Yes, Coordination Compounds is a highly scoring chapter because its concepts are systematic, and many questions in board and competitive exams are direct and formula-based.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 9 Coordination Compounds
Did you know why the colour of blood is red or how some medicines work so effectively? The answer lies in class 12 chemistry chapter 9 Coordination compounds.These are special compounds formed when a central metal atom combines with small atoms or ions called ligands.These compounds function like a well organised system, where each part plays a specific role to make the whole structure stable and useful. The structure and properties of these compounds that make them essential in nature and industry.
Electronic gadgets or devices such as Mobile phones, smartwatches, calculators, and more are not allowed inside the CBSE 2026 exam hall.
This Story also Contains
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: MCQ (Type 1)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: MCQ (Type 2)
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: Short Answer Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: Matching Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: Assertion and Reason Type
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: Long Answer Type
- Class 12 Chemistry NCERT Chapter 9: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 9
- Topics Covered in NCERT Exemplar Solutions Class 12 Chemistry Chapter 9
- NCERT Class 12 Chemistry Exemplar Chapter 9 Important Formulas
- Advantages of Class 12 Chemistry Solutions Chapter 9 coordination compounds NCERT Exemplar Solutions
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Solution subject-wise
- NCERT Exemplar Class 12 Solutions
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus

The important topics like crystal field theory, ligands, color and complex formation are all discussed in this chapter. The NCERT Exemplar Class 12 Chemistry Solutions are designed to present these concepts in the form of solved questions. These NCERT Exemplar solutions are valuable resources to enhance performance in board exams as well as in competitive exams as they provide well-explained answers to help you understand the concepts with ease. The higher-order thinking skills (HOTS) questions are also provided to develop your critical thinking. We have also added some points that will help you build a good problem-solving strategy. For more practice students can refer to NCERT Solutions.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: MCQ (Type 1)
The MCQ questions are covered in the Class 12 Chemistry NCERT Exemplar Solutions Chapter 9 to enhance your knowledge. The concepts are explained in detail in notes available on our website.
Question 1 Which of the following complexes formed by $\text {Cu}^{2+}$ ions is most stable?
(i) $\text {Cu}^{2+}+\text {4NH}_{3}\rightleftharpoons \left [ \text {Cu}\left ( \text {NH}_{3} \right )_{4} \right ]^{2+}\; \; \; \; \; \; \; \text {logK}= 11.6$
(ii) $\text {Cu}^{2+}+\text {4CN}^{-}\rightleftharpoons \left [ \text {Cu}\left ( \text {CN} \right )_{4} \right ]^{2-}\; \; \; \; \; \; \; \text {logK}= 27.3$
(iii) $\text {Cu}^{2+}+\text {2en}\rightleftharpoons \left [ \text {Cu}\left ( \text {en} \right )_{2} \right ]^{2+}\; \; \; \; \; \; \; \; \; \; \; \; \; \text {logK}= 15.4$
(iv) $\text {Cu}^{2+}+\text {4H}_{2}\text {O}\rightleftharpoons \left [ \text {Cu}\left ( \text {H}_{2}\text {O} \right )_{4} \right ]^{2+}\; \; \; \; \; \; \; \text {logK}= 8.9$
Answer:
Option (ii) is the correct answer.
Explanation:
The stability of a compound will be more when the value of log K increases.
For reaction,
$\text {Cu}^{2+}+\text {4CN}^{-}\rightleftharpoons \left [ \text {Cu}\left ( \text {CN} \right )_{4} \right ]^{2-}$
$\text {K}=\frac{\left [ \text {Cu}\left ( \text {CN}_{4} \right ) ^{2-}\right ]}{\left [ \text {Cu}^{2+} \right ]\left [ \text {CN}^{-} \right ]^{4}}\;\text {and} \; \text {log K}=27.3$
Log K has the highest value for this reaction amongst all the four reactions. The value of K will also be higher so, the stability of this complex will be highest.
Question 2: The colour of the coordination compounds depends on the crystal field splitting. What will be the correct order of absorption of wavelength of light in the visible region, for the complexes, $\left [ \text {Co}\left ( \text {NH}_{3} \right )_{6} \right ]^{3+}$, $\left [ \text {Co}\left ( \text {CN} \right )_{6} \right ]^{3-}$, $\left [ \text {Co}\left ( \text {H}_{2}\text {O} \right )_{6} \right ]^{3+}$
(i) $\left [ \text {Co}\left ( \text {CN} \right )_{6} \right ]^{3-}> \left [ \text {Co}\left ( \text {NH}_{3} \right )_{6} \right ]^{3+}> \left [ \text {Co}\left ( \text {H}_{2}\text {O} \right )_{6} \right ]^{3+}$
(ii) $\left [ \text {Co}\left ( \text {NH}_{3} \right )_{6} \right ]^{3+}> \left [ \text {Co}\left ( \text {H}_{2}\text {O} \right )_{6} \right ]^{3+}> \left [ \text {Co}\left ( \text {CN} \right )_{6} \right ]^{3-}$
(iii) $\left [ \text {Co}\left ( \text {H}_{2}\text {O} \right )_{6} \right ]^{3+}> \left [ \text {Co}\left ( \text {NH}_{3} \right )_{6} \right ]^{3+}> \left [ \text {Co}\left ( \text {CN} \right )_{6} \right ]^{3-}$
(iv) $\left [ \text {Co}\left ( \text {CN} \right )_{6} \right ]^{3-}> \left [ \text {Co}\left ( \text {NH}_{3} \right )_{6} \right ]^{3+}> \left [ \text {Co}\left ( \text {H}_{2}\text {O} \right )_{6} \right ]^{3+}$
Answer:
Option (iii) is the correct answer.
Explanation :
$\Delta _{0}$ values follow the order : $\left [ \text {Co}\left ( \text {H}_{2}\text {O} \right )_{6} \right ]^{3+}< \left [ \text {Co}\left ( \text {NH}_{3} \right )_{6} \right ]^{3+}< \left [ \text {Co}\left ( \text {CN} \right )_{6} \right ]^{3-}$ and therefore,absorption wavelength follows the order:
$\left [ \text {Co}\left ( \text {H}_{2}\text {O} \right )_{6} \right ]^{3+}> \left [ \text {Co}\left ( \text {NH}_{3} \right )_{6} \right ]^{3+}> \left [ \text {Co}\left ( \text {CN} \right )_{6} \right ]^{3-}$
Question 3 When 0.1 mol $\text {CoCl}_{3}\left ( \text {NH}_{3} \right )_{5}$ is treated with an excess of $\text {AgNO}_{3}$, 0.2 mol of $\text {AgCl}$ are obtained. The conductivity of the solution will correspond to
(i) 1:3 electrolyte
(ii) 1:2 electrolyte
(iii) 1:1 electrolyte
(iv) 3:1 electrolyte
Answer:
Option (ii) is the correct answer.
Explanation:
One mole of chloride ion gets precipitated by one mole of $\text {AgNO}_{3}$. $\text {AgCl}$, is obtained when 0.1 mole of $\text {CoCl}_{3}\left ( \text {NH}_{3} \right )_{5}$ is treated with $\text {AgNO}_{3}$ in excess. So this leaves two free chloride ions in the solution of electrolyte for every 1 mole reaction.
Hence the molecular formula should be $\left [ \text {Co}\left ( \text {NH}_{3} \right )_{5}\text {Cl} \right ]\text {Cl}_{2}$ and the solution of electrolyte should contain $\left [ \text {Co}\left ( \text {NH}_{3} \right )_{5}\text {Cl} \right ]^{2+}$ and two Cl as their constituent ions. Therefore, it is an 1:2 electrolyte.
$\left [ \text {Co}\left ( \text {NH}_{3} \right )_{5}\text {Cl} \right ]\text {Cl}_{2} \rightarrow \left [ \text {Co}\left ( \text {NH}_{3} \right )_{5}\text {Cl} \right ]^{2+} \text {(aq)}+\text {2Cl}^{-} \text {(aq)}$
Question 4 When 1 mol $\text {CrCl}_{3}.\text {6H}_{2}\text {O}$ is treated with an excess of $\text {AgNO}_{3}$, 3 mol of $\text {AgCl}$ are obtained. The formula of the complex is:
(i) $\left [ \text {CrCl}_{3}\left ( \text {H}_{2}\text {O}\right )_{3} \right ].\text {3H}_{2}\text {O}$
(ii) $\left [ \text {CrCl}_{2}\left ( \text {H}_{2}\text {O}\right )_{4} \right ]\text {Cl}.\text {2H}_{2}\text {O}$
(iii) $\left [ \text {CrCl}\left ( \text {H}_{2}\text {O}\right )_{5} \right ]\text {Cl}_{2}.\text {H}_{2}\text {O}$
(iv) $\left [ \text {Cr}\left ( \text {H}_{2}\text {O}\right )_{6} \right ]\text {Cl}_{3}$
Answer:
Option (iv) is the correct answer.
Explanation: 3 mol of $\text {AgCl}$ indicates that $\text {3Cl}^{-}$ ions are given in the solution. Therefore, the formula should be $\left [ \text {Cr}\left ( \text {H}_{2}\text {O}\right )_{6} \right ]\text {Cl}_{3}$.
Question 5: The correct $\text {IUPAC}$ name of $\left [ \text {Pt}\left ( \text {NH}_{3} \right )_{2}\text {Cl}_{2} \right ]$ is
(i) Diamminedichloridoplatinum (II)
(ii) Diamminedichloridoplatinum (IV)
(iii) Diamminedichloridoplatinum (0)
(iv) Dichloridodiammineplatinum (IV)
Answer:
Option (i) is the correct answer.
Explanation: diamminedichloridoplatinum (II) is $\left [ \text {Pt}\left ( \text {NH}_{3} \right )_{2}\text {Cl}_{2} \right ]$.
Question 6 The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species?
(i) $\left [ \text {Fe}\left ( \text {CO} \right ) _{5}\right ]$
(ii) $\left [ \text {Fe}\left ( \text {CN} \right ) _{6}\right ]^{3-}$
(iii) $\left [ \text {Fe}\left ( \text {C}_{2}\text {O}_{4} \right ) _{3}\right ]^{3-}$
(iv) $\left [ \text {Fe}\left ( \text {H}_{2}\text {O} \right ) _{6}\right ]^{3+}$
Answer:
Option (iii) is the correct answer.
Explanation: Chelation is the formation of cycle of linkages between the metal ion and ligands is responsible for stabilizing the coordination compounds. A ligand which chelates any metal ion is known as chelating ligand.
Here, oxalate ion is the chelating ligand and $\left [ \text {Fe}\left ( \text {C}_{2}\text {O}_{4} \right ) _{3}\right ]^{3-}$ is the coordination compound. Hence, the oxalate ions stabilize the coordination compound by chelating $\text {Fe}^{3+}$ ions.
Question 7 Indicate the complex ion which shows geometrical isomerism.
(i) $\left [ \text {Cr}\left ( \text {H}_{2}\text {O} \right )_{4}\text {Cl}_{2} \right ]^{+}$
(ii) $\left [ \text {Pt}\left ( \text {NH}_{3}\right )_{3}\text {Cl}\right ]$
(iii) $\left [ \text {Co}\left ( \text {NH}_{3}\right )_{6}\right ]^{3+}$
(iv) $\left [ \text {Co}\left ( \text {CN}\right )_{5}\left ( \text {NC} \right )\right ]^{3-}$
Answer:
Option (i) is the correct answer.
Explanation :
(a) $\left [ \text {Cr}\left ( \text {H}_{2}\text {O} \right )_{4}\text {Cl}_{2} \right ]^{+}$
Question 8 The CFSE for octahedral $\left [ \text {CoCl}_{6} \right ]^{4-}$ is 18,000 cm–1. The CFSE for tetrahedral $\left [ \text {CoCl}_{4} \right ]^{2-}$ will be
(i) 18,000 $\text {cm}^{-1}$
(ii) 16,000 $\text {cm}^{-1}$
(iii) 8,000 $\text {cm}^{-1}$
(iv) 20,000 $\text {cm}^{-1}$
Answer:
Option (iii) is the correct answer.
Explanation :
(c) CFSE for tetrahedral complex is $\Delta _{t}=\left ( \frac{4}{9} \right )\Delta _{0}$
$\Delta _{t}=\frac{4}{9} \times 18000=8,000 \; \text {cm}^{-1}$
Question 9 Due to the presence of ambidentate ligands coordination compounds show isomerism. Palladium complexes of the type $\left [ \text {Pd}\left ( \text {C}_{6}\text {H}_{5} \right )_{2}\left ( \text {SCN} \right )_{2} \right ]$ and $\left [ \text {Pd}\left ( \text {C}_{6}\text {H}_{5} \right )_{2}\left ( \text {NCS} \right )_{2} \right ]$ are
(i) linkage isomers
(ii) coordination isomers
(iii) ionisation isomers
(iv) geometrical isomers
Answer:
Option (i) is the correct answer.
Explanation: Ambident ligands are those ligands which have two different bonding sites. For example: $\text {NCS,NO}_{2}$ etc.
In this case, $\text {NCS}$ contains two binding sites at N and S. Therefore, $\text {NCS}$ (thiocyanate) will be bonding to the metal in these two ways; $\text {M}\leftarrow \text {NCS}$ or $\text {M}\rightarrow \text {SNC}$
Hence, the coordination compounds containing $\text {NCS}$ as ligands can show linkages isomerism; $\left [ \text {Pd}\left ( \text {C}_{6}\text {H}_{5} \right )_{2}\left ( \text {SCN} \right )_{2} \right ]$ and $\left [ \text {Pd}\left ( \text {C}_{6}\text {H}_{5} \right )_{2}\left ( \text {NCS} \right )_{2} \right ]$ are linkage isomers.
Question 10 The compounds $\left [ \text {Co}\left ( \text {SO}_{4}\right ) \left ( \text {NH}_{3} \right ) _{5}\right ]\text {Br}$ and $\left [ \text {Co}\left ( \text {SO}_{4}\right ) \left ( \text {NH}_{3} \right ) _{5}\right ]\text {Cl}$ represent
(i) linkage isomerism
(ii) ionisation isomerism
(iii) coordination isomerism
(iv) no isomerism
Answer:
Option (iv) is the correct answer.
Explanation:$\left [ \text {Co}\left ( \text {SO}_{4}\right ) \left ( \text {NH}_{3} \right ) _{5}\right ]\text {Br}$ and $\left [ \text {Co}\left ( \text {SO}_{4}\right ) \left ( \text {NH}_{3} \right ) _{5}\right ]\text {Cl}$ are different compounds and show no isomerism.
Question 11 A chelating agent has two or more than two donor atoms to bind to a single metal ion. Which of the following is not a chelating agent?
(i) thiosulphate
(ii) oxalate
(iii) glycinato
(iv) ethane-1,2-diamine
Answer:
Option (i) is the correct answer.
Explanation: Thiosulphate or $\text {S}_{2}\text {O}_{3}^{2-}$ is a monodentate ligand and not a chelating agent.
Question 12 Which of the following species is not expected to be a ligand?
(i) $\text {NO}$
(ii) $\text {NH}_{4}^{+}$
(iii) $\text {NH}_{2}\text {CH}_{2}\text {CH}_{2}\text {NH}_{2}$
(iv) $\text {CO}$
Answer:
Option (ii) is the correct answer.
Explanation: To form M-L bonds, the ligand must donate a pair of electrons or any loosely held electron pair to the metal.
eg:
Among all $\text {NH}_{4}^{+}$ does not have any pair of electrons.
Hence $\text {NH}_{4}^{+}$ is not a ligand.
Question 13 What kind of isomerism exists between $\left [ \text {Cr}\left ( \text {H}_{2}\text {O} \right )_{6} \right ]\text {Cl}_{3}\; \text {(violet)}$ and $\left [ \text {Cr}\left ( \text {H}_{2}\text {O} \right )_{5} \text {Cl}\right ]\text {Cl}_{2}.\text {H}_{2}\text {O}\; \text {(greyish-green)}$ ?
(i) linkage isomerism
(ii) solvate isomerism
(iii) ionisation isomerism
(iv) coordination isomerism
Answer:
Option (ii) is the correct answer.
Explanation: The compound has different water molecules in number inside and outside of the coordination sphere.
Question 14 IUPAC name of $\left [ \text {Pt}\left ( \text {NH}_{3} \right )_{2}\text {Cl}\left ( \text {NO}_{2} \right ) \right ]$ is :
(i) Platinum diaminechloronitrite
(ii) Chloronitrito-N-ammineplatinum (II)
(iii) Diamminechloridonitrito-N-platinum (II)
(iv) Diamminechloronitrito-N-platinate (II)
Answer:
Option (iii) is the correct answer.
Explanation: diamminechloridonitrito-N-platinum (II) is $\left [ \text {Pt}\left ( \text {NH}_{3} \right )_{2}\text {Cl}\left ( \text {NO}_{2} \right ) \right ]$
NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: MCQ (Type 2)
TheCoordination Compounds Class 12 Chemistry Chapter 9 NCERT Exemplar Solutions are provided here with simple explanations. Learn more through these advanced MCQs
Question 15 The atomic number of $\text {Mn, Fe and Co}$ are 25, 26 and 27 respectively. Which of the following inner orbital octahedral complexions are diamagnetic?
(i) $\left [ Co(NH_{3})_{6} \right ]^{3+}$
(ii) $\left [ Mn(CN)_{6} \right ]^{3-}$
(iii) $\left [ Fe(CN)_{6} \right ]^{4-}$
(iv) $\left [ Fe(CN)_{6} \right ]^{3-}$
Answer:
Option (i) and (iii) are the correct answers.
Explanation :
(a,c)
(a) Molecular orbital electronic configuration of $Co^{3+}$ in $\left [ Co(NH_{3})_{6} \right ]^{3+}$is
Number of unpaired electron = 0
Magnetic property = Diamagnetic
(b) Molecular orbital electronic configuration of $Mn^{3+}$ in $\left [ Mn(CN)_{6} \right ]^{3-}$
Number of unpaired electron = 2
Magnetic property = Paramagnetic
(c) Molecular orbital electronic configuration of $Fe^{2+}$ in $\left [ Fe(CN)_{6} \right ]^{4-}$
Number of unpaired electron = 0
Magnetic property = Diamagnetic
(d) Molecular orbital electronic configuration of $Fe^{3+}$ in $\left [ Fe(CN)_{6} \right ]^{3-}$
Number of unpaired electron = 1
Magnetic property = Paramagnetic
Question 16 The atomic number of $\text {Mn, Fe, Co and Ni}$ are 25, 26 27 and 28 respectively. Which of the following outer orbital octahedral complexes have the same number of unpaired electrons?
(i) $\left [ MnCl_{6} \right ]^{3-}$
(ii) $\left [ FeF_{6} \right ]^{3-}$
(iii) $\left [ CoF_{6} \right ]^{3-}$
(iv) $\left [ Ni(NH_{3})_{6} \right ]^{2+}$
Answer:
Option (i) and (iii) are the correct answer.
Explanation :
(a,c)
$[MnCl_{6}]^{3-}: Mn^{3+}(3d^{4})$
$[CoF_{6}]^{3-}: Co^{3+}(3d^{6})$
Question 17 Which of the following options are correct for $[Fe(CN)_{6}]^{3-}$ complex?
(i) $d^{2}sp^{3}$hybridisation
(ii) $sp^{3}d^{2}$hybridisation
(iii) paramagnetic
(iv) diamagnetic
Answer:
Option (i) and (iii) are the correct answers.
Explanation :
(a,c) $[Fe(CN)_{6}]^{3-}$
Magnetic nature -paramagnetic.
Question 18 An aqueous pink solution of cobalt(II) chloride changes to deep blue on the addition of an excess of $HCl$. This is because____________.
(i) $[Co(H_{2}O)_{6}]^{2+}$ is transformed into $[CoCl_{6}]^{4-}$
(ii) $[Co(H_{2}O)_{6}]^{2+}$ is transformed into $[CoCl_{4}]^{2-}$
(iii) tetrahedral complexes have smaller crystal field splitting than octahedral complexes.
(iv) tetrahedral complexes have larger crystal field splitting than octahedral complex.
Answer:
Option (ii) and (iii) are the correct answers.
Explanation: The transition of electrons from t2g to eg energy level gives an aqueous pink solution of cobalt (II) chloride in $[Co(H_{2}O)_{6}]^{2+}$ complex and when excess $HCl$ is added to the solution:
(i) $[Co(H_{2}O)_{6}]^{2+}$ is transformed into $[CoCl_{4}]^{2-}$
(ii) Tetrahedral complexes have smaller crystal field spliting than octahedral complexes because $\Delta _{r}=\frac{4}{9}\Delta _{0}$
Hence, options (b) and (c) are correct.
Question 19 Which of the following complexes is homoleptic?
(i) $\left [ Co\left ( NH_{3} \right )_{6} \right ]^{3+}$
(ii) $\left [ Co\left ( NH_{3} \right )_{4}Cl_{2} \right ]^{+}$
(iii) $\left [ Ni\left ( CN \right )_{4} \right ]^{2-}$
(iv) $\left [ Ni\left ( NH_{3} \right )_{4}Cl_{2} \right ]$
Answer:
Option (i) and (iii) are the correct answers.
Explanation: Both $\text {Co and Ni}$ are attached the same kind of ligands in the complexes $\left [ Co\left ( NH_{3} \right )_{6} \right ]^{3+}$ and $\left [ Ni\left ( CN \right )_{4} \right ]^{2-}$. Hence they are homoleptic.
Question 20 Which of the following complexes are heteroleptic?
(i) $\left [ Cr(NH_{3})_{6} \right ]^{3+}$
(ii) $\left [ Fe(NH_{3})_{4}Cl_{2} \right ]^{+}$
(iii) $\left [ Mn(CN)_{6}\right ]^{4-}$
(iv) $\left [ Co(NH_{3})_{4}Cl_{2} \right ]$
Answer:
Option (ii) and (iv) are the correct answers.
Explanation: Metal is bonded to more than one kind of ligands in complexes $\left [ Fe(NH_{3})_{4}Cl_{2} \right ]^{+}$and $\left [ Co(NH_{3})_{4}Cl_{2} \right ]$ therefore they are heteroleptic.
Question 21 Identify the optically active compounds from the following: -
(i) $[Co(en)_{3}]^{3+}$
(ii) $trans-[Co(en)_{2}Cl_{2}]^{+}$
(iii) $cis-[Co(en)_{2}Cl_{2}]^{+}$
(iv) $[Cr(NH_{3})_{5}Cl]$
Answer:
Option (i) and (iii) are the correct answers.
$[Co(en)_{3}]^{3+}$ and $cis-[Co(en)_{2}Cl_{2}]^{+}$ are optically active compounds because their mirror images are non superimposable isomer.
Non-superimposable isomers of $[Co(en)_{3}]^{3+}$
Non-superimosable isomers of $[Co(en)_{2}Cl_{2}]^{+}$
Question 22 Identify the correct statements for the behaviour of ethane-1, 2-diamine as a ligand.
(i) It is a neutral ligand.
(ii) It is a didentate ligand.
(iii) It is a chelating ligand.
(iv) It is a unidentate ligand.
Answer:
Option (i), (ii) and (iii) are the correct answers.
Explanation :
Ethane 1, 2-diamine is a neutral and didentate ligand. It is a chelating agent.
Question 23 Which of the following complexes show linkage isomerism?
(i) $[Co(NH_{3})_{5}(NO_{2})]^{2+}$
(ii) $[Co(H_{2}O)_{5}CO]^{3+}$
(iii) $[Cr(NH_{3})_{5}SCN]^{2+}$
(iv) $[Fe(en)_{2}Cl_{2}]^{+}$
Answer:
Option (i) and (iii) are the correct answers.
Explanation:$NO_{2}$ and $SCN$ show linkage isomerism because they are ambidentate ligands.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: Short Answer Type
The short-answer type questions are also given here in the NCERT Exemplar Class 12 Chemistry Solutions Chapter 9 coordination compounds for practice. This section contains important questions that are asked in the exams. Practice short answer types from the questions below
The correct order of increase in conductivity is given below:
$[Co(NH_{3})_{3}Cl_{3}] < [Co(NH_{3})_{4}Cl_{2}] Cl < [Cr(NH_{3})_{5}Cl]Cl_{2} < [Co(NH_{3})_{6}]Cl_{3}$
There should be one free chlorine atom left outside the coordination sphere if silver chloride is formed in the reaction. $\left [ Cr\left ( H_{2}O \right )_{4}Cl_{2} \right ]Cl$ has to be the formula and it is called tetraaquadichlorido chromium(III) chloride.
Question 26 A complex of the type $[M(AA)_2X_2]^{n+}$is known to be optically active. What does this indicate about the structure of the complex? Give one example of such complex.
Answer:
If the complex is optically active then its structure has to be cis-octahedral. $[Co(en)_{2}Cl_{2}]^{+}$ is one of the prominent examples of an optically stable compound.
Question 27 The magnetic moment of $\left [ MnCl_{4} \right ]^{2-}$ is $5.92\; BM$. Explain giving reason.
Answer:
A magnetic moment of 5.92 BM indicates that there are at least 5 unpaired electrons. The formula for Magnetic Moment $=\sqrt{n(n+2)}$
As four ligands are attached to $Mn^{2+}$, therefore the geometry tetrahedral with 5 unpaired electrons gives a magnetic moment of 5.92 BM.
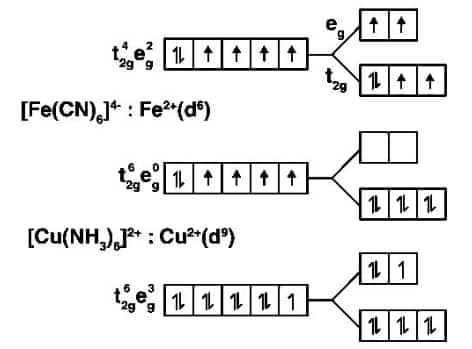
$t^{4}_{2g} e^{2} _{g}$ will be the electronic configuration where it indicates that it has 4 unpaired electrons and is paramagnetic, with a weak ligand $\Delta _{0}<p$. The configuration will be $t^{6}_{2g} e^{0}_{g}$ with strong field ligand and there will not be any unpaired electrons. Hence it is diamagnetic.
Question 29 Why are low spin tetrahedral complexes not formed?
Answer:
The crystal field splitting energy is very low for tetrahedral complexes. The crystal field splitting energy is lower than pairing energy, therefore pairing of electrons is not favorable. Therefore, the complexes may not form low spin complexes
Question 30 Give the electronic configuration of the following complexes based on the basis of Crystal Field Splitting theory.
$[CoF_{6}]^{3-}, [Fe(CN)_{6}]^{4-} and \; [Cu(NH_{3})_{6}]^{2+}.$
Answer:
$\left[\mathrm{CoF}_6\right]^{3-}$: $\mathrm{Co}^{3+}$(d6)
Question 31 Explain why $[Fe(H_{2}O)_{6}]^{3+}$ has a magnetic moment value of 5.92 BM whereas $[Fe(CN)_{6}]^{3-}$ has a value of only 1.74 BM.
Answer:
[Fe(CN)6]3- involves d2sp3 hybridisation with one unpaired electron and [Fe(H2O)6]3+ involves sp3d2 hybridisation with five unpaired electrons. This difference is due to the presence of strong ligand CN– and weak ligand H2O in these complexes
Answer:
The correct increasing order of crystal field is:
$[Cr(Cl)_{6}]^{3-}< [Cr(NH_{3})_{6}]^{3+} < [Cr(CN)_{6}]^{3-}$
According to the spectrochemical series, this is also the correct increasing of order of the field strength.
Question 33 Why do compounds having similar geometry have a different magnetic moment?
Answer:
The difference lies in the number of paired and unpaired electrons. Strong field ligand can easily cause the pairing of electrons while the weak field ligands are not able to form pairs. The magnetic moment of a compound depends on the number of unpaired or paired electrons. Hence it is different for compounds having similar geometry.
Question 34 $\text {CuSO}_{4}.\text {5H}_{2}\text {O}$ is blue while $\text {CuSO}_{4}$ is colourless. Why?
Answer:
In $\text {CuSO}_{4}.\text {5H}_{2}\text {O}$, the extra 5 water act as ligands. They apparently excite the electrons to the higher d orbital which shows the blue color. In $\text {CuSO}_{4}$, there are no water molecule that can act as ligands, so there is no crystal field slitting happening, hence there is no color.
Question 35 Name the type of isomerism when ambidentate ligands are attached to a central metal ion. Give two examples of ambidentate ligands.
Answer:
The ligands which have two binding sites are known as Ambidendate ligands. Some of the examples are: Nitrite-N, Nitrito-O and Isothiocyanato, Thiocyanato.
Linkage Isomerism is a type of isomerism when ambidentate ligands get attached to central metal ions. This happens because it only differs in the atom that they are linked the central metal ion.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: Matching Type
The chemistry chapter 9 important questions are discussed below. These are generally asked in exams to test your knowledge. These solutions are quite helpful for competitive exams.
|
Column I (Complex ion) |
Column II (Colour) |
|
(A) $\left [ Co\left ( NH_{3} \right ) _{6}\right ]^{3+}$ |
1. Violet |
|
(B) $\left [ Ti\left ( H_{2}O\right ) _{6}\right ]^{3+}$ |
2. Green |
|
(C) $\left [ Ni\left ( H_{2}O\right ) _{6}\right ]^{2+}$ |
3. Pale blue |
|
(D) $\left [ Ni\left ( H_{2}O\right ) _{4}(en)\right ]^{2+}(aq)$ |
4.Yellowish Orange |
|
|
5.Blue |
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (3) B (2) C (4) D (1)
(iv) A (4) B (1) C (2) D (3)
Answer:
Option (ii) is the correct answer.
(a) $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}$ is Yellowish Orange because the cobalt(III) ammine complex typically appears yellowish orange due to ligand field transitions.
(b) $\left[T i\left(H_2 O\right)_6\right]^{3+}$ is Pale Blue.
(c) $\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ is Green, the nickel(II) aqua complex is characteristically green in solution.
(d) $\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_4(\mathrm{en})\right]^{2+}$ is Violet because mixed-ligand nickel (II) complex can show a violet colour due to stronger ligand field splitting by ethylenediamine.
|
Column I (Coordination Compound) |
Column II ( Central metal atom) |
|
A. Chlorophyll |
1. rhodium |
|
B. Blood pigment |
2. Cobalt |
|
C.Wilkinson catalyst |
3.Calcium |
|
D Vitamin B12 |
4.Iron |
|
|
5. magnesium |
(i) A (5) B (4) C (1) D (2)
(ii) A (3) B (4) C (5) D (1)
(iii) A (4) B (3) C (2) D (1)
(iv) A (3) B (4) C (1) D (2)
Answer:
Option (i) is the correct answer.
(a) Chlorophyll contains a central magnesium $\left(\mathrm{Mg}^{2+}\right)$ ion.
(b) The blood pigment haemoglobin contains a central iron $\left(\mathrm{Fe}^{2+} / \mathrm{Fe}^{3+}\right)$ ion.
(c) Wilkinson's catalyst $\left[\mathrm{RhCl}\left(\mathrm{PPh}_3\right)_3\right]$ contains rhodium as the central atom.
(d) Vitamin $\mathrm{B}_{12}$ contains a central cobalt (Co) atom.
|
Column I (Complex ion) |
Column II (Hybridisation, number of unpaired electrons) |
|
A. $\left [ Cr\left ( H_{2}O \right )_{6} \right ]^{3+}$ |
1. $dsp^{2},1$ |
|
B. $\left [ Co\left ( CN \right )_{4} \right ]^{2-}$ |
2.$sp^{3}d^{2},5$ |
|
C. $\left [ Ni\left ( NH_{3} \right )_{6} \right ]^{2+}$ |
3. $d^{2}sp^{3},3$ |
|
D. $\left [ MnF_{6} \right ]^{4-}$ |
4. $sp^{3},4$ |
|
|
5.$sp^{3}d^{2},2$ |
(i) A (3) B (1) C (5) D (2)
(ii) A (4) B (3) C (2) D (1)
(iii) A (3) B (2) C (4) D (1)
(iv) A (4) B (1) C (2) D (3)
Answer:
Option (i) is the correct answer.
Explanation : (i) Strong field ligand forms an inner orbital complex with hybridization $d^{2}sp^{3}$
(ii) Weak field ligand forms an outer orbital complex with hybridization $d^{2}sp^{3}$.
According to VBT, hybridization and the number of unpaired electrons of coordination compounds can be calculated as
(a) $\left [ Cr\left ( H_{2}O \right )_{6} \right ]^{3+}$
MOEC (Molecular orbital electronic configuration) of $Cr^{3+}$ in $\left [ Cr\left ( H_{2}O \right )_{6} \right ]^{3+}$ is
Hybridisation = $d^{2}sp^{3}$
n (number of unpaired electrons) = 3
(b) $\left [ Co\left ( CN \right )_{4} \right ]^{2-}$ is
MOEC of $Co^{2+}$ in $\left [ Co\left ( CN \right )_{4} \right ]^{2-}$ is
Hybridisation = $dsp^{2}$
n (number of unpaired electrons) = 1
(c) $\left [ Ni\left ( NH_{3} \right )_{6} \right ]^{2+}$
MOEC of $Ni^{2+}$ in $\left [ Ni\left ( NH_{3} \right )_{6} \right ]^{2+}$ is
Hybridisation = $sp^{3}d^{2}$
n (number of unpaired electrons) = 2
(d) $\left [ MnF_{6} \right ]^{4-}$
MOEC of $Mn^{2+}$ in $\left [ MnF_{6} \right ]^{4-}$ is
Hybridisation = $sp^{3}d^{2}$
n (number of unpaired electrons) = 5
Hence , correct choice can be represented by (a).
|
Column I (Complex species) |
Column II (Isomerism) |
|
A. $[Co (NH_{3})_{4}Cl_{2}]^{+}$ |
1. Optical |
|
B. $cis-[Co (en)_{2}Cl_{2}]^{+}$ |
2. Ionisation |
|
C. $[Co (NH_{3})_{5}(NO_{2})]Cl_{2}$ |
3. Coordination |
|
D. $[Co (NH_{3})_{6}]\left [ Cr(CN)_{6} \right ]$ |
4. Geometrical |
|
|
5. Linkage |
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (4) B (1) C (5) D (3)
(iv) A (4) B (1) C (2) D (3)
Answer:
Option (iv) is the correct answer.
Explanation : The types of ligands, arrangement of ligands and the geometry of coordination decide the isomerism in coordination compounds.
(a) $[Co (NH_{3})_{4}Cl_{2}]^{+}$ shows geometrical isomerism due to presence of two types of ligand whoes $[Co (NH_{3})_{4}Cl_{2}]^{+}$ arrangement around central metal ion
(b) $cis-[Co (en)_{2}Cl_{2}]^{+}$ shows an optical isomer due to its non - superimposable mirror image relationship.
(c) $[Co (NH_{3})_{5}(NO_{2})]Cl_{2}$ shows an ionization isomer due to its interchanging ligand from outside the ionization sphere.
(d) $[Co (NH_{3})_{6}]\left [ Cr(CN)_{6} \right ]$ shows coordination isomer due to interchanging of ligand in between two metal ions from one coordination sphere to another coordination sphere.
Hence, correct choice is (iv)
|
Column I (Compound) |
Column II (Oxidation state of Co) |
|
A. $\left [ Co\left ( NCS \right )\left ( NH_{3} \right )_5 \right ]\left ( SO_{3} \right )$ |
1. +4 |
|
B. $\left [ Co\left ( NH_{3} \right )_{4}Cl_{2} \right ] SO_{4}$ |
2. 0 |
|
C. $Na_{4}\left [ Co(S_{2}O_{3}) _{3}\right ]$ |
3. +1 |
|
D. $[Co_{2}(CO)_{8}]$ |
4. +2 |
|
|
5. +3 |
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (5) B (1) C (4) D (2)
(iv) A (4) B (1) C (2) D (3)
Answer:
Option (iii) is the correct answer.
Explanation: The oxidation state of the whole molecule is considered to calculate the oxidation state of CMI (central metal ion).
(a) $\left [ Co\left ( NCS \right )\left ( NH_{3} \right )_5 \right ]\left ( SO_{3} \right )$
Let oxidation state of $Co$ be x.
$x-1+5\times 0=+2$
$x=+2+1=+3$
(b) $\left [ Co\left ( NH_{3} \right )_{4}Cl_{2} \right ] SO_{4}$
Let oxidation state of $Co$ = x.
$\Rightarrow x+4\times0+2\times(-1)=+2$
$x-2=+2$
$x=4$
(c) $Na_{4}\left [ Co(S_{2}O_{3}) _{3}\right ]$
Let oxidation state of $Co$ = x.
$x+3\times(-2)=-4$
$x-6=-4$
$x=-4+6=+2$
(d) $[Co_{2}(CO)_{8}]$
Let oxidation state of $Co$ = x.
$x-8\times 0=0$
$x=0$
NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: Assertion and Reason Type
This is one of the most important sections covered in the NCERT exemplar solutions Class 12 chemistry chapter 9. These questions will improve your critical thinking.
Question 41 In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Toxic metal ions are removed by the chelating ligands.
Reason: Chelate complexes tend to be more stable.
(i) Assertion and reason both are true, the reason is the correct explanation of assertion.
(ii) Assertion and reason both are true, but the reason is not the correct explanation of assertion.
(iii) An assertion is true, the reason is false.
(iv) The assertion is false, the reason is true.
Answer:
Option (i) is correct answer.
Explanation: Ligands chelate the metal ions by forming stable complex when solution of chelating ligands is added to a solution containing toxic metals.
Question 42 In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion:$[Cr(H_{2}O)_{6}]Cl_{2}$ and $[Fe(H_{2}O)_{6}]Cl_{2}$ are reducing in nature.
Reason: Unpaired electrons are present in their d-orbitals.
(i) Assertion and reason both are true, the reason is the correct explanation of assertion.
(ii) Assertion and reason both are true, but the reason is not the correct explanation of assertion.
(iii) An assertion is true, the reason is false.
(iv) The assertion is false, the reason is true.
Answer:
Option (ii) is correct answer.
Explanation: In both the complexes, $\text {Cr and Fe}$ Coexist as $\text {Cr}^{2+}$ and $\text {Fe}^{2+}$ respectively, these both complexes become stable when oxidation of metal ion happens to $\text {Cr}^{3+}$ and $\text {Fe}^{3+}$.
Question 43 In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Linkage isomerism arises in coordination compounds containing ambidentate ligand.
Reason: The Ambidentate ligand has two different donor atoms.
(i) Assertion and reason both are true, the reason is the correct explanation of assertion.
(ii) Assertion and reason both are true, but the reason is not the correct explanation of assertion.
(iii) An assertion is true, and the reason is false.
(iv) The assertion is false, the reason is true.
Answer:
Option (i) is correct answer.
Explanation: Two different donor atoms in ambidentate ligand gives rise to linkage isomerism.
Question 44 In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
(i) Assertion and reason both are true, the reason is the correct explanation of assertion.
(ii) Assertion and reason both are true, but the reason is not the correct explanation of assertion.
(iii) An assertion is true, the reason is false.
(iv) The assertion is false, the reason is true.
Answer:
Option (iii) is correct answer.
Explanation: A different arrangement of ligands is not possible for complexes of MX6 and MX5L type. The complexes MA4B2, M(AA)2B2 and MA3B3 with coordination number 6 show geometrical isomerism.
Question 45 In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion:$[Fe(CN)_{6}]^{3-}$ ion shows magnetic moment corresponding to two unpaired electrons.
Reason: Because it has $d^{2}sp^{3}$ type hybridisation.
(i) Assertion and reason both are true, the reason is the correct explanation of assertion.
(ii) Assertion and reason both are true, but the reason is not the correct explanation of assertion.
(iii) An assertion is true, the reason is false.
(iv) The assertion is false, the reason is true.
Answer:
Option (iv) is correct answer.
Explanation: Corresponding to one unpaired ion, $[Fe(CN)_{6}]^{3-}$ shows magnetic moment.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 9: Long Answer Type
The following are the long-answer type questions that needs more practice and learning. These are the chemistry chapter 9 important questions that are asked in the exams.
(i)
$[ CoF_{6}]^{3-}$ :
$\text {Co}^{3+}=\text {3d}^{6}$
Number of unpaired electrons =4
Magnetic moment
$=\sqrt{n(n+2)}=\sqrt{4(4+2)}=4.9 B.M$
$[Co(H_{2}O)_{6}]^{2+}$
$\text {Co}^{2+}=\text {3d}^{7}$
Number of unpaired electrons =3
Magnetic moment =$\sqrt{3(3+2)}=3.87 B.M$
$[Co(CN)_{6}]^{3-}$
$\text {Co}^{3+}=\text {3d}^{6}$
Number of unpaired electrons =0
Diamagnetic .
(ii)
$[ FeF_{6}]^{3-}$
$Fe^{3+}=3d^{5}$
Number of unpaired electrons =5
Magnetic moment = $\sqrt{5(5+2)}=5.92\; B.M$
$[Fe(H_{2}O)_{6}]^{2+}$
$Fe^{2+}=3d^{6}$
Number of unpaired electrons =4
Magnetic moment = $\sqrt{4(4+2)}=4.9 \; B.M$
$[Fe(CN)_{6}]^{4-} :$
$Fe^{2+}=3d^{6}$
Diamagnetic.
Answer:
$\left [ Mn(CN)_{6} \right ]^{3-} :$
$Mn^{3+}=3d^{4}$
(i) Hybridisation - $d^{2}sp^{3}$
(ii) Inner orbital complex
(iii) Paramagnetic
(iv) Magnetic moment $\sqrt{2(2+2)}=2.87 \; B.M$
$\left [ Co(NH_{3})_{6} \right ]^{3+}:$
$Co^{3+}=3d^{6}$
(i) Hybridisation - $d^{2}sp^{3}$
(ii) Inner orbital complex
(iii) Diamagnetic
(iv) Magnetic moment=0
$\left [ Cr(H_{2}O) _{6}\right ]^{3+}$
$Cr^{3+}=3d^{3}$
(i) Hybridisation - $d^{2}sp^{3}$
(ii) Inner orbital complex
(iii) Paramagnetic
(iv) Magnetic moment=$\sqrt{3(3+2)}=3.87 \; B.M$
$\left [ FeCl_{6} \right ]^{4-}$
$Fe^{2+}=3d^{6}$
(i) Hybridisation - $sp^{3}d^{2}$
(ii) Outer orbital complex
(iii) Paramagnetic
(iv) Magnetic moment=$\sqrt{4(4+2)}=4.9 \; B.M$
$\text {CoSO}_{4}\text {Cl}.\text {5NH}_{3}$ :
(i). The first isomer A reacts with $\text {AgNO}_{3}$ and not with $\text {BaCl}_{2}$, that means it has a $\text {Cl}^{-}$ ion outside the coordination sphere. Therefore, A is $\left [ Co\left ( NH_{3} \right )_{5}SO_{4} \right ]Cl$
B reacts with $\text {BaCl}_{2}$ and does not react with $\text {AgNO}_{3}$, that means it has $SO^{-}_{4}$ outside the coordination sphere. Therefore B is $\left [ Co\left ( NH_{3} \right )_{5}Cl\right ]SO_{4}$
(ii). Ionisation Isomerism
(iii). A is Pentaamminesulphatocobalt (III) chloride while B is Pentaamminechlorocobalt (III) sulphate.
Question 49 What is the relationship between the observed color of the complex and the wavelength of light absorbed by the complex?
Answer:
Some part of the white light is absorbed when it falls on a complex compound. The crystal field splitting is inversely proportional to the wavelength absorbed by the complex i.e. higher the crystal field splitting, lower is the wavelength absorbed by the complex. The observed color by the complex is the color generated from the wavelength left over.
Question 50 Why are different colors observed in octahedral and tetrahedral complexes for the same metal and same ligands?
Answer:
In an octahedral complex, the lower wavelength of light is absorbed more than tetrahedral complex for the same metal and ligand. The formation of the d-orbital splitting is inverted in the tetrahedral coordination entity and it is smaller than the octahedral field splitting. It can be shown below for the same metal ligand.
$\Delta _{t}=\left ( \frac{4}{9} \right )\Delta _{o}.$
Class 12 Chemistry NCERT Chapter 9: Higher Order Thinking Skills (HOTS) Questions
HOTS-type questions are covered to improve your problem-solving ability and conceptual thinking. The class 12 chemistry chapter 9 questions and answers are given below that will help you tackle complex problems. Students can follow coordination compounds class 12 notes to learn the concepts in detail. The questions below will help you evaluate your understanding of the concepts.
Question 1: Given below are two statements :
Statement (I) : In octahedral complexes, when $\Delta_{\mathrm{o}}<\mathrm{P}$ high spin complexes are formed. When $\Delta_{\mathrm{o}}>\mathrm{P}$ low spin complexes are formed.
Statement (II) : In tetrahedral complexes because of $\Delta_t<\mathrm{P}$, low spin complexes are rarely formed.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Statement I is correct but Statement II is incorrect.
(2) Both Statement I and Statement II are incorrect
(3) Statement I is incorrect but Statement II is correct
(4) Both Statement I and Statement II are correct
Answer:
In octahedral complex $(\mathrm{CN}=6)$
If $\Delta_0<$ P.E. , then high-spin complexes are formed
If $\Delta_0>$ P.E. , then low spin complexes are formed
But in tetrahedral complex $(\mathrm{CN}=4)$
$\Delta_{\mathrm{t}}<$ P.E. , then mainly high spin complexes are formed and rarely low spin complexes are formed.
Hence, the correct answer is the option (4).
Question 2: Identify the homoleptic complexes with odd number of $d$ electrons in the central metal.
(A) $\left[\mathrm{FeO}_4\right]^{2-}$
(B) $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$
(C) $\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NO}\right]^{2-}$
(D) $\left[\mathrm{CoCl}_4\right]^{2-}$
(E) $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_3 \mathrm{~F}_3\right]$
Choose the correct answer from the options given below :
(1) (B) and (D) only
(2) (C) and (E) only
(3) (A), (B) and (D) only
(4) (A), (C) and (E) only
Answer:
To determine the complexes that are both homoleptic and have an odd number of d-electrons, we analyze each option based on the oxidation state and electron count of the central metal.
1. $\left[\mathrm{FeO}_4\right]^{2-}$ : Fe is in +6 oxidation state (since $\mathrm{Fe}=[\mathrm{Ar}] 3 \mathrm{~d}^6$, and losing six electrons leaves $\mathrm{d}^2$ ). This is not an odd number
2. $\left[\mathrm{Fe}(\mathrm{CN})_6 \right]^{3-}$: Iron in $+3\left(\mathrm{Fe}^{3+}, \mathrm{d}^5\right)$. Since all ligands are $\mathrm{CN}^{-}$, the complex is homoleptic and has an odd number (5) of d-electrons.
3. $\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NO}\right]^{2-}$: The NO ligand introduces $\pi$-back bonding and redox ambiguity, making it a nonhomoleptic complex. Hence, it is not considered.
4. $\left[\mathrm{CoCl}_4\right]^{2-}:$ Cobalt is in $+2\left(\mathrm{Co}^{2+}, \mathrm{d}^7\right)$. Since 7 is odd and all ligands are $\mathrm{Cl}^{-}$(making it homoleptic), this satisfies both conditions.
5. $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_3 \mathrm{~F}_3\right]$ : This complex contains two different ligands $\left(\mathrm{H}_2 \mathrm{O}\right.$ and $\left.\mathrm{F}^{-}\right)$, so it is not homoleptic and does not qualify.
Hence, the correct answer is option (1).
Question 3: The number of paramagnetic metal complex species among $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}, \quad\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}$, $\left[\mathrm{MnCl}_6\right]^{3-},\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$ and $\left[\mathrm{FeF}_6\right]^{3-}$ with same number of unpaired electrons is___________.
Answer:
Number of paramagnetic complexes with same number of unpaired electrons = 2 pairs
2 complexes have 0 unpaired electrons; they are
$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+},\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}$
Now,
$\left[\mathrm{MnCl}_6\right]^{3-}$
$\mathrm{Cl}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Mn}=+3$
$M n$ atomic number $=25 \rightarrow \mathrm{Mn}^{3+}=3 \mathrm{~d}^4$
$\mathrm{Cl}^{-}$is a weak-field ligand $\rightarrow$ high-spin
$3 d^4$ high-spin $\rightarrow 4$ unpaired electrons
$\rightarrow$ Paramagnetic, 4 unpaired
$\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$
$\mathrm{CN}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Mn}=+3$
$\mathrm{Mn}^{3+}=3 \mathrm{~d}^4$
$\mathrm{CN}^{-}=$strong field $\rightarrow$ low-spin
$3 \mathrm{~d}^4$ low-spin $\rightarrow 2$ unpaired electrons
$\rightarrow$ Paramagnetic, 2 unpaired
$\left[\mathrm{CoF}_6\right]^{3-}$
- $\mathrm{F}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Co}=+3$
$\mathrm{Co}^{3+}=3 \mathrm{~d}^6$
$\mathrm{F}^{-}=$weak field $\rightarrow$ high-spin
$3 d^6$ high-spin $\rightarrow 4$ unpaired electrons
$\rightarrow$ Paramagnetic, 4 unpaired
$\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$
$\mathrm{CN}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Fe}=+3$
Fe atomic number $=26 \rightarrow \mathrm{Fe}^{3+}=3 \mathrm{~d}^5$
$\mathrm{CN}^{-}=$strong field $\rightarrow$ low-spin
- $3 d^5$ low-spin $\rightarrow 1$ unpaired electron
$\rightarrow$ Paramagnetic, 1 unpaired
$\left[\mathrm{FeF}_6\right]^{3-}$
$\mathrm{F}^{-}: 6 \times(-1)=-6 \rightarrow \mathrm{Fe}=+3$
-$\mathrm{Fe}^{3+}=3 \mathrm{~d}^5$
$\mathrm{F}^{-}=$weak field $\rightarrow$ high-spin
$3 \mathrm{~d}^5$ high-spin $\rightarrow 5$ unpaired electrons $\rightarrow$ Paramagnetic, 5 unpaired
2 paramagnetic species have the same number of unpaired electrons. $\left[\mathrm{Mn}(\mathrm{Cl})_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-}$
No other pairs have the same number.
Hence, the answer is 2.
Question 4: If $\mathrm{Ni}^{2+}$ is replaced by $\mathrm{Pt}^{2+}$ in the complex $\left[\mathrm{NiCl}_2 \mathrm{Br}_2\right]^{2-}$, which of the following properties are expected to get changed?
A. Geometry
B. Geometrical isomerism
C. Optical isomerism
D. Magnetic properties
(1) A, B and C
(2) A, B and C
(3) A and D
(4) B and C
Answer:
$\left[\mathrm{NiCl}_2 \mathrm{Br}_2\right]^{2-}$ is a tetrahedral complex due to presence of WFL. As Ni is replaced by Pt, Pt belongs to $5 d$-series it will form square planar complex no matter whether the ligand is SFL and WFL. and Square planar complexes show geometrical isomerism.
Hence, the correct answer is option (2).
Question 5: Match the LIST-I with LIST-II
| LIST-I (Complex/Species) | LIST-II (Shape & magnetic moment) | ||
| A. | $\left[\mathrm{Ni}(\mathrm{CO})_4\right]$ | I. | Tetrahedral, 2.8 BM |
| B. | $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ | II. | Square planar, 0 BM |
| C. | $\left[\mathrm{NiCl}_4\right]^2$ | III. | Tetrahedral, 0 BM |
| D. | $\left[\mathrm{MnBr}_4\right]^{2-}$ | IV. | Tetrahedral, 5.9 BM |
Choose the correct answer from the options given below :
(1) A-III, B-IV, C-II, D-I
(2) A-I, B-II, C-III, D-IV
(3) A-III, B-II, C-I, D-IV
(4) A-IV, B-I, C-III, D-II
Answer:
A) $\mathrm{Ni}(\mathrm{CO})_4 \rightarrow \mathrm{Ni}(0) \Rightarrow \mathrm{sp}^3$, tetrahedral, 0 BM
$\left(3 d^{10}\right)$ (pairing)
B) $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-} \rightarrow \mathrm{Ni}^{2+} \Rightarrow \mathrm{dsp}^2$, square planar, 0 BM
$\left(3 \mathrm{~d}^8\right)$ (pairing)
C) $\left[\mathrm{NiCl}_4\right]^{2-} \rightarrow \mathrm{Ni}^{2^{+}}$(no pairing) $\Rightarrow \mathrm{sp}^3$, tetrahedral,
2 unpaired electrons therefore, $2.8 \text { BM }$
D) $\left[\mathrm{MnBr}_4\right]^{2^{-}} \Rightarrow \mathrm{Mn}^{2+} \Rightarrow 3 \mathrm{~d}^5$ (no pairing)
Hence, the correct answer is option (3).
Approach to Solve Questions of Chapter 9
To effectively solve questions from Coordination Compounds Class 12 Chemistry Chapter 9 NCERT Exemplar Solutions students can follow the approaches given below:
1. This is the basic yet important step. Some of the important terminologies are given below:
- Types of ligands
- Coordination number
- Coordination complex/entity
- Central metal ion
- Counter ion
2. While solving questions related to nomenclature, identify the ligands, metal, and their oxidation states, and follow IUPAC rules like:
- Firstly, name the ligand
- Name the ligands in alphabetical order, and if the complex is an anion, then end the naming with 'ate'.
- Oxidation states of metals are written in Roman numerals.
Learn more from coordination compounds class 12 notes.
3. Theories like Werner's theory, valence bond theory, the crystal field theory, forms the basis of this chapter and are frequently asked in exams.
4. It's important to memorise some of the general and basic geometries like Linear, Trigonal planar, Tetrahedral, Square planar, Square pyramidal, Octahedral. The NCERT Exemplar Class 12 Chemistry Solutions Chapter 9 will help you understand these concepts better.
5. A Proper understanding of basic concepts and practice helps students clear their doubts and solve questions effectively. You can get these type of questions in the NCERT Class 12 solutions available on our website. Students can refer NCERT Exemplar textbooks for practice.
Topics Covered in NCERT Exemplar Solutions Class 12 Chemistry Chapter 9
The following are the important topics in the NCERT chemistry chapter 9.
- Coordination Compounds
- Werner's Theory of Coordination Compounds
- Definitions of Some Important Terms Pertaining to Coordination Compounds
- Nomenclature of Coordination Compounds
- Isomerism In Coordination Complexes
- Bonding in Coordination Compounds
- Bonding in Metal Carbonyls
- Stability of Coordination Compounds
- Importance and Applications of Coordination Compounds
NCERT Class 12 Chemistry Exemplar Chapter 9 Important Formulas
The following are the important trends covered in the Class 12 NCERT Chemistry chapter 9. These formulas are highly useful, as questions based on them are often asked directly in the exam.
1. Effective Atomic Number Formula:
$\mathrm{EAN}=Z-x+2 n$
2. Oxidation Number of Central Atom:
Oxidation number $=$ Total charge $-\sum$ charges of ligands
3. Crystal field Stabilisation Energy
For octahedral complexes
$\mathrm{CFSE}=\left(-0.4 \times n_{t_{2 g}}+0.6 \times n_{e_g}\right) \Delta_0$
Tetrahedral complexes
$\mathrm{CFSE}=\left(-0.6 \times n_{t_2}+0.4 \times n_e\right) \Delta_t$
5. Some basic geometries according to Valence Bond Theory
| Coordination Number | Type of Hybridisation | Shape |
| 4 | sp3 | Tetrahedral |
| 4 | dsp2 | Square Planar |
| 5 | sp3d | Trigonal Bipyramidal |
| 6 | sp3d2 | Octahedral |
| 6 | d2sp3 | Octahedral |
7. Magnetic moment
$\mu=\sqrt{\mathrm{n}(\mathrm{n}+2)}$
Advantages of Class 12 Chemistry Solutions Chapter 9 coordination compounds NCERT Exemplar Solutions
NCERT Exemplar Class 12 Chemistry Solutions Chapter 9 coordination compounds provide a clear explanation of concepts that helps in mastering topics and scoring well in board and competitive exams. The advantages of using these solutions of NCERT are given below:
- These coordination compound solutions help students to understand the topics like ligands, coordination number, Werner’s theory, isomerism, valence Bond Theory and Crystal Field Theory using solved questions.
- These NCERT Exemplar Class 12 Solutions are prepared by subject experts in a well structured and organised manner.
- These class 12 chemistry chapter 9 Coordination compounds solutions include important derivations, and examples commonly asked in board and competitive exams.
- Students can understand the formation, structure, and stability of coordination compounds using NCERT Exemplar Solutions.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Solution subject-wise
The NCERT subject-wise solutions will help you broaden your concepts and will also help in revision. Learn more from Class 12 NCERT notes.
NCERT Exemplar Class 12 Solutions
Students can refer to the links given below for the NCERT Exemplar subject-wise solutions for Class 12.
NCERT Notes subject-wise
You can follow the links given in the table below to get access to the Class 12 NCERT notes.
NCERT Books and NCERT Syllabus
You can find links to the Class 12 NCERT chemistry book and syllabus for the respective subjects.
Frequently Asked Questions (FAQs)
Coordination compounds are complexes consisting of a central metal atom or ion bonded to surrounding molecules or ions called ligands. They are formed through coordinate covalent bonds, where ligands donate a pair of electrons to the metal to form these bonds, resulting in a stable structure.
Properties of coordination compounds are determined with the help of ligands. Ligands affect properties like stability, colour of compounds and their reactivity.
The coordination number refers to the number of ligand atoms or ions that are directly bonded to the central metal atom in a coordination compound. It is usually determined by the spatial arrangement of ligands around the metal atom and can vary based on the size and charge of the metal ion as well as the type of ligands involved.
Strong field ligands create a larger splitting of d-orbitals while weak field ligands creates small splitting.Strong field ligands like CN⁻ and CO, tend to lead to low-spin complexes, while weak field ligands, such as I⁻ and Br⁻, lead to high-spin complexes.
Crystal field theory explains the electronic structure of transition metal complexes by considering the interactions between the central metal ion and surrounding ligands. The presence of ligands alters the energy levels of the d-orbitals, leading to the splitting of these orbitals based on the geometry of the complex. Learn morefrom our class 12 NCERT notes.
NCERT exemplar solutions Class 12 chemistry chapter 9 deals with complexes formed by metal ions bonded to ligands, covering their structures, nomenclature, bonding theories, isomerism, and applications in chemistry and biology.
The chemistry of coordination compounds studies metal ions bonded to ligands, focusing on their structures, bonding, isomerism, stability, and reactions in biological and industrial systems.
A ligand is an ion or molecule that donates a lone pair of electrons to the metal ion.
It refers to compounds with the same formula but different structures or arrangements.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters




























