Matching-type questions in Class 12 Chemistry Biomolecules usually involve pairing biomolecules like carbohydrates, proteins, enzymes, vitamins, and nucleic acids with their functions, structures, or examples to test conceptual understanding.
NCERT Exemplar Class 12 Chemistry Solutions chapter 14 Biomolecules
The distance between the sun and the earth is nearly 152.06 million kilometers, and this is how long your DNA is. The DNA in a single human cell is around 2 meters long, and if you combined the DNA from all the cells in your body, it could stretch to the sun and back multiple times! DNA is a biomolecule, and biomolecules are the essential organic compounds that form the basis of life. They include carbohydrates, proteins, lipids, vitamins, etc. This chapter of NCERT will teach you about the structure, classification, properties, and functions of biomolecules in real life.
The board provides 15 minutes of reading time for students from 10:15 am to 10:30 am for students to review the question paper.
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: Long Answer Type
- Class 12 Chemistry NCERT Chapter 14: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Exemplar Chapter 14
- Topics And Subtopics of NCERT Exemplar Class 12 Chemistry 14
- Advantages of Using Biomolecules Class 12 Chemistry NCERT Exemplar Solutions Chapter 14
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions subject-wise
- NCERT Exemplar Class 12 Solutions subject-wise
- NCERT Class 12 subject-wise notes
- NCERT Books and NCERT Syllabus

Seeing the need and importance of the chapter, our subject matter experts have designed the exemplar solutions. The NCERT Exemplar Class 12 Chemistry Solutions are designed in such a way that students get detailed explanations, conceptual clarity, and the confidence to attempt the problems. These NCERT exemplar solutions are beneficial for boards as well as competitive exams. In this article, the High order thinking skills (HOTs) are also added to deepen your understanding of the concepts. Students can also access the NCERT Solutions for better understanding.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: MCQ (Type 1)
At first, MCQ-type questions from NCERT Exemplar Class 12 Chemistry Chapter 14 Biomolecules are covered to improve your conceptual thinking. These questions also help in quick revision and enhance your problem-solving ability.
Question 1. Glycogen is a branched chain polymer of α-D-glucose units in which chain is formed by C1−C4 glycosidic linkage whereas branching occurs by the formation of C1−C6 glycosidic linkage. The structure of glycogen is similar to __________.
(i) Amylose
(ii) Amylopectin
(iii) Cellulose
(iv) Glucose
Answer:
The answer is the option (ii).
Structure of glycogen is very similar to the structure of amylopectin due to the C1−C4 glycosidic linkage in chain and C1−C6 glycosidic linkage present in branching. Thus, Option (ii) is the answer.
Question 2. Which of the following polymer is stored in the liver of animals?
(i) Amylose
(ii) Cellulose
(iii) Amylopectin
(iv) Glycogen
Answer:
The answer is the option (iv).
The liver of animals takes up excess glucose and converts it into glycogen for storage. So, option (iv) is the answer.
Question 3. Sucrose (cane sugar) is a disaccharide. One molecule of sucrose on hydrolysis gives _________.
(i) 2 molecules of glucose
(ii) 2 molecules of glucose + 1 molecule of fructose
(iii) 1 molecule of glucose + 1 molecule of fructose
(iv) 2 molecules of fructose
Answer:
The answer is the option (iii).
Each molecule of sucrose gives option (iii) 1 molecule of glucose + 1 molecule of fructose when hydrolysed.
Question 4. Which of the following pairs represents anomers?
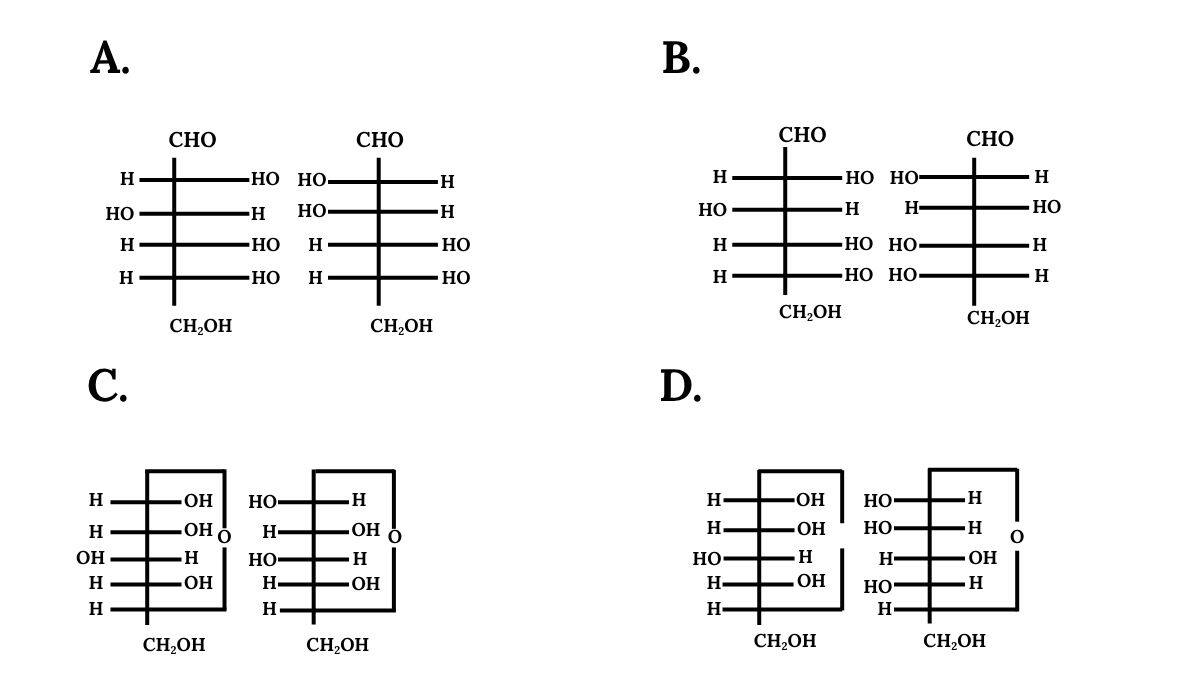
Answer:
The correct answer is Option (C) because it shows the characteristics of anomers that is it differs only in the configuration of the hydroxyl group at C-1 position.
Question 5. Proteins are found to have two different types of secondary structures viz. α-helix and β-pleated sheet structure. α-helix structure of the protein is stabilised by:
(i) Peptide bonds
(ii) van der Waals forces
(iii) Hydrogen bonds
(iv) Dipole-dipole interactions
Answer:
The answer is the option (iii).
As these proteins are twisted in the right-hand screw fashion so each -NH group is attached to an amino acid through option (iii) Hydrogen bonds
The answer is option (B).
In Option B, both anomeric carbons are linked through a 1,1-glycosidic bond that forms a non-reducing sugar.
In the other options (A, C, D), at least one anomeric carbon is free that makes them reducing sugars.
Question 7. Which of the following acids is a vitamin?
(i) Aspartic acid
(ii) Ascorbic acid
(iii) Adipic acid
(iv) Saccharic acid
Answer:
The answer is option (ii).
Ascorbic acid is the right answer as it is also known as Vitamin C.
Question 8. Dinucleotide is obtained by joining two nucleotides together by a phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are. Are these linkages present?
(i) 5′ and 3′
(ii) 1′ and 5′
(iii) 5′ and 5′
(iv) 3′ and 3′
Answer:
The answer is option (i).
As the linkage is between phosphoric acid with a nucleotide at the 5’ position, Option (i) 5′ and 3′ is the right answer.
Question 9. Nucleic acids are the polymers of ______________.
(i) Nucleosides
(ii) Nucleotides
(iii) Bases
(iv) Sugars
Answer:
The answer is the option (ii).
As nucleic acid is composed of units containing a sugar, a base and a phosphate molecule. It is a polymer of option (ii) Nucleotides
Question 10. Which of the following statements is not true about glucose?
(i) It is an aldohexose.
(ii) On heating with HI, it forms n-hexane.
(iii) It is present in furanose form.
(iv) It does not give 2,4-DNP test.
Answer:
The answer is the option (iii). It is present in furanose form is the right answer as all other properties are exhibited by a glucose molecule.
(i) primary structure of proteins.
(ii) secondary structure of proteins.
(iii) tertiary structure of proteins.
(iv) quaternary structure of proteins.
Answer:
The answer is option (i).
This type of structure in which each polypeptide is a protein has amino acids linked with each other in a specific sequence is termed as Option (i) primary structure of proteins.
Question 12. DNA and RNA contain four bases each. Which of the following bases is not present in RNA?
(i) Adenine
(ii) Uracil
(iii) Thymine
(iv) Cytosine
Answer:
The answer is the option (iii).
RNA is less stable genetic material due to the presence of uracil whose structure is less stable than thymine present in DNA. So, Option (iii) Thymine is the right choice.
Question 13. Which of the following B group vitamins can be stored in our body?
(i) Vitamin B1
(ii) Vitamin B2
(iii) Vitamin B6
(iv) Vitamin B12
Answer:
The correct option is (iv).
Although vitamin B12 is water-soluble, the body is capable of storing it mainly in the liver.
Question 14. Which of the following bases is not present in DNA?
(i) Adenine
(ii) Thymine
(iii) Cytosine
(iv) Uracil
Answer:
The answer is option (iv).
Due to DNA being more stable genetic material, it contains cytosine instead of uracil due to the higher stability of the molecule. So, Option (iv) Uracil is the answer.
Question 15. Three cyclic structures of monosaccharides are given below which of these are anomers.
(i) I and II
(ii) II and III
(iii) I and III
(iv) III is anomer of I and II
Answer:
The answer is the option (i). Anomers are the compounds are the cyclic structures of monosaccharides which differ at the carbon-1 in their structure. So, option (i) I and II is the right answer.
Question 16. Which of the following reactions of glucose can be explained only by its cyclic structure?
(i) Glucose forms pentaacetate.
(ii) Glucose reacts with hydroxylamine to form an oxime.
(iii) Pentaacetate of glucose does not react with hydroxylamine.
(iv) Glucose is oxidised by nitric acid to gluconic acid
Answer:
The answer is the option (iii). The property of glucose being non-reactive with hydroxylamine determines the absence of the -CHO group. This property is defined only by the cyclic structure of glucose. Therefore, option (iii) Pentaacetate of glucose does not react with hydroxylamine is the correct answer.
Question 17. Optical rotations of some compounds along with their structures are given below which of them have D configuration.
(i) I, II, III
(ii) II, III
(iii) I, II
(iv) III
Answer:
The answer is the option (i).
All of the given compounds have D-configuration due the presence of -OH group of asymmetric carbon on the right side. So, Option (i) I, II, III is the answer.
Question 18. Structure of a disaccharide formed by glucose and fructose is given below. Identify anomeric carbon atoms in monosaccharide units.
(i) ‘a’ carbon of glucose and ‘a’ carbon of fructose.
(ii) ‘a’ carbon of glucose and ‘e’ carbon of fructose.
(iii) ‘a’ carbon of glucose and ‘b’ carbon of fructose.
(iv) ‘f’ carbon of glucose and ‘f ’ carbon of fructose.
Answer:
The answer is the option (iii).
The carbon present in the glucose or fructose molecule right adjacent to the oxygen atom is known as anomeric carbon. This concludes that option (iii) is the answer.
Question 19 Three structures are given below in which two glucose units are linked. Which of these linkages between glucose, units are between C1 andC4 and which linkages are between C1 and C6?
(i) (A) is between C1 andC4 , (B) and (C) is between C1 and C6
(ii) (A) and (B) are between C1 andC4 , (C) is between C1 and C6
(iii) (A) and (C) is between C1 andC4 , (B) is between C1 and C6
(iv) (A) and (C) is between C1 and C6, (B) is between C1 andC4
Answer:
The correct option is option (iii)
This is because the numbering, in case of glucose, starts from the -O atom and ends at the last CH2OH. This means the (iii) (A) and (C) is between C1 andC4, (B) is between C1 and C6 is the right answer.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: MCQ (Type 2)
Class 12 Chemistry NCERT Exemplar Solutions Chapter 14 provides detailed explanations for all types of questions to strengthen student understanding. These solutions are highly useful for board exam preparation as well as competitive exams like NEET and JEE.
Question 20. Carbohydrates are classified on the basis of their behaviour on hydrolysis and also as reducing or non-reducing sugar. Sucrose is a __________.
(i) monosaccharide
(ii) disaccharide
(iii) reducing sugar
(iv) non-reducing sugar
Answer:
The answer is the option (ii) and (iv).
As sucrose gives an equimolar solution of glucose and fructose on hydrolysis and the of the sugar being non-reducing sugar due to its involvement in glycosidic bond formation it is a disaccharide. Making Option (ii) and (iv) as the correct answers.
Question 21. Proteins can be classified into two types on the basis of their molecular shape i.e., fibrous proteins and globular proteins. Examples of globular proteins are :
(i) Insulin
(ii) Keratin
(iii) Albumin
(iv) Myosin
Answer:
The answer is the option (i) and (iii).
Because of the ability to dissolve in water and having their polypeptide chain coiled around the spherical shape, Option (i) Insulin and (iii) Albumin are the correct answers.
Question 22. Which of the following carbohydrates are branched polymer of glucose?
(i) Amylose
(ii) Amylopectin
(iii) Cellulose
(iv) Glycogen
Answer:
Option (ii) and (iv) are the correct answers that is Amylopectin and Glycogen because it is a form of carbohydrate storing in animal bodies whereas amylose is constituted of 80-85% starch. It is also a branched polymer of glucose in which branching takes place by C1−C6 glycosidic linkage.
Question 23 Amino acids are classified as acidic, basic or neutral depending upon the relative number of amino and carboxyl groups in their molecule. Which of the following are acidic?
Answer:
The answer is the option (ii) and (iv). In case of amino acids, the number of amino and carboxyl groups in their structure determines their acidic nature, more carboxyl groups increase their acidic nature. From the compounds given above, only (ii) and (iv) have only one amino group and two carboxyl groups. Therefore option (ii) and (iv) are the answers.
Question 24
Lysine
(i) α-Amino acid
(ii) Basic amino acid
(iii) Amino acid synthesised in the body
(iv) β−Amino acid
Answer:
The answer is the option (i), (ii), and (iii).
As the structure of Lysine contains an amino group in its side chain, the compound is basic in nature. And since the amino acids present in the body have the ability to accept H+ more easily than water. This categorises Lysine as an Amino acid synthesised in the body. The correct options are option (i), (ii) and (iii) are the answers.
Question 25. Which of the following monosaccharides are present as five-membered cyclic structure (furanose structure)?
(i) Ribose
(ii) Glucose
(iii) Fructose
(iv) Galactose
Answer:
The answer is the option (i) and (iii).
Due to the presence of polyhydroxyl carbonyl compound of 5 carbon atoms, ribose and fructose show the furanose structure containing five-membered cyclic structure. Thus, Option (i) and (iii) are the answers.
Question 26. In fibrous proteins, polypeptide chains are held together by ___________.
(i) van der Waals forces
(ii) disulphide linkage
(iii) electrostatic forces of attraction
(iv) hydrogen bonds
Answer:
The answer is the option (ii) and (iv).
To obtain the characteristic fibre-like structure, the fibrous proteins are held together by) hydrogen bonds and disulphide linkage making Option (ii) and (iv) the right answers.
Question 27 Which of the following are purine bases?
(i) Guanine
(ii) Adenine
(iii) Thymine
(iv) Uracil
Answer:
The answer is the option (i) and (ii).
The compounds under purines contain a six membered and a five membered ring with nitrogen infused together to make the chemical structure. Thus, the right options are (i) Guanine and (ii) Adenine
Question 28 Which of the following terms are correct about enzyme?
(i) Proteins
(ii) Dinucleotides
(iii) Nucleic acids
(iv) Biocatalysts
Answer:
The answer is the option (i) and (iv).
Some of the main characteristics of enzymes are that they are the biocatalyst and comes under the protein category. Their role in biochemical reactions is extremely specific. So, the correct option is (i) Proteins and (iv) Biocatalysts.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: Short Answer Type
These Chapter 14 Biomolecules short answer type questions help students practise precise explanations and improve their conceptual clarity. They also prepare students to write to-the-point answers in exams, saving time and boosting accuracy.
Answer:
Milk contains lactose, an oligosaccharide sugar. It consists of two subunits of monosaccharide, namely glucose and galactose. Such oligosaccharides sugars composed of two monosaccharide units are known as disaccharides.
Question 30. How do you explain the presence of all the six carbon atoms in glucose in a straight chain?
Answer:
Glucose sugar contains six carbon atom chain, and this occurrence is due to the prolonged heating in the presence of hydrogen iodide (HI). This results in an n-hexane, thus forming a straight chain of six carbon atoms.
Answer:
The characteristic formation feature of nucleoside it that it requires five-carbon sugar with a nitrogenous base attached to its 1’ position. And in this case, the phosphoric acid present in the five-carbon sugar is linked to the 5’ position of the nucleoside molecules converting it into a nucleotide molecule.
Question 32. Name the linkage connecting monosaccharide units in polysaccharides.
Answer:
The linkage present between monosaccharides is a glycosidic linkage which constitutes a polysaccharide. In this linkage, an oxide replaces a water molecule and forms a bond between two monosaccharide units.
Question 33 Under what conditions glucose is converted to gluconic and saccharic acid?
Answer:
Glucose being a six-carbon sugar easily gets converted into gluconic acid when treated with mild oxidising agents such as Br2 water. To convert glucose into saccharic acid (dicarboxylic acid), it is treated with nitric acid.
Answer:
To categorise fructose, knowing its molecular formula and the functional group is important as they are categorised on the basis of the number of carbon atoms present in the molecule. The formula being C6H12O6 and as it has ketone group present, so it is placed in the class of ketohexoses
To identify whether the compound configuration is ‘D’ or ‘L’ we check the presence of the -OH group. As the -OH group is present on the left side of the fifth carbon atom, the compound configuration is ‘L’.
Question 36. Aldopentoses named as ribose and 2-deoxyribose are found in nucleic acids. What is their relative configuration?
Answer:
The compound being aldopentoses, the configuration of both of them is ‘D’. The naming of ribose would be β-D-ribose whereas IUPAC name for 2-deoxyribose is β-D-2-deoxyribose
Question 37. Which sugar is called invert sugar? Why is it called so?
Answer:
The common example of invert sugar is sucrose. It is found naturally in sugarcane and sugar beet. The reason for being named invert sugar is that due to hydrolysis of sucrose, there is a change in the sign of rotation from Dextro (+) to laevo (–)
Answer:
To form a polypeptide chain, the amino acid should be linked to the α-carbon in the molecule. The types of amino acids forming a polypeptide are α-amino acids and alpha-amino acids
Answer:
When a polypeptide chain is twisted in a right-handed screw fashion to make a stable α-Helix chain, the interaction taking place includes the bonding of each amino acid residues’ -NH group with the −C=O in the adjacent turn of the helix.
Answer:
Oxidoreductases are the enzymes named after their role in of oxidative reduction in redox reactions. They act as a catalyst, one of the examples of these enzymes is Alcohol Dehydrogenase that plays the role of reducing the human body’s’ alcohol levels during ingestion of alcohol
Question 41. During curdling of milk, what happens to sugar present in it?
Answer:
The process of curdling is initiated due to the presence of bacteria that converts the milk into curd and the sugar present in milk, lactose, gets converted into lactic acid.
Question 42. How do you explain the presence of five —OH groups in the glucose molecule?
Answer:
To prove the presence of 5 -OH groups present in glucose molecule, it is treated with acetic anhydride (CH3CO)2O in the presence of ZnCl2. This results in acetylation and forms glucose pentaacetate thus confirming the presence of 5 -OH groups
Question 43. Why does compound (A) give below not form an oxime?
Answer:
The given compound is glucose pentaacetate. Due to the presence of a free −C=O (carbonyl) group in the glucose molecule, it is able to form oxime. But after being converted into glucose pentaacetate, the no longer has the free carbonyl group is it is not able to make an oxime when treated with hydroxylamine.
Question 44. Why must vitamin C be supplied regularly in diet?
Answer:
There are two categories of vitamins, water-soluble and non-soluble. The water-soluble vitamins can’t be stored in the body, so we need a regular uptake these vitamins. Vitamin C is a water-soluble vitamin, so it is essential to include it in the daily diet plan.
Question 45. Sucrose is dextrorotatory but the mixture obtained after hydrolysis is laevorotatory. Explain.
Answer:
The pure nature of sucrose in aqueous solution is dextrorotatory, which means it rotates plane-polarised light entering the solution 66.5∘ to the right. But when it is hydrolysed, it gets converted into dextrorotatory D-(+)-glucose and laevorotatory D-(-)-fructose in equimolar concentration. Due to this, the sign of rotation shifts from Dextro (+) to laevo (–). Thus, this makes the overall hydrolysed solution laevorotatory.
Question 46 Amino acids behave like salts rather than simple amines or carboxylic acids. Explain.
Answer:
The basic structure of an amino acid consists of a −NH2 group and a −COOH group. When amino acids are in an aqueous solution, the −NH2 gains a proton whereas the −COOH group loses a proton [H]+ resulting in a zwitterion which is a salt. Thus, amino acids exhibit the properties of salt in aqueous solution.
Question 47. Structures of glycine and alanine are given below. Show the peptide linkage in glycylalanine.
Answer:
To form glycylalanine. Peptide linkage between the hydroxyl group of glycine is attached to the amine group of alanine takes place.
Answer:
When the protein is in its native form, it is intact with its secondary and tertiary structures along with primary structure. These amino acid residues are connected via hydrogen bonds and the intermolecular forces. But when it is subjected to changes like temperature and pH, these structures get destroyed except the primary structure due to the disturbance in the hydrogen bonds. This process unfolds the native protein and is called denaturation of proteins
Answer:
The energy required in a particular reaction mainly depends on the types of bond that needs to be broken; thus, it dictates the energy required. If the bonds are strong, the reaction requires a large sum of energy and time. These factors can be changed by the addition of a biocatalyst; enzymes are the most common type of biocatalysts. The catalyst reduces the activation energy of the reaction, thus the hydrolysis of the enzyme sucrose very less time compared to the traditional acidic hydrolysis.
Question 50 How do you explain the presence of an aldehydic group in a glucose molecule?
Answer:
To confirm the presence of an aldehyde group is glucose molecule, it is treated with a mild oxidising agent such as bromine water which forms carboxylic acid gluconic acid. This confirms the presence of the aldehyde group
Answer:
A nucleotide is a polymer of multiple subunits of nucleosides held together by linkage of phosphoric acid at 5′-position of pentose sugar. These nucleotides are further joined at the 5′ and 3′ carbon atoms of the sugar moiety, this linkage is known as phosphodiester linkage, and it results in dinucleotide. The key involvement in this linkage is of phosphoric acid.
Question 52 What are glycosidic linkages? In which type of biomolecules are they present?
Answer:
Glycosidic linkage is very common in saccharides; it occurs when the loss of water molecule happens due to an oxide linkage formed between two molecules of monosaccharides. This type of joining of two monosaccharides via an oxygen atom is termed as glycosidic linkage. The linkage is mostly observed in trisaccharides, polysaccharides, disaccharides, etc.
Answer:
Starch contains a-glucose, while cellulose has β-D glucose units. In the linking of starch and glucose, glycosidic a-linkage is seen, and in case of cellulose, the monosaccharide glucose units are joined by glycosidic β-linkage
Question 54 How do enzymes help a substrate to be attacked by the reagent effectively?
Answer:
Functioning of enzymes in the human body, the interaction between substrate and reagent is crucial to start the process. This is done by the active site of enzyme which holds the substrate for effective interaction with the reagent.
Question 55 Describe the term D- and L-configuration used for amino acids with examples.
Answer:
There are only two types of sugar present in the amino acid family: definitively configured D-Family and L-Family. The key difference in the configuration of these families is decided by the glyceraldehyde. The presence of glyceraldehyde is taken as the standard, and it can be found in two forms:
L-(-)-Glyceraldehyde configuration and the D-(+)-Glyceraldehyde. The positioning of the -OH group of the carbon adjacent to CH2OH categorises the configuration. If the group is attached to the right side, then it would be of D-configuration but the positioning of -OH group on the left makes it of L-configuration. The natural sugars present are all of D-configuration.
Question 56 How will you distinguish 1∘ and 2∘ hydroxyl groups present in glucose? Explain with reactions.
Answer:
To distinguish between the1∘ and 2∘ hydroxyl groups of glucose via the chemical method, presence of 5-OH groups can indicate. The reaction involves adding a few drops of a cone. H2SO4 in the presence of pyridine, this results in the formation of forms Penta-acetyl derivative. This also indicates that from 5-OH groups, one group is primary (1°) alcoholic while others are secondary (2°) alcoholic groups.
The other way to determine their presence is by oxidising gluconic acid (Glucose) with HNO3, this forms a dicarboxylic acid named saccharic acid. The result indicates that only one of the -OH group oxidised and formed -COOH group and the rest of the secondary -OH groups are unable to oxidise due to absence of drastic conditions.
Answer:
An egg contains a soluble globular protein called albumin, which, when heated, transforms into insoluble fibrous protein. This denaturation results in the loss of biological activity, as well as the secondary and the tertiary structures of proteins, gets destroyed. But the primary structure of α-amino acids does not get denatured.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: Matching Type
Biomolecules Class 12 Chemistry NCERT Exemplar Solutions Chapter 14 matching type questions are designed to test your understanding of the concepts . They enhance analytical skills and strengthen understanding for exams.
Question 58. Match the vitamins given in Column I with the deficiency disease they cause given in Column II.
|
COLUMN I (Vitamins) |
COLUMN II (Diseases) |
|
i. Vitamin A |
a. Pericious anameia |
|
ii. Vitamin B1 |
b.Increased blood clotting time |
|
iii. Vitamin B12 |
c.Xerophthalamia |
|
iv. Vitamin C |
d. Rickets |
|
v. Vitamin D |
e. Muscular weakness |
|
vi. Vitamin E |
f. Night blindness |
|
vii. Vitamin K |
g. Beri beri |
|
|
h. Bleeding gums |
|
|
i. Ostemomalacia |
Answer:
The above-mentioned columns describe the types of vitamins and the resulting diseases caused by their deficiency. The correct match of the options is-
(i→c,f),(ii→g),(iii→a),(iv→h),(v→d,i),(vi→e),(vii→b)
Question 59. Match the following enzyme given in Column I with the reactions they catalyse given in Column II.
|
Column I |
Column II |
|
i. Invertase |
a. Decomposition of urea into NH3 and CO2 |
|
ii. Maltase |
b. Conversion of glucose into ethyl alcohol |
|
iii. Pepsin |
c.Hydrolysis of maltase into glucose |
|
iv. Urease |
d. Hydrolysis of cane sugar |
|
v. Zymase |
e. Hydrolysis of proteins of peptides |
Answer:
All the given enzymes have their specific roles in biochemical cycles in the human body. The correct roles of these enzymes are-
(i→d),(ii→c),(iii→e),(iv→a),(v→b)
Invertase catalyzes the hydrolysis of cane sugar into glucose and fructose. Maltase breaks down maltose into two glucose molecules. Pepsin is a digestive enzyme that hydrolyzes proteins into peptides. Urease facilitates the breakdown of urea into ammonia and carbon dioxide. Zymase is involved in the fermentation of glucose to produce ethyl alcohol.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: Assertion and Reason Type
Assertion and Reason type questions of NCERT Class 12 chemistry Biomolecules help students enhance knowledge and improve critical thinking. These are so important for exams and it will clear most of your doubts.
Question 60. In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:(i) Assertion and reason both are correct statements and reason explains the
assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not
explain assertion.
Assertion (A): D(+) – Glucose is dextrorotatory in nature.
Reason (R): ‘D’ represents its dextrorotatory nature.
Answer:
The answer is the option (iii)
The correct reason is that the nature of glucose is dextrorotatory due to the presence of -OH group og asymmetric carbon attached towards the right side.
Question 61. In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
(i) Assertion and reason both are correct statements and reason explains the
assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not
explain assertion.
Assertion (A): Vitamin D can be stored in our body.
Reason (R): Vitamin D is fat soluble vitamin.
Answer:
The answer is the option (i).
Vitamin D being fat soluble is the key reason for its storage in the body.
Question 62 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
(i) Assertion and reason both are correct statements and reason explains the
assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not
explain assertion.
Assertion (A):β-glycosidic linkage is present in maltose.
Reason (R): Maltose is composed of two glucose units in which C-1 of one glucose unit is linked to C-4 of another glucose unit.
Answer:
The answer is the option (iv) The linkage present in maltose is α-glycosidic in place of β-glycosidic linkage
Maltose
Question 63. In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
(i) Assertion and reason both are correct statements and reason explains the
assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not
explain assertion.
Assertion (A): All naturally occurring α-amino acids except glycine are optically active.
Reason (R): Most naturally occurring amino acids have L-configuration.
Answer:
The answer is option (v).
This exception is due to the presence of at least one chiral carbon in all α-amino acids except glycine
Question 64. In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:(i) Assertion and reason both are correct statements and reason explains the
assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not
explain assertion.
Assertion (A): Deoxyribose, C5H10O4 is not a carbohydrate.
Reason (R): Carbohydrates are hydrates of carbon so compounds which follow Cx(H2O)y formula are carbohydrates.
Answer:
The answer is the option (ii).
The assertion is wrong as Deoxyribose is categorised under carbohydrates and is present as the pentose sugar in DNA. The reasoning is incorrect as carbohydrates are the substances that results into polyhydroxy aldehyde or polyhydroxy ketone and they are also optically active compounds.
Question 65. In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:(i) Assertion and reason both are correct statements and reason explains the
assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not
explain assertion.
Assertion (A): Glycine must be taken through diet.
Reason (R): It is an essential amino acid.
Answer:
The answer is the option (ii) Glycine being naturally synthesised in the body indicates assertion is incorrect as well as due to this property it is a non-essential amino acid.
Question 66. In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:(i) Assertion and reason both are correct statements and reason explains the
assertion.
(ii) Both assertion and reason are wrong statements.
(iii) Assertion is correct statement and reason is wrong statement.
(iv) Assertion is wrong statement and reason is correct statement.
(v) Assertion and reason both are correct statements but reason does not
explain assertion.
Assertion (A): In presence of enzyme, substrate molecule can be attacked by the reagent effectively.
Reason (R): Active sites of enzymes hold the substrate molecule in a suitable position.
Answer:
The answer is the option (i) The presence of enzyme makes the process much effective as it hold onto the substrate while it is attacked by the reagent.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 14: Long Answer Type
The long answer type questions of Chemistry Class 12 NCERT Exemplar Solutions Chapter 14 enhances students ability to present concepts systematically. They also help in strengthening theoretical understanding for board examinations.
Answer:
Some of the short comings of the open chain structure of glucose are mentioned below, these are the facts unexplained by the open chain structure –
(i)Even after the presence of (-CHO) an aldehydic group, it is unable to react some of the characteristic reactions of aldehydes such as,
(a) Non-reactive with ammonia.
(b) No addition product formation when added in sodium bisulphite (NaHSO3).
(c) The group indicator tests like Schiff’s test and 2, 4-DNP test are not valid
(ii) Due to the absence of -CHO groups in glucose pentaacetate, it does not react with hydroxylamine (−NH2OH) but the reaction with hydroxylamine is feasible for glucose.
(iii) The isomerism shown by D (+) – Glucose is, α-D-glucose and β-D-glucose. Both forms are crystalline and differ in melting points and optical rotations.
(v) Its reaction with methanol in the presence of dry hydrogen chloride gas, results into two isomers namely methyl α -D-glucoside (m.p. = 438 K or 1650C) and methyl β-D-glucoside (m.p. = 380 K or 1070C).
All the above points clarify that glucose does not have an open chain structure.
Question 68. On the basis of which evidences D-glucose was assigned the following structure?
Answer:
1. Molecular formula : Molecular formula of glucose is has been found to be C6H12O6
2. Straight chain sructure:
(i) When aqueous solution of glucose is treated with sodium amalgam (Na/Hg) or sodium borohydride, it is reduced to sorbitol (or glucocitol) a hexahydric alcohol.
(ii) Prolonged heating with hydriodic acid and red phosphorous at 1000 C gives a mixture of n-hexane and 2-iodohexane.
The formation of n-hexane suggests that all the six carbon atoms in glucose are arranged in a straight chain structure of glucose.
3. Presence of five hydroxyl (-OH) groups: The formation of pentaacetate after acetylation with acetic anhydride indicated the presence of 5 -OH groups. As glucose is a stable compound the 5-OH groups are supposed to be present on different carbon atoms.
4. Presence of one primary alcoholic group: The oxidation reaction of glucose and gluconic acid with conc. Nitric acid yields the same dicarboxylic acid, saccharic acid or glucaric acid.
5. Presence of an aldehyde (-CHO) group: The reaction of glucose with hydroxylamine is feasible it indicated the presence of carbonyl (CHOH)4 (>C = O) groups.
The above-mentioned observations, the open chain structure of glucose can be described as:
Answer:
The storage molecules of carbohydrates present in both plants and animals are given below –
(i) The major molecules in plants are starch, cellulose, sucrose, etc.
(ii) Presence of glycogen as known as animal starch in liver, brain, muscle of animals
(iii) Wood and clothe fibres contain cellulose.
Answer:
Primary structure: Every protein contains one or more polypeptide chains. These chains are made up of a-amino acids linked in a specific sequence. This specificity of sequence attachment is called primary structure.
The successive hydrolysis in the presence of mineral acids leads to the determination of the primary structure of proteins. The reaction results in products with decreasing molecular mass in each step, as shown below:
Proteins → Proteoses → Peptones → Polypeptides →Simple Peptides → α-Amino acids
Secondary structure: The folding and arrangement of polypeptide chains result in secondary structures. It also provides a specific shape to the protein molecule. There are two types of structures that arise from this folding -
α-Helix structure: this structure is a very common folding way of polypeptide chains, in this, the h-bonds are twisted in a right-handed manner, with additional -NH group bonded with -C = O group at adjacent turns. Thus, the structure is commonly known as 3.613 helix. The pitch of helix contains 3.16 amino acids. Hydrogen bonds are the backbone of this structure present in between one amide group and a carbonyl group.
β-pleated sheet structure: The arrangement of polypeptide chains in in a zigzag manner adjacent to each other and fixed at a common distance. The intermolecular H-bonds hold the chains together, and numerous chains combine to make a sheet. A 3-D arrangement of these sheet on top of each other takes place and due to its resemblance to drapery the structure is named β-pleated sheet structure.

Answer:
During the hydrolyses, the main constituents of DNA include a pentose sugar, nitrogenous bases and phosphoric acid
Attachment of base to I ‘-position of sugar results in formation of a nucleoside. The linkage of two nucleotides through phosphoric acid at 5′-position of sugar makes a nucleotide. Multiple nucleotides joined at 5’-and 3’carbon atoms of the pentose sugar by phosphodiester linkage makes a chain of nucleotides.
The coiling of two chains of nucleotides and held by the H-bond formation between bases makes DNA.
Class 12 Chemistry NCERT Chapter 14: Higher Order Thinking Skills (HOTS) Questions
Higher Order Thinking Skills (HOTS) Questions of NCERT Exemplar Class 12 Chemistry Solutions chapter 14 Biomolecules are designed to enhance problem-solving. These questions are very helpful for competitive exams like JEE and NEET.
Question 1. Given below are two statements :
Statement (I) : On hydrolysis, oligo peptides give rise to fewer number of $\alpha$-amino acids while proteins give rise to a large number of $\beta$-amino acids.
Statement (II) : Natural proteins are denatured by acids which convert the water soluble form of fibrous proteins to their water insoluble form.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Both statement I and statement II are correct
(2) Statement I is incorrect but Statement II is correct
(3) Both statement I and statement II are incorrect
(4) Statement I is correct but Statement II is incorrect
Answer.
Protein give rise to alpha amino acid, so the above statement is incorrect.
Natural proteins are denatured by acid. Due to this, globules unfold and helices get uncoiled.
Hence, the correct answer is option (3).
Question 2. Fat-soluble vitamins are :
A. Vitamin $\mathrm{B}_{1}$
B. Vitamin C
C. Vitamin E
D. Vitamin $\mathrm{B}_{12}$
E. Vitamin K
Choose the correct answer from the options given
below :
(1) C & D Only
(2) A & B Only
(3) B & C Only
(4) C & E Only
Answer.
Fat soluble vitamins are : A, D, E and K.
In the given question we are given:
A. Vitamin $\mathrm{B}_{1}$
C. Vitamin E
D. Vitamin $\mathrm{B}_{12}$
E. Vitamin K
So, out of these, only vitamin E and Vitamin K are fat soluble
Hence, the correct answer is option (4)
Question 3. Given below are two statements :
Statement-I: D-(+)- Glucose and D-(+)- fructose are formed on hydrolysis of sucrose.
Statement II : Invert sugar is formed during sucrose hydrolysis.
In the light of the above statements, choose the correct answer from the options given below -
(1) Both Statement I and Statement II are true.
(2) Statement I is false but Statement II are true.
(3) Statement I is true but Statement II is false.
(4) Both Statement I and Statement II are false.
Answer.
On hydrolysis of sucrose gives $\mathrm{D}-(+)$-glucose and D-(-)-fructose while in St. (1) D-(+)-fructose is given; hence, Statement-(1) is incorrect.
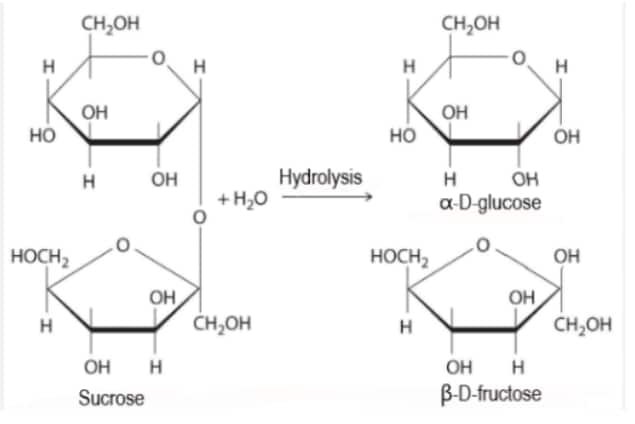
St. II - It is correct because sucrose on hydrolysis gives invert sugar
Hence, the correct answer is option (2).
Question 4: Match List I with List II
| LIST I | LIST II | ||
| A. | Glucose $/ \mathrm{NaHCO}_3 / \Delta$ | I. | Gluconic acid |
| B. | Glucose $/ \mathrm{HNO}_3 $ | II. | No reaction |
| C. | Glucose $/ \mathrm{HI}/ \Delta$ | III. | n-hexane |
| D. | Glucose/Bromine Water | IV. | Saccharic acid |
Choose the correct answer from the options given below:
(1) A-I, B-IV, C-III, D-II
(2) A-IV, B-I, C-III, D-II
(3) A-II, B-IV, C-III, D-I
(4) A-III, B-II, C-I, D-IV
Answer:
Glucose $/ \mathrm{NaHCO}_3 / \Delta$
Glucose has no - COOH group, so it cannot react with $\mathrm{NaHCO}_3$ (only carboxylic acids release $\mathrm{CO}_2$ ).
$\mathrm{A} \rightarrow \mathrm{II}$ (No reaction)
B. Glucose / $\mathrm{HNO}_3$
Hot nitric acid is a strong oxidising agent.
It oxidises both the aldehyde group and the primary alcohol group dicarboxylic acid.
Product $=$ Saccharic acid
B → IV (Saccharic acid)
C. Glucose / HI/ $\Delta$
Hot $\mathrm{HI}+$ red $\mathrm{P} \rightarrow$ complete reduction of glucose (all $\mathrm{C}-\mathrm{O}$ bonds reduced).
This gives n -hexane.
C → III (n-hexane)
D. Glucose / Bromine water
$\mathrm{Br}_2$ water is a mild oxidising agent; it oxidises only the aldehyde group gluconic acid.
D → I (Gluconic acid)
Hence, the correct answer is option (3).
Question 5: Identify the pair of reactants that upon reaction, with elimination of HCl will give rise to the dipeptide Gly-Ala.
(1)
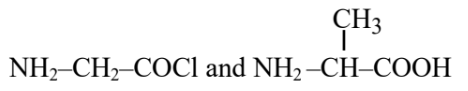
(2)
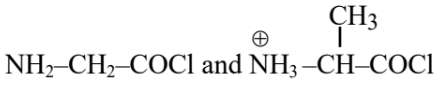
(3)
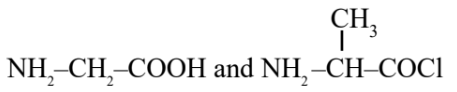
(4)
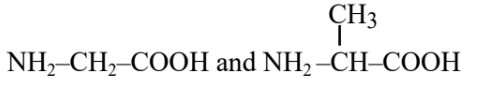
Answer:
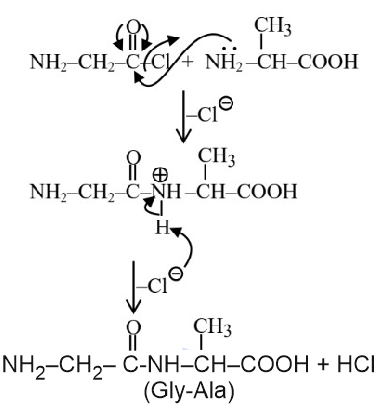
Hence, the correct answer is option (1).
Approach to Solve Questions of Exemplar Chapter 14
To solve the questions from Class 12 NCERT Exemplar Chapter 14 Biomolecules, it is important to first understand the basic concepts thoroughly. Focus on practising different types of questions, revise key formulas, the approaches given below helps you to solve questions from Biomolecules Class 12 Chemistry effectively.
1. Focus on the basics of carbohydrates, proteins, vitamins, enzymes and nucleic acids. Learn their types, functions and examples.
2. Memorize classifications of carbohydrates, that is, monosaccharides, disaccharides, polysaccharides.
The reactions like osazone formation, reducing sugar test and mutarotation are often asked in exams. Practice them regularly.
Also, memorize open chain and cyclic (Haworth) forms of Glucose and Fructose by drawing them regularly. Also, learn the key reactions that proves the type of structure.
3. Know the classification of amino acids, zwitterionic nature, isoelectric point and types of protein structure (primary to quaternary).
Also, study the enzyme action (lock and key model), factors affecting enzyme activity, and the classification of water-soluble and fat-soluble vitamins along with their deficiency diseases. For understanding these concepts better students can also follow NCERT Class 12 Biomolecules notes.
4. Understand the structure of DNA and RNA, components like nucleotides and base-pairing rules.
5. Focus on definitions, structure-based questions and reasoning-type questions like Why glucose is an aldehyde?, etc. Also, practice drawing structures of amino acids, peptides, and nucleotides as these often come in 2-3 mark questions. These NCERT Exemplar Class 12 Chemistry Solutions chapter 14 Biomolecules are very helpful for exams.
Topics And Subtopics of NCERT Exemplar Class 12 Chemistry 14
Following are the important topics from the chapter 14 biomolecules class 12 Chemistry:
- Carbohydrates
- Classification Of Carbohydrates
- Monosaccharides
- Glucose
- Fructose
- Disaccharides
- Polysaccharides
- Importance of Carbohydrates
- Proteins
- Amino Acids
- Classification of Amino Acids
- Structure of Proteins
- Denaturation of Proteins
- Enzymes
- Mechanism of Enzyme Action
- Vitamins
- Classification of Vitamins
- Nucleic Acids
- Chemical Composition of Nucleic Acids
- Structure of Nucleic Acids
- Biological Functions of Nucleic Acids
- Hormones
Advantages of Using Biomolecules Class 12 Chemistry NCERT Exemplar Solutions Chapter 14
These Class 12 Chemistry NCERT Exemplar Solutions Chapter 14 cover all questions from the NCERT book in a very simple way. The advantages of using these solutions are given below:
- Students can refer to these solutions to get clear explanations of carbohydrates, proteins, vitamins, and nucleic acids.
- Using these NCERT Exemplar Class 12 Solutions students can understand the structure, function, and classification of various biomolecules.
- These NCERT Exemplar Class 12 Chemistry Solutions chapter 14 Biomolecules include diagrams and tables.
- These solutions of NCERT are prepared by subject experts in a very clear and comprehensive manner that helps students for board and competitive exams.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions subject-wise
The NCERT subject-wise solutions will help you cover the intext and exercise questions. Follow the links below
NCERT Exemplar Class 12 Solutions subject-wise
Make your preparation better by solving the NCERT exemplar solutions for other subjects as well.
NCERT Class 12 subject-wise notes
You can now do quick revision by following the NCERT notes. Click on the link given in the table
NCERT Books and NCERT Syllabus
Follow the links to the syllabus and books for the respective subjects.
Frequently Asked Questions (FAQs)
Biomolecules are organic molecules that are essential for the functioning of living organisms. They include carbohydrates, proteins, lipids, and nucleic acids. These macromolecules play critical roles in biological processes, such as energy storage, cellular structure, signaling, and genetic information transfer.
Vitamins are typically classified into two groups based on their solubility.
Fat-soluble vitamins- Vitamins A, D, E, and K. They are stored in the body's fatty tissues.
Water-soluble vitamins- Vitamin C and the B-complex vitamins (e.g., B₁, B₂, B₆, B₁₂, niacin, folic acid, biotin, pantothenic acid). They are not stored in the body and are excreted in urine.
Vitamins are organic compounds that are essential for normal growth and nutrition and are required in small quantities in the diet because they cannot be synthesized by the body. They act as coenzymes or cofactors in various metabolic reactions.
A nucleotide is the monomer unit of nucleic acids. It consists of three components
A nitrogenous base (adenine, guanine, cytosine, thymine, or uracil).
A pentose sugar (deoxyribose in DNA, ribose in RNA).
A phosphate group.
Carbohydrates are biomolecules made up of carbon, hydrogen, and oxygen, typically with a ratio of 1:2:1. They are categorized into simple carbohydrates and complex carbohydrates.
Simple carbohydrates: Glucose and sucrose are examples. They provide quick energy.
Complex carbohydrates: Starch and cellulose are examples. They serve longer-term energy storage and structural functions, respectively.
A glycosidic linkage is a type of covalent bond that joins a carbohydrate molecule to another group, which may or may not be another carbohydrate. It is formed by the removal of a water molecule.
You can get the NCERT Exemplar Class 12 Chemistry Chapter 14 Biomolecules Solutions from the official NCERT website’s textbook/exemplar section or through authorised educational platforms that provide free downloadable PDFs.
Nucleic acid chemistry in Class 12 NCERT Exemplar Chapter 14 Biomolecules explains the structure, components, and functions of DNA and RNA, highlighting how they store and transmit genetic information.
Long-answer questions in NCERT Exemplar Solutions for Class 12 Chemistry Chapter 14 provide detailed explanations of carbohydrates, proteins, enzymes, vitamins and nucleic acids to help students understand biological importance and mechanisms clearly.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters






















