You should not skip NCERT Chemistry Chapter 13 – Amines questions, as they are frequently asked in CBSE and JEE-level exams and help strengthen reaction mechanisms.
NCERT Exemplar Class 12 Chemistry Solutions chapter 13 Amines
What is the first thing that comes to your mind when you are bored or sleepy? Coffee right? do you know that coffee contains caffeine, an alkaloid with amine groups? They block sleep-inducing receptors in the brain. This is how active amines are in our daily lives. Amines are one of the most important classes of organic compounds playing a significant role in biological systems, medicines, and industries. They are widely found in Proteins, Vitamins , and hormones, and are derivatives of ammonia, where one or more hydrogen atoms are replaced by alkyl or aryl groups.
Electronic gadgets or devices such as Mobile phones, smartwatches, calculators, and more are not allowed inside the CBSE 2026 exam hall.
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: Long Answer Type
- Class 12 Chemistry NCERT Chapter 13: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 13 Amines
- Topics and Subtopics of NCERT Exemplar Solutions for Class 12 Chemistry Amines
- Advantages of Using Class 12 Chemistry NCERT Exemplar Solutions Chapter 13 Amines
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Exemplar Class 12 Solutions
- NCERT Solution subject-wise
- NCERT Notes Subject-Wise
- NCERT Books and the NCERT Syllabus
NCERT Exemplar Solutions Class 12 Chemistry includes topics like the classification of amines, their nomenclature and physical and chemical properties. Students will also learn about the synthesis and named reaction of amines, which are essential in the production of pharmaceutical drugs, dyes, and polymers. This conceptual clarity is maintained by our subject matter expert in the creation of NCERT Exemplar solutions. The solutions strictly align with the syllabus and curriculum of CBSE class 12 and the step-by-step approach to the solution of the numerical and reaction-based questions ensures that the students can acquire maximum knowledge. Follow NCERT Solutions for better understanding of these concepts.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: MCQ (Type 1)
The first section containes MCQs for quick revision of concepts of Class 12 Chemistry chapter 13 Amines are provided which are important for both boards and competitive exams and helps you to improve your conceptual thinking and problem-solving ability.
Question 1. Which of the following is a $3^{o}$ amine?
(i) 1-methylcyclohexylamine
(ii) Triethylamine
(iii) tert-butylamine
(iv) N-methyl aniline
Answer:
Option (ii) is the correct answer.
Triethylamine is a tertiary $\left(3^{\circ}\right)$ amine because the nitrogen atom is bonded to three ethyl groups and no hydrogen atoms.
Question 2. The correct IUPAC name for $CH_{2}=CHCH_{2} NHCH_{3}$ is
(i) Allylmethylamine
(ii) 2-amino-4-pentene
(iii) 4-aminopent-1-ene
(iv) N-methylprop-2-en-1-amine
Answer:
Option (iv) is the correct answer.
The amine group is considered a functional group that modifies the parent hydrocarbon chain. In the compound $\mathrm{CH}_2=\mathrm{CHCH}_2 \mathrm{NHCH}_3$, the amine is a substituted primary amine, so the longest chain is numbered from the end closer to the $-\mathrm{NHCH}_3$ group to give it the lowest possible number.
Question 3. Amongst the following, the strongest base in aqueous medium is ____________.
(i) $CH_{3}NH_{2}$
(ii) $NCCH_{2}NH_{2}$
(iii) $(CH_{3})_{2}NH$
(iv) $C_{6}H_{5}NHCH_{3}$
Answer:
Option (iii) is the correct answer.
$(CH_{3})_{2}NH$ is a $2^{o}$ amine and $CH_{3}NH_{2}$ is a $1^{o}$ amine, therefore $(CH_{3})_{2}NH$ is more basic than $CH_{3}NH_{2}$. And because of –I effect of CN group, $NC-CH_{2}NH_{2}$ is less basic than $CH_{3}NH_{2}$. Moreover, $C_{6}H_{5}NHCH_{3}$ is the least basic and less basic than $CH_{3}NH_{2}$ and $(CH_{3})_{2}NH$; it is due to delocalisation of lone pair electrons that are present in nitrogen atom into the benzene ring. Therefore, the decreasing order of amines will be:
$\left ( CH_{3} \right )_{2}NH>CH_{3}NH_{2}>C_{6}H_{5}NHCH_{3}>NC-CH_{2}NH_{2}$
Question 4. Which of the following is the weakest Brönsted base?
Answer:
Option (A) is the correct answer.
The lone pair of electrons on the N-atom has delocalised into the benzene ring; therefore $C_{6}H_{5}NH_{2}$ s the weakest base.
Question 5. Benzylamine may be alkylated, as shown in the following equation:
$C_{6}H_{5}CH_{2}NH_{2} + R-X \rightarrow C_{6}H_{5}CH_{2}NHR$
Which of the following alkyl halides is best suited for this reaction through $S_{N}1$ mechanism?
(i) $CH_{3}Br$
(ii) $C_{6}H_{5}Br$
(iii) $C_{6}H_{5}CH_{2}Br$
(iv) $C_{2}H_{5}Br$
Answer:
Option (iii) is the correct answer.
$S_{N}1$ is a two-step reaction. At first, $R-X$ bond is broken, which produces carbocation. Then nucleophile attacks the carbocation. The more the stability of carbocation, the more will be the rate of reaction. Benzylic halides are highly reactive towards $S_{N}1$ reactions.
Question 6. Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
(i) $\text {H}_{2}$ (excess)/Pt
(ii) $\text {LiAIH}_{4}$ in ether
(iii) $\text {Fe}$ and $\text {HCl}$
(iv) $\text {Sn}$ and $\text {HCl}$
Answer:
Option (ii) is the correct answer.
$\mathrm{LiAlH}_4$ in ether is not suitable for reducing aryl nitro compounds to amines because it is ineffective in reducing the nitro group directly on an aromatic ring. Catalytic hydrogenation ( $\mathrm{H}_2 / \mathrm{Pt}$ ) or metal-acid reductions ( $\mathrm{Fe} / \mathrm{HCl}$ or $\mathrm{Sn} / \mathrm{HCl}$ ) are more effective for such conversions.
Question 7. In order to prepare a 1° amine from an alkyl halide with simultaneous addition of one $\text {CH}_{2}$ group in the carbon chain, the reagent used as source of nitrogen is ___________.
(i) Sodium amide, $\text {NaNH}_{2}$
(ii) Sodium azide, $\text {NaN}_{3}$
(iii) Potassium cyanide, $\text {KCN}$
(iv) Potassium phthalimide, $\text {C}_{6}\text {H}_{4}\left ( \text {CO}_{2} \right )\text N^{-}K^{+}$
Answer:
Option (iii) is the correct answer.
The number of carbon atoms increases with the help of $\text {KCN}$. The alkyl halide reacts with KCN to form a nitrile $(\mathrm{R}-\mathrm{CN})$, which is then reduced to a primary amine.
Question 8. The source of nitrogen in Gabriel synthesis of amines is _____________.
(i) Sodium azide, $\text {NaN}_{3}$
(ii) Sodium nitrite, $\text {NaNO}_{2}$
(iii) Potassium cyanide, $\text {KCN}$
(iv) Potassium phthalimide, $\text {C}_{6}\text {H}_{4}\left ( \text {CO} \right )_{2}\text N^-K^+$
Answer:
Option (iv) is the correct answer.
In Gabriel's synthesis, Potassium Phthalimide is the source of nitrogen.
Steps in Gabriel's synthesis-
1. Potassium phthalimide $+R-X \rightarrow \mathrm{~N}$-alkyl phthalimide
2. N-alkyl phthalimide $+\mathrm{H}_2 \mathrm{O} / \mathrm{H}^{+} \rightarrow$ Primary amine $\left(\mathrm{R}-\mathrm{NH}_2\right)+$ Phthalic acid
Question 9. Amongst the given set of reactants, the most appropriate for preparing 2° amine is _____.
(i) 2° $\text {R-Br+NH}_{3}$
(ii) 2° $\text {R-Br+NaCN}$ followed by $\text {H}_{2}/\text {Pt}$
(iii) 1° $\text {R-NH}_{2}+\text {RCHO}$ followed by $\text {H}_{2}/\text {Pt}$
(iv) 1° $\text {R-Br}\left ( \text {2 mol} \right )+$ potassium phthalimide followed by $\text {H}_{3}\text {O}^{+}$/heat
Answer:
Option (iii) is the correct answer.
A primary amine reacts with an aldehyde (RCHO) to form an imine (Schiff base) which is then reduced (e.g., by $\mathrm{H}_2 / \mathrm{Pt}$ ) to form a secondary amine. This is an efficient method for synthesizing $2^{\circ}$ amines.
Question 10. The best reagent for converting 2–phenylpropanamide into 2-phenylpropanamine is _____.
(i) excess $\text {H}_{2}$
(ii) $\text {Br}_{2}$ in aqueous $\text {NaOH}$
(iii) iodine in the presence of red phosphorus
(iv) $\text {LiAlH}_{4}$ in ether
Answer:
Option (iv) is the correct answer.
$\mathrm{LiAlH}_4$ in ether is a strong reducing agent that converts amides to amines efficiently. So, 2-phenylpropanamide is reduced to 2-phenylpropanamine using this reagent.
Question 11 The best reagent for converting, 2-phenylpropanamide into 1- phenylethanamine is ____.
(i) excess $\text {H}_{2}/\text {Pt}$
(ii) $\text {NaOH}/\text {Br}_{2}$
(iii) $\text {NaBH}_{4}$/methanol
(iv) $\text {LiAlH}_{4}$/ether
Answer:
Option (ii) is the correct answer.
$\mathrm{NaOH} / \mathrm{Br}_2$ (Hofmann bromamide reaction) removes one carbon from the amide and forms a primary amine. So, 2-phenylpropanamide (3 carbon chain) is converted to 1phenylethanamine (2 carbon chain amine).
Question12. Hoffmann Bromamide Degradation reaction is shown by __________.
(i) $\text {ArNH}_{2}$
(ii) $\text {ArCONH}_{2}$
(iii) $\text {ArNO}_{2}$
(iv) $\text {ArCH}_{2}\text {NH}_{2}$
Answer:
Option (ii) is the correct answer.
The Hoffmann Bromamide Degradation is a reaction where a primary amide is converted to a primary amine with one less carbon atom in the presence of Br2 and a strong base like NaOH or KOH.
$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CONH}_2 \xrightarrow[\text { Heat }]{\mathrm{Br}_2+4 \mathrm{NaOH}} \mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2+\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{NaBr}+2 \mathrm{H}_2 \mathrm{O}$
Question13. The correct increasing order of basic strength for the following compounds is______________.
(i) II < III < I
(ii) III < I < II
(iii) III < II < I
(iv) II < I < III
Answer:
Option (iv) is the correct answer.
Explanation: The basic strength is decreased with electron-withdrawing groups, and it is increased with electron releasing groups of aniline.
Question 14. Methylamine reacts with $\text {HNO}_{2}$ to form _________.
(i) $\text {CH}_{3}\text {-O-N=O}$
(ii) $\text {CH}_{3}\text {-O-CH}_{3}$
(iii) $\text {CH}_{3}\text {OH}$
(iv) $\text {CH}_{3}\text {CHO}$
Answer:
Option (iii) is the correct answer.
Explaination : $(c)\; \text {CH}_{3}\text {NH}_{2}+\text {HNO}_{3}\overset{0^{o}-5^{o}C}{\rightarrow}\text {CH}_{3}\text {OH}+\text {N}_{2}+\text {H}_{2}\text {O}$
Question 15. The gas evolved when methylamine reacts with nitrous acid is __________.
(i) $\text {NH}_{3}$
(ii) $\text {N}_{2}$
(iii) $\text {H}_{2}$
(iv) $\text {C}_{2}\text {H}_{6}$
Answer:
Option (ii) is the correct answer.
Explanation: The chemical reaction of methylamine with nitrous acid is as follows:
Question 16. In the nitration of benzene using a mixture of conc. $\text {H}_{2}\text {SO}_{4}$ and conc. $\text {HNO}_{3}$, the species which initiates the reaction is __________.
(i) $\text {NO}_{2}$
(ii) $\text {NO}^{+}$
(iii) $\text {NO}_{2}^+$
(iv) $\text {NO}_{2}^{-}$
Answer:
Option (iii) is the correct answer.
Explanation: The process of nitration is initiated by $\text {NO}_{2}^+$ (Nitronium Ion) electrophile. It is then obtained as:
$H_{2}SO_{4}\left ( conc. \right )\rightarrow H^{+}+HSO_{4}^{-}$
$H^{+}+HNO_{3}\rightarrow H_{2}NO_{3}^{+}$
$H_{2}NO_{3}^{+}\rightarrow NO_{2}^{+}+H_{2}O$
Question 17. Reduction of aromatic nitro compounds using $\text {Fe and HCl}$ gives __________.
(i) aromatic oxime
(ii) aromatic hydrocarbon
(iii) aromatic primary amine
(iv) aromatic amide
Answer:
Option (iii) is the correct answer.
Explanation :
Question 18. The most reactive amine towards dilute hydrochloric acid is ___________.
Answer:
Option (ii) is the correct answer.
Explanation: The reactivity towards dilute $HCl$ is more if the strength of the base is more. Therefore,$\left ( CH_{3} \right )_{2}NH$ has the highest basic strength.
Question 19. Acid anhydrides on reaction with primary amines give ____________.
(i) amide
(ii) imide
(iii) secondary amine
(iv) imine
Answer:
Option (i) is the correct answer.
Explanation:
$\\(a)C_{2}H_{5}NH_{2}+\left ( CH_{3}CO \right )_{2}O\rightarrow CH_{3}CONHC_{2}H_{5}+CH_{3}COOH \\$
N-Ethylacetamide
Question 20. The reaction $ArN_{2}^+Cl^{-} \overset{Cu/HCl}{\rightarrow} ArCl + N_2+ CuCl$ is named as _________.
(i) Sandmeyer reaction
(ii) Gatterman reaction
(iii) Claisen reaction
(iv) Carbylamine reaction
Answer:
Option (ii) is the correct answer.
Explanation:
Question 21. The best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is
(i) Hoffmann Bromamide reaction
(ii) Gabriel phthalimide synthesis
(iii) Sandmeyer reaction
(iv) Reaction with $NH_{3}$
Answer:
Option (ii) is the correct answer.
Explanation: To get primary amines from alkyl halide without changing the number of carbon atoms, Gabriel Phthalimide synthesis is used.
Question 22. Which of the following compound will not undergo an azo coupling reaction with benzene diazonium chloride.
(i) Aniline
(ii) Phenol
(iii) Anisole
(iv) Nitrobenzene
Answer:
Option (iv) is the correct answer.
Explanation: Diazonium Chloride is a very weak electrophile; therefore, it reacts with any electron rich compound which contains electron donating groups; $-OH, -NO_{2}, -OCH_{3}$. The compounds should not contain electron withdrawing groups like: $-NO_{2}$, etc.
Question 23. Which of the following compounds is the weakest Brönsted base?
Answer:
Option (iii) is the correct answer.
Explanation: All the amines have a tendency to accept protons, therefore are stronger Bronsted Bases than phenols and alcohols. And since phenol is much more acidic than alcohol, Phenols have fewer tendencies to accept any proton. Hence the weakest.
Question 24. Among the following amines, the strongest Brönsted base is __________.
Answer:
Option (iv) is the correct answer.
Explanation:$NH_{3}$ is a stronger base than aniline because of the delocalisation of the lone pair of electrons at N-atom and into the Benzene Ring. And in Pyrrole, as the lone pair electrons on N-atom donate to aromatic sextet formation, it is not basic at all. Hence, Pyrrolidine accepts the proton readily while also being the strongest base.
Question 25. The correct decreasing order of basic strength of the following species is _______. $H_{2}O, NH_{3}, OH^{-}, NH_{2}^{-}$
$(i)\; NH_{2}^{-}>OH^{-}>NH_{3}>H_{2}O$
$(ii)\; OH^{-}>NH_{2}^{-}>H_{2}O>NH_{3}$
$(iii)\;NH_{3}>H_{2}O>NH_{2}^{-}>OH^{-}$
$(iv)\;H_{2}O>NH_{3}>OH^{-}>NH_{2}^{-}$
Answer:
Option (i) is the correct answer.
Explanation: The electronegativity of $O$ is more than $N$ atom; therefore, $O-H$ bond is more polar than $N-H$ bond. Therefore, $O-H$ is more acidic than the $N-H$ bond. $NH_{2}^-$ and $OH^-$, both have a negative charge, and because of that, they are more basic than $NH_{3}$ and $H_{2}O$.
Question 26. Which of the following should be most volatile?
(i) II
(ii) IV
(iii) I
(iv) III
Answer:
Option (ii) is the correct answer.
Explanation: The boiling points of 1° and 2° amines are higher than 3° amines because of intermolecular H-Bonding. They are also less volatile than 3° amines and hydrocarbons of similar molecular mass.
Question 27. Which of the following methods of preparation of amines will not give the same number of carbon atoms in the chain of amines as in the reactant?
(i) The reaction of nitrite with $LiAlH_{4}$.
(ii) The reaction of the amide with $LiAlH_{4}$ followed by treatment with water.
(iii) Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis.
(iv) Treatment of amide with bromine in the aqueous solution of sodium hydroxide.
Answer:
Option (iv) is the correct answer.
Hoffmann degradation of amides results in the loss of one carbon atom as CO2. Hence, the amine formed has one carbon less than the reactant.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: MCQ (Type 2)
MCQ-type questions are covered to improve your conceptual thinking and problem-solving ability. These questions from NCERT Exemplar Class 12 Chemistry chapter 13 Amines further enhance exam preparation.
Question 28. Which of the following cannot be prepared by Sandmeyer's reaction?
(i) Chlorobenzene
(ii) Bromobenzene
(iii) Iodobenzene
(iv) Fluorobenzene
Answer:
Option (iii) and (iv) are the correct answers.
Explanation: Using Sandmeyer's reaction, Chloro and Bromo arenes are prepared. And by simply warming Diazonium salt solution with aqueous $KI$ solution, Iodarenes can be prepared. Fluoroarenes are made using Balz-Schiemann reaction. All other reagents give off aniline.
Question 29. Reduction of nitrobenzene by which of the following reagent gives aniline?
(i) ${Sn}/{HCl}$
(ii) ${Fe}/{HCl}$
(iii) $H_{2}-Pd$
(iv) ${Sn}/{NH_{4}OH}$
Answer:
Option (i), (ii) and (iii) are the correct answers.
$\mathrm{Sn} / \mathrm{HCl}, \mathrm{Fe} / \mathrm{HCl}$, and $\mathrm{H}_2 / \mathrm{Pd}$ effectively reduce nitrobenzene to aniline via complete reduction of the NO2 group. $\mathrm{Sn} / \mathrm{NH}_4 \mathrm{OH}$ being mildly reducing and in basic medium, does not efficiently convert nitrobenzene to aniline.
Question 30. Which of the following species are involved in the carbylamine test?
(i) $R-NC$
(ii) $CHCl_{3}$
(iii) $COCl_{2}$
(iv) $NaNO_{2}+HCl$
Answer:
Option (i) and (ii) are the correct answers.
Explanation: Amine when reacts with a mixture of $\text {CHCl}_{3}$ and $\text {KOH}$ gives out alkyl isocyanate. This reaction is called a Carbylamine reaction.
Here, $\text {R-NH}_{2}+\text {CHCl}+\text {3KOH}\rightarrow \text {RNC}+\text {3KCl}+\text {3H}_{2}\text {O}$ Only $\text {RNC}$ and $\text {CHCl}_{3}$ are involved in carbylamine reaction.
Question 31. The reagents that can be used to convert benzene diazonium chloride to benzene are __________.
(i) ${SnCl_{2}}/{HCl}$
(ii) $CH_{3}CH_{2}OH$
(iii) $H_{3}PO_{2}$
(iv) $LiAlH_{4}$
Answer:
Option (ii) and (iii) are the correct answers.
Explanation :
Question 32. The product of the following reaction is __________.
Answer:
Option (A) and (B) is the correct answer.
Explanation :
Question 33. Arenium ion involved in the bromination of aniline is __________.
Answer:
Option (i), (ii) and (iii) are the correct answers.
Explanation: Bromination of aniline involves Arenium is as follows:
Question 34. Which of the following amines can be prepared by Gabriel synthesis.
$\\(i) Isobutyl amine\\ (ii) 2-Phenylethylamine\\ (iii) N-methyl benzylamine\\ (iv) Aniline$
Answer:
Option (i) and (ii) are the correct answers.
Explanation:$(CH_{3})_{2}CH-CH_{2}NH_{2}$ and $C_{6}H_{5}CH_{2}NH_{2}$ are the primary aliphatic amines that can be prepared by Gabriel synthesis. $2^{o}$ amines are $C_{6}H_{5}CH_{2}NHCH_{3} (C)$ and $1^{o}$ amine, $C_{6}H_{5}NH_{2}$ cannot be prepared by this process.
Question 35. Which of the following reactions are correct ?
Answer:
Options (i) and (iii) are the correct answers.
Explanation : $CH_{3}CH_{2}NH_{2}+NH_{4}Cl$
Question 36. Under which of the following reaction conditions, aniline gives p-nitro derivative as the major product?
(i) Acetyl chloride/pyridine followed by reaction with $conc. H_{2}SO_{4} + conc. HNO_{3}$
(ii) Acetic anhydride/pyridine followed by $conc. H_{2}SO_{4} + conc. HNO_{3}$
(iii) Dil. HCl followed by reaction with $conc. H_{2}SO_{4} + conc. HNO_{3}$
(iv) Reaction with $conc. HNO_{3}+conc. H_{2}SO_{4}$
Answer:
Option (i) and (ii) are the correct answers.
Explanation: When aniline reacts with acetyl chloride or acetic anhydride in the presence of pyridine produces N-acetyl aniline. This is a ortho, para directing group which produces p-nitroaniline on reaction with nitrating mixtures. As shown below:
Question 37. Which of the following reactions belong to electrophilic aromatic substitution?
(i) Bromination of acetanilide
(ii) Coupling reaction of aryldiazonium salts
(iii) Diazotisation of aniline
(iv) Acylation of aniline
Answer:
Option (i) and (ii) are the correct answers.
Explanation: Nucleophilic substitution reaction where $-NH_{2} \; and\; H$$�NH_{2} \; and\; H$ atoms are replaced by acyl groups is known as Acylation. Diazotisation is also a type of nucleophilic substitution reaction.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: Short Answer Type
Short-answer type questions are covered to improve your problem-solving ability. These Chapter 13 Amines important questions help in building a strong foundation for board and competitive exams.
Question 38. What is the role of $\text {HNO}_{3}$ in the nitrating mixture used for nitration of benzene?
Answer:
The 1:1 solution of $\text {HNO}_{3}$ and $\text {H}_{2}\text {SO}_{4}$ is a nitrating mixture, and it is used for nitration of organic compounds. This mixture acts as a base which provides electrophile in the nitration process of benzene.
Question 39. Why is $\text {NH}_{2}$ group of aniline acetylated before carrying out nitration?
Answer:
The $\text {NH}_{2}$ group of aniline is acetylated before carrying out the nitration for controlling the nitration reaction, carry oxidation products and nitro derivative products formation.
As the acetyl group is an electron-withdrawing group, it attracts the lone pair of the electrons in the N atom towards the carbonyl group.
Question 40. What is the product when $\text {C}_{6}\text {H}_{5}\text {CH}_{2}\text {NH}_{2}$ reacts with $\text {HNO}_{2}$ ?
Answer:
$\text {HNO}_{2}$ is reacted $\text {C}_{6}\text {H}_{5}\text {CH}_{2}\text {NH}_{2}$ which forms unstable diazonium salt, and in turn, alcohol is given out. This is the reaction:
$\text {C}_{6}\text {H}_{5}\text {CH}_{2}\text {NH}_{2}+\text {HNO}_{2}\rightarrow \text {C}_{6}\text {H}_{5}\text {CH}_{2}\text {OH}+\text {N}_{2}+\text {H}_{2}\text {O}$
Question 41. What is the best reagent to convert nitrile to primary amine?
Answer:
$\text {LiAlH}_{4}$ and Sodium/Alcohol are the best reagents for the conversion of nitrile to a primary amine. And nitriles can be converted into a corresponding primary amine using reduction.
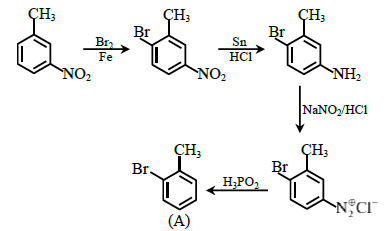
Question 42. Give the structure of 'A' in the following reaction.
Answer:
3-Methylnitrobenzene is the product formed in the above reaction.
Question 43. What is Hinsberg reagent?
Answer:
$\text {C}_{6}\text {H}_{5}\text {SOCl}$ aka Benzene sulphonylchloride is known as Hinsberg's reagent. It is used to differentiate between primary, secondary and tertiary amines.
Question 44. Why is benzene diazonium chloride not stored and is used immediately after its preparation?
Answer:
Benzene Diazonium is very stable at low temperatures and highly soluble in water at high temperatures. It is recommended to use it immediately after its preparation as it is unstable.
Question 45. Why does the acetylation of $\text {-NH}_{2}$ group of aniline reduce its activating effect?
Answer:
The activating effect of $\text {-NH}_{2}$ group of aniline is reduced by its acetylation. It happens because of the lone pair electrons on the nitrogen of acetanilide react with oxygen atom because of resonance.
Question 46. Explain why $\text {MeNH}_{2}$ is a stronger base than $\text {MeOH}$ ?
Answer:
$\text {MeOH}$ is a weaker base than $\text {MeNH}_{2}$ because $\text {MeNH}_{2}$ has lower electronegativity and has lone pair electrons present on the nitrogen atom.
Question 47. What is the role of pyridine in the acylation reaction of amines?
Answer:
By acylation of $\text {-NH}_{2}$ group with acetic anhydride in the presence of pyridine and after that carrying substitution followed with hydrolysis of the substituted amide with the substituted amine, the activating effect of $\text {-NH}_{2}$ group can be controlled. $\text {HCl}$ is a side product, which is removed with the help of pyridine as a base.
Question 48. Under what reaction conditions (acidic/basic), the coupling reaction of aryldiazonium chloride with aniline is carried out?
Answer:
The reaction is performed in a mild basic medium and known as electrophilic substitution reaction. Aniline and Aryldiazonium chloride react to form a yellow dye of p-Aminoazobenzene.
Question 49. Predict the product of the reaction of aniline with bromine in a non-polar solvent such as $\text {CS}_{2}$.
Answer:
When aniline and bromine react in a non-polar solvent, such as $\text {CS}_{2}$, non-polar products are formed that are 4-Bromoaniline and 2-Bromoaniline in which 4-Bromoaniline is present in the majority.
$\text {CH}_{3}\text {CH}_{2}\text {CH}_{3}<\text {CH}_{3}\text {CH}_{2}\text {NH}_{2}<\text {CH}_{3}\text {CH}_{2}\text {OH}$
$\text {CH}_{3}\text {CH}_{2}\text {OH}$ has dipole moment greater than $\text {CH}_{3}\text {CH}_{2}\text {NH}_{2}$. Whereas,$\text {CH}_{3}\text {CH}_{2}\text {CH}_{3}$ has the least dipole moment amongst the three compounds. $\text {CH}_{3}\text {CH}_{2}\text {CH}_{3}$ is an almost non-polar molecule.
Question 51. What is the structure and IUPAC name of the compound, allyl amine?
Answer:
The structure of allyl amine is 
Question 52. Write down the IUPAC name of
Answer:
IUPAC name of
Question 53. A compound Z with molecular formula $\text {C}_{3}\text {H}_{9}\text {N}$ reacts with $\text {C}_{6}\text {H}_{5}\text {SO}_{2}\text {Cl}$ to give a solid, insoluble in alkali, identify Z.
Answer:
Solid, insoluble alkali is given off when compound Z with molecular formula $\text {C}_{3}\text {H}_{9}\text {N}$ and is an aliphatic amine on treatment with $\text {C}_{6}\text {H}_{5}\text {SO}_{2}\text {Cl}$. And hence, the product does not have any replaceable hydrogen on its nitrogen atom. The amine (Z) should be a secondary amine which means Z is ethyl methylamine $(\text {C}_{2}\text {H}_{5}\text {NHCH}_{3})$.
$\begin{array}{lcc}\mathrm{R}-\mathrm{NH}_2+\mathrm{CHCl}_3+3 \mathrm{KOH} \rightarrow \mathrm{R}-\mathrm{NC} \xrightarrow[\text { (Reduction) }]{\mathrm{H}_2 / \mathrm{Pd}} \mathrm{R}-\mathrm{NH}-\mathrm{CH}_3 \\ 1^{\circ} \text { Amine } \quad \text { Alkyl isocyanide } & 2^{\circ} \text { Amine }\end{array}$
Carbylamine reaction is shown by $1^{o}$ amine only which result in the replacement of two hydrogen atoms attached to N atom of $-\text {NH}_{2}$ group by one carbon atom to form isocyanide. On catalytic reduction, the isocyanide will give a secondary amine with one methyl group.
Question 56. Why is aniline soluble in aqueous $\text {HCl}$ ?
Answer:
Anilinium chloride salt is formed when aniline reacts with aqueous $\text {HCl}$, and it is soluble in water.
Question 57. Suggest a route by which the following conversion can be accomplished.
Answer:
Question 58. Identify A and B in the following reaction.
Answer:
This reaction is an example of electrophilic aromatic substitution. Phenol forms phenoxide ion in an alkaline medium, which is more electron-rich than phenol and therefore more reactive in the electrophilic attack. Arydiazonium cation is electrophile in this reaction, and p-Nitrophenyldiazonium cation is a stronger electrophile than p-toluene diazonium cation. Hence, it pairs preferentially with phenol.
Question 62. How will you bring out the following conversion?
Answer:
Question 63. How will you carry out the following conversion?
Answer:
Question 65. How will you carry out the following conversion?
Answer:
NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: Matching Type
Matching-type questions are covered to improve conceptual clarity and topic awareness. Such exercises in NCERT Exemplar Class 12 Chemistry chapter 13 Amines strengthen understanding.
Question 66. Match the reactions given in Column I with the statements given in Column II.
|
Column I |
Column II | ||
|
(i) |
Ammonolysis |
(a) |
Amine with lesser number of carbon atoms |
|
(ii) |
Gabriel phthalimide synthesis |
(b) |
Detection test for primary amines |
|
(iii) |
Hofmann Bromamide reaction |
(c) |
Reaction of phthalimide with $\text {KOH}$ and $\text {R-X}$ |
|
(iv) |
Carbylamine reaction |
(d) |
Reaction of alkyl halides with $\text {NH}_{3}$ |
Answer:
(i $\rightarrow$ d), (ii $\rightarrow$ c), (iii $\rightarrow$ a), (iv $\rightarrow$ b)
Ammonolysis $\rightarrow$ (d)
Alkyl halides react with ammonia to give amines
Gabriel phthalimide synthesis $\rightarrow$ (c)
Phthalimide is deprotonated by KOH to potassium phthalimide, which does SN2 with an alkyl halide (R–X); hydrolysis then releases a primary amine
Hofmann bromamide reaction $\rightarrow$ (a)
An amide treated with $\mathrm{Br}_2 / \mathrm{NaOH}$ rearranges to a primary amine with one fewer carbon
Carbylamine reaction $\rightarrow$ (b)
Only primary amines with chloroform and alcoholic KOH form foul-smelling isocyanides used as a detection test for primary amines.
Question 67. Match the compounds given in Column I with the items given in Column II.
|
Column I |
Column II | ||
|
(i) |
Benzene sulphonyl Chloride |
(a) |
Zwitter ion |
|
(ii) |
Sulphanilic acid |
(b) |
Hinsberg reagent |
|
(iii) |
Alkyl diazonium salts |
(c) |
Dyes |
|
(iv) |
Aryl diazonium salts |
(d) |
Conversion to alcohols |
Answer:
(i $\rightarrow$ b), (ii $\rightarrow$ a), (iii $\rightarrow$ d), (iv $\rightarrow$ c)
Benzene sulphonyl chloride $\rightarrow$ Hinsberg reagent because it is used in the Hinsberg test to distinguish amines.
Sulphanilic acid $\rightarrow$ Zwitter ion, Exists as $\mathrm{H}_3 \mathrm{~N}^{+}-\mathrm{C}_6 \mathrm{H}_4-\mathrm{SO}_3^{-}$
Alkyl diazonium salts $\rightarrow$ Conversion to alcohols because it is Very unstable
Aryl diazonium salts $\rightarrow$ Dyes, Undergo azo coupling to form brightly coloured azo dyes.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: Assertion and Reason Type
Assertion and Reason type questions are covered to improve your critical thinking and problem-solving solving ability.These are an important part of Chemistry Class 12 NCERT Exemplar Chapter 13 Amines, helping students prepare effectively for board and competitive exams.
Question 68. In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Acylation of amines gives a monosubstituted product whereas alkylation of amines gives polysubstituted product.
Reason (R): Acyl group sterically hinders the approach of further acyl groups.
(i) Both assertion and reason are wrong.
(ii) Both assertion and reason are correct statements but the reason is not the correct explanation of the assertion.
(iii) Assertion is a correct statement, but the reason is wrong statement.
(iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(v) Assertion is wrong statement but reason is correct statement.
Answer:
(iii) Assertion is correct statement but reason is wrong statement.
The assertion is correct because acylation of an amine converts it into an amide, and amides are much less nucleophilic at nitrogen, so further acylation is discouraged and you end up with a monosubstituted product.
The given reason is incorrect. The prevention of further acylation is mainly electronic (resonance delocalisation of the lone pair into the carbonyl and electron withdrawal by the acyl group), not primarily due to steric hindrance.
Question 69. In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Hoffmann's bromamide reaction is given by primary amines.
Reason (R): Primary amines are more basic than secondary amines.
(i) Both assertion and reason are wrong.
(ii) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(v) Assertion is wrong statement but reason is correct statement.
Answer:
(iii) Assertion is correct statement but reason is wrong statement.
Hofmann’s bromamide reaction is given by amides, not by amines. Moreover, primary amines are basic than secondary amines.
Question 70. In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): N-Ethylbenzene sulphonamide is soluble in alkali.
Reason (R): Hydrogen attached to nitrogen in sulphonamide is strongly acidic.
(i) Both assertion and reason are wrong.
(ii) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(v) Assertion is wrong statement but reason is correct statement.
Answer:
Ethylbenzene sulphonamide is soluble in alkali because it has acidic hydrogen.
Hence (iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
Question 71. In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): N, N-diethyl benzene sulphonamide is insoluble in alkali.
Reason (R): Sulphonyl group attached to the nitrogen atom is a strong electron-withdrawing group.
(i) Both assertion and reason are wrong.
(ii) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(v) Assertion is wrong statement but reason is correct statement.
Answer:
N, N-diethyl benzene sulphonamide is not soluble in alkali as it there is no acidic hydrogen present on this. Sulphonyl group is an electron-withdrawing group which is attached to a nitrogen atom.
Hence, (ii) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Question 72. In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Only a small amount of $\text {HCl}$ is required in the reduction of nitro compounds with iron scrap and $\text {HCl}$ in the presence of steam.
Reason (R):$\text {FeCl}_{2}$ formed gets hydrolysed to release $\text {HCl}$ during the reaction.
(i) Both assertion and reason are wrong.
(ii) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(v) Assertion is wrong statement but reason is correct statement.
Answer:
$\text {Fe}+\text {2HCl}\rightarrow \text {FeCl}_{2}+\text {2[H]}$
Nacent hydrogen is reduced into nitro compounds.
$\text {FeCl}_{2}+\text {H}_{2}\text {O}\text {(g)}\rightarrow \text {FeO}+\text {2HCl}$
Hence, (iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
Question 73. In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Aromatic $1^{o}$ amines can be prepared by Gabriel phthalimide synthesis.
Reason (R): Aryl halides undergo nucleophilic substitution with anion formed by phthalimide.
(i) Both assertion and reason are wrong.
(ii) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(v) Assertion is wrong statement but reason is correct statement.
Answer:
(i) Both assertion and reason are wrong
Aromatic amines are never obtained by Gabriel phthalimide synthesis.
Aryl halides are resistant to nucleophilic substitution under normal Gabriel synthesis conditions.
Question 74. In the following question, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
Assertion (A): Acetanilide is less basic than aniline.
Reason (R): Acetylation of aniline results in the decrease of electron density on nitrogen.
(i) Both assertion and reason are wrong.
(ii) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
(iii) Assertion is correct statement but reason is wrong statement.
(iv) Both assertion and reason are correct statements and reason is correct explanation of assertion.
(v) Assertion is wrong statement but reason is correct statement.
Answer:
(iv) Both assertion and reason are correct statements and reason is correct explanation of assertion. Both are correct acetylation withdraws nitrogen’s lone pair via resonance, hence acetanilide is less basic than aniline.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 13: Long Answer Type
Long-answer type questions are covered to improve your subject knowledge and conceptual thinking. These Amines NCERT Exemplar Class 12 Chemistry Solutions enhance analytical skills and strengthen exam preparation.
(i) Addition of $\text {HCl}$ to compound 'A' shows that compound 'A' is alkene. Compound 'B' is $\text {C}_{4}\text {H}_{9}\text {Cl}$
(ii) Compound 'B' reacts with $\text {NH}_{2}$, it forms amine 'C'.
$\\\text {C}_{4}\text {H}_{8}\overset{\text {HCl}}{\rightarrow}\text {C}_{4}\text {H}_{9}\text {Cl}\overset{\text {NH}_{3}}{\rightarrow}\text {C}_{4}\text {H}_{11}\text {N}\; \text {Or}\; \text {C}_{4}\text {H}_{9}\text {NH}_{2}\\\text {(A)}\; \; \; \; \; \; \; \; \; \; \; \; \text {(B)}\; \; \; \; \; \; \; \; \; \; \; \; \; \text {(C)}$
(iii) 'C' gives diazonium salt with $\text {NaNO}_{2}/\text {HCl}$, which yields an optically active alcohol. So, 'C' is aliphatic amine.
(iv) 'A' on Ozonolysis produces 2 moles of $\text {CH}_{3}\text {CHO}.$ So, 'A' is $\text {CH}_{3}-\text {CH}=\text {CH}-\text {CH}_{3}\left ( \text {But-2-ene} \right )$
Reactions :
Question 77. Predict the reagent or the product in the following reaction sequence.
Answer:
Class 12 Chemistry NCERT Chapter 13: Higher Order Thinking Skills (HOTS) Questions
Class 12 Chemistry NCERT Exemplar Solutions chapter 13 Amines also includes HOTS-type questions that are covered to improve your problem-solving ability and conceptual thinking. These advanced questions help in developing higher-level analytical skills for exams.
Question 1: Consider the following sequence of reactions to produce major product (A)
Molar mass of product (A) is _______ $\mathrm{g} \mathrm{mol}^{-1}$. (Given molar mass in $\mathrm{g} \mathrm{mol}^{-1}$ of $\mathrm{C}: 12, \mathrm{H}: 1$, $\mathrm{O}: 16, \mathrm{Br}: 80, \mathrm{~N}: 14, \mathrm{P}: 31)$
Answer.
Molar mass of product $\left(\mathrm{C}_7 \mathrm{H}_7 \mathrm{Br}\right)(\mathrm{A})$ is $171 \mathrm{~g} \mathrm{~mol}^{-1}$
Hence, the answer is 171.
Question 2: 9.3 g of pure aniline is treated with bromine water at room temperature to give a white precipitate of the product ' P '. The mass of product ' P ' obtained is 26.4 g. The percentage yield is ......... %.
Answer.

93 g of aniline produces 330 g of 2,4,6-tribromoaniline. Hence, 9.3 g of aniline should produce 33 g of 2, 4, 6-tribromoaniline. Hence percentage yield $\frac{26.4 \times 100}{33}=80 \%$
Hence, the answer is (80%).
Question 3: The correct order of basic nature on aqueous solution for the bases $\mathrm{NH}_3, \mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2$, $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$ and $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}$ is :
(1) $\mathrm{NH}_3<\mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2<\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2<$ $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$
(2) $\mathrm{NH}_3<\mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2<\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}<$ $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}$
(3) $\mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2<\mathrm{NH}_3<\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2<$ $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$
(4) $\mathrm{NH}_2-\mathrm{NH}_2<\mathrm{NH}_3<\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2<\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}<$ $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$
Answer.
Basic strength of amine depends on hydrogen bonding and electronic inductive effect.
$\mathrm{NH}(\mathrm{Et})_2>\mathrm{N}(\mathrm{Et})_3>\mathrm{NH}_2 \mathrm{Et}>\ddot{\mathrm{N}}_3>\mathrm{NH}_2-\mathrm{NH}_2$
Hence, the correct answer is option (4).
Question 4: The major product of the reaction is
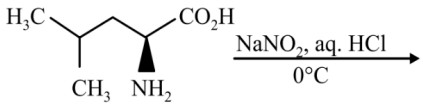
(1)
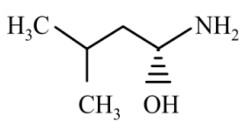
(2)
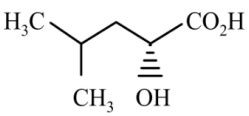
(3)
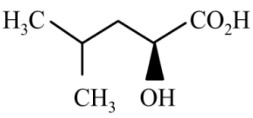
(4)
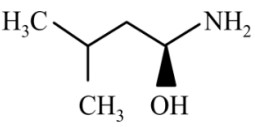
Answer:
Reaction proceeds via diazonium salt with neighbouring group participation
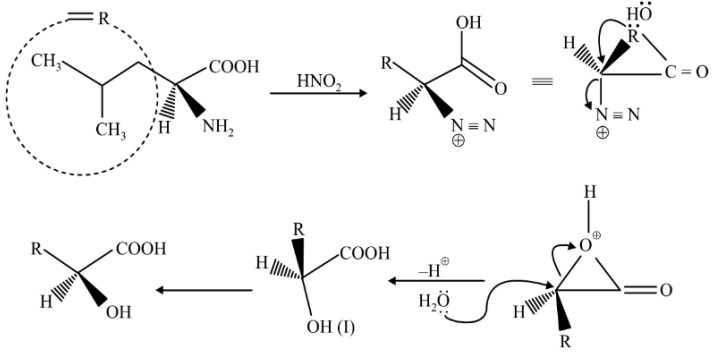
Hence, the correct answer is option (3).
Question 5: The descending order of basicity of following amines is :
(A)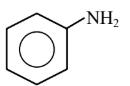
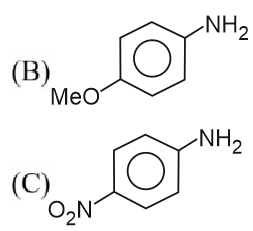
(D) $\mathrm{CH}_3 \mathrm{NH}_2$
(E) $\left(\mathrm{CH}_3\right)_2 \mathrm{NH}$
Choose the correct answer from the options given below :
(1)B $>$ E $>$ D $>$ A $>$ C
(2)E $>$ D $>$ B $>$ A $>$ C
(3) E $>$ D $>$ A $>$ B $>$ C
(4)E $>$ A $>$ D $>$ C $>$ B
Answer:
Aliphatic amines are more basic than aromatic amines, as the lone pair on nitrogen is localized.

E > D > B > A > C
Hence, the correct answer is option (2).
Approach to Solve Questions of Chapter 13 Amines
To effectively solve questions from NCERT Exemplar Class 12 Chemistry chapter 13 Amines, follow a structured and concept-driven approach. Here is a concise and structured approach to follow:
1) Firstly, the understanding of basic concepts is necessary to solve the problems related to amines like
- Classification of primary, secondary and tertiary amines.
- Classification of Aliphatic and aromatic amines.
- IUPAC and common names of compounds
- Distinguishing amines from other functional groups
2) Preparation methods are quite important from the exam point of view. Some of them include, Reduction of nitriles, amides, and nitro compounds, Hoffmann Bromamide degradation, Reduction of Nitrobenzene. Students can refer to NCERT Class 12 Chemistry Chapter 13 Amines Notes for better understanding.
3) Carefully study the physical properties of compounds like boiling point, melting point, solubility trends, physical properties and the effect of Hydrogen bonding on them is important.
4) Students can make use of short notes or charts to remember the concepts like Basicity of Amines and the reactions involving amines. Also, the test to distinguish the amines is too crucial, such as the Carbylamine test, Hinsberg's test and Nitrous acid reaction.
5) Solve the problems involving molecular weight, percentage yield and reaction stoichiometry. Also, solve the in-text and exercise problems from NCERT Exemplar Solutions for Class 12 Chemistry Chapter 13 Amines.
Topics and Subtopics of NCERT Exemplar Solutions for Class 12 Chemistry Amines
Important topics of NCERT Exemplar Class 12 Chemistry Chapter 13 are given below:
- Structure of Amines
- Classification
- Nomenclature
- Preparation Of Amines
- Reduction of Nitro Compounds
- Ammonolysis of Alkyl Halides
- Reduction of nitrates
- Reduction of Amides
- Gabriel phthalimide synthesis
- Hoffmann bromamide degradation reaction
- Physical Properties
- Chemical Reactions
- Basic character of amines
- Alkylation
- Acylation
- Carbylamine reaction
- Reaction with nitrous acid
- Reaction with aryl sulphonyl chloride
- Electrophilic substitution
- Method of Preparation of Diazonium Salts
- Physical Properties
- Chemical Reaction
- Reactions involving the displacement of nitrogen
- Reactions involving retention of the diazo group
- Importance of Diazonium Salts in the Synthesis of Aromatic Compounds
Advantages of Using Class 12 Chemistry NCERT Exemplar Solutions Chapter 13 Amines
NCERT Exemplar Solutions for Class 12 Chemistry Chapter 13 Amines cover all important concepts from the NCERT book in a simple and organised manner. The advantages of using these solutions of NCERT are given below:
- Students can get a clear understanding of the structure, classification, and preparation methods of amines.
- These NCERT Exemplar Class 12 Solutions include mechanisms of important reactions like diazotisation and Hoffmann bromamide reaction.
- The Class 12 Chemistry NCERT Exemplar Solutions chapter 13 Amines contain key points, tables, and formulas for easy learning.
- They are created by subject experts in a very clear and comprehensive way that are helpful in both board and competitive exams.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Exemplar Class 12 Solutions
Students can refer to the links given below for the NCERT Exemplar subject-wise solutions for Class 12:
NCERT Solution subject-wise
Refer to the links given below for the NCERT subject-wise solutions:
NCERT Notes Subject-Wise
Do a quick revision by following the NCERT notes. Click on the link given in the table
NCERT Books and the NCERT Syllabus
Follow the links to access the syllabus and books
Frequently Asked Questions (FAQs)
Class 12 Chemistry NCERT exemplar solutions chapter 13 covers the topic of amines which is a crucial part of organic chemistry. Those who are planning to take up engineering or biochemistry should not skip this chapter.
Students can make the most of these questions and NCERT exemplar solutions for Class 12 Chemistry chapter 13 by understanding the topic better and also these solutions will help in answering the questions asked in the exam.
The entire chapter covers the topic of amines, its properties, types, classification, applications and uses, and the chemical reactions.
Amines are organic compounds derived from ammonia (NH₃) by replacing one or more hydrogen atoms with alkyl or aryl groups. They are characterized by the presence of a nitrogen atom with a lone pair of electrons.
Amines are classified based on the number of alkyl or aryl groups attached to the nitrogen atom:
- Primary (1°) amines: One alkyl/aryl group attached to the nitrogen. (R-NH₂)
- Secondary (2°) amines: Two alkyl/aryl groups attached to the nitrogen. (R₂-NH)
- Tertiary (3°) amines: Three alkyl/aryl groups attached to the nitrogen. (R₃-N)
- Quaternary Ammonium Salts: Four alkyl/aryl groups attatched to the nitrogen with a positive charge on the nitrogen atom. (R₄N⁺)
The structure of an amine consists of a nitrogen atom bonded to hydrogen and/or carbon atoms. The presence of lone pair electrons on the nitrogen makes amines basic, as they can accept protons (H+). Basicity varies among types of amines; for example, tertiary amines are generally more basic than primary ones due to steric hindrance affecting the ability to donate their lone pair for protonation.
Class 12 Chemistry chapter 13 Amines explains several methods for preparing amines, including the reduction of nitriles or amides, the alkylation of ammonia, and the reduction of nitro compounds. Each method has its own advantages and is chosen based on the availability of starting materials and desired alkyl groups in the final amine product.
Amine take part in various reactions such as acylation, alkylation, and reactions with acids to form ammonium salts. They can be protonated under acidic conditions, forming ammonium ions. The chapter highlights these reactions, showcasing their utility in synthesizing different organic compounds and the way they interact with other functional groups.
The nature of alkyl groups attached to the nitrogen in amines can significantly influence their physical and chemical properties, including boiling points, solubility, and reactivity. For example, larger alkyl groups can lead to increased steric hindrance, affecting the basicity and reactivity of the amine. Understanding these influences helps in predicting the behavior of amines in various chemical reactions.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters