You can get Class 12 Chemistry NCERT Exemplar Solutions chapter 16 Chemistry in Everyday life from the official NCERT website or through trusted educational platforms that provide free PDF solutions.
NCERT Exemplar Class 12 Chemistry Solutions chapter 16 Chemistry in Everyday life
Do you know how painkillers work, why toothpaste protects teeth from cavities, and what makes packaged food fresh for weeks? The answer to all these questions lies in NCERT Exemplar Class 12 Chemistry Solutions chapter 16 Chemistry in Everyday life. This chapter explains the role of various chemicals in different sectors such as cosmetics, pharmaceuticals, detergents, and various other products. Chemistry in Everyday Life also deals with various chemical reactions that are involved in day-to-day processes.
CBSE has implemented on-screen marking for evaluating Class 12 board exam answer sheets. However, it has clarified that Class 10 answer scripts will continue to be assessed in the traditional physical mode.
Before the start of the formal evaluation process, the first day will be set aside for reviewing the marking scheme, carrying out mock assessments to minimise discrepancies in scoring, and training evaluators to use the digital platform effectively.
Read More: CBSE explains how two Class 10 board exams 2026, on-screen marking for Class 12 will work
This Story also Contains
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: MCQ (Type 1)
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: MCQ (Type 2)
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: Short Answer Type
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: Matching Type
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: Assertion and Reason Type
- NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: Long Answer Type
- NCERT Exemplar Class 12 Chemistry Chapter 16: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Class 12 Chemistry Chapter 16
- Topics and Subtopics Covered in the NCERT Exemplar Class 12 Chemistry Chapter 16
- Advantages of Using Class 12 Chemistry NCERT Exemplar Solutions chapter 16 Chemistry in Everyday life
- NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions subject-wise
- NCERT Exemplar Class 12 Solutions subject-wise
- NCERT Class 12 subject-wise notes
- NCERT Books and NCERT Syllabus

NCERT Exemplar Solutions of Class 12 Chemistry help students develop a clear understanding of critical concepts through a series of solved examples and conceptual explanations. These NCERT Exemplar Solutions are designed by our subject experts which helps students to enhance performance in board exams as well as in competitive exams like NEET, JEE Mains, etc. This article also includes some higher-order thinking skills questions that help to build confidence in Class 12 Chemistry chapter 16.
NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: MCQ (Type 1)
The MCQ questions are covered in the Class 12 Chemistry NCERT Exemplar Solutions chapter 16 Chemistry in Everyday life helps to enhance your knowledge. The concepts are explained in detail in Chemistry in everyday life notes available on our website. Students can also follow NCERT Solutions for conceptual clarity and better understanding of important topics through a series of solved examples.
Question 1. Which of the following statements is not correct.
(i) Some antiseptics can be added to soaps.
(ii) Dilute solutions of some disinfectants can be used as antiseptics.
(iii) Disinfectants are antimicrobial drugs.
(iv) Antiseptic medicines can be ingested.
Answer:
Antiseptic drugs are used to kill any microbes on cuts or wounds to prevent infections, but they are only for external use; therefore, Option (iv) Antiseptic medicines can be ingested is the answer.
Question 2. Which is the correct statement about birth control pills?
(i) Contain estrogen only.
(ii) Contain progesterone only.
(iii) Contain a mixture of estrogen and progesterone derivatives.
(iv) Progesterone enhances ovulation.
Answer:
Birth control pills are used for ovulation, and they contain female hormones to act scientifically. The pills contain (iii) Contain a mixture of estrogen and progesterone derivatives.
Question 3. Which statement about aspirin is not true
(i) Aspirin belongs to narcotic analgesics.
(ii) It is effective in relieving pain.
(iii) It has an anti-blood-clotting action.
(iv) It is a neurologically active drug.
Answer:
Aspiring is a very common drug used to relieve headaches or other tissue pains, so it comes under the class of non-steroidal anti-inflammatory drugs. And it also used to prevent heart attack due to the thinning of blood; therefore, Option (i) Aspirin belongs to narcotic analgesics is the right answer.
Question 4. The most useful classification of drugs for medicinal chemists is _________.
(i) On the basis of chemical structure.
(ii) On the basis of drug action.
(iii) On the basis of molecular targets.
(iv) On the basis of the pharmacological effect
Answer:
There are multiple ways of classifying drugs, but for a medical chemist, the most convenient mode of drug classification is (iii) on the basis of molecular targets
Question 5. Which of the following statements is correct?
(i) Some tranquilizers function by inhibiting the enzymes that catalyze the degradation of noradrenaline.
(ii) Tranquilizers are narcotic drugs.
(iii) Tranquillizers are chemical compounds that do not affect the message transfer from the nerve to the receptor.
(iv) Tranquilizers are chemical compounds that can relieve pain and fever.
Answer:
Tranquilizers are drugs used for the proper synthesis of a mood-controlling hormone named noradrenaline and treating depression. The correct answer is (i) Some tranquilizers function by inhibiting the enzymes that catalyze the degradation of noradrenaline
Question 6. Salvarsan is an arsenic-containing drug which was first used for the treatment of ____________.
(i) syphilis
(ii) typhoid
(iii) meningitis
(iv) dysentery
Answer:
Salvarsan is a very old drug used in the treatment of trypanosomiasis as well as syphilis. So, Option (i) is the answer.
Question 7. A narrow-spectrum antibiotic is active against _______________.
(i) Gram-positive or Gram-negative bacteria.
(ii) Gram-negative bacteria only.
(iii) single organism or one disease.
(iv) both gram-positive and gram-negative bacteria.
Answer:
The antibiotics are mainly divided into two categories depending on their target organisms. The narrow-range antibiotics kill both (i) gram-positive or gram-negative bacteria.
Question 8. The compound that causes general antidepressant action on the central nervous system belongs to the class of _____________.
(i) analgesics
(ii) tranquillizers
(iii) narcotic analgesics
(iv) antihistamines
Answer:
Analgesics are used for pain-relieving purposes, but tranquilizers are the drug category used to treat the imbalanced neurotransmitters present in the brain. Therefore, option (ii), tranquilizers are the right answer.
Question 9. Compound which is added to soap to impart antiseptic properties is __________.
(i) sodium lauryl sulfate
(ii) sodium dodecylbenzenesulphonate
(iii) rosin
(iv) bithional
Answer:
Properties of soap not only include cleansing agents but numerous other features like antiseptic properties. This is introduced by the addition of (iv) bithional as it is an antiseptic, thus safe for living tissues.
Question 10. Equanil is __________.
(i) artificial sweetener
(ii) tranquilizer
(iii) antihistamine
(iv) antifertility drug
Answer:
The common name of Equanil, being meprobamate, it has the properties of a sleeping pill thus classifying under option (ii) tranquilizer
Question 11. Which of the following enhances the leathering property of soap?
(i) Sodium carbonate
(ii) Sodium resinate
(iii) Sodium stearate
(iv) Trisodium phosphate
Answer:
To increase the leaching property of soaps, a gum named rosin is added. So, option (ii) Sodium resinate is the correct answer.
Question 12. Glycerol is added to soap. It functions ______________.
(i) as a filler.
(ii) to increase leathering.
(iii) to prevent rapid drying.
(iv) to make soap granules.
Answer:
The common use of glycerol is in shaving soap as it prevents instant drying of the soap. So the correct option is (iii) to prevent rapid drying.
Question 13 Which of the following is an example of liquid dishwashing detergent?

Answer:
The liquid dishwashing detergents come under the category of non-ionic detergents making Option (ii) the correct answer due to being non-ionic.
Question 14. Polyethyleneglycols are used in the preparation of which type of detergents?
(i) Cationic detergents
(ii) Anionic detergents
(iii) Non-ionic detergents
(iv) Soaps
Answer:
Due to the presence of the -OH group, it contributes information of non-ionic detergents. Thus, option (iii) Non-ionic detergents is the answer.
Question 15. Which of the following is not a target molecule for drug function in the body?
(i) Carbohydrates
(ii) Lipids
(iii) Vitamins
(iv) Proteins
Answer:
Some of the most common drug targets in the human body include carbohydrates, lipids, and proteins so, Option (iii) Vitamins is the answer.
Question 16. Which of the following statements is not true about enzyme inhibitors?
(i) Inhibit the catalytic activity of the enzyme.
(ii) Prevent the binding of substrate.
(iii) Generally, a strong covalent bond is formed between an inhibitor and an enzyme.
(iv) Inhibitors can be competitive or non-competitive.
Answer:
Enzyme inhibitors are very useful for balancing the chemical reactions in the human body, they have the ability to bind reversibly and irreversibly. While in irreversible binding, a strong covalent bond is created between the two. Therefore, option (iii), generally, a strong covalent bond is formed between an inhibitor and an enzyme, is the answer.
Question 17. Which of the following chemicals can be added for the sweetening of food items at cooking temperature and does not provide calories?
(i) Sucrose
(ii) Glucose
(iii) Aspartame
(iv) Sucralose
Answer:
There are numerous types of sweeteners present in the market with no calorific value, but they have limitations like temperature range. The name of a chemically stable artificial sweetener with no calories is (iv) Sucralose.
Question 18. Which of the following will not enhance the nutritional value of food?
(i) Minerals
(ii) Artificial sweeteners
(iii) Vitamins
(iv) Amino acids
Answer:
The artificial sweeteners are created to provide the essence of sweetness but do not contain any calories. Thus, Option (ii) Artificial sweeteners are the answer.
NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: MCQ (Type 2)
NCERT Exemplar Solutions for Chemistry in Everyday Life for MCQ (Type 2) are provided here with simple explanations. Learn these Important Questions through these advanced MCQs.
Question 19. Which of the following statements are incorrect about receptor proteins?
(i) The majority of receptor proteins are embedded in the cell membranes.
(ii) The active site of receptor proteins opens on the inside region of the cell.
(iii) Chemical messengers are received at the binding sites of receptor proteins.
(iv) The shape of the receptor doesn’t change during attachment of the messenger.
Answer:
Receptor proteins are found in the plasma membrane, they are responsible of receiving the chemical signals from other cells and reacting accordingly from changing electrical activity to accepting pharmaceutical drugs. Therefore, option (ii) The active site of receptor proteins opens on the inside region of the cell, and (iv) The shape of the receptor doesn’t change during attachment of the messenger are the answers.
Question 20. Which of the following are not used as food preservatives?
(i) Table salt
(ii) Sodium hydrogen carbonate
(iii) Cane sugar
(iv) Benzoic acid
Answer:
Food preservatives are designed to prevent the growth of microbes in food and increase its shelf life. These are safe for consumption, and common examples are (ii) Sodium hydrogen carbonate, and (iii) Cane sugar.
Question 21. Compounds with antiseptic properties are ______________.
(i) CHCl3
(ii) CHI3
(iii) Boric acid
(iv) 0.3 ppm aqueous solution of Cl2
Answer:
Antiseptic drugs apply the concept of killing infectious microbes using non-infectious microbes when applied to cuts or wounds. Common antiseptics include (ii) CHI3 and (iii) Boric acid.
Question 22. Which of the following statements are correct about barbiturates?
(i) Hypnotics or sleep-producing agents.
(ii) These are tranquillizers.
(iii) Non-narcotic analgesics.
(iv) Pain reducing without disturbing the nervous system.
Answer:
Barbiturates are composed of barbituric acid and its derivatives, classified under tranquilizers. They are used as sleep-producing agents termed as hypnotics. So, Option (i) Hypnotics or sleep producing agents, and (ii) These are tranquilizers are the answers
Question 23. Which of the following are sulpha drugs?
(i) Sulphapyridine
(ii) Prontosil
(iii) Salvarsan
(iv) Nardil
Answer:
The functioning of sulpha drugs includes killing bacteria and fungi by destroying their metabolism. Some of the most common sulpha drugs are (i) Sulphapyridine, and (ii) Prontosil.
Question 24. Which of the following are antidepressants?
(i) Iproniazid
(ii) Phenelzine
(iii) Equanil
(iv) Salvarsan
Answer:
Antidepressant drugs are administered to control the imbalanced neurotransmitters of the brain that cause mood swings and hinder the brain functioning. From the mentioned drugs, (i) Iproniazid, (ii) Phenelzine, and (iii) Equanil are commonly used antidepressants.
Question 25. Which of the following statements are incorrect about penicillin?
(i) An antibacterial fungus.
(ii) Ampicillin is its synthetic modification.
(iii) It has a bacteriostatic effect.
(iv) It is a broad-spectrum antibiotic
Answer:
Penicillin is one of the world’s first antibiotic group of drugs. It contains a fungus that kills the unwanted bacteria present in the body, causing disease. Thus, option (iii) It has a bacteriostatic effect and (iv) It is a broad-spectrum antibiotic, is a right answer
Question 26. Which of the following compounds are administered as antacids?
(i) Sodium carbonate
(ii) Sodium hydrogen carbonate
(iii) Aluminium carbonate
(iv) Magnesium hydroxide
Answer:
Antacids are used to control the excess release of HCl in the stomach that causes acidity and hyperacidity. From the given options, (ii) Sodium hydrogen carbonate and (iv) Magnesium hydroxide are types of antacids.
Question 27. Amongst the following antihistamines, which are antacids?
(i) Ranitidine
(ii) Brompheniramine
(iii) Terfenadine
(iv) Cimetidine
Answer:
Some of the antacids are also helpful in treating hyperacidity as they prevent the interaction of histamine in the stomach, thus categorized under antihistamines. Some of the most common antacids used as antihistamines are option (i) Ranitidine and (iv) Cimetidine
Question 28. Veronal and luminal are derivatives of barbituric acid which are __________.
(i) Tranquilizers
(ii) Non-narcotic analgesic
(iii) Antiallergic drugs
(iv) Neurologically active drugs
Answer:
Being derivatives of barbituric acid, Veronal and luminal is part of barbiturate drugs. These are tranquilizers and used as antiallergic drugs.
Hence (i), (iv) are correct option
Question 29. Which of the following are anionic detergents?
(i) Sodium salts of sulfonated long-chain alcohol.
(ii) Ester of stearic acid and polyethylene glycol.
(iii) Quaternary ammonium salt of an amine with acetate ion.
(iv) Sodium salts of sulfonated long-chain hydrocarbons.
Answer:
Detergents are basically cleansing agents used in the washing of clothes and for cleansing purposes. They are similar to soap in purpose but different in chemical structure. Detergents are of three types, namely cationic, anionic, and non-ionic. So, the correct options are option (i) Sodium salts of sulfonated long-chain alcohol (anionic detergents) and (iv) Sodium salts of sulfonated long-chain hydrocarbons.
Question 30. Which of the following statements are correct?
(i) Cationic detergents have germicidal properties
(ii) Bacteria can degrade the detergents containing highly branched chains.
(iii) Some synthetic detergents can give foam even in ice-cold water.
(iv) Synthetic detergents are not soaps
Answer:
The use of detergents is very prominent. They clear out dirt, grease, and impurities by making them more soluble. But some other properties of detergents include germicidal property in cationic detergents, strong detergents can also give foam in ice-cold water and synthetic detergents are not soaps. Therefore, Option (i), (iii), and (iv) are the answers.
NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: Short Answer Type
Some short answer type questions are also covered in NCERT Exemplar Class 12 Chemistry Solutions chapter 16 Chemistry in Everyday life for practice. This section contains Important Questions that are frequently asked in the exams.
Question 31. What is the average molecular mass of drugs?
Answer:
The average estimated molecular mass of drugs ranges from 100-500u
Question 32. Write the uses of medicines.
Answer:
Medicines are a crucial part of the modern world; they are used to treat diseases and maintain overall health in our daily lives. Medicines are of numerous types depending on their usage, such as infections, viral diseases, etc.
Question 33. What are antiseptics?
Answer:
In the case of a wounds or cuts, antiseptics are used to prevent infection due to microbial growth at the place. They can kill a wide range of microorganisms from bacteria, fungus, and even viruses in some cases.
Question 34. Which type of drugs comes under antimicrobial drugs?
Answer:
The drugs used to treat the growth of microorganisms for both external and internal body areas are classified as antibacterial drugs. The most common categories of this medicine are antiseptics, antibiotics, and sulfa drugs.
Question 35. Where are receptors located?
Answer:
Receptors are the biological transducers responsible for accepting the various medicinal substrates. They are present on the surface of cells or in the cytoplasm of cells.
Question 36. What is the harmful effect of hyperacidity?
Answer:
Hyperacidity is a very serious stomach condition caused due to the release of a large amount of acid, it can cause chronic pains due to the development of ulcers or gastric reflux.
Question 37. Which site of an enzyme is called allosteric site?
Answer:
Enzymes contain two binding sites; one is used in the binding of drugs to take their action in the body. The other site available for various chemical reactions of the human body is termed an allosteric site.
Question 38. What type of forces are involved in binding of substrate to the active site of an enzyme?
Answer:
The binding of the substrate to the active site of the enzyme is a significant step in all chemical reactions of the body. The forces involved in their binding are
i) hydrogen bonding
ii) ionic bonds
iii) Van der Waal force
iv) dipole interaction
Question 39. What is the commonality between the antibiotic arsphenamine and azodye?
Answer:
Antibiotics arsphenamine and azodye are similar in terms of their linkage type. The linkage in arsphenamine is –As=As- linkage which is very similar to –N=N- linkage in azodye
Question 40. Which class of drugs is used in sleeping pills?
Answer:
The category of sleeping pills is termed as tranquilizers in the medical world. These drugs are designed and used for treating fear, anxiety, and mental distractions.
Question 41. Aspirin is a pain relieving antipyretic drug but can be used to prevent heart attack. Explain.
Answer:
Other than being a pain-relieving antipyretic drug, aspirin also has a side effect on the thickness of blood. It prevents blood clotting and makes the consistency thin, thus helping in the prevention of a heart attack.
Question 42. Both antacids and antiallergic drugs are antihistamines, but they cannot replace each other. Explain why?
Answer:
Both antacids and antiallergic drugs are categorized under antihistamines due to their working pathway, but they cannot be replaced. Since antacids are responsible for neutralizing excess acid present in the stomach, antiallergic drugs act to prevent the action of histamine produced in the body in case of an allergic reaction. Having different body receptors and purposes, they cannot be replaced.
Question 43. What is a soft soap?
Answer:
Soft soaps are mainly constituted of potassium salts of fatty acid, and they dissolve very easily.
Question 44. If the soap has high alkali content it irritates the skin. How can the amount of excess alkali be determined? What can be the source of excess alkali?
Answer:
To determine the excess presence of alkali content in the soap, acid-base titration can be used. The common source of excess alkali in soaps is the hydrolysis of oil during soap preparation results in alkali formation that might irritate the skin.
Question 45. Explain why sometimes foaming is seen in river water near the place where sewage water is poured after treatment?
Answer:
Detergents, which are water-soluble cleaning agents, are used that combine with the impurities and dirt. The non-biodegradable detergents used in washing clothes to bathing cause foam formation in water bodies as they cannot be treated in the sewage treatment methods.
Question 46. Which category of the synthetic detergents is used in toothpaste?
Answer:
The synthetic detergents present in toothpaste come under the anionic category. Common examples include sodium or ammonium lauryl sulfate
Question 47. Hair shampoos belong to which class of synthetic detergent?
Answer:
The shampoo contains cationic detergents such as cetyltrimethylammonium bromide. The chemical nature of these cationic detergents is quaternary ammonium salts of acetates, chlorides, or bromides.
Question 48. Dishwashing soaps are synthetic detergents. What is their chemical nature?
Answer:
The dishwashing soaps contain synthetic detergents which are of non-ionic nature. They have good cleansing properties by combining with the impurities and dirt, making them more soluble and easier to wash away.
Question 49. Draw the diagram showing micelle formation by the following detergent. $CH_3(CH_2)_{10}CH_2OSO_3^-Na^{+}$
Answer:
The functioning of every detergent and soap involves micelle formation. The micelle structure formed by the detergent is -
$CH_3(CH_2)_{10}CH_2OSO_3^-Na^{+}$

Question 50. How does the branching of the hydrocarbon chain of synthetic detergents affect their biodegradability?
Answer:
Detergents are made up of long chains of hydrocarbons with extensive branching, which makes the molecule difficult to degrade or break b natural processes. Thus, it becomes a major cause of water pollution. The smaller number of chains and branches makes the molecule easier to degrade through biological processes.
Question 51. Why is it safer to use soap from the environmental point of view?
Answer:
Due to smaller hydrocarbon chains and minimal branching, soaps are easily biodegradable compared to detergents, as their molecules are easier to break. So, from an environmental point of view, soaps are a better option.
Question 52. What are analgesics?
Answer:
Analgesics are the drugs used to reduce pain; these are neurologically active drugs and do not have any side effects, unlike other drugs
Question 53. What is the scientific explanation for the feeling of depression?
Answer:
When seen from a scientific point of view, this state is caused by a hormone named noradrenaline, whose major purpose is to control mood swings. When the levels of noradrenaline drop, it hampers with the signal activities of brain functioning.
Question 54. What is the basic difference between antiseptics and disinfectants?
Answer:
Antiseptics and disinfectants are very similar in their end purpose, but the path followed and the target is different. Antiseptics are safe for living tissues, unlike disinfectants that are used on non-living substances. They both are antimicrobial, but antiseptics are used to prevent microbial growth on cuts and wounds, but the target of disinfectants are surfaces like floors and toilets to kill microbes.
Question 55. Between sodium hydrogen carbonate and magnesium hydroxide, which is a better antacid and why?
Answer:
Magnesium hydroxide is a hydroxide antacid that completely treats acidity by maintaining the pH of the stomach and is also insoluble in the stomach, whereas carbonated antacids like sodium hydrogen carbonate make the nature of the stomach alkaline, which results in releasing larger amounts of acid.
Question 56. Which analgesics are called opiates
Answer:
The medicine obtained from the opium poppy is known as opiates and are also known as narcotic analgesics. The examples include morphine and its derivatives such as codeine and morphine diacetate (heroin).
Question 57. What is the medicinal use of narcotic drugs?
Answer:
Narcotics are administered to produce sleep and relieve pain. They are prescribed to patients for postoperative pain, terminal cancer, childbirth, and cardiac pain.
Question 58. What are antagonistic drugs?
Answer:
Antagonistic drugs inhibit the natural function of the receptor site by binding to it. The most common example is cimetidine antacid drug.
Question 59. What is the mode of action of antimicrobial drugs?
Answer:
As the name indicates, antimicrobial drugs are designed to eradicate microorganisms such as bacteria, viruses, fungi, or other parasites. The pathogenic action caused by the microbes is also inhibited by the drugs.
Question 60. What is the side product of soap industry? Give reactions showing soap formation.
Answer:
The key side product of soap formation in the soap industry is glycerol.

Question 61. What is the difference between bathing soap and washing soaps?
Answer:
The major difference between bathing soaps and washing soaps is their chemical composition. Bathing soaps are the potassium salts of long-chain fatty acids, but washing soaps are sodium salts of long-chain fatty acids. Bathing soaps do not contain any unused alkali and are soft at touch. Washing soaps are hard and have residual alkali in them.
Question 62. How are transparent soaps manufactured?
Answer:
When the soap is dissolved and the excess solvent is evaporated, it results in the formation of transparent soaps.
Question 63. What is the advantage of using antihistamines over antacids in the treatment of acidity
Answer:
The target of antacid drugs is the excess acid produced in the stomach and neutralizing it; thus, they only control the symptoms, but not the cause of excess acid production. When antihistamine drugs are taken instead of antacids, they directly attack the receptors and prevent histamine from attaching to the active site responsible for acid production, and inhibit their functions. This way, the medicine used to cure the problem rather than treating only the symptoms. That’s why antihistamines are preferred over antacids.
Question 64. What are the functions performed by histamine in the body?
Answer:
Histamine plays many vital human body functions; it controls the muscles of the gut and bronchi. It controls the muscle relaxation of fine blood vessels and is also responsible for congestion related to the common cold and allergies in the nose. It regulates the production of hydrochloric acid in the stomach and releases pepsin.
Question 65. With the help of an example explain how do tranquillizers control the feeling of depression?
Answer:
The chemical responsible for the mood swings in the state of depression is called noradrenaline. Low level of this compound results in a person feeling the imbalance in emotions, and this prolonged state can result in depression.
Question 66. Why are certain drugs called enzyme inhibitors?
Answer:
Enzymes control all the human body functions at the ground state, and they contain multiple active sites that are substrate-specific and carry out the normal biochemical reactions. There are many drugs available that can block the active site interaction between the enzyme and substrate. This eradicates the catalytic property of the enzyme, thus making the reactions slow. Therefore, these types of drugs are called inhibitors.
Question 67. What are fillers and what role these fillers play in soap?
Answer:
Due to the diverse application of soaps, each type contains specific constituents to fulfil its role. These substances, which provide a soap, have these specificity properties and are known as fillers. In shaving soaps, glycerol acts as a filler to stop the rapid drying of the soap. In laundry soaps, many fillers are present to increase the ability to form leather-like sodium rosinate, sodium silicate, borax, and sodium carbonate. In medical soaps, fillers are added to give it properties like antibacterial, antifungal, etc. Soap deodorants also include the addition of fillers.
Question 68. Sugar is the main source of energy as it produces energy on metabolic decomposition. But these days low chloride drinks are more popular, why?
Answer:
People are very conscious about their intake of calories in today’s world, but the intake of sweet beverages is on the rise even though it contain sugar content. This is due to the usage of artificial sweetening agents produced synthetically in these drinks. Due to their non-metabolizing property, they do not increase calorie intake.
Question 69. Pickles have a long shelf life and do not get spoiled for months, why?
Answer:
To increase the shelf life of food products, they contain preservatives to avoid spoilage. The preservatives present in pickles include Table salt, sugar, vegetable oils, and sodium benzoate etc. Hence, moisture or air is not able to enter the material, and pickles can stay edible for months
Question 70. What is the difference between saccharin and saccharic acid?
Answer:
The name is very similar, but they are two completely different compounds. Saccharin is an artificial sweetener also known as (o-sulpho benzoicimide), but saccharic acid is an inorganic compound obtained by oxidation of glucose in the presence of a cone. HNO3 is known as dicarboxylic acid.

Question 71. Name an artificial sweetener which is derivative of sucrose.
Answer:
The artificial sweetener named sucralose is a trichloroglucoside derivative. It is widely used due to its no-calorie production or tooth decay risk; it is 600 times sweeter than sucrose.
Question 72. Name two α-amino acids which form a dipeptide which is 100 times more sweet than cane sugar?
Answer:
Two a-amino acids forming the methyl ester of the dipeptide are Aspartic acid and phenylalanine. The name of the ester is aspartame, which is an artificial sweetener and is 100 times sweeter than cane sugar.
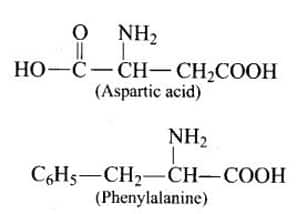
Question 73. Aspartame is unstable at cooking temperature, where would you suggest aspartame to be used for sweetening?
Answer:
When aspartame is added at cooking temperature, it gets denatured due to less stable composition. Thus, it can be used only as a sweetener in soft drinks and cold foods.
Question 74. Sodium salts of some acids are Very useful as food preservatives. Suggest a few such acids.
Answer:
Some commonly used sodium acid salts as food preservatives include salts of benzoic acid, sorbic acid, and propanoic acid etc.
Question 75. Explain the role of allosteric site in enzyme inhibition?
Answer:
Some drugs bind to the enzyme to inhibit its natural functioning, but the mechanism followed may differ in the drugs. Sometimes, instead of the active site, the drugs bind to the second site, known as the allosteric site, which results in changing the shape of the active site. This change makes the active site unrecognizable to the substrate; hence, no bonding can take place. This is a rather permanent approach if the attachment is by covalent bond formation, as it eradicates the affinity between the site and its substrate. To overcome this situation, sometimes the body generates a completely new enzyme and discards the previous one.

Question 76. How are receptor proteins located in the cell membrane?
Answer:
The receptor proteins are arranged in a specific manner so as to project out their active sites towards the surface, and they open in the outer region of the membrane. The proteins are embedded in the cell membrane
Question 77. What happens when the bond formed between an enzyme and an inhibitor is a strong covalent bond?
Answer:
The inhibitor drugs act by bonding with the enzyme at either the active site or the allosteric site. If they are connected via a covalent bond, the bond is exceedingly difficult to break and requires a large amount of energy, thus blocking the enzyme permanently.
NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: Matching Type
The Chemistry in Everyday Life Class 12 Important Questions are discussed below. These are generally asked in exams to test your knowledge. These Solutions are quite helpful for competitive exams.
Question 78. Match the medicines given in Column I with their use given in Column II.
|
Column I |
Column II |
|
(i) Ranitidin |
(a) Tranquilizer |
|
(ii) Furacine |
(b) Antibiotic |
|
(iii) Phenelzine |
(c) Antihistamine |
|
(iv) Chloramphenicol |
(d) Antiseptic |
|
|
(e) Antifertility drug |
Answer:
The correct match of the medicines and their role is- $(\mathrm{i} \longrightarrow \mathrm{c}),(\mathrm{ii} \longrightarrow>\mathrm{d}),(\mathrm{iii} \longrightarrow » \mathrm{a}),(\mathrm{iv} \longrightarrow » \mathrm{~b})$
(i) Ranitidine – It is used to inhibit the histamine interaction with enzyme receptors in the stomach wall. It directly regulates the production of hydrochloric acid and pepsin.
(ii) Furacine – It is applied to kill the microbes on the living tissues and also prevent their growth. Thus, it is an antiseptic.
(iii) Phenelzine – Commonly known as Nardil. It is used for treating depression, and its structure is

(iv) Chloramphenicol – It is used to cure a wide range of diseases like acute fever, dysentery, certain urinary infections, meningitis, and pneumonia. It is classified as a broad-spectrum antibiotic.
Question 79. Match the soaps given in Column I with items given in Column II.

Answer:
The right match of the above options is - $(\mathrm{i}->\mathrm{b}),(\mathrm{ii} \longrightarrow>\mathrm{a}),(\mathrm{iii} \longrightarrow>\mathrm{d}),(\mathrm{iv} \longrightarrow \mathrm{c})$
(i) Soap chips – as the name suggests, are formed by spreading and cooling the melted soap and then cutting it into small pieces.
(ii) Soap granules are made due to the drying of small soap bubbles.
(iii) To enhance the action of soaps, the soap powder contains builders such as sodium carbonate and trisodium phosphate. (iv) The components present in scouring soaps include Scouring soaps contain soap powder, a scouring agent (abrasive) such as powdered pumice, or finely divided sand and builders.
Question 80. Match the structures given in Column I with the type of detergents given in Column II.

Answer:
The correct match of the structure with their type of detergent are - $(\mathrm{i} \longrightarrow \mathrm{c}),(\mathrm{ii} \longrightarrow \mathrm{d}),(\mathrm{iii} \longrightarrow \mathrm{b}),(\mathrm{iv}->\mathrm{a})$
Question 81. Match the detergents given in Column I with their uses given in Column II.

Answer:
The detergent components and their use are matches as follows -$(\mathrm{i} \longrightarrow \mathrm{c}),(\mathrm{ii} \longrightarrow \mathrm{d}),(\mathrm{iii} \longrightarrow \mathrm{b}),(\mathrm{iv} \longrightarrow \mathrm{a})$
(i) The detergents used in hair shampoos/conditioners are cationic, containing quaternary ammonium salts of amines along with chlorides, bromides or acetates, etc. Examples of cationic detergents are cetyltrimethyl ammonium bromide.
(ii) Toothpaste contains anionic detergents for e.g., sodium dodecyl benzene sulphonate. Their preparation is as follows

(iii) The fillers in laundry soaps enhance the leather forming ability, and the examples include sodium rosinate, Sodium silicate, borax, and sodium carbonate.
(iv) Dishwashing powder contains non-ionic detergents.

Question 82. Match the class of compounds given in Column I with their functions given in Column II.

Answer:
The correct match of compounds with their functions is - $(\mathrm{i} \longrightarrow \mathrm{b}),(\mathrm{ii} \longrightarrow \mathrm{d}),(\mathrm{iii} \longrightarrow \mathrm{a}),(\mathrm{iv} \longrightarrow \mathrm{e}),(\mathrm{v} \longrightarrow \mathrm{c})$
(i) Antagonist drugs are used to inhibit the functioning of specific enzyme receptors. For example, to block the dopamine receptors by receptor antagonism, the drug used is of a dopamine antagonist.
(ii) Agonist drugs are used and effective only in the absence of a chemical messenger, e.g. heroin
(iii) Chemical messengers are responsible for communicating the message from two neurons and between neurons to muscles. These compounds are present at the site of binding in receptors.
(iv) The role of inhibitors is to block the binding site of the receptors, hence hindering the catalytic activity of the enzymes
(v) Receptors are very significant for the body as these proteins are present in the cell membrane to transmit and communicate with other cells.
Question 83. Match the classes of drugs given in Column I with their action given in Column II.

Answer:
The correct match for the mentioned drugs and their actions is -$(\mathrm{i} \longrightarrow \mathrm{e}),(\mathrm{ii}->\mathrm{f}),(\mathrm{iii} \longrightarrow \mathrm{d}),(\mathrm{iv} \longrightarrow \mathrm{g}),(\mathrm{v}->\mathrm{b}),(\mathrm{vi} \longrightarrow \mathrm{a}),(\mathrm{vii} \longrightarrow \mathrm{c})$
(i) Analgesics are commonly called pain killers; they reduce or abolish pain without any major side effects like mental confusion. E.g. aspirin
(ii) These are the class of drugs which kill and stop the growth of microbes on living tissues during wound healing, e.g. tincture of iodine.
(iii) Antihistamines are prescribed for allergic reactions. They inhibit the natural functioning of histamine by binding to the receptor site, e.g. Seldane.
(iv) Antacids are used to neutralize the excess production of acid in the stomach, causing acidity, e.g., a mixture of Mg(OH)2 and Al(OH)3.
(v) These drugs are administered to treat mental illnesses like stress and anxiety, e.g., Equanil.
(vi) Antibiotics have used the concept of killing bacteria by other bacteria; they are used for mild infections, and due to less toxicity, they are considered very effective, e.g., chloramphenicol.
(vii) Disinfectants also have the same role as antiseptic, but these are applied onto non-living components due to unsafe nature, e.g., 1 percent solution of phenol.
NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: Assertion and Reason Type
The Assertion and Reason Type questions included in Chemistry in Everyday life form an important section that tests students concept clarity and reasoning ability of Chemistry in Everyday Life Class 12 NCERT Exemplar.
(i) Both assertion and reason are correct statements but the reason does not explain the assertion.
(ii) Assertion and reason both are correct and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is a correct statement, reason is a wrong statement.
(v) Assertion is a wrong statement, reason is the correct statement.
Answer:
(iii) Penicillin (G) does not kill gram-positive and gram-negative bacteria as it’s not a broad-spectrum antibiotic so both assertion and reason are wrong statements.
Assertion (A): Sulpha drug contain sulphonamide group.
Reason (R): Salvarsan is a sulpha drug.
(i) Both assertion and reason are correct statements, but the reason does not explain the assertion.
(ii) Assertion and reason both are correct, and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is a correct statement, reason is a wrong statement.
(v) Assertion is a wrong statement, reason is the correct statement.
Answer:
(iv) Assertion is a correct statement, reason is a wrong statement.
Sulpha drugs contain the sulphonamide group, which is essential for their antibacterial activity. Salvarsan is an organometallic compound of arsenic. It was used to treat syphilis.
Assertion (A): Receptors are crucial to body’s communication process.
Reason (R): Receptors are proteins.
(i) Both assertion and reason are correct statements but the reason does not explain the assertion.
(ii) Assertion and reason both are correct, and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is a correct statement; the reason is a wrong statement.
(v) Assertion is a wrong statement, reason is the correct statement.
Answer:
i) The human body contains a specific system for message transfer from cell to cell. And the factors transferring the messages are Neurotransmitters, small molecules. After message transfer, there is no change in the shape of neurotransmitters, and the receptors present are proteins.
Assertion (A): Enzymes have active sites that hold substrate molecule for a chemical reaction.
Reason (R): Drugs compete with natural substrate by attaching covalently to the active site of enzyme.
(i) Assertion and reason both are correct statements but the reason does not explain the assertion.
(ii) Assertion and reason both are correct and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is correct statement, reason is wrong statement.
(v) Assertion is wrong statement reason is the correct statement.
Answer:
(iv) To compete for binding at the active site of receptors, inhibitor drugs compete with the natural substrate by forming bonds like H-bonding, van der Waals interaction, etc.
(i) Assertion and reason both are correct statements,s but the reason does not explain the assertion.
(ii) Assertion and reason both are correct and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is a correct statement; the reason is a wrong statement.
(v) Assertion is wrong statement, reason is the correct statement.
Answer:
(iv) The chemical messengers communicate by attaching to the active site of enzymes on the cell membrane, but they cannot enter the cell through the receptors.
(i) Assertion and reason both are correct statements,s butthe reason does not explain the assertion.
(ii) Assertion and reason both are correct and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is a correct statement, reason is a wrong statement.
(v) Assertion is a wrong statement, reason is a correct statement.
Answer:
(iv) Assertion is a correct statement, reason is a wrong statement.
Transparent soaps are prepared by dissolving regular soap in a mixture of ethanol, glycerol, and sometimes sugar.
The ethanol helps dissolve the soap and makes the mixture clear and uniform.
Ethanol does not make objects invisible. It can make some substances transparent if their refractive index matches ethanol, but it doesn’t make objects disappear. That’s a misconception
(i) Both assertion and reason are correct statements but the reason does not explain the assertion.
(ii) Assertion and reason both are correct, and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is a correct statement, reason is a wrong statement.
(v) Assertion is a wrong statement, reason is a correct statement.
Answer:
(ii) When esters of long-chain fatty acids are hydrolyzed with an alkali, it results in saponification. The process of soap formation but the soap produced as the end result is in colloidal form due to the addition of sodium chloride for precipitation purposes.
Assertion (A): Competitive inhibitors compete with natural substrate for their attachment on the active sites of enzymes.
Reason (R): In competitive inhibition, inhibitor binds to the allosteric site of the enzyme.
(i) Assertion and reason both are correct statement but reason does not explain the assertion.
(ii) Assertion and reason both are correct and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is a correct statement, reason is a wrong statement.
(v) Assertion is wrong statement reason is the correct statement.
Answer:
(iv) When the inhibitor drugs use the competitive mechanism of action, they bind to the active site of the enzyme.
Assertion (A): Non-competitive inhibitor inhibits the catalytic activity of enzyme by binding with its active site.
Reason (R): Non-competitive inhibitors changes the shape of the active site in such a way that substrate cannot recognize it.
(i) Both assertion and reason are correct statements, but the reason does not explain the assertion.
(ii) Assertion and reason both are correct, and the reason explains the assertion.
(iii) Both assertion and reason are wrong statements.
(iv) Assertion is a correct statement, reason is a wrong statement.
(v) Assertion is a wrong statement, reason is a correct statement.
Answer:
(v) Assertion is a wrong statement, reason is a correct statement.
A non-competitive inhibitor inhibits the catalytic activity of an enzyme but does not bind to its active site. Non-competitive inhibitors bind to a site other than the active site. This binding causes a conformational change in the enzyme's structure, including the active site.
Assertion (A): Chemical messenger gives message to the cell without entering the cell.
Reason (R): Chemical messenger is received at the binding site of receptor proteins.
(i) Assertion and reason both are correct statement but reason does not explain assertion.
(ii) Assertion and reason both are correct and reason explains the assertion.
(iii) Both assertion and reason are wrong statement.
(iv) Assertion is correct statement reason is wrong statement.
(v) Assertion is wrong statement reason is correct statement.
Answer:
(ii) while transferring messages to cells, the chemical messengers do not enter in the cell as they attach to the active site of receptors in the cell membrane and detach after message transmission.
Assertion (A): Receptor proteins show selectivity for one chemical messenger over the other.
Reason (R): Chemical messenger binds to the receptor site and inhibits its natural function.
(i) Assertion and reason both are correct statement but reason does not explain assertion.
(ii) Assertion and reason both are correct and reason explains the assertion.
(iii) Both assertion and reason are wrong statement.
(iv) Assertion is correct statement reason is wrong statement.
(v) Assertion is wrong statement reason is correct statement.
Answer:
(iv) the message is transferred by the chemical messenger after its binding to the receptor site with going in the cell.
Assertion (A): All chemicals added to food items are called food preservatives.
Reason (R): All these chemicals increase the nutritive value of the food.
(i) Assertion and reason both are correct statement but reason does not explain assertion.
(ii) Assertion and reason both are correct and reason explains the assertion.
(iii) Both assertion and reason are wrong statement.
(iv) Assertion is correct statement reason is wrong statement.
(v) Assertion is wrong statement reason is correct statement.
Answer:
(iii) To increase the shelf life of food products and prevent any microbial growth like bacteria, mould, or fungus, some chemicals are added that are known as a preservative. They do not contain any nutritive value.
Assertion (A): Preservative are added to food items.
Reason (R): Preservatives inhibit the growth of microorganisms.
(i) Assertion and reason both are correct statement but reason does not explain assertion.
(ii) Assertion and reason both are correct and reason explains the assertion.
(iii) Both assertion and reason are wrong statement.
(iv) Assertion is correct statement reason is wrong statement.
(v) Assertion is wrong statement reason is correct statement.
Answer:
(ii) Preservatives protect the food from spoilage due to microbial growth and increase the shelf life.
Assertion (A): Artificial sweeteners are added to the food to control the intake of calories.
Reason (R): Most of the artificial sweeteners are inert and do not metabolize in the body.
(i) Assertion and reason both are correct statement but reason does not explain assertion.
(ii) Assertion and reason both are correct and reason explains the assertion.
(iii) Both assertion and reason are wrong statement.
(iv) Assertion is correct statement reason is wrong statement.
(v) Assertion is wrong statement reason is correct statement.
Answer:
(ii) Because of the inert nature of artificial sweeteners, they do not metabolize; thus, they don’t have any calorific value.
NCERT Exemplar Solutions Class 12 Chemistry Chapter 16: Long Answer Type
Following are the long-answer type questions from Chapter 16 that need more practice and learning. These are the Chemistry in Everyday Life Class 12 Important Questions that are asked in the exams.
Question 98. In what respect do prontosil and Salvarsan resemble. Is there any resemblance between azo dye and prontosil? Explain.
Answer:
Both Prontosil and Salvarsan have been classified under the same category, i.e., antibacterial (antimicrobial) drugs, they were discovered by Paul Ehrlich. The other name for Salvarsan is arsphenamine and its molecule being organoarsenic has a -As = As- double bond.

Due to the same category, they have similarities in their structure. The linkage in Salvarsan is -As = As-, and prontosil contains -N = N- linkage.

Question 99. How do enzymes catalyze a chemical reaction in the living system? Explain drug target interaction taking the example of an enzyme as target.
Answer:
Enzymes play the main role in biochemical reactions as they are the catalysts; their types of mechanisms of catalysis are -
(i) The active site of the enzyme captures the substrate and holds it in a particular position so as to react effectively with the substrate. Some of the types of forces involved in binding of the substrate with the active site include ionic bonding, hydrogen bonding, van der Waals, and dipole-dipole interactions.
(ii) Enzymes also work as a catalyst by providing functional groups to attack the substrate in order to complete the chemical reactions. This role is acted out by amino acid residues of protein found on the enzyme’s active site. They attack the substrate to carry out chemical reactions. The enzyme inhibitor drugs hinder this process by blocking the active site attachment of the substrate.

Synthetically manufactured detergents have the characteristic properties of soap, but they do not contain soap. And due to their ability to work in both hard water and soft water, they are highly preferred. Detergents are divided into three categories depending on their chemical nature -
(i) Anionic detergents – These are constituted of long chains of hydrocarbons or alcohols and sodium alkylbenzene. They are designed for cleansing purposes. e.g., $CH_3(CH_2)_{10}CH_2OSO_3^{-} Na^{+}$
(ii) Cationic detergents – cationic detergents contain the quaternary ammonium salts of chlorides, bromides, or acetates as anions.

(iii) Non-ionic detergents – These detergents do not have any ions present in their composition, for example, $CH_3(CH_2)_{16}COO(CH_2CH_2O)_nCH_2CH_2OH$, formed using stearic acid and polyethylene glycol.
To combat the pollution caused by detergents, the hydrocarbon chains can be made shorter and less branched to make the compound biodegradable
The role of enzyme inhibitors is to bind to the enzyme site so as to block the attachment of the substrate to the active site. -This overall inhibits the catalytic activity of the enzyme. Their mode of action is -
(i) By following the competitive mechanism approach, they challenge the substrate for attachment to the active site of the enzyme. These drugs are known as competitive inhibitors.
(ii) The second mechanism includes the bonding of the inhibitor drug with the allosteric site. It changes the shape of the active site completely, thus making it unrecognizable to the substrate. These are called a non-competitive inhibitor. Due to the bond formed between the two is a strong covalent bond, it is a rather permanent inhibition of the substrate.

In the NCERT exemplar solutions for Class 12 Chemistry chapter 16, we would learn the different types of drugs and their specifications, their interaction with several receptors and enzymes, the medicinal relief offered by different classes of drugs, the preservatives, antioxidants present in our food, and the cleansing action of soaps and detergents.
NCERT Exemplar Class 12 Chemistry Chapter 16: Higher Order Thinking Skills (HOTS) Questions
The Higher Order Thinking Skills (HOTS) questions of NCERT Exemplar Class 12 Chemistry Chapter 16 Solutions are provided below; they are designed to test your understanding to Class 12 Chemistry Chapter 16.
Question 1. The $\mathrm{F}^{-}$ions make the enamel on teeth much harder by converting hydroxyapatite (the enamel on the surface of teeth) into much harder fluoroapatite, having the formula.
(1) $\left[3\left(\mathrm{Ca}_3\left(\mathrm{PO}_4\right)_2\right) \cdot \mathrm{CaF}_2\right]$
(2) $\left[3\left(\mathrm{Ca}_2\left(\mathrm{PO}_4\right)_2\right) \cdot \mathrm{Ca}(\mathrm{OH})_2\right]$
(3) $\left[3\left(\mathrm{Ca}_3\left(\mathrm{PO}_4\right)_3\right) \cdot \mathrm{CaF}_2\right]$
(4) $\left[3\left(\mathrm{Ca}_3\left(\mathrm{PO}_4\right)_2\right) \cdot \mathrm{Ca}(\mathrm{OH})_2\right]$
Answer:
Fluoroapatite $\Rightarrow\left[3 \mathrm{Ca}_3\left(\mathrm{PO}_4\right)_2 \cdot \mathrm{CaF}_2\right]$
Hence, the correct answer is option (1).
Question 2. Which of the following compounds are not used as disinfectants?
A. Chloroxylenol B. Bithional C. Veronal D. Prontosil E. Terpineol
Choose the correct answer from the options given below :
(1) C, D
(2) B, D, E
(3) A, B
(4) A, B, E
Answer:
- Veronal is a tranquilizer
- Prontosil is an antibiotic drug.
Hence, the correct answer is option (1).
Question 3. Soaps do not work in hard water due to:
(1) Formation of scum
(2) Formation of Lather
(3) Presence of scouring agents
(4) Absorption of water on the cloth
Answer:
Soaps do not work effectively in hard water because it contains calcium (Ca²⁺) and magnesium (Mg²⁺) ions, which react with soap molecules to form insoluble scum (calcium and magnesium salts of fatty acids). This prevents soap from producing lather, reducing its cleansing ability.
Hence, the correct answer is option (1).
Question 4: Match Iist I with Iist II :
|
LIST I (Compound) |
LIST II (Uses) | ||
| A. | Iodoform | I. | Fire Extinguisher |
| B. | Carbon Tetrachloride | II. | Insecticide |
| C. | CFC | III. | Antiseptic |
| D. | DDT | IV. | Refrigerants |
Choose the correct answer from the options given below :
(1) A-I, B-II, C-III, D-IV
(2) A-III, B-II, C-IV, D-I
(3) A-III, B-I, C-IV, D-II
(4) A-II, B-IV, C-I, D-III
Answer:
Iodoform - Antiseptic
$\mathrm{CCl}_4$ - Fire extinguisher
$\mathrm{CFC}$ - Refrigerants
DDT - Insecticide
Hence, the answer is the option (3).
Question 5: Which of the following artificial sweeteners has the highest sweetness value in comparison to cane sugar?
(1) Sucralose
(2) Aspartame
(3) Alitame
(4) Saccharin
Answer:
Alitame has 2000 times more sweetener as compared to cane sugar.
Hence, the answer is the option (3).
Approach to Solve Questions of Class 12 Chemistry Chapter 16
To effectively solve questions of Chemistry in Everyday Life Class 12 NCERT Exemplar, systematic and structured approach are given below:
1) Before solving questions of NCERT it is important to have a proper knowledge of definitions and understanding of the classification of drugs based on structure, mechanism, etc. Students can refer to Chemistry in everyday life notes.
2) Questions related to mechanisms of drugs are often asked in exams
- Keep focus on how drugs interact with receptors and enzymes
- Learn key terms like competitive inhibition
3) These key differences help clarify fundamental concepts and are often asked in exams to test conceptual understanding.
- Antibiotic, antiseptic, and disinfectant
- Detergent and soap
- Natural sweeteners and synthetic sweeteners
4) Proper understanding of basic concepts and practice helps students clear their doubts and solve Chemistry in Everyday Life Important Questions effectively. Follow the NCERT Exemplar Class 12 Chemistry Solutions chapter 16 Chemistry in Everyday life for detailed explanations.
Topics and Subtopics Covered in the NCERT Exemplar Class 12 Chemistry Chapter 16
Given below the topics covered in NCERT Exemplar Solutions for Chemistry in Everyday Life. These topics are essential for solving questions and Answers. Go through these topics carefully.
- Drugs and Their Classification
- Classification of Drugs
- Drug-Target Interaction
- Enzymes as Drug Targets
- Receptors as Drug Targets
- Therapeutic Action of Different Classes of Drugs
- Antacids
- Antihistamines
- Neurologically Active Drugs
- Antimicrobials
- Antifertility Drugs
- Chemicals in Food
- Artificial Sweetening Agents
- Food Preservatives
- Antioxidants in Food
- Cleansing Agents
- Soaps
- Synthetic Detergents
Advantages of Using Class 12 Chemistry NCERT Exemplar Solutions chapter 16 Chemistry in Everyday life
TheseChemistry in Everyday Life Class 12 NCERT Exemplar Solutions cover all questions from the NCERT book in a very simple way. The advantages of using these solutions are given below:
- These solutions help students to understand topics like the role of drugs, cleansing agents, and chemicals used daily.
- These NCERT Exemplar Class 12 Solutions include detailed answer of every questions included in textbook.
- These NCERT Exemplar Solutions for Chemistry in Everyday Life are prepared by subject experts in a very clear and comprehensive manner that helps students for board and competitive exams.
- They offers systematic solutions that help students to write accurate, concise and detailed answers in exams.
NCERT Exemplar Solutions Class 12 Chemistry Chapter-Wise
These NCERT Exemplar Solutions for Class 12 Chemistry are designed to help students strengthen their conceptual understanding and problem-solving skills. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry are prepared to help students understand complex concepts with ease. Here is a list of NCERT chapter-wise solutions:
NCERT Solutions subject-wise
The NCERT subject-wise solutions will help you broaden your concept and will also help in revision.
NCERT Exemplar Class 12 Solutions subject-wise
Excel your preparation with NCERT exemplar solutions. Click on the link below
NCERT Class 12 subject-wise notes
You can follow the links given in the table below to get access to the Class 12 NCERT notes.
NCERT Books and NCERT Syllabus
Also, you can find links to the Class 12 NCERT chemistry book and syllabus for the respective subjects.
Frequently Asked Questions (FAQs)
NCERT Exemplar Chapter 16 focuses on the role of chemistry in our daily lives, highlighting how various chemical compounds are used in medicines, household products, and even in food.
The chapter categorizes drugs based on their therapeutic uses, such as analgesics , antibiotics, and antihistamines. Each category has specific characteristics and modes of action, allowing students to understand the importance of these drugs and their applications.
Acid rain is precipitation that is unusually acidic. It has a high concentration of hydrogen ions or low pH. It is primarily caused by emissions of sulfur dioxide and nitrogen oxides from burning fossil fuels, industrial processes, and vehicle exhaust. These gases react with water, oxygen, and other chemicals in the atmosphere to form sulfuric acid and nitric acid, which then fall to the earth as acid rain.
Medications that are unstable at room temperature and can degrade or decompose, need to be stored in a refrigerator. Refrigeration slows down these chemical reactions, preserving the drug's potency.
Antibiotics kill or inhibit bacteria, while antiseptics prevent infection.
Enzymes are biological catalysts that speed up chemical reactions in living organisms. According to the chapter, they are vital in processes such as digestion, metabolism, and biochemical reactions necessary for life. Enzymes also have practical applications, such as in detergents and food processing, showcasing their importance beyond just biological contexts.
Antioxidants are substances that inhibit oxidation and can prevent damage to our cells caused by free radicals. The chapter emphasizes the significance of antioxidants found in everyday foods as they help protect against chronic diseases, support overall health, and play a vital role in aging and maintaining cellular integrity.
Preservatives are substances added to food to prevent spoilage and extend shelf life. The chapter discusses their importance in preventing microbial growth and maintaining food safety, which is essential for public health. By understanding the types of preservatives and their functions, consumers can make informed choices about the foods they consume.
Soaps and detergents are important because they serve as cleaning agents that help remove dirt, grease, and microbes from surfaces. The chapter explains their chemical structure and how they function to emulsify oils and suspend dirt, making them indispensable in personal hygiene and household cleaning.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Hello,
Here is your Final Date Sheet Class 12 CBSE Board 2026 . I am providing you the link. Kindly open and check it out.
https://school.careers360.com/boards/cbse/cbse-class-12-date-sheet-2026
I hope it will help you. For any further query please let me know.
Thank you.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
