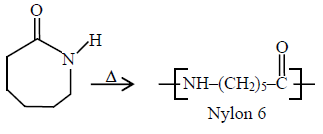Nylon in NCERT Exemplar Solutions Polymers refers to a synthetic polyamide formed by condensation polymerisation of diamines and diacids like nylon-6,6 or by ring-opening polymerisation like nylon-6, known for high strength and durability.
NCERT Exemplar Class 12 Chemistry Solutions Chapter 15 Polymers
What made our daily lives convenient and much more colourful? Who played a significant role in many industries? The answer to these questions lies in NCERT Exemplar Class 12 Chemistry Polymers. This chapter provides a detailed explanation of monomers, types of polymers, application of polymers and the principles and theories that govern their behaviour. This chapter also deals with different types of polymers such as Natural And Synthetic Polymer, homopolymers and copolymers, Linear, Branched, and Cross-linked Polymers, Thermoplastics and Thermosets, addition and condensation polymers. The adaptability of polymers to different functions makes them indispensable in various industries, improving safety, convenience, and sustainability in our daily lives.
Students can check the CBSE Class 10 Marking Scheme 2026 below:
| Subjects | Theory marks | Internal assessment |
| English | 80 | 20 |
| Hindi | 80 | 20 |
| Maths | 80 | 20 |
| Science | 80 | 20 |
| Social Science | 80 | 20 |
This Story also Contains
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: MCQ (Type 1)
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: MCQ (Type 2)
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: Short Answer Type
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: Matching Type
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: Assertion and Reason Type
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: Long Answer Type
- NCERT Exemplar Class 12 Chemistry Chapter 15: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Class 12 Chemistry Chapter 15
- Topics and Subtopics Covered in the NCERT Exemplar Class 12 Chemistry Chapter 15
- Advantages of Using NCERT Exemplar Solutions Class 12 Chemistry Chapter 15 Polymers
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions subject-wise
- NCERT Exemplar Class 12 Solutions subject-wise
- NCERT Class 12 subject-wise notes
- NCERT Books and NCERT Syllabus

Our subject experts designed the NCERT Exemplar Solutions to offer a systematic and structured approach to these important concepts and help students to develop a clear understanding of critical concepts through the series of solved examples and conceptual explanations, these NCERT Exemplar Solutions of Class 12 Chemistry provide a valuable resource to enhance performance in board exams as well as in the competitive exams like NEET, JEE Mains, etc. This article includes some higher-order thinking skills (HOTS) questions that are beyond memorization and promote conceptual understanding, improve analytical thinking, enhance application skills, and build confidence in chemistry. Students can also refer NCERT Solutions for better understanding of Polymers.
NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: MCQ (Type 1)
MCQ-type questions are covered in the first section of NCERT Exemplar Class 12 Chemistry Chapter 15 Polymers to improve your conceptual thinking. You can also follow notes, available on our website, to understand these concepts in detail.
Question 1. Which of the following polymers of glucose is stored by animals?
(i) Cellulose
(ii) Amylose
(iii) Amylopectin
(iv) Glycogen
Answer:
The answer is the option (iv). Amongst the given options, glycogen is a polymer of glucose that is found in liver, brain and muscles of animals.Question 2. Which of the following is not a semisynthetic polymer?
(i) cis-polyisoprene
(ii) Cellulose nitrate
(iii) Cellulose acetate
(iv) Vulcanised rubber
Answer:
The answer is the option (i). Of all the given options, Cis-polyisoprene is not a semisynthetic polymer. m-polyisoprene is a natural polymer while the others are semisynthetic polymers.Question 3. The commercial name of polyacrylonitrile is ______________.
(i) Dacron
(ii) Orlon (Acrilan)
(iii) PVC
(iv) Bakelite
Answer:
The answer is the option (ii). The commercial name of polyacrylonitrile is Orion (acrilan). It is used as a substitute for wool in making commercial fibres.
Question 4. Which of the following polymer is biodegradable?
Answer:
The answer is the option (iii). PHBV is biodegradable in nature. It is formed by the copolymerisation of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid.
Question 5. In which of the following polymers ethylene glycol is one of the monomer units?
Answer:
The answer is the option (i). The polymer given below is obtained on condensation polymerisation of ethylene glycol and phthalic acid. The water molecule gets eliminated in the process.
Question 6. Which of the following statements is not true about low-density polythene?
(i) Tough
(ii) Hard
(iii) Poor conductor of electricity
(iv) Highly branched structure
Answer:
The answer is the option (iii). We can obtain low-density polythene by polymerisation of ethane under high pressure. It tough but flexible (not too hard) in nature and has a highly branched structure.Question 8. Which of the following polymer can be formed by using the following monomer unit?
(i) Nylon 6, 6
(ii) Nylon 2–nylon 6
(iii) Melamine polymer
(iv) Nylon-6
Answer:
The answer is the option (iv). Nylon -6 is the polymer formed by heating caprolactam with water at a high temperature.
NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: MCQ (Type 2)
The MCQ (Type 2) of Polymers is designed to evaluate students ability to apply the concepts. Chapter 15 Polymers Class 12 Questions and Answers helps to test student understanding of concepts.
Question 9. Which of the following polymers, need at least one diene monomer for their preparation?
(i) Dacron
(ii) Buna-S
(iii) Neoprene
(iv) Novolac
Answer:
The answer is the option (ii, iii) Buna-S and neoprene need at least one diene monomer in their preparation. Buna-S is prepared by copolymerisation of 1, 3-butadiene and styrene in the presence of peroxide catalyst.
Neoprene is formed by the free radical polymerisation of chloroprene.

Question 10. Which of the following are characteristics of thermosetting polymers?
(i) Heavily branched cross-linked polymers.
(ii) Linear slightly branched long-chain molecules.
(iii) Become infusible on moulding so cannot be reused.
(iv) Soften on heating and harden on cooling, can be reused.
Answer:
The answer is the option (i, iii) Thermosetting polymers or thermoset are heavily branched and cross-linked molecules. They cannot be reused as they become infusible on the moulding.Question 11. Which of the following polymers are thermoplastic?
(i) Teflon
(ii) Natural rubber
(iii) Neoprene
(iv) Polystyrene
Answer:
The answer is the option (i, iv) Thermoplastic polymers can be repeatedly softened on heating and hardened on cooling. Thus, they can be remoulded again and again. Teflon and polystyrene are common examples of thermoplastics.Question 12. Which of the following polymers are used as fibre?
(i) Polytetrafluoroethane
(ii) Polychloroprene
(iii) Nylon
(iv) Terylene
Answer:
The answer is the option (iii, iv) Fibres have high tensile strength and modulus due to strong intermolecular forces like H-bonding. This leads to close packing in chain resulting in crystalline nature. Polyamides (Nylon) and Polyesters (terylene) are used as fibres.Question 13. Which of the following are addition polymers?
(i) Nylon
(ii) Melamine formaldehyde resin
(iii) Orlon
(iv) Polystyrene
Answer:
The answer is the option (iii, iv) Orion and polystyrene are examples of addition polymers formed by repeated addition of monomer molecules. Orion is made by polymerisation of $\text {CH }_{2}=\text { CH - CN (acrylonitrile)}$ and polystyrene by $\text {C}_{6}\text {H}_{5}-\text {CH=CH}_{2}\; \text {(styrene)}$Question 14. Which of the following polymers are condensation polymers?
(i) Bakelite
(ii) Teflon
(iii) Butyl rubber
(iv) Melamine formaldehyde resin
Answer:
The answer is the option (i, iv) Phenol when heated with formaldehyde gives an infusible solid mass known as bakelite. On condensation polymerisation of melamine and formaldehyde, melamine formaldehyde resin is obtained.Question 15. Which of the following monomers form biodegradable polymers?
(i) 3-hydroxybutanoic acid + 3-hydroxypentanoic acid
(ii) Glycine + aminocaproic acid
(iii) Ethylene glycol + phthalic acid
(iv) Caprolactam
Answer:
The answer is the option (i, ii) The polymers which are easily decomposed are known as biodegradable polymer. PHBV is biodegradable in nature. It is formed by the copolymerisation of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid. Glycine + amino caproic acid on copolymerisation give Nylon-2-nylon 6 which is also biodegradable.
Question 16. Which of the following is an example of a synthetic rubber?
(i) Polychloroprene
(ii) Polyacrylonitrile
(iii) Buna-N
(iv) cis-polyisoprene
Answer:
The answer is the option (i, iii)Polychloroprene is a polymer of chloroprene and it is synthetic rubber.

Question 17. Which of the following polymers can have strong intermolecular forces?
(i) Nylon
(ii) Polystyrene
(iii) Rubber
(iv) Polyesters
Answer:
The answer is the option (i, iv). Polyamides (Nylon) and Polyesters (terylene) have high tensile strength and modulus due to strong intermolecular forces like H-bonding. This leads to close packing in the chain resulting in crystalline nature. That is why they are used as thread forming fibres.Question 18. Which of the following polymers have vinylic monomer units?
(i) Acrilan
(ii) Polystyrene
(iii) Nylon
(iv) Teflon
Answer:
The answer is the option (i, ii, iv) Amongst the given options, acrilan, polystyrene and Teflon has vinylicMonomer units.

Question 19. Vulcanisation makes rubber ______________.
(i) more elastic
(ii) soluble in inorganic solvent
(iii) crystalline
(iv) more stiff
Answer:
The answer is the option (i, iv). Vulcanisation is a chemical process wherein rubber is heated with Sulphur to form cross-links between rubber molecules. The process is carried to improve the physical properties like elasticity of natural rubber. It makes the rubber stiffer.NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: Short Answer Type
Some short answer type questions are also given here in the Class 12 NCERT Exemplar Chapter 15 Polymers for practice. This section contains Important Questions that are asked in the exams.
Answer:
The structures of the Vulcanised rubber molecules are as given below.
Question 21. Identify the type of polymer.
$\text {-A-A-A-A-A-A-}$
Answer:
Polymerisation of single monomeric species (one type of monomer unit) gives homopolymers. In this case the unit is A.Question 22. Identify the type of polymer.
$\text {-A-B-B-A-A-A-B-A-}$
Answer:
Polymers wherein repeating structural unit has 2 types of monomer units (let’s say A and B) are known as copolymers.Question 23. Out of chain growth polymerisation and step-growth polymerisation, in which type willyou place the following.
Answer:
It is a type of chain growth polymerisation because only addition occurs in this.Question 24. Identify the type of polymer given in the following figure.
Answer:
The polymer given in the diagram is a cross linked polymer because a 3- D network structure (giant molecule) is formed from various polymer chains.Question 25. Identify the polymer given below :
Answer:
The polymer given is cis-polyisoprene (natural rubber) and has a coiled structure. It shows elastic properties as well.Question 26. Why are rubbers called elastomers?
Answer:
Rubber is a natural polymer with elastic properties, i.e. it stretches and relaxes with the application or removal of external force. That is why these are called elastomers.Question 27. Can the enzyme be called a polymer?
Answer:
Since enzymes are biocatalysts that are basically proteins, they can be called polymers.Question 28. Can nucleic acids, proteins and starch be considered as step growth polymers?
Answer:
Step growth polymers are formed by condensation polymerisation reaction resulting in the loss of simple molecule like water, alcohol that in turn, results in the formation of high molecular mass polymers. Nucleic acids, proteins and starch are formed in the same way. Hence, they can be considered as step-growth polymers.Question 29. How is the following resin intermediate prepared and which polymer is formed by this monomer unit?
Answer:
The given intermediate is formed by the condensation polymerisation of melamine and formaldehyde. Its polymerisation gives melamine formaldehyde.
Question 30. To have practical applications, why are cross-links required in rubber?
Answer:
Cross-links are formed in the process of vulcanisation which is done to improve the physical properties of natural rubber. By cross-linking, rubber gets hard and tough due to increased tensile strength. The vulcanised rubber has higher elasticity, increased stiffness and low water absorption tendency.Question 31. Why does cis-polyisoprene possess elastic property?
Answer:
The cis-polyisoprene molecule has a coiled structure. In it, several chains are held together by weak van der Waals interactions. This is why they possess elastic property.Answer:
$\text {LDP}$ or Low-density polythene has a highly branched molecular structure. It has low density (0.92 g/cm 3) and low melting point, as the molecules do not pack well due to branching. It is transparent and chemically inert with moderate toughness.$\text {HDP}$ or High-density polythene has linear chains, and thus the molecules are closely packed in space. Therefore, it has a higher density and melting point. In comparison to $\text {LDP}$, it is harder, tougher and has greater tensile strength.
Answer:
The role of Benzoyl peroxide in free radical addition polymerisation of alkenes is to generate free radicals and act as an initiator of the reaction by providing chain initiation.
The free radical is added to the double bond of an alkene molecule forming the polymer.

The new free radical is added to a double bond of monomer to form a larger free radical, which when added to other alkene molecules forms the polymer.

Lastly chain termination step-

Question 34. Which factor imparts crystalline nature to a polymer like nylon?
Answer:
Polymers like nylon have strong intermolecular forces like hydrogen bonding that lead to close packing of polymer chains. This, along with the linear structure, gives a crystalline nature to the polymer.Question 35. Name the polymers used in laminated sheets and give the name of monomeric units involved in its formation.
Answer:
The polymer is a urea-formaldehyde resin and the monomeric units are urea and formaldehyde.Question 36. Which type of biomolecules has some structural similarity with synthetic polyamides? What is this similarity?
Answer:
The biomolecules having some structural similarity with synthetic polyamides are protein. The similarity is that the polyamides and proteins both contain amide linkage.Question 37. Why should the monomers used in addition polymerisation through free radical pathway be very pure?
Answer:
The monomers used in addition polymerisation through free radical should be very pure because even a tiny amount of impurities may act as initiators leading to the formation of polymers with small chain lengths.NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: Matching Type
The Chemistry NCERT Exemplar Class 12 Polymers important questions are discussed below. These are generally asked in exams to test your knowledge these questions and answers are quite helpful for competitive exams.
Question 38. Match the polymer of column I with a correct monomer of column II.
|
Column I
|
Column II
|
|
(i) High density polythene
|
(a) Isoprene
|
|
(ii) Neoprene
|
(b) Tetrafluoroethene
|
|
(iii) Natural rubber
|
(c) Chloroprene
|
|
(iv) Teflon
|
(d) Acrylonitrile
|
|
(v) Acrilan
|
(e) Ethene
|
Answer:
(i $\rightarrow$ e), (ii $\rightarrow$ c), (iii $\rightarrow$a), (iv $\rightarrow$ b), (v $\rightarrow$d)
Question 39. Match the polymers given in Column I with their chemical names given in Column II.
|
Column I
|
Column II
|
|
(i) Nylon 6
|
(a) Polyvinyl chloride
|
|
(ii) PVC
|
(b) Polyacrylonitrile
|
|
(iii) Acrilan
|
(c) Polycaprolactum
|
|
(iv) Natural rubber
|
(d) Low density Polythene
|
|
(v) LDP
|
(e) cis-polyisoprene
|
Answer:
(i $\rightarrow$ c), (ii $\rightarrow$ a), (iii $\rightarrow$ b), (iv $\rightarrow$ e), (v $\rightarrow$ d)Question 40. Match the polymers given in Column I with their commercial names given in Column II.
|
Column I
|
Column II
|
|
(i) The polyester of glycol and phthalic acid
|
(a) Novolac
|
|
(ii) The copolymer of 1, 3-butadiene and styrene
|
(b) Glyptal
|
|
(iii) Phenol and formaldehyde resin
|
(c) Buna-S
|
|
(iv) The polyester of glycol and terephthalic acid
|
(d) Buna -N
|
|
(v) The copolymer of 1, 3-butadiene and acrylonitrile
|
(e) Dacron
|
Answer:
(i $\rightarrow$ b), (ii $\rightarrow$ c), (iii $\rightarrow$ a), (iv $\rightarrow$ e), (v $\rightarrow$ d)Question 41. Match the polymers given in Column I with their main applications given in Column II.
|
Column I
|
Column II
|
|
i) Bakelite
|
(a) Unbreakable crockery
|
|
ii) Low-density polythene
|
(b) Non-stick cookware
|
|
iii) Melamine-formaldehyde resin
|
(c) Packaging material for shock absorbance
|
|
iv) Nylon 6
|
(d) Electrical switches
|
|
v) Polytetrafluoroethane
|
(e) Squeeze bottles
|
|
vi) Polystyrene
|
(f) Tyre, cords
|
Answer:
(i $\rightarrow$ d), (ii $\rightarrow$ e), (iii $\rightarrow$ a), (iv $\rightarrow$ f), (v $\rightarrow$ b), (vi $\rightarrow$ c)
Question 42. Match the polymers given in Column I with the preferred mode of polymerisation followed by their monomers.
|
Column I
|
Column II
|
|
(i) Nylon -6,6
|
(a) Free radical polymerisation
|
|
(ii) PVC
|
(b) Ziegler-Natta polymerisation or Coordination polymerisation
|
|
(iii) HDP
|
(c) Anionic Polymerisation
|
|
|
(d) Condensation polymerisation
|
Answer:
(i $\rightarrow$ d), (ii $\rightarrow$ a), (iii $\rightarrow$ b)
(i) Nylon-6,6 follows condensation polymerisation. Each time a molecule of the diamine reacts with a molecule of the diacid, an amide bond (–CO–NH–) is formed and a water molecule is eliminated.
(ii) The monomer unit of PVC is vinyl chloride. Under pressure, heat, and the presence of a free radical initiator, vinyl chloride monomers undergo free radical addition polymerisation.
(iii) High-Density Polyethene (HDP) is a widely used plastic made by the polymerisation of ethene (ethylene) monomers. HDP follows Coordination polymerisation, carried out using Ziegler–Natta catalyst.
Question 43. Match the polymers given in Column I with the type of linkage present in they have given in Column II.
|
Column I
|
Column II
|
|
(i) Terylene
|
(a) Glycosidic linkage
|
|
(ii) Nylon
|
(b) Ester linkage
|
|
(iii) Cellulose
|
(c) Phosphodiester linkage
|
|
(iv) Protein
|
(d) Amide linkage
|
|
(v) RNA
|
|
Answer:
(i $\rightarrow$ b), (ii $\rightarrow$ d), (iii $\rightarrow$ a), (iv $\rightarrow$ d), (v $\rightarrow$ c)
(i) Terylene is formed by condensation polymerisation of terephthalic acid and ethylene glycol. Each unit forms an ester linkage with the elimination of water.
(ii) Nylon is formed by condensation polymerisation of hexamethylenediamine and adipic acid, forming an amide bond (–CO–NH–) with the elimination of water. Therefore, amide linkage is present in nylon.
(iii) Cellulose is a natural polysaccharide made up of repeating units of β-D-glucose. The hydroxyl group (-OH) on carbon 1 (C1) of one glucose reacts with the –OH on carbon 4 (C4) of the next glucose. This forms a glycosidic bond.
(iv) Proteins are natural polymers made up of amino acids. A (–CO–NH–) bond is formed between the carboxyl group of one amino acid and the amino group of another. Therefore, amide linkage is present in proteins.
(v) RNA is a nucleic acid polymer made up of nucleotides. It consists of ribose sugar, a phosphate group and a nitrogenous base. The phosphate group of one nucleotide forms two ester bonds, one with the 3′-OH group and another with the 5′-OH group. Therefore, 3′-5′ phosphodiester linkage is present in RNA.
Question 44. Match materials are given in Column I with the polymers given in Column II.
|
Column I
|
Column II
|
|
(i) Natural rubber latex
|
(a) Nylon
|
|
(ii) Wood laminates
|
(b) Neoprene
|
|
(iii) Ropes and fibres
|
(c) Dacron
|
|
(iv) Polyester fabric
|
(d) Melamine formaldehyde resins
|
|
(v) Synthetic rubber
|
(e) Urea-formaldehyde resins
|
|
(vi) Unbreakable crockery
|
(f) cis-polyisoprene
|
Answer:
(i $\rightarrow$ f), (ii $\rightarrow$ e), (iii $\rightarrow$ a), (iv $\rightarrow$ c), (v $\rightarrow$ b), (vi $\rightarrow$ d)Question 45. Match the polymers given in Column I with their repeating units given in Column II
|
Column I
|
Column II
|
|
(i) Acrilan
|
(a)
 |
|
(ii) Polystyrene
|
(b)
 |
|
(iii) Neoprene
|
(c)
 |
|
(iv) Novolac
|
(d)
 |
|
(v) Buna-N
|
(e)
 |
|
|
(f)
 |
Answer:
(i $\rightarrow$ d), (ii $\rightarrow$ a), (iii $\rightarrow$ b), (iv $\rightarrow$ e),(v $\rightarrow$ c)NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: Assertion and Reason Type
The Assertion and Reason Type questions included in Class 12 NCERT Exemplar Chapter 15 Polymers form an important section that tests students concept clarity and reasoning ability.
Question 46. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Rayon is a semi-synthetic polymer and is taken as a better choice than cotton fabric.
Reason: Mechanical and aesthetic properties of cellulose can be improved by acetylation.
(i) Assertion and reason both are correct statements, but reason does not explain the assertion.
(ii) Assertion and reason both are correct statements, and reason explain the assertion.
(iii) Both assertion and reason are the wrong statements.
(iv) The assertion is correct statement and reason is the wrong statement.
(v) The assertion is the wrong statement and reason is the correct statement.
Answer:
The answer is the option (ii). Rayon is semi-synthetic polymer and is taken as a better choice than cotton fabric because mechanical and aesthetic properties of cellulose can be improved by acetylation. Thus, both assertion and reason are correct with the latter being the correct explanation.Question 47. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Most of the Synthetic polymers are not biodegradable.
Reason: Polymerisation process induces toxic character in organic molecules.
(i) Assertion and reason both are correct statements, but reason does not explain the assertion.
(ii) Assertion and reason both are correct statements, and reason explain the assertion.
(iii) Both assertion and reason are the wrong statements.
(iv) The assertion is correct statement and reason is the wrong statement.
(v) The assertion is the wrong statement and reason is the correct statement.
Answer:
The answer is the option (iv). Most of the synthetic polymers are non-biodegradable in nature i.e. they are not degraded by enzymatic, hydrolytic and environmental oxidation. Polymerisation process does not induce toxic characters in organic molecules.Question 48. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Olefinic monomers undergo addition polymerisation.
Reason: Polymerisation of vinyl chloride is initiated by peroxides/persulphates.
(i) Assertion and reason both are correct statements, but reason does not explain the assertion.
(ii) Assertion and reason both are correct statements, and reason explain the assertion.
(iii) Both assertion and reason are the wrong statements.
(iv) The assertion is correct statement and reason is the wrong statement.
(v) The assertion is the wrong statement and reason is the correct statement.
Answer:
The answer is the option (i). Olefinic monomers like ethene undergo addition polymerisation. The monomers used in addition polymerisation reaction are unsaturated compounds like alkenes, alkadienes etc. The assertion and reason both are correct statements but the reason does not explain the assertion.Question 49. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Polyamides are best used as fibres because of high tensile strength.
Reason: Strong intermolecular forces (like hydrogen bonding within polyamides) lead to close packing of chains and increase the crystalline character, hence, provide high tensile strength to polymers.
(i) Assertion and reason both are correct statements, but reason does not explain the assertion.
(ii) Assertion and reason both are correct statements, and reason explain the assertion.
(iii) Both assertion and reason are the wrong statements.
(iv) The assertion is correct statement and reason is the wrong statement.
(v) The assertion is the wrong statement and reason is the correct statement.
Answer:
The answer is the option (ii). Polyamides are best-used fibers because they have high tensile strength. They have closely packed chains and increased crystalline character due to presence of strong inter molecular forces like hydrogen bonding.Question 50. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: For making rubber synthetically, isoprene molecules are polymerised.
Reason: Neoprene (a polymer of chloroprene) is a synthetic rubber.
(i) Assertion and reason both are correct statements, but reason does not explain the assertion.
(ii) Assertion and reason both are correct statements, and reason explain the assertion.
(iii) Both assertion and reason are the wrong statements.
(iv) The assertion is correct statement and reason is the wrong statement.
(v) The assertion is the wrong statement and reason is the correct statement.
Answer:
(v) Isoprene (2-methyl-1,3-butadiene) molecule is the monomer for natural rubber. Neoprene (a polymer of chloroprene) is a synthetic rubber.Question 51. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Network polymers are thermosetting.
Reason: Network polymers have high molecular mass.
(i) Assertion and reason both are correct statements, but reason does not explain the assertion.
(ii) Assertion and reason both are correct statements, and reason explain the assertion.
(iii) Both assertion and reason are the wrong statements.
(iv) The assertion is correct statement and reason is the wrong statement.
(v) The assertion is the wrong statement and reason is the correct statement.
Answer:
The answer is the option (i). Network polymers are thermosetting and have high molecular mass. However, the reason they are thermosetting is because of extensive cross-linking during polymerisation and not because of higher molecular mass.Question 52. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Polytetrafluoroethene is used in making non-stick cookware.
Reason: Fluorine has the highest electronegativity.
(i) Assertion and reason both are correct statements, but reason does not explain the assertion.
(ii) Assertion and reason both are correct statements, and reason explain the assertion.
(iii) Both assertion and reason are the wrong statements.
(iv) The assertion is correct statement and reason is the wrong statement.
(v) The assertion is the wrong statement and reason is the correct statement.
Answer:
The answer is the option (i). Polytetrafluoroethene or Teflon is used in making nonstick cookware, as it is chemically inert, resistant to attack by corrosive reagents and thermally stable.NCERT Exemplar Solutions Class 12 Chemistry Chapter 15: Long Answer Type
The Chapter 15 Polymers Class 12 Questions and Answers Long Answer Type section is an important part of this chapter, where detailed explanations are required. These solutions given below will help you to understand complex concepts clearly.
Answer:
Synthetic polymers are non-biodegradable as they are resistant to the environmental degradation process. They form a major share of the polymer solid waste material.Biopolymers are natural polymers of amino acids or carbohydrates, linked together by peptide or glycosidic linkages. They are found in plants and animals.

Biopolymers may or may not be biodegradable. For example, while protein and starch are biodegradable, keratin is not. Biodegradable polymer is a polymer which are not resistant to the environmental degradation process, e.g., PHBV nylon-2, nylon-6. Both the biodegradable polymers and biopolymers contain similar functional groups.
Question 54. Differentiate between rubbers and plastics on the basis of intermolecular forces.
Answer:
|
Rubber
|
Plastics
|
|
The molecule consists of chains held together by weak Van Der Waals interaction.
|
Intermolecular forces of attarction intermediate between elastomers and fibre.
|
|
Coiled structure
|
Linear or slightly branched long chain
|
|
Returns to its original shape, size and length after being stretched.
|
Not really elastic in nature but capable of softening on heating and hardening on cooling.
|
Answer:
Polymer ‘A’ in the question is Novalac and ‘B’ is bakelite. The reactions involved are:
The main structural difference between them is that novolac is a linear chain polymer while bakelite is a cross-linked polymer.
Answer:
|
LDP
|
HDP
|
|
Formed at a temperature of 350 K to 570 K and very high pressure of about 1000-2000 atm.
|
The temperature of about 333 K to 343 K and a pressure of 6-7 atm is required.
|
|
It is obtained by free-radical addition and has a highly branched structure.
|
It has straight linear molecules and a high density.
|
|
Chemically inert and tough but flexible.
|
Chemically inert.
Tougher and harder than LDP
|
Answer:
Thermoplastic polymers soften on heating and harden on cooling. Polythene, polyvinyl and polystyrene are thermoplastic polymers. Their intermolecular force of attraction is somewhere between elastomers and fibres. They soften on heating and harden on cooling.Bakelite, urea-formaldehyde resin are examples of thermosetting polymers. They are cross-linked and cannot be reused.
NCERT Exemplar Class 12 Chemistry Chapter 15: Higher Order Thinking Skills (HOTS) Questions
Some Class 12 Chemistry Polymers Important Questions are given below that will help you tackle complex problems. Students can follow Polymers Notes to learn the concepts in detail.
Question 1. Given below are two statements:
Statement I: Wet cotton clothes made of cellulose based carbohydrate takes comparatively longer time to get dried than wet nylon polymer-based clothes.
Statement II: Intermolecular hydrogen bonding with water molecule is more in nylon-based clothes than in the case of cotton clothes.
In the light of above statements, choose the Correct answer from the options given below
(1) Statement I is false but Statement II is true
(2) Statement I is true but Statement II is false
(3) Both Statement I and Statement II are true
(4) Both Statement I and Statement II are false
Answer:
Wet cellulose-based cotton clothes take more time to dry than wet nylon-based clothes due to the greater number of H-bonds between cellulose and water molecules.
So, Statement I is correct
Statement II is incorrect.
Hence, the correct answer is option (2).
Question 2. Which of the following polymers are used as fibre?
(i) Polytetrafluoroethylene
(ii) Polychloroprene
(iii) Nylon
(iv) Terylene
Options
(1) (i) and (ii)
(2) (iii) and (iv)
(3) (ii) and (iii)
(4) (i) and (iv)
Answer:
Fibres have high tensile strength and modulus due to strong intermolecular forces like H-bonding. This leads to close packing in chains, resulting in a crystalline nature. Polyamides (Nylon) and Polyesters (Terylene) are used as fibres.
Hence, the correct answer is option (2).
Question 3. Which of the following is a condensation polymer?
(i) Buna-S
(ii) Neoprene
(iii) Teflon
(iv) Nylon-6,6
Answer:
Condensation Polymers -Formed by repeated condensation reaction between two different bi-functional or tri-functional monomers. Nylon 6,6 is a condensation polymer of hexamethylene diamine and adipic acid. Buna-S, Teflon & Neoprene are not condensation polymers.
Question 4: Match List I with List II
| List 1 | List 2 |
| A. Elastomeric polymer | I. Urea formaldehyde resin |
| B. Fibre Polymer | II. Polystyrene |
| C. Thermosetting Polymer | III. Polyester |
| D. Thermoplastic Polymer | IV. Neoprene |
Choose the correct answer from the options given below :
(1) A-II, B-III, C-I, D-IV
(2) A-IV, B-III, C-I, D-II
(3) A-IV, B-I, C-III, D-II
(4) A-II, B-I, C-IV, D-III
Answer:
Neoprene : Elastomer
Polyester Fibre
Polstyrene : Thermoplastic
Urea–Formaldhyde Resin: Thermosetting polymer
Hence, the answer is the option (2).
Question 5: Caprolactam when heated at high temperature in presence of water, gives
(1) Nylon 6, 6
(2) Nylon 6
(3) Teflon
(4) Dacron
Answer:
Hence, the answer is the option (2).
Approach to Solve Questions of Class 12 Chemistry Chapter 15
To solve the questions from Class 12 NCERT Exemplar Chapter 15 Polymers, it is important to first understand the basic concepts thoroughly. Focus on practising different types of questions, revise key formulas, the approaches given below helps you to solve questions effectively.
1. Before solving questions it is important to understand the basic concepts of Polymers like
- Classification of Polymers
- Classification based on structure, sources and mode of polymerisation
- Difference between synthetic and natural polymers
- Uses and properties of some important polymers
2. Important polymers like PVC, Teflon, Nylon-6,6, and Bakelite are important and used often.
3. Reaction mechanisms are often asked in exams, so to solve questions of these follow the steps
- Understand the reactions
- Practice writing polymerisation reactions
- Addition and condensation processes are important
4. Key differences between Thermoplastic vs Thermosetting and Addition polymerisation vs condensation polymerisation are often asked in exams
5. While solving Chapter 15 Polymers Class 12 Questions and Answers include structures in the solutions, it will help students an extra edge. Solve NCERT examples, back exercises, and exemplar problems .Do previous year questions from NEET and JEE to get used to question patterns. Follow NCERT Exemplar Solutions Polymers for more practice.
Topics and Subtopics Covered in the NCERT Exemplar Class 12 Chemistry Chapter 15
Given below the topics and subtopics covered in NCERT Exemplar Class 12 Chemistry Polymers.
- Classification of Polymers
- Classification Based on Source
- Classification Based on the Structure of Polymers
- Classification Based on Mode of Polymerisation
- Classification Based on Molecular Forces
- Classification Based on Growth Polymerisation
- Types of Polymerisation Reactions
- Addition Polymerisation or Chain Growth Polymerisation
- Condensation Polymerisation or Step Growth polymerization
- Copolymerisation
- Rubber
- Molecular Mass of Polymers
- Biodegradable Polymers
- Polymers of Commercial Importance
Advantages of Using NCERT Exemplar Solutions Class 12 Chemistry Chapter 15 Polymers
These NCERT Exemplar Solutions Class 12 Chemistry Chapter 15 Polymers cover all questions from the NCERT book in a very simple way. The advantages of using these solutions are given below:
- These solutions help students to understand topics like types of polymer, properties, and mechanisms with the help of solved questions.
- These NCERT Exemplar Class 12 Solutions include detailed answer of every questions included in textbook.
- These NCERT Exemplar Class 12 Chemistry Chapter 15 Polymers are prepared by subject experts in a very clear and comprehensive manner that helps students for board and competitive exams.
- They offers systematic solutions that help students to write accurate, concise and detailed answers in exams.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions subject-wise
The NCERT subject-wise solutions will help you broaden your concept and will also help in revision.
NCERT Exemplar Class 12 Solutions subject-wise
Excel your preparation with NCERT exemplar solutions. Click on the link below
NCERT Class 12 subject-wise notes
You can follow the links given in the table below to get access to the Class 12 NCERT notes.
NCERT Books and NCERT Syllabus
Also, you can find links to the Class 12 NCERT chemistry book and syllabus for the respective subjects.
Frequently Asked Questions (FAQs)
A polymer is a large molecule composed of repeating structural units called monomers, connected by covalent chemical bonds, and it is discussed in detail NCERT Exemplar Solutions Polymers.
Monomers are the building blocks of a polymer chain. They are small molecules that join together to form a polymer. Examples include ethylene, which forms polyethylene, and amino acids, which form proteins.
The glass transition temperature (Tg): At this temperature, an amorphous polymer transitions from a hard, glassy state to a soft, rubbery state. It's an important property for thermoplastics.
It refers to the degree of ordered arrangement of polymer chains. Amorphous regions are more disordered, while crystalline regions are tightly packed and aligned. Highly crystalline polymers tend to be stronger, more rigid, and less transparent.
The differences between thermoplastics and thermosets:
- Thermoplastics: Can be repeatedly melted and reshaped. The process is reversible when heated. Examples: polyethylene, polypropylene.
- Thermosets: Once set, they cannot be melted and reshaped. Undergo irreversible chemical change when heated, forming a rigid structure. Examples: epoxy resins, phenolic resins.
Chemistry NCERT Exemplar Class 12 Polymers important questions provides detailed, step-by-step answers to advanced questions on polymer types, properties, and reactions, helping students prepare for board and competitive exams.
Compare and compile Class 12 Chemistry Polymers Notes by organising topics under clear headings like polymer types, classification, preparation methods, and applications, and then cross-checking each point with NCERT and exemplar questions for accuracy.
In NCERT, a polymer is a large macromolecule made of repeating structural units, while a monomer is the simple small molecule that joins together to form the polymer.
Polymerisation is the chemical process in which monomers combine to form polymers through addition or condensation reactions.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Hello,
Here is your Final Date Sheet Class 12 CBSE Board 2026 . I am providing you the link. Kindly open and check it out.
https://school.careers360.com/boards/cbse/cbse-class-12-date-sheet-2026
I hope it will help you. For any further query please let me know.
Thank you.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters