Biomolecules class 12th notes- Free ncert class 12 Chemistry Chapter 14 Notes- Download PDF
How do simple sugars give you energy? What makes food nutritious? How do proteins build your body? Biomolecules uncover the chemistry behind carbohydrates, proteins, nucleic acids, and enzymes, which form the foundation of every living organism; they play a unique role in the functionality and health of organisms. Biomolecules, or biological molecules, are made by living organisms and are crucial for various life functions. All biomolecules are naturally occurring compounds. They include large macromolecules, such as proteins, carbohydrates, lipids, and nucleic acids, as well as smaller molecules, including vitamins and hormones. These organic molecules are key to all life forms and support many biological processes. From simply eating food to the occurrence of all the processes inside our body, Biomolecules play an important role.
This Story also Contains
- NCERT Notes for Class 12 Chapter 10 Biomolecules: Download PDF
- NCERT Notes for Class 12 Chapter 10 Biomolecules
- Biomolecules Previous Years' Questions With Answers
- How to Master Class 12 Chemistry Chapter 10 Biomolecules
- Advantages of Using Class 12 Chemistry Chapter 10 Notes
- NCERT Class 12 Notes Chapter-Wise
- NCERT Exemplar Solutions Subject-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions Subject-Wise
The NCERT Notes for Class 12 Chemistry will be helpful for a quick revision of topics. These class 12 chemistry chapter 10 biomolecules notes are designed by our subject experts, which ensures the credibility of the content provided. It becomes difficult and time consuming for students to read the NCERT textbooks point to point. So, to solve this problem, we are providing these NCERT notes that cover all the topics and concepts provided in the NCERT textbook in a very clear and comprehensive way. These notes are also valuable resources for various competitive exams like JEE, NEET, etc. Also, check the NCERT Solutions for all the chapters.
NCERT Notes for Class 12 Chapter 10 Biomolecules: Download PDF
Download the biomolecules Class 12 Chemistry Chapter 10 CBSE notes pdf to access clear explanation, important reactions. These NCERT notes for Class 12 cover all the key concepts of Biomolecules. You can download the PDF from the button given below:
Also Read:
NCERT Notes for Class 12 Chapter 10 Biomolecules
The class 12 chemistry chapter 10 biomolecules notes includes topics like the structure, classification, and functions of essential biomolecules like carbohydrates, proteins, nucleic acids, and vitamins. It shows how biomolecules are linked to our health and influence everything from what we eat to the medicines we take. Carbohydrates provide energy for everyday activities, Proteins are essential for growth, Lipids serve the purpose of energy storage, and Nucleic Acids, like DNA and RNA, are essential for genetic information transmission. The biomolecules class 12 ncert notes pdf is the best resource for quick revision, also they help build a clear understanding of fundamental principles and their real-life applications.
Biomolecules
Biomolecules are organic substances that form a basis for the growth and maintenance of the human body.
Biomolecules discussed here are carbohydrates, proteins, enzymes, vitamins, nucleic acids, and hormones.
A) Carbohydrates
- Manufactured in plants by performing photosynthesis.
- Optically active polyhydroxy aldehydes/ polyhydroxy ketones.
- Food, clothing, and shelter.
Classification of carbohydrates:
Classification of carbohydrates is based on their structure and the number of sugar units they contain. You can download these biomolecules Class 12 Chemistry Chapter 10 CBSE notes pdf for offline access, making it easier to study at your own pace and convenience.
1. Monosaccharides
Monosaccharides are the simplest carbohydrates that cannot be hydrolyzed into simpler compounds.
Examples are glucose, fructose, and ribose.
General formula- $\left(\mathrm{CH}_2 \mathrm{O}\right)_5$
Glucose:
Present in honey and fruits.
Belongs to D-family.
Preparation of glucose
a)From sucrose-
$\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}+\mathrm{H}_2 \mathrm{O} \xrightarrow{\mathrm{H}^{+}} \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6$
b)From starch-
$\left(\mathrm{C}_6 \mathrm{H}_{10} \mathrm{O}_5\right)_{\mathrm{n}}+\mathrm{nH}_2 \mathrm{O} \xrightarrow[393 \mathrm{~K}, 2-3 \text { bar }]{\mathrm{H}^{+}} \mathrm{nC}_6 \mathrm{H}_{12} \mathrm{O}_6$
Structure Of Glucose
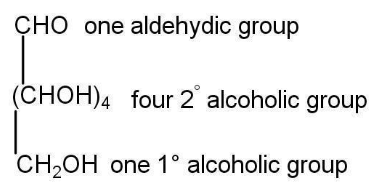
Here are a few reactions to find the structure of glucose-
a)Straight chain structure-
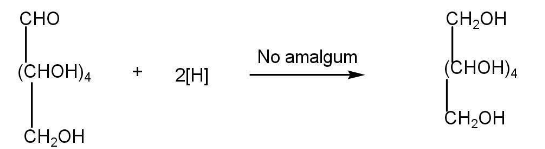
b)Presence of five hydroxyl groups-
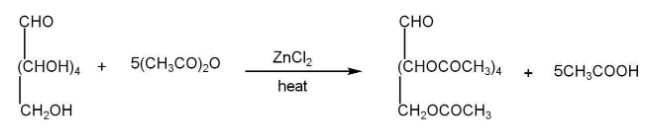
c)Presence of an aldehyde group-
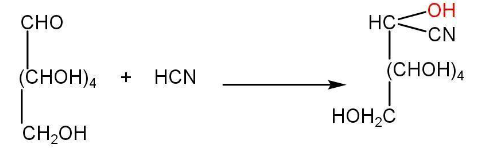
d) Oxidation of glucose-
Oxidation of glucose indicates the presence of the primary alcoholic group.
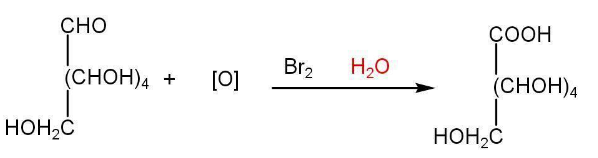
e) Open chain structure of Glucose-
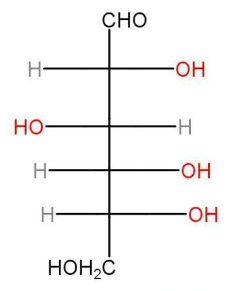
D-(+)-Glucose
The Cyclic Structure of Glucose
Limitations of open chain structure of glucose-
- Glucose does not undergo some of the characteristic reactions of aldehydes.
- No reaction with ammonia
- No reaction with hydroxylamine.
(+)-Glucose exists in two stereoisomeric forms i.e., α-D-glucose and β-D-Glucose.
Mutarotation-the two forms of glucose, convert into each other at equilibrium when glucose is dissolved in water and allowed to stand.
Anomers
Cyclization in the structure of glucose has been observed by the formation of hemiacetal between -CHO group and -OH group. On C5 carbon.
Optical isomers exist when configuration around only one of the carbons C1 takes place. These are known as anomers.
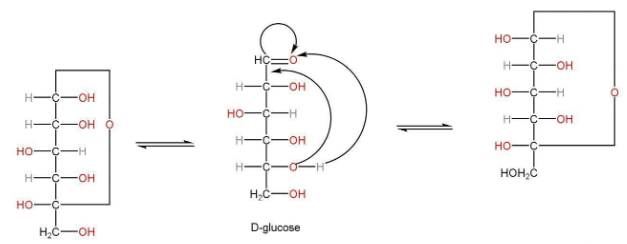
α-D-glucose β-D-Glucose
Fructose
Fructose is obtained when disaccharides are hydrolyzed. Fructose has a six-member hemiacetal ring structure.
Structure of fructose
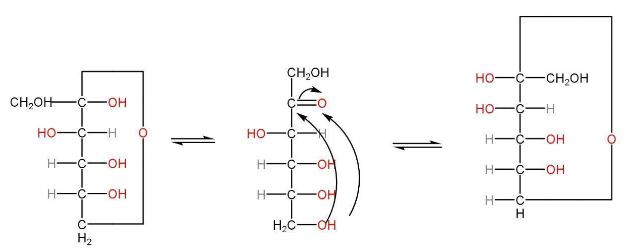
α-D-fructose β-D-fructose
Haworth structure of fructose
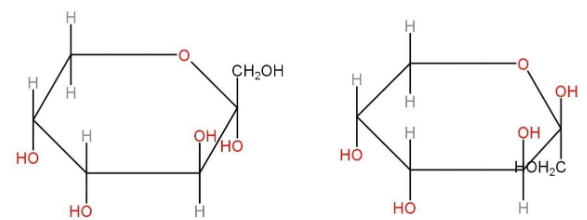
α-D-fructose β-D-fructose
2. Disaccharides
These are the types of carbohydrates that give more than one (can be the same or not) monosaccharide.
Examples-
$\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}+\mathrm{H}_2 \mathrm{O} \xrightarrow{\mathrm{H}^{+}} \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6$
$\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11 .}+\mathrm{H}_2 \mathrm{O} \xrightarrow{\mathrm{H}^{+}} \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6$
3. Polysaccharides
These are polymer compounds that are formed by many numbers of monosaccharide units which are joined together by glycosidic linkages.
a)Starch
- Found in plants.
- Found in wheat, rice, maize, potatoes, barley, etc.
- Starch reacts with iodine solution to give a blue color.
- It is a non-reducing saccharide.
- Does not reduce Fehling’s solution.
b)Amylose
- Linear polymer
- It contains 200-300 α-D-glucose units linked together by glycosidic linkages.
- Molecular mass (10,000-500,000)
c)Amylopectin
- It is a highly branched polymer.
- Do not react with iodine solution to give a blue color.
d)Cellulose
- It is the most abundant organic polymer found on earth.
- It is a straight-chain polymer joined by β-glycosidic linkages.
- Used in the manufacturing of paper, textiles, and plastic industries.
e)Glycogen
- It is a polysaccharide found in animal cells occurring in muscles, and the liver.
- It is known as animal starch.
- It is a polymer made up of a thousand glucose units.
Importance of Carbohydrates
- Plays a major role for both plants as well as animals.
- Carbohydrates are the major source of energy (except cellulose)
- Carbohydrates store energy for the functioning of living organisms.
- Carbohydrates are used as raw materials in the production of textiles, papers, lacquers, breweries, etc.
B) Proteins:
All living cells are made up of biomolecules having a high molecular mass, known as amino acids. Students can also refer biomolecules ncert notes to understand these concepts better through a series of solved questions.
1. Amino acids
Amino acids are the building block units of proteins. These are the organic compounds that contain amino as well as a carboxyl group.
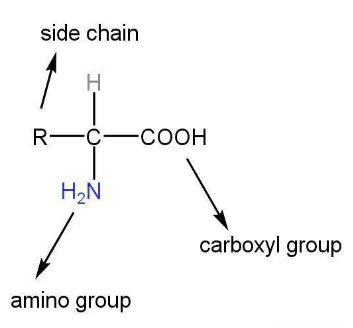
The above unit may be attached to any other carbon atom other than that of the -COOH group.
2. Classification of Amino Acids
Amino acids can be broadly classified as – acidic, basic, or neutral.
Neutral amino acids- These are the amino acids that contain an equal number of amino and carboxyl groups. Examples- glycine, alanine, valine, etc.
Acidic neutral acids- These are amino acids that contain more carboxyl groups than amino groups. Examples- are aspartic acid, asparagine acid, and glutamic acid, which contain two -COOH groups and one -NH3 group.
Basic amino acids- These contain more amino groups than carboxyl groups. Examples- lysine, arginine, and histidine.
3. Structure of Proteins
Amino acids exist as zwitterion which is dipolar.
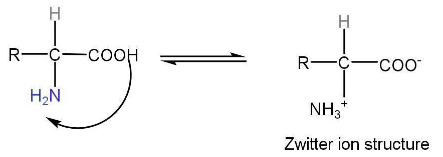
The basic nature of zwitterion is due to -COO- ion.
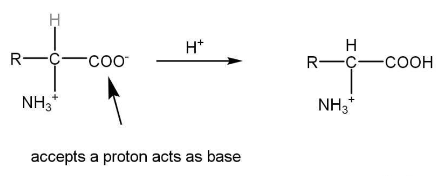
The acidic character of zwitterion is due to the -NH3+ group.
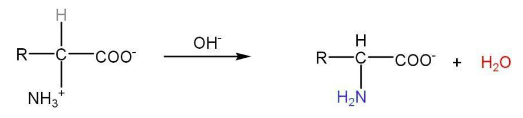
Peptide linkage- When two or more amino acids condense, the resulting -CO-NH- link is called peptide linkage of the peptide bond.
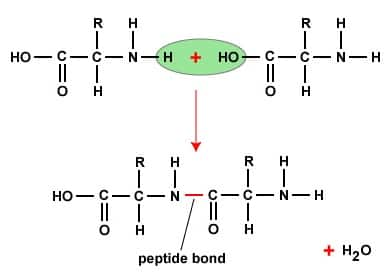
The structure of proteins is classified into four hierarchical levels based on different structural parameters such as the folding patterns, the sequence of amino acids, 3D conformation, and functional arrangement.
- Primary structure of proteins- amino acids are linked in one or more polypeptide chains, which are known as the primary structure of proteins.
- Secondary structure of proteins- the folding and arrangement of polypeptide chains give the shape or conformation of the protein.
- The secondary structure can be of α-helix structure type or β-pleated sheet structure.
- Tertiary structure- the tertiary structure of the protein arises due to the folding, bending, and coiling, which results in three-dimensional structures.
- Quaternary structure- many of the proteins exist as a grouping of two or more polypeptide chains. These polypeptide chains are called the sub-units.
Proteins can be classified into two types based on their molecular shape.
(a) Fibrous proteins: When the polypeptide chains run parallel and are held together by hydrogen and disulfide bonds, then fiber-like structure is formed. Such proteins are generally insoluble in water. Some common examples are keratin (present in hair, wool, silk) and myosin (present in muscles), etc
4. Denaturation of Proteins
- Proteins can be denatured (physical changes and biological changes) but there is no chemical change in the protein structure.
- Denaturation can arise due to many factors such as changes in temperature, pH, or certain chemical agents.
Enzymes
Enzymes are biological catalysts that catalyze biochemical reactions in living organisms. For example- hydrolysis of maltose is catalyzed by maltase.
Mechanism of enzyme
The mechanism is given as
1. The enzyme (E) binds to the substrate(s)
E+S→ES
2. Product Formation
ES→EP
3. Products released from the above complex.
EP→E+P
C) Vitamins
These are the biomolecules that are not produced by the body and hence, need to be supplied in small amounts for necessary biological functions of the body.
There are A, B, C, D, E, and K vitamins.
Classification of Vitamins
Water-soluble vitamins- water-soluble vitamins are vitamin B, vitamin C, etc.
These vitamins need to be supplied to the body from time to time.
Fat-soluble vitamins- vitamins that are only soluble in fat are called fat-soluble vitamins. A, D, E, and, K vitamins are soluble in fat.
Vitamin Deficiency Associated Diseases:
Vitamin A - Night blindness, Xeropthalmia
Vitamin (Thiamine) B1 - Beriberi
Vitamin (Riboflavin) B2 - Cheilosis
Vitamin (Niacin) B3 - Pellagra
Vitamin (Pyridoxine) B2 - Convulsions , Anaemia
Vitamin B12 - Pernicious anaemia
Vitamin C (Ascorbic acid) - Scurvy
Vitamin D - Rickets ( in children)
Osteomalacia ( in adults )
Vitamin E - Increased RBC fragility, muscular weakness
Vitamin K - Poor blood clotting
D) Nucleic Acids
- Nucleic acids are polymers that are present in all human bodies.
- Nucleic acids play an important role in the development and reproduction of all life forms.
- Nucleic acids have nucleotides as their repeating units.
There are two types of nucleic acids-
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid)
1. Chemical composition of nucleic acids-
Nucleotides consist of three chemical components such as a heterocyclic base, a five-carbon sugar, and a phosphate group.
2. Structure of Nucleic acids-
a) Nitrogen-containing heterocyclic base- Purines and pyrimidines are two types of heterocyclic bases. Example- Adenine and guanine are purines. Cytosine, thymine, and uracil are pyrimidines.
b) Sugars- the two types of sugars are RNA and DNA.
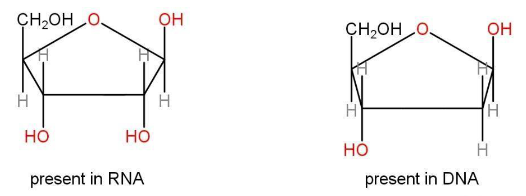
c) phosphate group- nucleotides are joined by these linkages.
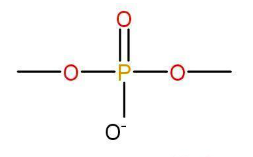
d)Nucleoside- When a nitrogen base is attached to a sugar molecule a nucleoside unit is produced.

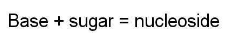
e) Nucleotide-
Base+Sugar+phosphate →nucleotide
-
The biological function of nucleic acids-
Some of the biological functions of nucleic acids are-
Replication- It is the property of a biomolecule to synthesize another molecule.
For example, DNA has a unique property to replicate itself.
Protein synthesis- genetic information stored in DNA in a specific base sequence is expressed in the form of a specific base sequence.
E) Hormones
Hormones are molecules that act as intercellular messengers. These are produced by endocrine glands in the body and are poured directly in the blood stream, which transports them to the site of action.
In terms of chemical nature, some of these are steroids, e.g., estrogens and androgens; some are poly peptides for example insulin and endorphins; and some others are amino acid derivatives such as epinephrine and norepinephrine. Hormones have several functions in the body. They help to maintain the balance of biological activities in the body.
Biomolecules Previous Years' Questions With Answers
Selected Previous Years Questions with Answers are given below that help students revise important exam topics and understand the type of questions asked. The concepts used to solve these questions are explained in ncert class 12 chemistry chapter 10 biomolecules notes.
Question 1:Given below are two statements :
Statement (I) : On hydrolysis, oligo peptides give rise to fewer number of $\alpha$-amino acids while proteins give rise to a large number of $\beta$-amino acids.
Statement (II) : Natural proteins are denatured by acids which convert the water soluble form of fibrous proteins to their water insoluble form.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Both statement I and statement II are correct
(2) Statement I is incorrect but Statement II is correct
(3) Both statement I and statement II are incorrect
(4) Statement I is correct but Statement II is incorrect
Answer:
Protein give rise to alpha amino acid, so the above statement is incorrect.
Natural proteins are denatured by acid. Due to this, globules unfold and helices get uncoiled.
Hence, the correct answer is option (3).
Question 2: Fat-soluble vitamins are :
A. Vitamin $\mathrm{B}_{1}$
B. Vitamin C
C. Vitamin E
D. Vitamin $\mathrm{B}_{12}$
E. Vitamin K
Choose the correct answer from the options given below :
(1) C & D Only
(2) A & B Only
(3) B & C Only
(4) C & E Only
Answer:
Fat soluble vitamins are : A, D, E and K.
In the given question we are given:
A. Vitamin $\mathrm{B}_{1}$
C. Vitamin E
D. Vitamin $\mathrm{B}_{12}$
E. Vitamin K
So, out of these, only vitamin E and Vitamin K are fat soluble
Hence, the correct answer is option (4)
Question 3. Explain what is meant by:
(i) Peptide linkage
(ii) Glycosidic linkage
Answer:
(i) Peptide linkage: In this type of linkage, an amide bond is formed between the COOH group of one amino acid and the $\mathrm{NH}_2$ group of another, releasing a molecule of water.
(ii) Glycosidic linkage: By the elimination of water, an ether (–O–) bond is formed between two monosaccharide units.
Question 4. Name two water‑soluble vitamins, their sources, and deficiency diseases.
Answer:
Vitamin B2: Found in milk, yeast, and leafy vegetables. Deficiency causes glossitis, dermatitis.
Vitamin C: Found in citrus fruits and green veggies. Deficiency leads to scurvy.
Question 5. Name the four bases in DNA. Which one is not present in RNA?
Answer:
Four bases of DNA: Adenine, Guanine, Cytosine, Thymine.
Thymine is not present in RNA; it is replaced by Uracil.
How to Master Class 12 Chemistry Chapter 10 Biomolecules
These ncert class 12 chemistry chapter 10 biomolecules notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 10.
- In order to master this chapter, first understand the basics of carbohydrates, proteins, vitamins, nucleic acids, and lipids.
- Make notes on structures, classifications, and functions of different biomolecules.
- Then students need to learn about reactions and tests used to identify biomolecules, such as Biuret test, Molisch test, and iodine test.
- The questions related to structure–function relationship, especially for proteins and nucleic acids, are often asked in exams. To understand these concepts better they can refer to biomolecules ncert notes.
- After that students can solve previous year questions from this chapter.
Advantages of Using Class 12 Chemistry Chapter 10 Notes
These biomolecules Class 12 Chemistry Chapter 10 CBSE notes cover all topics from the NCERT book in a very simple way. The advantages of using these notes are given below:
- Students can refer to these notes to get clear explanations of carbohydrates, proteins, vitamins, and nucleic acids.
- Using these notes students can understand the structure, function, and classification of various biomolecules.
- These biomolecules class 12 ncert notes include diagrams and tables.
- These notes are prepared by subject experts in a very clear and comprehensive manner that helps students for board and competitive exams.
NCERT Class 12 Notes Chapter-Wise
Along with the class 12 chemistry chapter 10 biomolecules notes , students can also refer to the NCERT Class 12 notes of other chapters through the links given below.
NCERT Exemplar Solutions Subject-Wise
NCERT Class 12 exemplar solutions for each subject are given below:
NCERT Solutions for Class 12 Chemistry
Along with NCERT notes Class 12 Chemistry Chapter 10 Biomolecules, students can also refer to solutions of NCERT for Class 12 Chemistry chapters.
NCERT Solutions Subject-Wise
NCERT Solutions Class 12 for each subject is given below:
| NCERT Solutions for Class 12 Mathematics |
| NCERT Solutions for Class 12 Chemistry |
| NCERT Solutions for Class 12 Physics |
| NCERT Solutions for Class 12 Biology |
Frequently Asked Questions (FAQs)
Enzymes are biological catalysts that speed up chemical reactions in the body without being consumed in the process. They usually have a specific shape that fits their substrates, and they lower the activation energy required for reactions. Factors like temperature and pH can influence their activity.
Nucleic acids, primarily DNA and RNA, are vital for the storage and transmission of genetic information. DNA holds the genetic blueprint for all living organisms, while RNA is involved in protein synthesis. The sequence of nucleotides in these molecules encodes the information necessary for life processes.
Lipids are a diverse group of hydrophobic molecules, including fats, oils, waxes, and steroids. They are significant for energy storage, cell membrane structure, and signaling molecules within the body. Lipids help in insulating and protecting organs and are crucial for hormone production.
Saturated fatty acids contain no double bonds between carbon atoms, which means they are saturated with hydrogen atoms. They typically are solid at room temperature, such as butter. Unsaturated fatty acids have one or more double bonds, causing kinks in their structure, making them liquid at room temperature, like olive oil.
Proteins serve as enzymes, hormones, structural components, and antibodies. They are made up of amino acids linked by peptide bonds and can take on complex three-dimensional shapes that determine their function.
Carbohydrates are classified into three main categories based on the number of sugar units they contain:
- Monosaccharides: These are the simplest sugars, consisting of a single sugar unit. Examples include glucose, fructose, and galactose.
- Disaccharides: These are composed of two monosaccharides joined together by a glycosidic bond. Examples include sucrose, lactose, and maltose.
- Polysaccharides: These are complex carbohydrates made up of many monosaccharide units linked together. Examples include starch, glycogen, and cellulose.
NCERT notes Class 12 Chemistry Chapter 10 Biomolecules are concise study materials that summarise the chapter on carbohydrates, proteins, lipids, nucleic acids, and vitamins. They cover structures, classifications, properties, reactions, and biological functions, along with important tests and mechanisms, making it easier for students to revise and prepare effectively for exams.
Student should go through Class 12 Chemistry chapter 10 notes containing all the headings and subheadings with their brief explanation and solve questions from each topic.
Proteins are made up of amino acids. Amino acids are organic molecules that contain an amino group, a carboxyl group , and a side chain, all attached to a central carbon atom. 20 common amino acids are used to build proteins.
The four major types of biomolecules are:
- Carbohydrates
- Proteins
- Nucleic acids
- Lipids
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
