{+}\rightarrow 2 \mathrm{HCrO}_4^{-}$(Hydrogen chromate)
$2 \mathrm{HCrO}^{4-}\rightarrow \mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{H}_2 \mathrm{O}$ Dichromate (orange)
Properties
-
In an acidic media, potassium dichromate is a powerful oxidizing agent.
$\mathrm{Cr}_2 \mathrm{O}_7{ }^{2-}+14 \mathrm{H}^{+}+6$ electron $\rightarrow$ $2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}$
-
Heat causes potassium dichromate to decompose, resulting in the formation of potassium chromate, chromic oxide, and oxygen
$4 \mathrm{~K}_2 \mathrm{Cr}_2 \mathrm{O}_7$-Heat $\rightarrow$ $4 \mathrm{~K}_2 \mathrm{CrO}_4+2 \mathrm{CrO}_3+3 \mathrm{O}_2$
.png)
2. Potassium permanganate ($\mathrm{KMnO}_4$)
-
Potassium Permanganate is a dark purple solid made up of two ions: potassium and permanganate. Because it is a strong oxidizing agent with medicinal qualities, it is frequently used to clean wounds and treat dermatitis.
-
In the presence of air or an oxidizing agent, fusion of powdered Pyrolusite ore with an alkali metal hydroxide like KOH results in the formation of dark green potassium Manganate, which disproportionates in a neutral or acidic solution and results in the formation of potassium permanganate.
$\begin{aligned} & 2 \mathrm{MnO}_2+4 \mathrm{KOH}+\mathrm{O}_2 \rightarrow 2 \mathrm{~K}_2 \mathrm{MnO}_4+2 \mathrm{H}_2 \mathrm{O} \\ & 3 \mathrm{MnO}_4^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O}\end{aligned}$
-
Commercially, potassium permanganate is made by alkaline oxidative fusion of Pyrolusite ore followed by electrolytic oxidation of manganate (4) ion.
$\begin{aligned} & 2 \mathrm{MnO}_2+4 \mathrm{KOH}+\mathrm{O}_2 \rightarrow 2 \mathrm{~K}_2 \mathrm{MnO}_4+2 \mathrm{H}_2 \mathrm{O} \\ & \mathrm{MnO}_4{ }^{2+}+(\text { electrolytic oxidation }) \rightarrow \mathrm{MnO}_4{ }^{-}+\mathrm{e}^{-}\end{aligned}$
.jpg)
f-block Elements
f-block elements are those that have gradually filled f orbitals. The elements of the inner transition metals' 4f series are known as lanthanoids, while the elements of the 5f series are known as actinoids.
Lanthanoid
- Lanthanoid has an electronic configuration of$[X e] 4 f^{+1} 5 d^1 6 s^2$ or [Xe] $[\mathrm{Xe}] 4 \mathrm{fn}^5 \mathrm{~d}^1 6 \mathrm{~s}^2$, with a valence shell electronic configuration of $4 f^{1-14} 6 s^2$
- They have oxidation states of +3, +2, and +4
| Atomic Number | Name | Symbol | Electronic Configuration | Radii/pm | ||||
|---|---|---|---|---|---|---|---|---|
| Ln | Ln²⁺ | Ln³⁺ | Ln⁴⁺ | Ln | Ln³⁺ | |||
| 57 | Lanthanum | La | 5d¹6s² | – | 4f⁰ | – | 187 | 106 |
| 58 | Cerium | Ce | 4f¹5d¹6s² | – | 4f¹ | 4f⁰ | 183 | 103 |
| 59 | Praseodymium | Pr | 4f³6s² | – | 4f² | 4f¹ | 182 | 101 |
| 60 | Neodymium | Nd | 4f⁴6s² | – | 4f³ | 4f² | 181 | 99 |
| 61 | Promethium | Pm | 4f⁵6s² | – | 4f⁴ | 4f³ | 181 | 98 |
| 62 | Samarium | Sm | 4f⁶6s² | – | 4f⁵ | 4f⁴ | 180 | 96 |
| 63 | Europium | Eu | 4f⁷6s² | 4f⁷ | 4f⁶ | – | 199 | 95 |
| 64 | Gadolinium | Gd | 4f⁷5d¹6s² | – | 4f⁷ | 4f⁷5d¹ | 180 | 94 |
| 65 | Terbium | Tb | 4f⁹6s² | – | 4f⁸ | 4f⁷ | 178 | 92 |
| 66 | Dysprosium | Dy | 4f¹⁰6s² | – | 4f⁹ | 4f⁸ | 177 | 91 |
| 67 | Holmium | Ho | 4f¹¹6s² | – | 4f¹⁰ | 4f⁹ | 176 | 89 |
| 68 | Erbium | Er | 4f¹²6s² | – | 4f¹¹ | 4f¹⁰ | 175 | 88 |
| 69 | Thulium | Tm | 4f¹³6s² | – | 4f¹² | 4f¹¹ | 174 | 87 |
| 70 | Ytterbium | Yb | 4f¹⁴6s² | 4f¹⁴ | 4f¹³ | – | 173 | 86 |
| 71 | Lutetium | Lu | 4f¹⁴5d¹6s² | – | 4f¹⁴ | – | – | 86 |
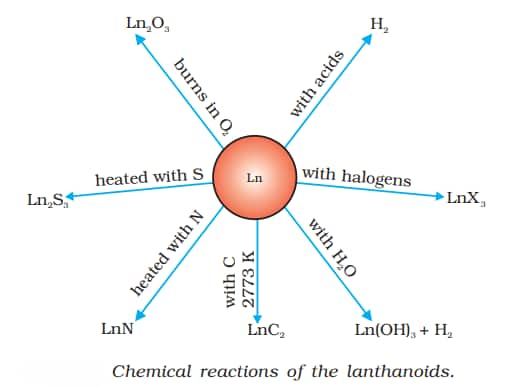
- The first members of this series are mildly reactive to calcium, and their behaviour gradually resembles that of aluminium as the atomic number increases.
- Lanthanides mix with hydrogen when exposed to mild heat.
- Carbides and halides are formed when they are heated with carbon (in the presence of halogens while burning).
- Lanthanides react with dilute acids to produce hydrogen gas.
General Characteristics:
Lanthanoids are soft, silvery-white metals that tarnish in air and show increasing hardness with atomic number (samarium is steel-hard). Their melting points range from $1000-1200 \mathrm{~K}$, with Sm melting at 1623 K . They have metallic structures, conduct heat and electricity well, and show smooth trends in propertiesexcept Eu, Yb, Sm, and Tm. Most $\mathbf{L n}^{\mathbf{3}+}$ ions are coloured due to f-electron transitions, except $\mathbf{L a}^{\mathbf{3}+}$ and $\mathbf{L u}^{\mathbf{3}+}$. The absorption bands are narrow. All $\mathbf{L n}^{\mathbf{3}+}$ ions (except $\mathbf{f}^{\mathbf{0}}$ and $\mathbf{f}^{\mathbf{1 4}}$ ) are paramagnetic.
Their first and second ionisation enthalpies ( $\sim 600$ and $\sim 1200 \mathrm{~kJ} / \mathrm{mol}$ ) are similar to calcium. Variations in third ionisation enthalpy reflect stability of empty, half-filled, and full f-orbitals, seen in La, Gd, and Lu. Chemically, early lanthanoids resemble calcium, while heavier ones behave more like aluminium. The standard electrode potentials lie between -2.2 and -2.4 V , with Eu at -2.0 V . They form hydrides, oxides $\left(\mathrm{Ln}_2 \mathrm{O}_3\right)$, hydroxides $\left(\mathrm{Ln}(\mathrm{OH})_3\right)$, halides, carbides, and liberate $\mathrm{H}_2$ from acids. Hydroxides are strongly basic, like those of alkaline earth metals.
Uses:
- Mischmetall ( $95 \% \mathrm{Ln}+\mathrm{Fe}$ ) is used in Mg-alloys for bullets and lighters.
- Mixed oxides act as petroleum cracking catalysts.
- Some Ln oxides serve as phosphors in TVs and fluorescent displays.
Lanthanide Contraction
As we move through the lanthanoid series, the atomic number, or the number of electrons and protons, gradually increases by one. The effective nuclear charge increases as electrons are added to the same shell. As the atomic number rises, so does the number of electrons in the 4f orbital, which has a weak shielding effect and increases the effective nuclear charge on the outside electrons. As a result, the size of lanthanoids reduces with increasing atomic number, a phenomenon known as lanthanoid contraction. The features of the second and third transition series are comparable as a result of lanthanoid contraction.
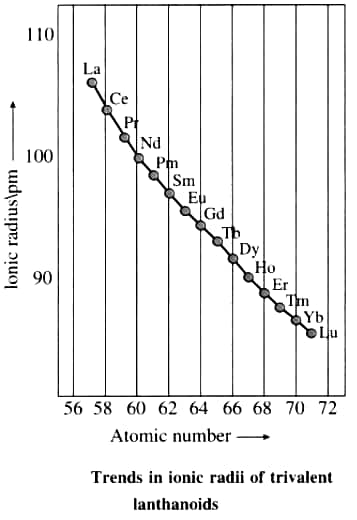
Actinoids
The elements Th to Lr are members of the Actinoids series, which consists of 14 elements. They are radioactive substances. The former elements have extended half-lives, whereas the subsequent elements, such as lawrencium with atomic number 103, have half-lives ranging from one day to three minutes. The electronic configuration of actinoids is 7s2, with inconstant occupancy of the 5f and 6d subshells. Because of the poor screening effect of nuclear charge exerted by the f electrons, the ionic radii gradually decrease across the series. Actinoid contraction is the term for this.
Comparison Between Lanthanoid and Actinoid
-
Atomic and ionic sizes
Actinoids' ionic radii gradually decrease across the series, similar to lanthanoids, due to the poor screening effect of nuclear charge exerted by the f electrons.
-
Oxidation states
The lanthanide have oxidation states of +3. Due to the additional stability of fully-filled and half-filled orbitals, some elements may show + 2 and + 4 oxidation states.
Actinoids, on the other hand, have a + 3 oxidation state. Due to the similar energies of 5f, 6d, and 7s, they also have different oxidation states.
| Element | Symbol | Atomic Number | Common Oxidation States | Most Stable Oxidation State(s) |
|---|---|---|---|---|
| Actinium | Ac | 89 | +3 | +3 |
| Thorium | Th | 90 | +4 | +4 |
| Protactinium | Pa | 91 | +3, +4, +5 | +5 |
| Uranium | U | 92 | +3, +4, +5, +6 | +6 |
| Neptunium | Np | 93 | +3, +4, +5, +6, +7 | +5 |
| Plutonium | Pu | 94 | +3, +4, +5, +6, +7 | +4, +5 |
| Americium | Am | 95 | +2, +3, +4, +5, +6 | +3 |
| Curium | Cm | 96 | +3, +4 | +3 |
| Berkelium | Bk | 97 | +3, +4 | +3 |
| Californium | Cf | 98 | +3, +4 | +3 |
| Einsteinium | Es | 99 | +3 | +3 |
| Fermium | Fm | 100 | +2, +3 | +3 |
| Mendelevium | Md | 101 | +2, +3 | +3 |
| Nobelium | No | 102 | +2, +3 | +2 |
| Lawrencium | Lr | 103 | +3 | +3 |
-
Chemical reactivity
The lanthanide series' earlier members are more reactive. With rising atomic numbers, they resemble Al. Finely split actinoids are highly reactive metals that produce a mixture of oxide and hydride when introduced to boiling water. Actinoids mix with the majority of non-metallic elements at moderate temperatures. The action of alkalies has little effect on actinides, while nitric acid has a minor effect due to the creation of a protective oxide layer.
General Characteristics and Comparison with Lanthanoids
The actinoids are silvery, highly reactive metals with varied structures due to greater irregularities in metallic radii compared to lanthanoids. They readily react with boiling water, non-metals, and HCl, but are less affected by HNO₃ due to protective oxide layers. Alkalis have no effect. Their magnetic properties are complex, and though the variation follows the number of unpaired 5f electrons, lanthanoids show higher magnetic susceptibilities.The early actinoids have lower ionisation enthalpies than lanthanoids, as 5f electrons are more shielded and less tightly held, making them more available for bonding.
While later actinoids resemble lanthanoids more closely, even early actinoids show gradual property trends. Both lanthanoid and actinoid contractions affect the elements that follow, though the lanthanoid contraction has a greater chemical impact due to better-known properties of subsequent elements.
Some Applications of d- and f-Block Elements
Iron and steel are among the most vital construction materials, produced by reducing iron oxides, removing impurities, and adding carbon along with alloying elements like $\mathrm{Cr}, \mathrm{Mn}$, and Ni . Some metal compounds serve specialized roles-for example, TiO is used in pigments, and $\mathrm{MnO}_2$ powers dry cells. The battery industry depends on Zn and $\mathrm{Ni} / \mathrm{Cd}$ systems. Group 11 elements (Cu, Ag, Au) are traditionally known as coinage metals, though today's UK coins use copper-coated steel or $\mathrm{Cu} / \mathrm{Ni}$ alloys, while Ag and Au coins are mostly for collectors.
Many transition metals and their compounds are key catalysts in industry:
- $\mathbf{V}_{\mathbf{2}} \mathbf{O}_{\mathbf{5}}$ for oxidizing $\mathbf{S O}_{\mathbf{2}}$ in sulphuric acid production
- $\mathrm{TiCl}_4+\mathrm{Al}\left(\mathrm{CH}_3\right)_3$ (Ziegler catalyst) for making polythene
- Fe in the Haber process $\left(\mathrm{NH}_3\right.$ production from $\left.\mathrm{N}_2 / \mathrm{H}_2\right)$
- Ni for hydrogenating fats
- $\mathrm{PdCl}_2$ in the Wacker process (oxidation of ethyne to ethanal)
- Ni complexes in polymerizing alkynes and aromatics
- The photographic industry relies on AgBr for its light sensitivity
The d- and f- block Elements Previous Year Question and Answer
These previous year questions and answers from this chapter are given below that will help students understand the exam pattern and practise important concepts effectively. To solve these questions effectively refer to class 12 chemistry chapter 4 d and f block elements notes.
Question 1: Consider the following reactions
$\begin{aligned}
& \mathrm{A}+\mathrm{NaCl}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{CrO}_2 \mathrm{Cl}_2+\text { Side Products } \\
& \quad \text { Little } \\
& \text { amount } \\
& \mathrm{CrO}_2 \mathrm{Cl}_{2(\text { Vapour })}+\mathrm{NaOH} \rightarrow \mathrm{~B}+\mathrm{NaCl}+\mathrm{H}_2 \mathrm{O} \\
& \mathrm{~B}+\mathrm{H}^{+} \rightarrow \mathrm{C}+\mathrm{H}_2 \mathrm{O}
\end{aligned}$
The number of terminal ' $O$ ' present in the compound ' C ' is______ .
Answer:
$\mathrm{Cr}_2 \mathrm{O}_7^{2-}+\mathrm{NaCl}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{CrO}_2 \mathrm{Cl}_2$
$\begin{aligned} & \mathrm{CrO}_2 \mathrm{Cl}_2(\text { Vapour })+\mathrm{NaOH} \rightarrow \\ & \mathrm{Na}_2 \mathrm{CrO}_4+\mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}\end{aligned}$
$
\mathrm{Na}_2 \mathrm{CrO}_4+\mathrm{H}^{\oplus} \rightarrow \mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7+\mathrm{H}_2 \mathrm{O}
$

$\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7 \rightarrow 2 \mathrm{Na}^{\oplus}+\mathrm{Cr}_2 \mathrm{O}_7^{2-}$
No of terminal " O " $=6$
Hence, the answer is 6.
Question 2: The metal ions that have the calculated spin only magnetic moment value of 4.9 B.M. are
A. $\mathrm{Cr}^{2+}$
B. $\mathrm{Fe}^{2+}$
C. $\mathrm{Fe}^{3+}$
D. $\mathrm{Co}^{2+}$
E. $\mathrm{Mn}^{3+}$
Choose the correct answer from the options given below:
(1) A, C and E only
(2)n A, D and E only
(3) B and E only
(4) A, B and E only
Answer: Given magnetic moment $=4.9 \mathrm{~B} . \mathrm{M}$.
We know M.M $=\sqrt{\mathrm{n}(\mathrm{n}+2)}$ B.M.
Where, $\mathrm{n} \rightarrow$ No. of unpaired $\mathrm{e}^{-}$
$4.9=\sqrt{n(n+2)}$
Solving this equation, we find that $n=4$.
(A) ${ }_{24} \mathrm{Cr}^{2+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^4 \quad$ (4 unpaired $\mathrm{e}^{-}$)
(B) ${ }_{26} \mathrm{Fe}^{2+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^6 \quad$ (4 unpaired $\mathrm{e}^{-}$)
(C) ${ }_{26} \mathrm{Fe}^{3+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^5 \quad$ (5 unpaired $\mathrm{e}^{-}$)
(D) ${ }_{27} \mathrm{Co}^{2+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^7 \quad\left(3\right.$ unpaired $\left.\mathrm{e}^{-}\right)$
(E) ${ }_{25} \mathrm{Mn}^{3+} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^4 \quad(4$ unpaired e $)$
The metal ions with a spin-only magnetic moment of approximately 4.9 B.M. are $\mathrm{Cr}^{2+}, \mathrm{Fe}^{2+}$, and $\mathrm{Mn}^{3+}$. Because, the value of n=4 for these ions.
Hence, the correct answer is option (4).
Question 3: The element having [Ar] $3 \mathrm{~d}^{10} 4 \mathrm{~s}^1$ electronic configuration is
(1) Cu
(2) Zn
(3) Cr
(4) Mn
Answer:
Given that the electronic configuration is: [Ar] 3d104s1
The number of electrons in Argon is 18, there are 10 electrons in the d orbital and 1 electron in the s orbital.
According to this configuration, the number of electrons is 29.
The given electronic configuration and the number of electrons correspond to the element Copper.
Copper has an atomic number of 29, and its electronic configuration is [Ar] 3d104s1.
Hence, the correct answer is option (1).
Question 4: The correct decreasing order of spin only magnetic moment values $(\mathrm{BM})$ of $\mathrm{Cu}^{+}, \mathrm{Cu}^{2+}, \mathrm{Cr}^{2+}$ and $\mathrm{Cr}^{3+}$ ions is:
(1) $\mathrm{Cu}^{+}>\mathrm{Cu}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cr}^{2+}$
(2) $\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}>\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}$
(3) $\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}$
(4) $\mathrm{Cr}^{3+}>\mathrm{Cr}^{2+}>\mathrm{Cu}^{+}>\mathrm{Cu}^{2+}$
Answer:
$\mathrm{Cu}^{+} \Rightarrow 3 \mathrm{~d}^{10} \Rightarrow$ 
$\mathrm{Cu}^{2+} \Rightarrow 3 \mathrm{~d}^9 \Rightarrow$ 
$\mathrm{Cr}^{2+} \Rightarrow 3 \mathrm{~d}^4 \Rightarrow$ 
$\mathrm{Cr}^{3+} \Rightarrow 3 \mathrm{~d}^3 \Rightarrow$ 
So order :
$\mathrm{Cr}^{2+}>\mathrm{Cr}^{3+}>\mathrm{Cu}^{2+}>\mathrm{Cu}^{+}$
Hence, the correct answer is option (3).
Question 5: The number of unpaired electrons responsible for the paramagnetic nature of the following complex species are respectively :
$\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-},\left[\mathrm{FeF}_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$
(1) $1,5,4,2$
(2) $1,5,5,2$
(3) $1,1,4,2$
(4) $1,4,4,2$
Answer:
$\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-} \Rightarrow \mathrm{Fe}^{3+}, \mathrm{d}^5, \mathrm{t}_{2 \mathrm{~g}}^5 \mathrm{e}_{\mathrm{g}}^0$
$\quad$ $\Rightarrow 1$ unpaired electron
$\left[\mathrm{FeF}_6\right]^{3-} \Rightarrow \mathrm{Fe}^{3+}, \mathrm{d}^5, \mathrm{t}_{2 \mathrm{~g}}^3 \mathrm{e}_{\mathrm{g}}^2 \Rightarrow 5$ unpaired electrons $\left[\mathrm{CoF}_6\right]^{3-} \Rightarrow \mathrm{Co}^{3+}, \mathrm{d}^6, \mathrm{t}_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^2 \Rightarrow 4$ unpaired electrons
$\begin{aligned} {\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-} \Rightarrow } & \mathrm{Mn}^{3+}, \mathrm{d}^4,\end{aligned} \mathrm{t}_{2 \mathrm{~g}}^4 \mathrm{e}_{\mathrm{g}}^0 \mathrm{C}$.
$\quad$ $\Rightarrow 2$ unpaired electron
Hence, the correct answer is option (1).
How to Master Class 12 Chemistry Chapter 4 The d- and f-Block Elements
These ncert class 12 chemistry chapter 4 d and f block elements notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 4.
- Firstly students need to understand the periodic trends like general properties, electronic configurations, and trends in atomic and ionic sizes, ionisation enthalpies, and oxidation states of d- and f-block elements.
- After that, students must learn about transition and inner transition elements. To understand these concepts better they can also refer to d and f-block elements class 12 notes.
- Students must understand the concepts of colour, magnetic properties, and key reactions of compounds like potassium dichromate, potassium permanganate, and their preparation methods.
- To make learning easy and interesting, create tables for properties of the first-row transition metals, lanthanide contraction, and oxidation states to easily compare and recall during exams.
- After that students can solve previous year questions from this chapter.
Advantages of Class 12 Chemistry Chapter 4 The d- and f-Block Elements Notes
NCERT Notes for Class 12 Chemistry Chapter 4 The d and f-Block Elements provide a clear explanation of concepts that helps in mastering topics and scoring well in board and competitive exams. The advantages of using these notes are given below:
- The d and f block elements notes cover all important concepts like electronic configuration, oxidation states, magnetic properties, and complex formation of transition and inner transition elements.
- These notes are prepared by subject experts and they are well organised that help students quick revision.
- Some important questions are also provided in these notes to help you in scoring good marks.
- The d and f block elements class 12 ncert notes are prepared in a concise and structured manner that are very helpful for last minute revision.
CBSE Class 12 Chemistry Chapter-wise Notes
Besides NCERT Notes for Class 12 Chemistry Chapter 4 The d and f-Block Elements here is a list of notes for Class 12 chemistry. Students can use the link to access the notes of other chapters as well.
NCERT Solutions for Class 12 Chemistry
Along with NCERT Solutions for Class 12 Chemistry Chapter 4 The d- and f-Block Elements, students can also refer to solutions of other Class 12 Chemistry chapters of NCERT.
Subject-Wise NCERT Exemplar Solutions
The hyperlinks of subject wise exempler solutions are given below:
Subject-Wise NCERT Solutions
The hyperlinks of subject-wise ncert solutions are given below:
Frequently Asked Questions (FAQs)
D-block elements form various important compounds, including coordination complexes, oxides, halides, and sulfates.
d-block elements are crucial in various applications such as catalysis, metallurgy, and the production of alloys. Metals like iron, copper, and nickel are significant in construction and electronics, while transition metals also play vital roles in biological systems, such as hemoglobin in oxygen transport.
Because the energy difference between the (n-1) d-orbital and the ns-orbital is so small, transition elements have a wide range of oxidation states. Because the orbitals have such a little energy difference, both energy levels can be utilized to establish a bond.
Common properties of d-block elements include high melting and boiling points, good electrical and thermal conductivity, the ability to form colored ions and compounds, and variable oxidation states.
F-block elements, particularly the lanthanides, are crucial in modern technology. They are used in making strong permanent magnets, phosphors for LED lights, catalysts in petroleum refining, and in various electronic devices.
The screening effect refers to the phenomenon where inner electrons shield outer electrons from the full charge of the nucleus. In d and f-block elements, this effect is significant because the inner f or d electrons can reduce the effective nuclear charge felt by the outer electrons, impacting their reactivity and bonding behaviour.
In Class 12 Chemistry, d-block elements are the elements found in groups 3 to 12 of the periodic table, also called transition elements, which have partially filled d-orbitals.
f-block elements are the inner transition elements that include lanthanides and actinides, where the last electron enters the f-orbital.
Class 12 Chemistry Chapter 4 covers the general properties, electronic configurations, oxidation states, and trends in atomic and ionic sizes, ionisation enthalpies, and magnetic behaviour of d-block, transition elements and f-block inner transition elements.
Each horizontal row in the d-block has ten d-subshell elements and can hold a maximum of ten electrons. Transition metals are d-block elements having a partially filled d-subshell.They exhibit variable valency. They have the ability to form coloured compounds.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
