Class 12 NCERT solutions of Amines are detailed answers to the questions. These solutions help students understand the structure, properties, preparation methods, and chemical reactions of amines, and provide step-by-step explanations to strengthen their conceptual clarity and exam preparation.
NCERT Solutions for Class 12 Chemistry Chapter 9 Amines
CBSE Class 12th Exam Date:01 Jan' 26 - 14 Feb' 26
Ever wondered what happens when ammonia meets alkyl or aryl groups? Let us find out the answer through amines! They are a class of organic compounds which is derived by replacing one or more hydrogen atoms of ammonia with alkyl or aryl groups. NCERT Solutions for Class 12 Chemistry Chapter 9 Amines will make students learn about different types, structures, properties and reactions of amines. The topics like Hofmann bromamide degradation, Gabriel phthalimide synthesis and how amines react in various situations are well discussed in this chapter.
This Story also Contains
- NCERT Solutions for Class 12 Chemistry Chapter 9: Download PDF
- NCERT Solutions Of Class 12 Amines (Intext Questions Ex 9.1 to 9.9)
- NCERT Solutions for Class 12 Chemistry Chapter 9 Amines (Exercise Questions)
- Class 12 Chemistry NCERT Chapter 9: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 9: Amines
- Topics of Chapter 9 In NCERT Book Class 12 Chemistry
- What Extra Should Students Study Beyond the NCERT for JEE/NEET?
- What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 9 Amines
- Importance of Class 12 Chemistry Chapter 9 Amines Solutions
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for Class 12 Subject-wise
- NCERT Exemplar subject-wise
- NCERT Books and NCERT Syllabus
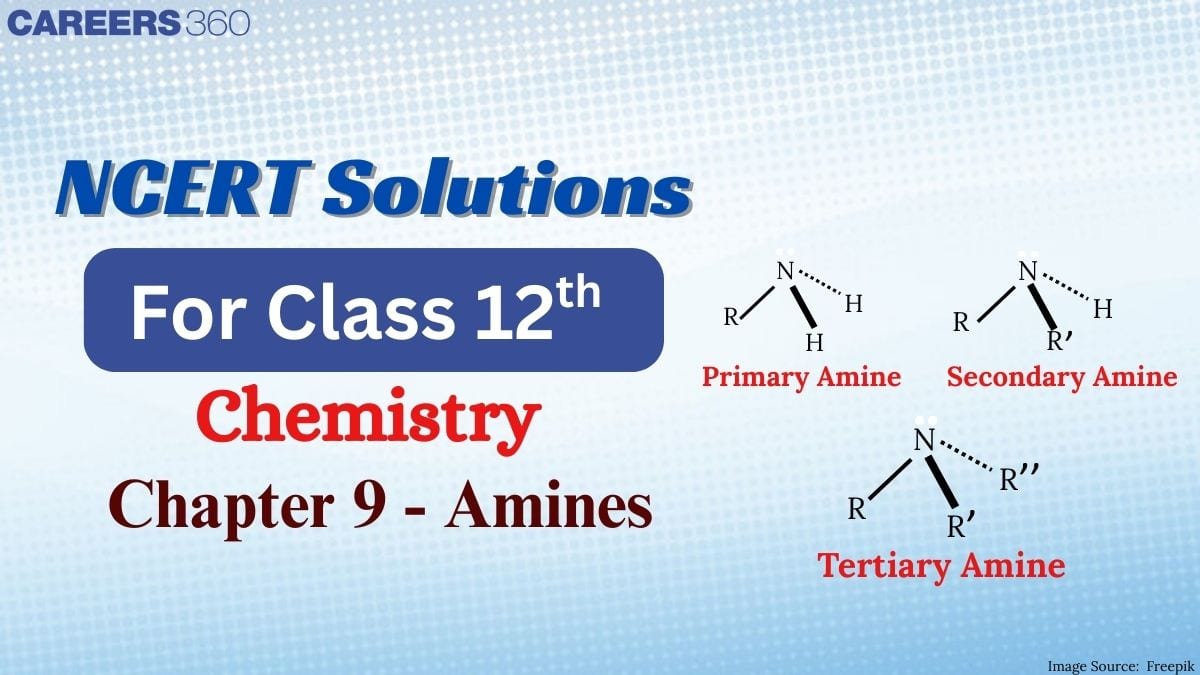
The chapter is important for anyone looking to do well in organic chemistry. The NCERT solutions for class 12 chemistry offer clear explanations that will help students understand Chapter 9 Amines Class 12 Chemistry topics and improve their problem solving skills. These solutions will help you prepare for boards as well as for competitive exams. We have also included higher-order thinking skills (HOTS) questions to check your critical thinking and deepen your understanding of the topics.
NCERT Solutions for Class 12 Chemistry Chapter 9: Download PDF
Students can download the amines ncert solutions pdf for free. These solutions are designed to help you understand the fundamental concepts and solve textbook questions with ease. Get comprehensive and accurate solutions to all exercises.
Also Read
NCERT Solutions Of Class 12 Amines (Intext Questions Ex 9.1 to 9.9)
These class 12 chemistry chapter 9 amines solutions are given below. They are designed provide step by step explanations, making it easier to learn concepts, apply formulas, and prepare effectively for exams. These NCERT Solutions for Class 12 help students grasp fundamental concepts, practise reactions, and build a strong foundation for exams.
Page No. 262
Question 9.1 Classify the following amines as primary, secondary or tertiary:
Answer :
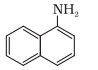
The nitrogen atom is directly connected with only one carbon atom, so it is a primary aromatic amine.
Question 9.1 Classify the following amines as primary, secondary or tertiary:
Answer :
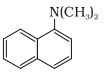
In this compound, the nitrogen atom is directly connected to 3 carbon atoms. So, it is a tertiary aromatic amine
Question 9.1 Classify the following amines as primary, secondary or tertiary:
(iii) $(C_{2}H_{5})_{2}CHNH_{2}$
Answer :
$(C_{2}H_{5})_{2}CHNH_{2}$
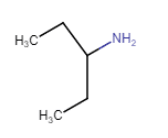
Here, the N atom is directly connected to only one carbon atom. So it is a primary aliphatic amine.
Question 9.1 Classify the following amines as primary, secondary or tertiary:
Answer :
$(C_{2}H_{5})_{2}NH$
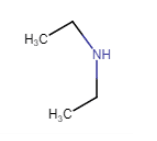
In structure, we can see that N is directly connected to 2 carbon atoms. So, it is a secondary amine.
Question 9.2 (i) Write structures of different isomeric amines corresponding to the molecular formula,
Answer :
different isomeric amines corresponding to the molecular formula, $C_{4}H_{11}N$
(i)$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NH}_2 \quad (v) \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NHCH}_3$
(ii)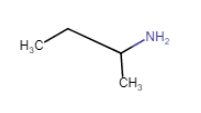 (vi)
(vi) 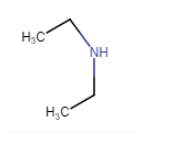
(iii) (vii)
(vii) 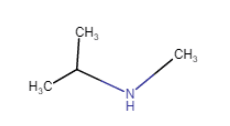
(iv)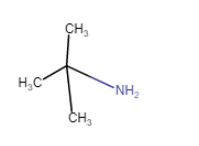 (viii)
(viii) 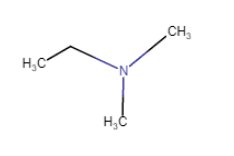
Question 9.2 (ii) Write IUPAC names of all the isomers.
Answer :
IUPAC name of all the isomers-
|
$CH_{3}CH_{2}CH_{2}CH_{2}NH_{2}$
|
Butanamine
|
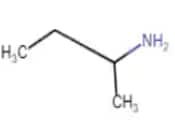 | Butan-2-amine |
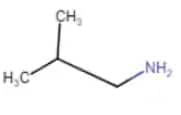 |
2-Methylpropanamine
|
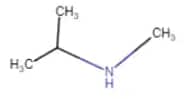 |
N-Methylpropan-2-amine
|
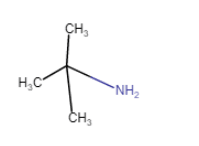 |
2-Methylpropan-2-amine
|
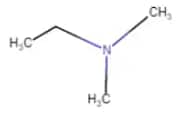 |
N,N-Dimethylethanamine
|
| $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NHCH}_3$ |
N-Methylpropanamine
|
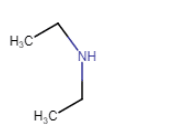 | N-Ethylethanamine |
Question 9.2 (iii) What type of isomerism is exhibited by different pairs of amines?
Answer :
|
$CH_{3}CH_{2}CH_{2}CH_{2}NH_{2}$
|
Butanamine
(Chain isomerism + position isomerism) |
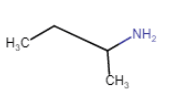 |
Butan-2-amine
(chain isomerism + position isomerism) |
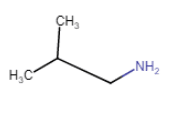 |
2-Methylpropanamine
(chain isomerism) |
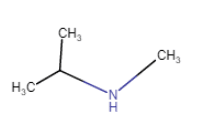 |
N-Methylpropan-2-amine
(position isomerism + metamerism) |
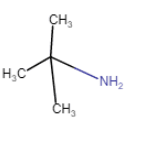 |
2-Methylpropan-2-amine
(chain isomerism) |
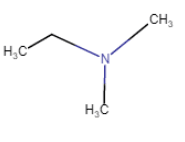 |
N,N-Dimethylethanamine
|
|
$CH_{3}CH_{2}CH_{2}CH_{2}NHCH_{3}$
|
N-Methylpropanamine
(Position isomerism) |
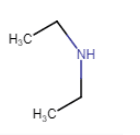 |
N-Ethylethanamine
(no isomerism) |
Question 9.3 (i) How will you convert
Answer :
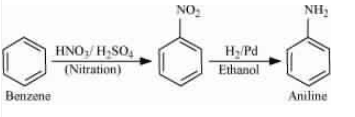
Nitration of benzene gives nitrobenzene. And after that, reduce the nitro group by catalytic hydrogenation.
Question 9.3 (ii) How will you convert
Benzene into N, N-dimethylaniline
Answer :
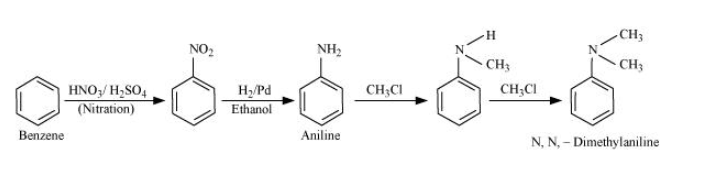
Nitration of benzene gives nitrobenzene, and after catalytic hydrogenation of nitrobenzene, it gives aniline. Aniline reacts with two moles of chloromethane to form N, N-dimethylaniline.
Question 9.3(iii) How will you convert
$Cl-(CH_{2})_{4}-Cl$ into hexan-1,6-diamine
Answer :
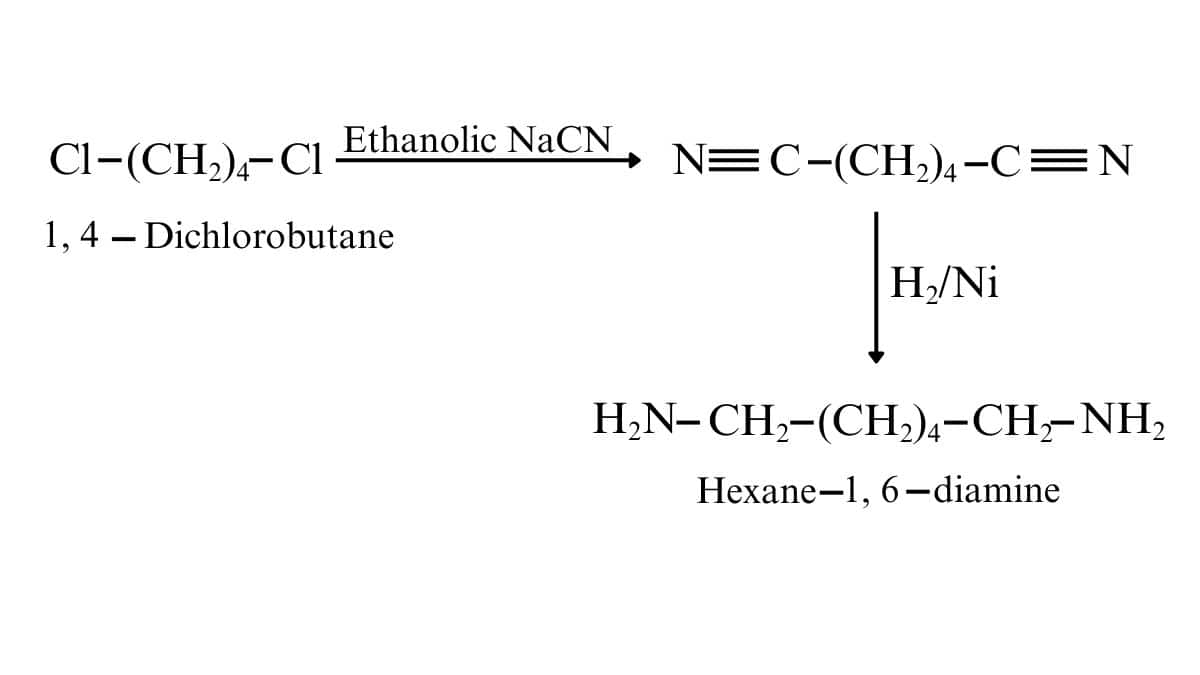
On reacting the given reactant with ethanolic sodium cyanide, the CN molecules replace both chlorine atoms. And after that catalytic hydrogenation, we get our desired product. (reduce the CN to $-CH_{2}NH_{2}$ in both sides)
Question 9.4 (i) Arrange the following in increasing order of their basic strength:
$C_{2}H_{5}NH_{2},$ $C_{6}H_{5}NH_{2},$ $NH_{3},$ $C_{6}H_{5}CH_{2}NH_{2}$ and $(C_{2}H_{5})_{2}NH$
Answer :
Considering the inductive effect, solvation effect and steric hindrance of the alkyl group, which decides the basic strength of alkylamines. The order of basic strength in ethyl-substituted amine is-
$NH_{3}<C_{2}H_{5}NH_{2}<(C_{2}H_{5})_{2}NH$
and order in the benzene-substituted ring-
$C_{6}H_{5}NH_{2}<C_{6}H_{5}CH_{2}NH_{2}$
Due to the -R effect of benzene $C_{6}H_{5}NH_{2}$ has less electron density than ammonia. So, the final order is -
$C_{6}H_{5}NH_{2}<NH_{3}<C_{6}H_{5}CH_{2}NH_{2}<C_{2}H_{5}NH_{2}<(C_{2}H_{5})_{2}NH$
Question 9.4 (ii) Arrange the following in increasing order of their basic strength:
$C_{2}H_{5}NH_{2},$ $(C_{2}H_{5})_{2}NH,$ $(C_{2}H_{5})_{3}N,$ $C_{6}H_{5}NH_{2}$
Answer :
$C_{6}H_{5}NH_{2}<C_{2}H_{5}NH_{2}<(C_{2}H_{5})_{3}N<(C_{2}H_{5})_{2}NH$
On considering the inductive effect, solvation effect and steric hindrance of the alkyl group, the increasing order of basicity in ethyl as a substituted group is shown above.
Question 9.4 (iii) Arrange the following in increasing order of their basic strength:
Answer :
On considering the inductive effect, solvation effect and steric hindrance of the alkyl group, which decides the basic strength of alkyl amines in the aqueous state. The order of basic strength in the case of methyl-substituted amines is -
$C_{6}H_{5}NH_{2}<C_{6}H_{5}CH_{2}NH_{2}<(CH_{3})_{3}N<CH_{3}NH_{2}<(CH_{3})_{2}NH$
Question 9.5 (i) Complete the following acid-base reactions and name the products:
$CH_{3}CH_{2}CH_{2}NH_{2}+HCl\rightarrow$
Answer :
The above-given reaction is an acid-base reaction. So salt is formed.
$CH_{3}CH_{2}CH_{2}NH_{2}+HCl\rightarrow$ $CH_{3}(CH_{2})_{2}NH_{3}^{+}Cl^{-}$
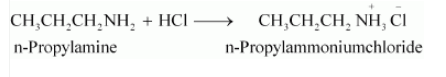
Question 9.5 (ii) Complete the following acid-base reactions and name the products:
$(C_{2}H_{5})_{3}N+HCl\rightarrow$
Answer :
$(C_{2}H_{5})_{3}N+HCl\rightarrow$ $(C_{2}H_{5})_{3}N^{+}HCl^{-}$
It is an acid-base reaction, so the N will accept H from $HCl$ and form a salt.
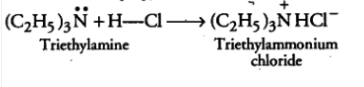
Answer :
The methyl iodide reacts with aniline to give N, N-dimethylaniline.
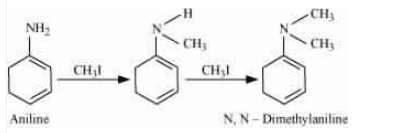
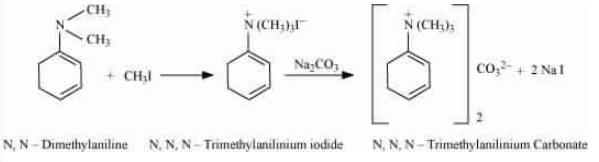
With the excess of methyl iodide in the presence of sodium carbonate solution ($Na_{2}CO_{3}$), N,N-dimethylaniline produces N,N,N-trimethyl anilinium carbonate.
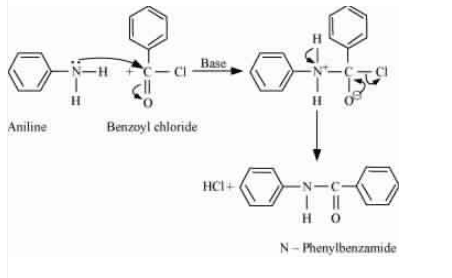
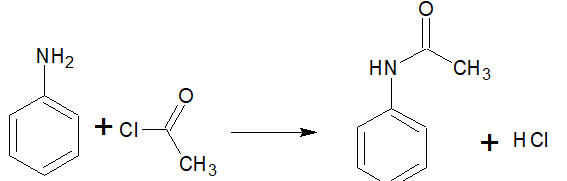
Question 9.7 Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Answer :
When aniline reacts with benzoyl chloride, HCl is produced as a by-product and N-phenylbenzamide is produced as a major product. The lone pair of the nitrogen atom attacks the acidic carbon of benzoyl chloride.
Answer :
Structures of different isomers corresponding to the molecular formula $C_{3}H_{9}N$ and their IUPAC name-
|
(i)
|
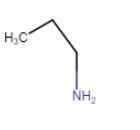 |
Propan-1-amine
|
|
(ii)
|
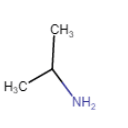 |
Propan-2-amine
|
|
(iii)
|
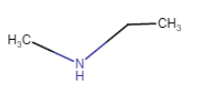 |
N – Methylethanamine
|
|
(iv)
|
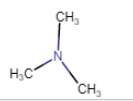 |
N,N-Dimethylmethanamine
|
The structures (i) and (ii) will liberate nitrogen gas ($N_{2}$) on treatment with nitrous acid.
Question 9.9 (i) Convert
3-Methylaniline into 3-nitrotoluene
Answer :
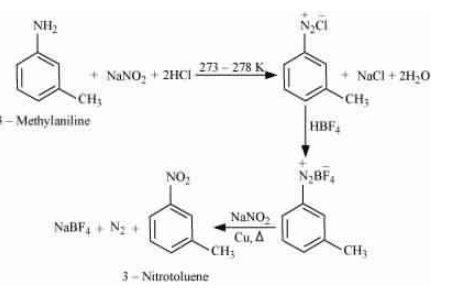
On diazotisation, reaction $NH_{2}$ will convert to $N_{2}Cl$ . And then reacts with fluoroboric acid, followed by $NaNO_{2}/Cu/\Delta$ that converts $N_{2}Cl$ into the nitro group ($-NO_{2}$) and evolution of dinitrogen gas.
Question 9.9 (ii) Convert
Aniline into 1,3,5-tribromobenzene.
Answer :
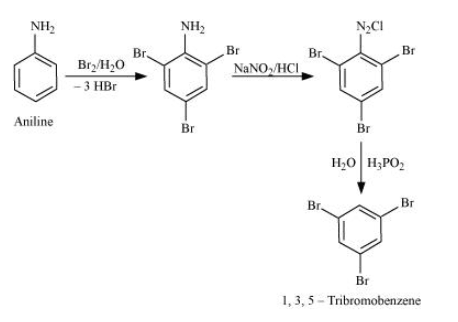
Aniline on bromination in the presence of water gives 2,4,6 tribromoaniline, which on further reacting with nitrous acid converts $NH_{2}$ into $N_{2}Cl$. Now, with the help of $H_{3}PO_{2}/water$, it removes the $N_{2}Cl$ group from the benzene ring and we get our final product 1,3,5-tribromobenzene.
NCERT Solutions for Class 12 Chemistry Chapter 9 Amines (Exercise Questions)
These class 12 chemistry chapter 9 amines question answer offer detailed and accurate answers to the exercise questions, these NCERT solutions helps students to revise key concepts and enhance their problem-solving skills.
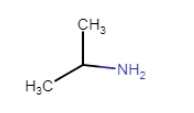
Question 9.1 Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
The IUPAC name of the compound is Propan-2-amine
Since $N$ (nitrogen) is connected to only one carbon. Hence, it is a primary amine.
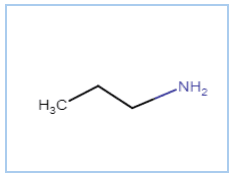
Question 9.1 (ii) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
The IUPAC name is Propan-1-amine
It is a primary amine (nitrogen connects with only one carbon atom)
Here is the structure of the compound $CH_{3}(CH_{2})_{2}NH_{2}$ -
Question 9.1 (iii) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
$CH_{3}NHCH(CH_{3})_{2}$
N-Methylpropan-2-amine
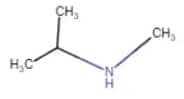
Since N atom is connected with two carbon atoms, it is a two-degree amine.
Question 9.1(iv) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
$(CH_{3})_{3}CNH_{2}$
2-methyl-propan-2-amine
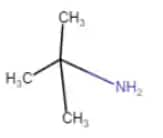
It is a one-degree amine
Question 9.1 (v) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
$C_{6}H_{5}NHCH_{3}$
N-Methylbenzamine or N-methylaniline
Here N atom is connected to two C atoms. So, it is a secondary amine.
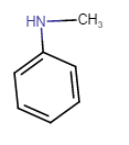
Question 9.1 (vi) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer:
$(CH_{3}CH_{2})_{2}NCH_{3}$
N,N-Diethylmethylamine
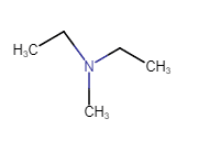
N is connected with three carbon atoms so that it is a tertiary amine
Question 9.1 (vii) Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
Answer :
$m-BrC_{6}H_{4}NH_{2}$
3-bromoaniline or 3-bromobenzenamine
It is a primary amine
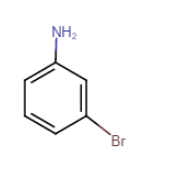
Question 9.2 Give one chemical test to distinguish between the following pairs of compounds.
(i) Methylamine and dimethylamine
Answer :
Carbylamine test
This test is used to distinguish between aliphatic and aromatic primary amines. Heating with chloroform $(CHCl_{3})$ and potassium hydroxide ( $KOH$ ) gives isocyanides or carbylamines, which have a foul smell. Two-degree and 3-degree amines do not show this reaction.
Here, methylamine is primary and dimethylamine is a secondary amine.
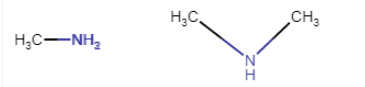
The first one is 1-degree and the second one is 2-degree amine.
Question 9.2 Give one chemical test to distinguish between the following pairs of compounds.
(ii) Secondary and tertiary amines
Answer :
Secondary and tertiary amines can be distinguished by reacting them with Hinsberg's reagent, which is also called benzenesulphonyl chloride. $(C_{6}H_{5}SO_{2}Cl)$ In the case of primary amine, the product is soluble in alkali but not in the secondary amine case. And tertiary amine does not react with this reagent.
In the case of a secondary amine
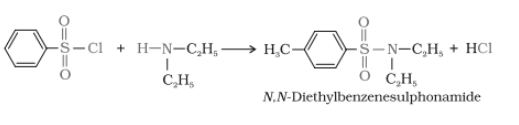
Tertiary amine + benzenesulphonyl chloride $\rightarrow$ no reaction
Question 9.2 Give one chemical test to distinguish between the following pairs of compounds.
Answer :
Ethylamine and aniline can be differentiated by the azo-dye test.
On reacting aniline with $(NaNO_{2}+dil.HCl)$ followed by 2-naphthol, gives a colored product. But when it is ethylamine, it gives brisk effervescence due to the evolution of $N_{2}$ gas under the same conditions.
Question 9.2 Give one chemical test to distinguish between the following pairs of compounds.
Answer :
Benzylamine reacts with nitrous acid to form a diazonium salt, which is unstable, and it also gives alcohol with the evolution of nitrogen gas. On the other hand, aniline reacts with nitrous acid to form a stable diazonium salt.
Question 9.2 Give one chemical test to distinguish between the following pairs of compounds.
(v) Aniline and N-methylaniline.
Answer :
Aniline and N-methylaniline can both be distinguished by the carbylamine test. Aniline is the primary aromatic compound so it gives s positive test, but N-methyl aniline is secondary and does not give this test.
Structure of both compounds-

Question 9.3 Account for the following:
(i) pKb of aniline is more than that of methylamine.
Answer:
Here is the structure of aniline (left) and methylamine
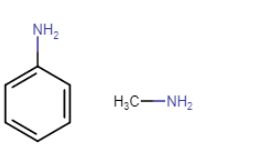
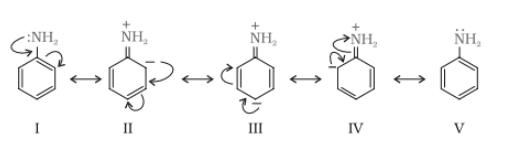
Due to the resonance in aniline, the electrons of the nitrogen atoms are delocalised over the benzene ring. So, because of that, the electron density at the nitrogen atom decreases and is less available for donation.
On the other hand, in the case of methylamine, the methyl ($CH_{3}$ ) group is an electron-donating group (+I effect) which increases the electron density at the N atom. Hence $pK_{b}$ value of aniline is higher than that of methylamine.
Question 9.3 Account for the following:
(ii) Ethylamine is soluble in water whereas aniline is not.
Answer:
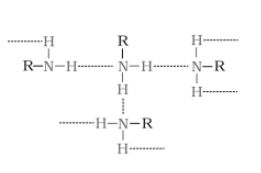 $R = C_{2}H_{5}$
$R = C_{2}H_{5}$
The extent of intermolecular hydrogen bonding in ethylamine is very high. Hence, it is soluble in water. But aniline does not undergo hydrogen bonding with water to a large extent due to the presence of a bulky hydrophobic group $(C_{6}H_{5})$. Hence, it is insoluble in water.
Question 9.3 Account for the following:
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
Answer :
$\mathrm{H}_3 \mathrm{C}-\mathrm{NH}_2$ $\mathrm{}-\mathrm{OH}$
Due to the +I effect of the methyl ($CH_{3}$) group, methylamine is more basic than water. So in water $CH_{3}NH_{2}$ produces hydroxide ion by accepting $H^{+}$ ions from water.
$CH_{3}NH_{2}+H_{2}O\rightarrow CH_{3}N^{+}H_{3}+OH^{-}$
Ferric chloride ($FeCl_{3}$) dissociates in water into- $FeCl_{3}\rightarrow Fe^{3+}+3Cl^{-}$
The hydroxide ion ($OH^{-}$) react with $Fe^{3+}$ and form a precipitate of $Fe(OH)_{3}$ (reddish brown ppt)
$Fe^{3+}+3OH^{-}\rightarrow Fe(OH)_{3}$
Question 9.3 Account for the following:
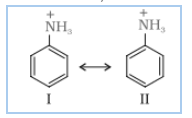
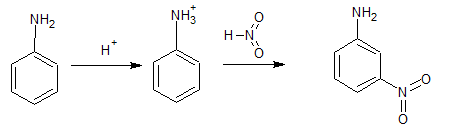
Answer :
Nitration is carried out in a strongly acidic medium, and aniline is protonated to form the anilinium ion, which is meta-directing. Because of this reason, aniline on nitration gives a substantial amount of meta-nitroaniline.
Question 9.3 Account for the following:
(v) Aniline does not undergo Friedel-Crafts reaction.
Answer :
In the Friedel-Crafts reaction, we use $AlCl_{3}$, which is a Lewis acid, and aniline has basic nature. Thus, there is an acid-base reaction between them and they form a salt.
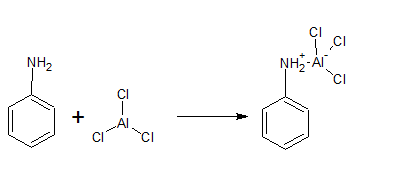
Due to the positive charge on the N-atom. The electrophilic substitution in the benzene ring is deactivated.
Question 9.3 Account for the following:
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Answer :
In diazonium salt, the structure undergoes resonance due to which the dispersal of positive charge is more and we know that the higher the resonance, the higher is the stability. Therefore, the diazonium salt of aromatic amines is more stable than that of aliphatic amines.
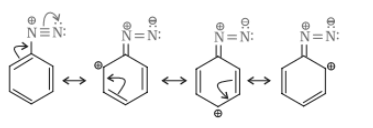
Question 9.3 Account for the following:
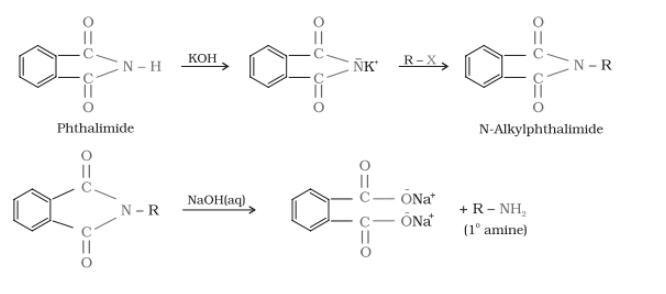
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Answer :
Gabriel synthesis is used for primary amines. Secondary and tertiary amines are not formed by this method. Therefore, to obtain pure and only 1-degree amine, the Gabriel phthalimide reaction is preferred.
Question 9.4 Arrange the following:
(i) In decreasing order of the pKb values:
$C_{2}H_{5}NH_{2},$ $C_{6}H_{5}NHCH_{3},$ $(C_{2}H_{5})_{2}NH$ and $C_{6}H_{5}NH_{2}$
Answer :
$C_{2}H_{5}NH_{2}$ and $(C_{2}H_{5})_{2}NH$ are aliphatic amines, so they are more basic than the other two compounds. In ethylamine, there is only one electron-donating group (+I effect), but in diethylamine, there are two electron-donating groups, so the electron density is much higher than in ethylamine.
Between $C_{6}H_{5}NHCH_{3}$ and $C_{6}H_{5}NH_{2}$, they are weak bases because of delocalisation of lone pair electrons in the benzene ring. Aniline is less basic than N-methylaniline( $C_{6}H_{5}NHCH_{3},$ ) due to the absence of an electron-donating group. Thus, the decreasing order of pKb values is-
$C_{6}H_{5}NH_{2}>C_{6}H_{5}NHCH_{3}>C_{2}H_{5}NH_{2}>(C_{2}H_{5})_{2}NH$
Question 9.4 Arrange the following:
(ii) In increasing order of basic strength:
$C_{6}H_{5}NH_{2},$ $C_{6}H_{5}N(CH_{3})_{2},$ $(C_{2}H_{5})_{2}NH$ and $CH_{3}NH$
Answer :
The increasing order of basic strength-
$C_{6}H_{5}NH_{2}<C_{6}H_{5}N(C_{2}H_{5})_{2}<CH_{3}NH_{2}<(C_{2}H_{5})_{2}NH$
Aliphatic amines are more basic than aromatic amines due to the presence of a high electron density of electron at the nitrogen atom so that it can donate an electron. On the other hand, in aromatic amines, the lone pair of electrons is delocalised in the benzene ring, so the availability of electrons is less.
$(C_{2}H_{5})_{2}NH$ is more basic because of the +I effect of the two ethyl groups (so electron density is highest in this compound), while methylamine has one ethyl group. In aromatic amines, diethylaniline is more basic due to the presence of a greater number of electron-donating groups.
Question 9.4 Arrange the following:
(iii) In increasing order of basic strength:
(a) Aniline, p-nitroaniline and p-toluidine
Answer :
Structure of the following compounds-
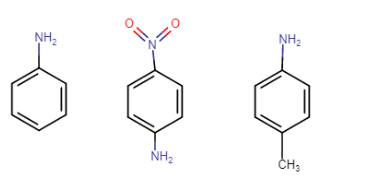
Aniline, p-nitroaniline, p-toluidine
In p-toluidine, the methyl group increases the electron density at the N atom (+I effect of methyl), so it is more basic than aniline. In p-nitroaniline, the nitro group is an electron-withdrawing group, so it decreases the electron density at nitrogen, so it is less basic than aniline.
Question 9.4 Arrange the following:
(iii) In increasing order of basic strength:
(b) $C_{6}H_{5}NH_{2},$ $C_{6}H_{5}NHCH_{3},$ $C_{6}H_{5}CH_{2}NH_{2}.$
Answer :
The increasing order of basic strength- $C_{6}H_{5}NH_{2}<C_{6}H_{5}NHCH_{3}<C_{6}H_{5}CH_{2}NH_{2}$
$C_{6}H_{5}NHCH_{3}$ is more basic than aniline due to the presence of one electron-donating group, which increases the electron density at nitrogen atom. But in this compound N atom is directly attached with the benzene ring, so due to -R effect $C_{6}H_{5}NHCH_{3}$ is less basic than $C_{6}H_{5}CH_{2}NH_{2}.$
Question 9.4 Arrange the following:
(iv) In decreasing order of basic strength in gas phase:
$C_{2}H_{5}NH_{2},$ $(C_{2}H_{5})_{2}NH,$ $(C_{2}H_{5})_{3}N,$ and $NH_{3}$
Answer :
Decreasing order of basic strength in the gas phase-
$(C_{2}H_{5})_{3}N>(C_{2}H_{5})_{2}NH>C_{2}H_{5}NH_{2}>NH_{3}$
In the gas phase, there is no solvation effect. So, the basicity directly depends on the number of electron-donating groups. More is the electron electron-donating groups, the higher the +I effect and if the size of the donating group is large +I effect should also be high.
Question 9.4 Arrange the following:
(v) In increasing order of boiling point:
$C_{2}H_{5}OH,$ $(CH_{3})_{2}NH,$ $C_{2}H_{5}NH_{2}$
Answer :
The boiling point of the compounds depends on the extent of hydrogen bonding of the compound. The more the extent, the higher the boiling point. $C_{2}H_{5}NH_{2}$, it contains two H atoms, so it has a higher boiling point than $(CH_{3})_{2}NH$. On the other hand, the Oxygen atom is more electronegative than the N atom, so the strength of hydrogen bonding is higher in $C_{2}H_{5}OH$ than in $C_{2}H_{5}NH_{2}$.
Thus, the increasing order of boiling point is-
$(C_{2}H_{5})_{2}NH<C_{2}H_{5}NH_{2}<C_{2}H_{5}OH$
Question 9.4 Arrange the following:
(vi) In increasing order of solubility in water:
$C_{6}H_{5}NH_{2},$ $(C_{2}H_{5})_{2}NH,$ $C_{2}H_{5}NH_{2}.$
Answer :
The more extensive the hydrogen bonding, the greater is the solubility in water. $C_{2}H_{5}NH_{2}$ contains two H atom while $(C_{2}H_{5})_{2}NH$ contains only one. So, the $C_{2}H_{5}NH_{2}$ undergoes extensive hydrogen bonding. Hence its solubility is more than $(C_{2}H_{5})_{2}NH$ in water. An increase in the molecular mass of amines decreases their solubility in water because of the increase in bulky hydrophobic groups.
Thus, the increasing order of solubility in water is-
$C_{6}H_{5}NH_{2<}(C_{2}H_{5})NH<C_{2}H_{5}NH_{2}$
Question 9.5 How will you convert:
(i) Ethanoic acid into methanamine
Answer :
Ethanoic acid on reacting with $SOCl_{2}$ replaces the OH by $Cl$ and then reacts with an excess of ammonia molecule to produce methanamide ($CH_{3}CONH_{2}$), which, by the Hoffmann bromamide degradation reaction, gives us desired product (methenamine)
$\mathrm{CH}_3 \mathrm{COOH}+\mathrm{SOCl}_2 \rightarrow \mathrm{CH}_3 \mathrm{COCl} \xrightarrow{\mathrm{NH}_3 \text { (excess) }} \mathrm{CH}_3 \mathrm{CONH}_2 \xrightarrow{\mathrm{Br}_2+\mathrm{KOH}} \mathrm{CH}_3 \mathrm{NH}_2$
Question 9.5 How will you convert:
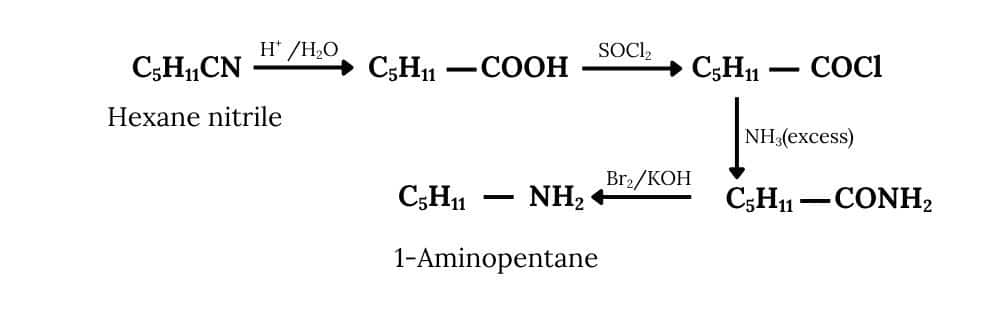
(ii) Hexanenitrile into 1-aminopentane
Answer :
First, hexanenitrile undergoes acid hydrolysis, converting the nitrile group (-CN) into a carboxylic acid $(-\mathrm{COOH})$. This carboxylic acid is then treated with thionyl chloride $\left(\mathrm{SOCl}_2\right)$ to form the corresponding acid chloride $(-\mathrm{COCl})$. Reacting this with excess ammonia $\left(\mathrm{NH}_3\right)$ replaces the chlorine atom with an amine group, yielding a primary amide ($-\mathrm{CONH}_2$).
Finally, the Hofmann bromamide degradation is carried out, which removes one carbon atom and produces the desired primary amine as the final product.
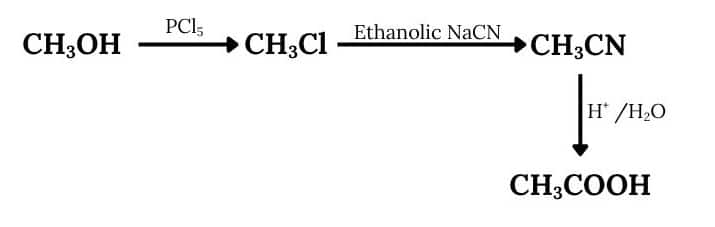
Question 9.5 How will you convert:
(iii) Methanol to ethanoic acid
Answer :
When methanol reacts with phosphorus pentachloride, OH- is reduced by Cl- to form chloromethane, which on reacting with ethanolic sodium cyanide, gives $CH_{3}CN$ and followed by acidic hydrolysis, it produces ethanoic acid ($CH_{3}COOH$)
Question 9.5 How will you convert:
(iv) Ethanamine into methanamine
Answer :
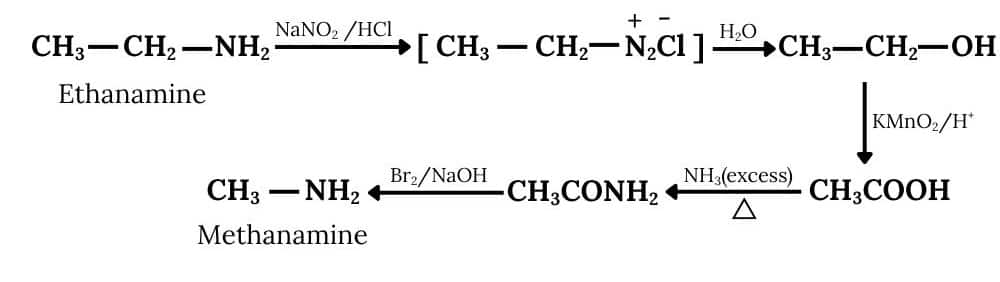
Ethanamine reacts with nitrous acid to form an azo compound, which further reacts with water to form ethanol and this on oxidation gives ethanoic acid. After treating with an excess of ammonia, the ethanoic acid becomes ethanamide, which further reacts with bromine and a strong base (Hoffmann bromamide degradation reaction), to form methanamine.
Question 9.5 How will you convert:
(v) Ethanoic acid into propanoic acid
Answer :
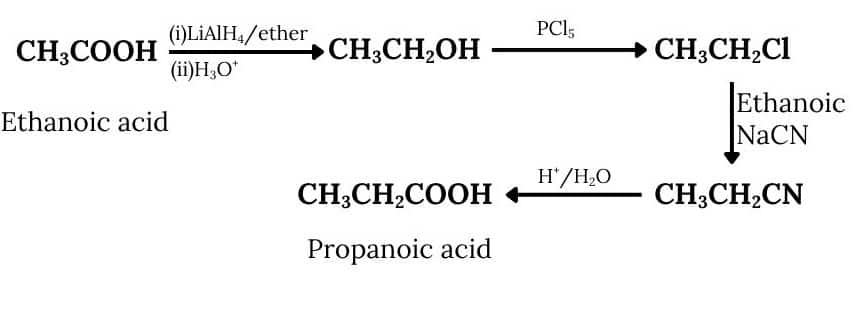
Acetic acid, on reduction with lithium aluminium hydride followed by acid hydrolysis, gives ethanol, which on reacting with $PCl_{5}$ gives ethyl chloride. Ethyl chloride further reacts with ethanolic sodium cyanide to form propanenitrile, which on acidic hydrolysis gives the desired product.
Question 9.5 How will you convert:
(vi) Methanamine into ethanamine
Answer :
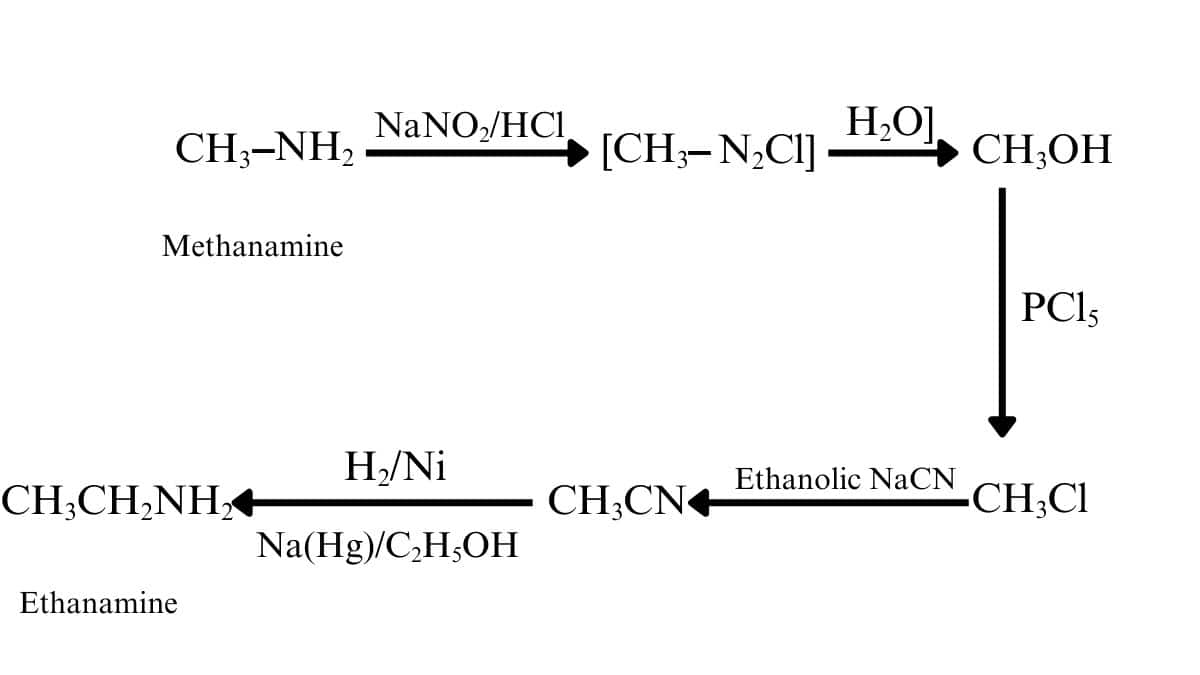
Methanamine on diazotisation gives methane diazonium salt, which on further hydrolysis forms methanol. Methanol on reacting with $PCl_{5}$, followed by ethanolic sodium cyanide ($NaCN$), produces ethane nitrile. This on reduction with $H_{2}/Pd$ gives ethanamine.
Question 9.5 How will you convert:
(vii) Nitromethane into dimethylamine
Answer :
Conversion of Nitromethane into dimethylamine is shown below:
$\mathrm{CH}_3 \mathrm{NO}_2 \xrightarrow[\mathrm{H}]{\mathrm{Sn} / \mathrm{HCl}} \mathrm{CH}_3 \mathrm{NH}_2 \xrightarrow{\mathrm{CHCl}_3 / \mathrm{KOH}} \mathrm{CH}_3 \mathrm{NC}$
Reduction of Isocyanide
$
\mathrm{CH}_3 \mathrm{NC} \xrightarrow[\mathrm{H}]{\mathrm{Na} / \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}} \mathrm{CH}_3 \mathrm{NHCH}_3
$
Question 9.5 How will you convert:
(viii) Propanoic acid into ethanoic acid?
Answer :
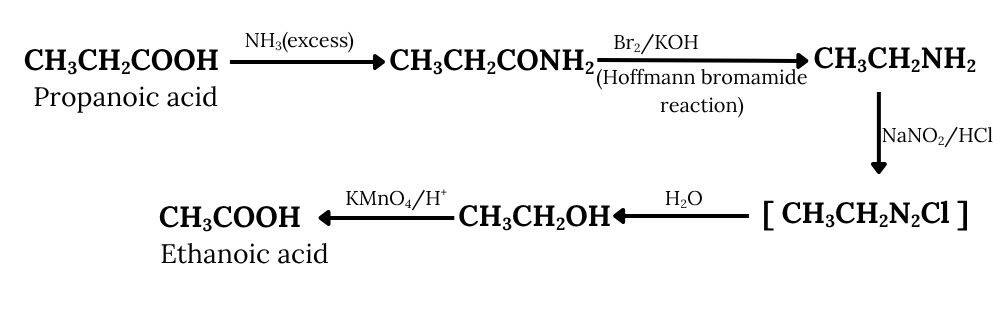
Propanoic acid on reacting with an excess of ammonia, gives propanamide, which further reacts with bromine and potassium hydroxide (Hoffmann bromamide degradation reaction) to give ethanamine. On diazotisation, ethanamine is converted into an azo salt, which on treatment with water forms ethanol. Ethanol on oxidation, provides ethanoic acid.
Answer :
Primary, secondary, and tertiary amines are distinguished by Hinsberg's reagent test. In this test, the amines are allowed to react with benzene sulphonyl chloride (Hinsberg's reagent). All three types of amines give different products.
1.Primary amine reacts with benzene sulphonyl chloride $(C_{6}H_{5}SO_{2}Cl)$ gives N-ethylbenzene sulphonamide which is soluble in alkali.
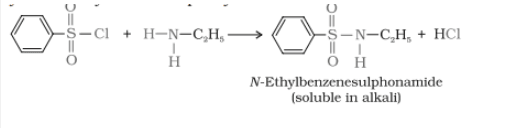
2. When secondary amines react, it gives N,N-diethylbenzene sulphonamide, which is insoluble in alkali.
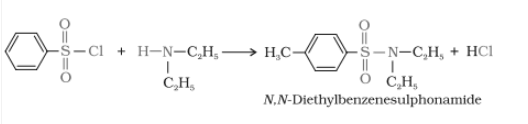
3. Tertiary amine does not react with Hinsberg's reagent.
Question 9.7 Write short notes on the following:
Answer :
Carbylamine reaction-
Aliphatic and aromatic primary amines on reacting with chloroform and ethanolic KOH form isocyanides or carbylamine, which is a foul-smelling substance. Secondary and tertiary amines do not give this reaction. This reaction is known as the carbylamine reaction or isocyanide test. It is used to distinguish primary amines.
$R-NH_{2}+CHCl_{3}+KOH\xrightarrow[heat]{^\Delta }R-NC+3KCl+3H_{2}O$
Question 9.7 Write short notes on the following:
Answer :
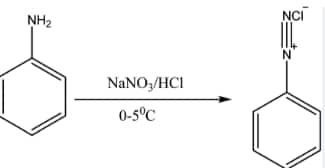
Aromatic primary amines react with nitrous acid ($NaNO_{2}+2HCl$) at low temperature to form diazonium salts. This conversion of aromatic primary amines into diazonium salts is known as diazotisation.
$\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2+\mathrm{NaNO}_2+2 \mathrm{HCl} \xrightarrow{273-278 \mathrm{~K}} \mathrm{C}_6 \mathrm{H}_5 \mathrm{~N}_2^{+} \mathrm{Cl}^{-}+\mathrm{NaCl}+2 \mathrm{H}_2 \mathrm{O}$
Question 9.7 Write short notes on the following:
(iii) Hofmann’s bromamide reaction
Answer :
Hofmann’s bromamide reaction-
When an amide is treated with bromine in aqueous solution or an ethanolic solution of sodium hydroxide, it produces primary amines with one less carbon atom than the parent compound. This reaction is known as the Hoffmann bromide degradation reaction.
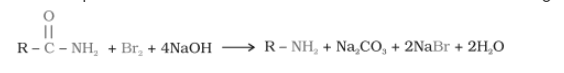
R = alkyl group like $CH_{3},C_{2}H_{5}....etc$
Question 9.7 Write short notes on the following:
Answer :
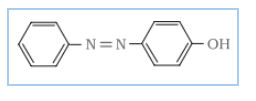
The reaction of joining diazonium salt to the aromatic ring (like phenol) through a $-N=N-$ bond is known as a coupling reaction. Benzene diazonium chloride reacts with phenol in which the phenol molecule at its para position is coupled with the diazonium salt ($C_{6}H_{5}N^{+}_{2}Cl^{-}$) to give p-hydroxyazobenzene (orange in colour).
Reactions-
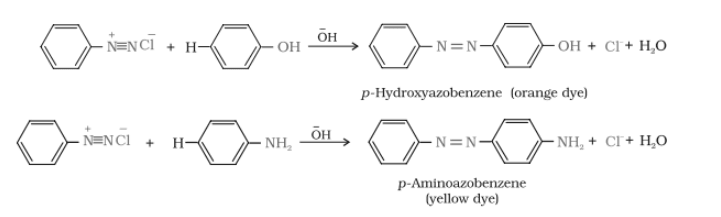
Diazonium salt reacts with aniline to give p-aminoazobenzene (yellow colour)
Question 9.7 Write short notes on the following:
Answer :
Ammonolysis-
When an alkyl or benzyl halide is going to react with the solution of ammonia(an ethanolic solution), it undergoes a nucleophilic substitution reaction in which the halogen atom is replaced by an amino ($-NH_{2}$) group. This process of cleavage of the carbon-halogen (C-X) bond by the ammonia molecule is known as ammonolysis.
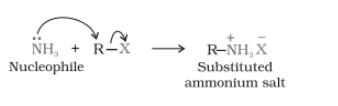
When this substituted ammonium salt is treated with a strong base, it gives a primary amine
$RN^{+}H_{3}X^{-}+NaOH\rightarrow R-NH_{2}+H_{2}O+NaX$
- Here, primary amine behaves as a nucleophile and can further react with an alkyl halide to form secondary and tertiary amines and finally quaternary ammonium salt.
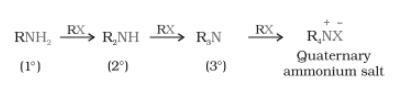
Question 9.7 Write short notes on the following:
Answer :
Acetylation-
The introduction of an acetyl group ( $CH_{3}CO$ )in a molecule is known as acetylation.
Example-
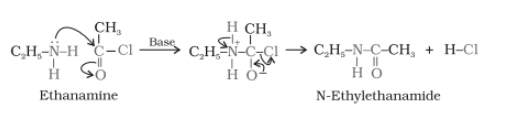
Aliphatic and aromatic primary or secondary amines undergo acetylation reaction when treated with acid chlorides, anhydrides, or esters by nucleophilic substitution. Here Hydrogen atom of $NH_{2}$ or $NH$ is replaced by $CH_{3}CO$ the group.
Question 9.7 Write short notes on the following:
(vii) Gabriel phthalimide synthesis.
Answer :
Gabriel phthalimide synthesis.-
It is mainly used for aliphatic primary amine preparation. Phthalimide reacts with ethanolic potassium hydroxide to produce the potassium salt of phthalimide. Upon heating with an alkyl halide and subsequent alkaline hydrolysis, this salt yields the corresponding primary amine. Aromatic amines cannot be produced by this method due to the inability of aryl halides to undergo nucleophilic substitution reaction with the anion produced by phthalimide.
Question 9.8 Accomplish the following conversions:
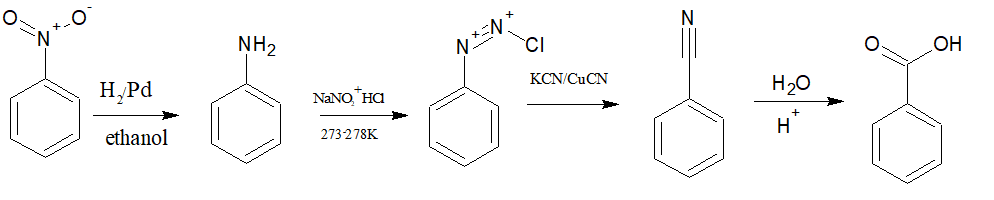
(i) Nitrobenzene to benzoic acid
Answer :
First, reduce nitrobenzene into aniline with the help of $H_{2}/Pd$. Now convert aniline to diazonium salt, followed by Sandmeyer's reaction ($CuCN/KCN$) to obtain benzonitrile, and after acid hydrolysis, this benzonitrile becomes benzoic acid.
Question 9.8 Accomplish the following conversions:
Answer :
First, benzene is nitrated to form nitrobenzene, which is a meta-directing group and thus directs incoming substituents to the meta position. Next, bromination of nitrobenzene is carried out, introducing a bromine atom at the meta position relative to the nitro group.
The nitro group is then reduced to an amine using $\mathrm{Sn}/\mathrm{HCl}$ that forms m-bromoaniline. This is followed by diazotization (reaction with $\mathrm{NaNO}_2$ and HCl at $0-5^{\circ} \mathrm{C}$) to form the diazonium salt, which is finally subjected to hydrolysis with warm water that gives m-bromophenol as the final product.
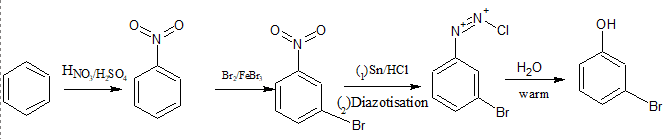
Question 9.8 Accomplish the following conversions:
Answer :
On reacting benzoic acid with $SOCl_{2}$, we get benzene sulphonyl chloride and when it reacts with ammonia the chlorine is replaced by $NH_{2}$. Then the Hoffmann bromide degradation reaction is carried out to remove -CO group.
Question 9.8 Accomplish the following conversions:
(iv) Aniline to 2,4,6-tribromofluorobenzene
Answer :
First, we will do the bromination of aniline which gives 2,4,6-tribromoaniline. After that, we will perform diazotisation reaction which converts $NH_{2}$ group into $N_{2}Cl$ and then we will react it with fluoroboric acid( $HBF_{4}+\Delta$) to give us the desired product.
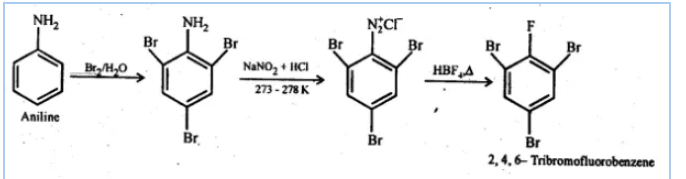
Question 9.8 Accomplish the following conversions:
(v) Benzyl chloride to 2-phenylethanamine
Answer :
On reacting benzyl chloride with the ethanolic sodium cyanide ($NaCN$), it replaces the Chlorine with the CN molecule. And then reduction with the help of $H_{2}/Ni$
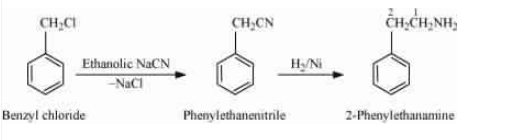
Question 9.8 Accomplish the following conversions:
(vi) Chlorobenzene to $p-chloroaniline$
Answer :
On nitration of chlorobenzene, it gives p-nitrochlorobenzene, which is on further reaction with $H_{2}/Pd$, reduces $NO_{2}$ to $NH_{2}$
Question 9.8 Accomplish the following conversions:
(vii) Aniline to $p-bromoaniline$
Answer :
Aniline on reacting with the acetic anhydride in the presence of pyridine, gives N-phenylethanamide, which on further reaction with $Br_{2}/CH_{3}COOH$, directs the bromine to the para-position. After hydrolysis, we can recover the original $NH_{2}$ group at the same position
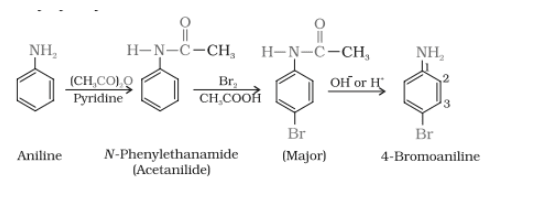
Question 9.8 Accomplish the following conversions:
Answer :
Use the Hoffmann bromamide degradation reaction so that it gives aniline and then do diazotisation reaction so that $NH_{2}$ group is replaced by $N_{2}Cl$ and then reduce it with the help of ($H_{2}O/H_{3}PO_{2}$) so that it reduced into the benzene ring. And then alkylation of benzene will give toluene as a final product.
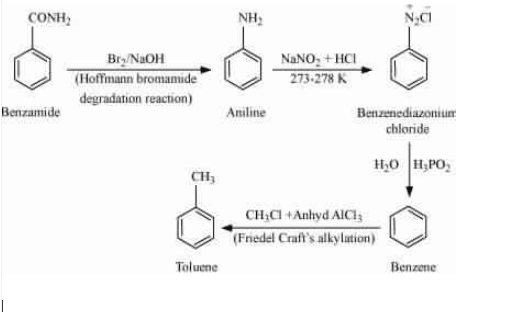
Question 9.8 Accomplish the following conversions:
(ix) Aniline to benzyl alcohol
Answer :
Aniline on treating with nitrous acid followed by sandmeyers reaction gives benzonitrile ( $C_{6}H_{5}C\equiv N$), which on acidic hydrolysis form benzoic acid ($C_{6}H_{5}COOH$). The benzoic acid is then reduced by $LiAlH_{4}$ (lithium aluminium hydride) to get the desired product.
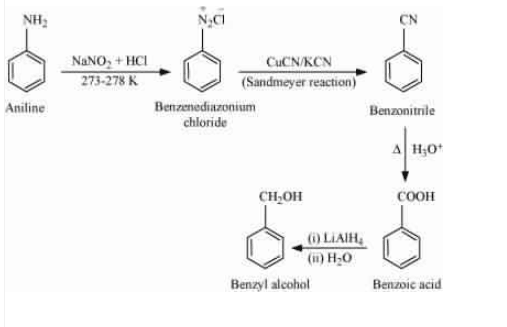
Question 9.9 Give the structures of A, B and C in the following reactions:
Answer :
In the first reaction, the iodine is replaced by CN, so the structure of (A) is $CH_{3}CH_{2}CN$.
On partial hydrolysis, the CN converted in to $CONH_{2}$, so the structure of B is $CH_{3}CH_{2}CONH_{2}$ (propanamide). The third reaction is the Hoffmann bromide degradation reaction. So, the -CO group will be removed and it will become a primary aliphatic amine. Thus the structure of C is $CH_{3}CH_{2}NH_{2}$ (ethanamine).
Question 9.9 Give the structures of A, B and C in the following reactions:
Answer :
The first part is the Sandmeyers reaction so CN replaces the $N_{2}Cl$ of the reactant and the product (A) is cyanobenzene $(C_{6}H_{5}CN)$. On acidic hydrolysis of cyanobenzene give product (B), benzoic acid $(C_{6}H_{5}COOH)$ and benzoic acid reacts with ammonia (heat) to produce
(C) benzamide $(C_{6}H_{5}CONH_{2})$.
Question 9.9 Give the structures of A, B and C in the following reactions:
Answer :
The structure of A,B and C is
$CH_{3}CH_{2}CN,CH_{3}CH_{2}CH_{2}NH_{2}$ and $CH_{3}CH_{2}CH_{2}OH$, respectively.
In the first reaction, the bromine is replaced by a cyanide molecule ($CN$) and after a reduction by $LiAlH_{4}$, the CN group becomes $CH_{2}NH_{2}$.
Question 9.9 Give the structures of A, B and C in the following reactions:
Answer :
A- $C_{6}H_{5}NH_{2}$
B- $C_{6}H_{5}N^{+}_{2}Cl^{-}$
C- $C_{6}H_{5}OH$
The first nitrobenzene reduced to aniline and then it reacts with $(NaNO_{2}/HCl)$ to form benzene diazonium salt and is hydrolysed to form phenol( $C_{6}H_{5}OH$).
Question 9.9 Give the structures of A, B and C in the following reactions:
(v) $CH_{3}COOH\xrightarrow[\Delta ]{NH_{3}}A\xrightarrow[]{NaOBr}B\xrightarrow[]{NaNO_{2}/HCl}C$
Answer :
A- ethanamide ($CH_{3}CONH_{2}$)
B- methenamine ($CH_{3}NH_{2}$)
C- methanol ($CH_{3}OH$)
Acetic acid reacts with ammonia, giving ethanamide and the second reaction is the Hoffmann bromamide degradation reaction, so it removes the -CO group and forms methanamine. The reaction with NaNO2/HCl gives methanol.
Question 9.9 Give the structures of A, B and C in the following reactions:
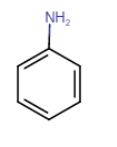
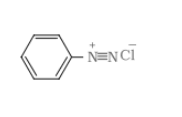
Answer :
Initially, product A is the reduction of nitrobenzene means it is an aniline. When A react with $HNO_{2}$ it gives benzene diazonium chloride (B). And when B reacts with another aromatic ring (phenol), it's a coupling reaction, so it gives azodye (p-Hydroxyazobenzene)
A= Aniline
B = benzene diazonium chloride
C= p-Hydroxyazobenzene
Answer :
The molecular formula of C = $C_{6}H_{7}N$
$A\xrightarrow[aq NH_{3}]{\Delta }B$
$B+Br_{2}+KOH\rightarrow C(C_{6}H_{7}N)$
It is a Hoffmann bromamide degradation reaction so the product should be $C_{6}H_{5}NH_{2}$ (aniline).
So B should be benzamide ($C_{6}H_{5}CONH_{2}$) and A should be benzoic acid ( $C_{6}H_{5}COOH$ )
Therefore, the above reaction proceeds as
Question 9.11 Complete the following reactions:
(i) $C_{6}H_{5}NH_{2}+CHCl_{3}+alc.KOH\rightarrow$
Answer :
The reaction given above is carbylamine reaction in which primary aromatic amines react with chloroform and alcoholic potassium hydroxide to give isocyanide or carbylamine as a product, which is a foul-smelling substance.
$C_{6}H_{5}NH_{2}+CHCl_{3}+alc.KOH\rightarrow$ $C_{6}H_{5}NC+KCl +H_{2}O$
Question 9.11 Complete the following reactions:
(ii) $C_{6}H_{5}N_{2}Cl+H_{3}PO_{2}+H_{2}O\rightarrow$
Answer :
When benzene diazonium chloride reacts with $H_{3}PO_{2}$ it reduced into benzene and $H_{3}PO_{2}$ oxidised and HCl produced as a by-product in this reaction.
$C_{6}H_{5}N_{2}Cl+H_{3}PO_{2}+H_{2}O\rightarrow$ $C_{6}H_{6}+H_{3}PO_{3}+HCl$
Question 9.11 Complete the following reactions:
(iii) $C_{6}H_{5}NH_{2}+H_{2}SO_{4}(conc.)\rightarrow$
Answer :
Aniline is a base and $H_{2}SO_{4}$ is acid. So, basically, it is an acid-base reaction. Aniline accepts $H^{+}$ ions from sulphuric acid and forms a product anilinium hydrogen sulphate.
$C_{6}H_{5}NH_{2}+H_{2}SO_{4}(conc.)\rightarrow$ $C_{6}H_{5}NH_{3}^{+}HSO_{4}^{-}$
Question 9.11 Complete the following reactions:
(iv) $C_{6 } H_{5}N_{2}Cl+C_{2}H_{5}OH\rightarrow$
Answer :
Ethanol reduces the benzene diazonium chloride to benzene and oxidises into ethanal ( $CH_{3}CHO$), hydrochloric acid is produced as a by-product, and the evolution of nitrogen gas also occurs.
$C_{6 } H_{5}N_{2}Cl+C_{2}H_{5}OH\rightarrow$ $C_{6}H_{6}+CH_{3}CHO+N_{2}+HCl$
Question 9.11 Complete the following reactions:
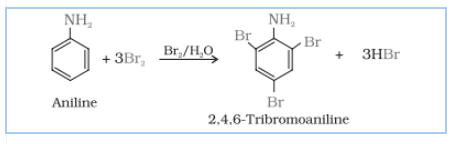
(v) $C_{6}H_{5}NH_{2}+Br_{2}(aq)\rightarrow$
Answer :
Aniline on bromination in the presence of water at room temperature, gives a white precipitate of 2,4,6-tribromoaniline
Question 9.11 Complete the following reactions:
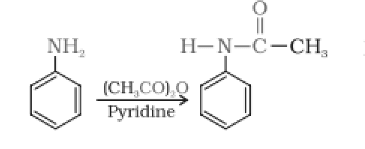
(vi) $C_{6}H_{5}NH_{2}+(CH_{3}CO)_{2}O\rightarrow$
Answer :
Aniline reacts with acetic anhydride by a nucleophilic substitution reaction. Here, the H atom from $NH_{2}$ group is replaced by an acyl group. The product formed is N-phenylethanamide. The reaction is carried out in the presence of a base stronger than the amine, like pyridine
$C_{6}H_{5}NH_{2}+(CH_{3}CO)_{2}O\rightarrow$ $C_{6}H_{5}NHCOCH_{3}+CH_{3}COOH$
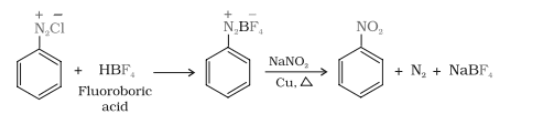
Question 9.11 Complete the following reactions:
(vii) $C_{6}H_{5}N_{2}Cl\xrightarrow[(ii)NaNO_{2}/Cu,\Delta )]{(i) HBF_{4})}$
Answer :
In this reaction, the chloride ion is replaced by $BF_{4}^{-}$ ion. When diazonium fluoroborate is heated with aqueous sodium nitrite in the presence of copper, the diazonium group is replaced by the –NO2 group.
Question 9.12 Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Answer :
Gabriel phthalimide synthesis is mainly used for aliphatic primary amine preparation. Phthalimide reacts with ethanolic potassium hydroxide and produces the potassium salt of phthalimide, which on heating with alkyl halide, followed by alkaline hydrolysis, gives the corresponding primary amine.
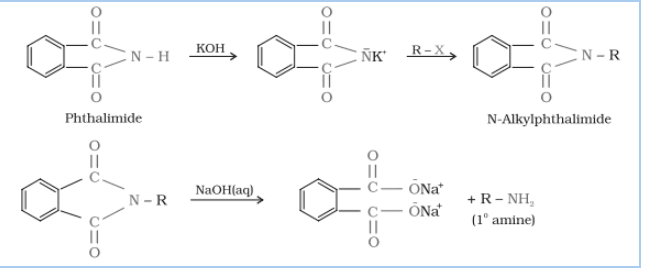
Aromatic amines cannot be produced by this method due to the inability of aryl halides to undergo nucleophilic substitution reaction with the anion produced by phthalimide.
Hence, aromatic amines cannot be produced by this method.
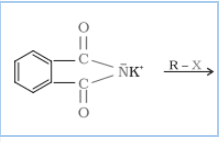
$No \ reaction$ {if R = Aromatic compound}
Question 9.13 Write the reactions of
(i) aromatic primary amines with nitrous acid.
Answer :
Aromatic primary amines react with nitrous acid( $NaNO_{2}+HCl$) at 273-278 K to form stable diazonium salts. Aniline reacts with nitrous acid to form benzene diazonium chloride.
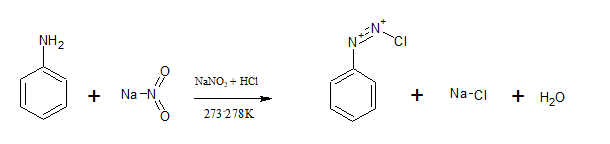
Question 9.13 Write the reactions of
(ii) aliphatic primary amines with nitrous acid.
Answer :
When aliphatic primary amines react with nitrous acid to form unstable diazonium salts, which on further reaction produce alcohol and hydrochloric acid ($HCl$) with the evolution of dinitrogen gas ($N_{2}$).
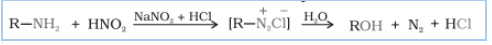
Question 9.14 Give a plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable molecular masses?
Answer :
Protonation of amines gives-
$RNH_{2}\rightarrow RNH^{-}+H^{+}$ (amide ion)
Protonation of alcohol gives -
$R-OH\rightarrow RO^{-}+H^{+}$ (alkoxide ion)
The negative charge is easily accommodated by the O atom because it is more electronegative than N atom. So, the amide ion is less stable than the alkoxide ion. Hence, amines are less acidic than alcohols.
Question 9.14 Give a plausible explanation for each of the following:
(ii) Why do primary amines have a higher boiling point than tertiary amines?
Answer :
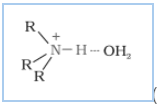
The extent of intermolecular hydrogen bonding in primary amines is higher than that of secondary amines. Secondary amine has only one hydrogen, and tertiary amines have no hydrogen atom for intermolecular hydrogen bonding, whereas primary amine has two hydrogen atoms available for intermolecular hydrogen bonding. And we know that the higher the extent of hydrogen bonding, the higher is the boiling point.
In $1^{0}$ amine
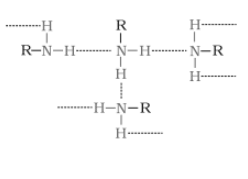
(In $2^{0}$ amine),
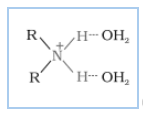
(In $3^{0}$ amines)
Question 9.14 Give plausible explanation for each of the following:
(iii) Why are aliphatic amines stronger bases than aromatic amines?
Answer :
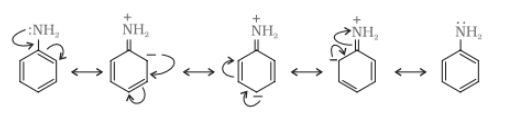
Aliphatic amines are stronger bases than aromatic amines due to resonance. In aromatic amines, the lone pair of electrons at N atom is delocalised over the benzene ring, which makes it less available for electron donation. On the other hand, in aliphatic amines ($R-NH_{2}$), the electron donor group is attached which increases the electron density at the N atom. Thus, the aliphatic amines are more basic than aromatic amines.
Class 12 Chemistry NCERT Chapter 9: Higher Order Thinking Skills (HOTS) Questions
Given below the class 12 chemistry chapter 9 amines question answer. These HOTS questions make you think deeper about the topic of amines and help you to understand it in a better and easier way.
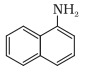
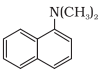
Question 1. When a concentrated solution of sulphanilic acid and 1-naphthylamine is treated with nitrous acid (273 K) and acidified with acetic acid, the mass (g) of 0.1 mole of product formed is : (Given molar mass in g mol–1 H : 1, C : 12, N : 14,O : 16, S : 32)
1) 343
2) 330
3) 33
4) 66
Answer:
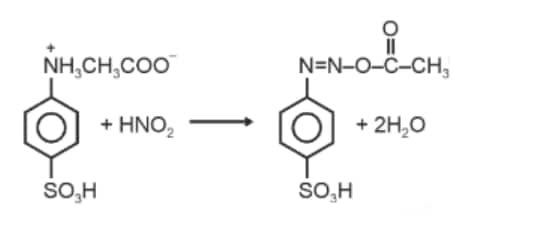
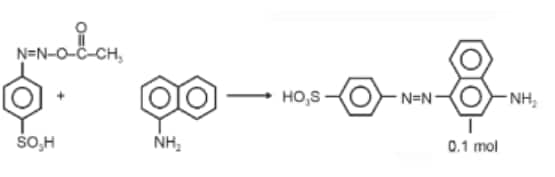
Molar mass of the product formed
$\begin{aligned}
& =(16 \times 12)+(14 \times 3)+(16 \times 3)+32+13 \\
& =327 \mathrm{~g}
\end{aligned}$
Mass of 0.1 mol product
$32.7 \mathrm{~g} \approx 33 \mathrm{~g}$
Hence, the correct answer is option (3).
Question 2. The major product (A) formed in the following reaction sequence is

1)

2)

3)

4)

Answer:

Hence, the correct answer is option (1).
Question 3.

The ratio of the number of oxygen atoms to bromine atoms in the product $\mathrm{Q}$ is ______ $\times 10^{-1}$.
Answer:

No. of oxygens/ No.of bromine atoms = 3/2 = 1.5
= 15 x 10-1.
Hence, the answer is (15).
Question 4. The descending order of basicity of following amines is :
(A)

(D) $\mathrm{CH}_3 \mathrm{NH}_2$
(E) $\left(\mathrm{CH}_3\right)_2 \mathrm{NH}$
Choose the correct answer from the options given below :
(1) B $>$ E $>$ D $>$ A $>$ C
(2) E $>$ D $>$ B $>$ A $>$ C
(3) E $>$ D $>$ A $>$ B $>$ C
(4) E $>$ A $>$ D $>$ C $>$ B
Answer:
Aliphatic amines are more basic than aromatic amines, as the lone pair on nitrogen is localized.

E > D > B > A > C
Hence, the correct answer is option (2).
Question 5. Which of the following amine (s) show (s) positive carbylamines test ?

Choose the correct answer from the options given below :
(1) A and E Only
(2) C Only
(3) A and C Only
(4) B, C and D Only
Answer:
Only $1^{\circ}$ or primary amines gives positive carbylamines test.

Option (A) and (C) are primary amine and will give positive carbyl amine test
Hence, the correct answer is option (3).
Question 6: The correct order of basicity for the following molecules is :
(1) P > Q > R
(2) R > P > Q
(3) Q > P > R
(4) R > Q > P
Answer:
In Q, the lone pair on nitrogen will be more available than P due to cross conjugation.

Hence, the correct answer is option (4).
Approach to Solve Questions of Chapter 9: Amines
To effectively solve class 12 chemistry amines question answer, one should follow a structured approach. Here are some points that can help you with problem-solving strategies
1) Firstly, the understanding of basic concepts is necessary to solve the problems related to amines like
- Classification of primary, secondary, and tertiary amines.
- Classification of Aliphatic and aromatic amines.
- IUPAC and common names of compounds
- Distinguishing amines from other functional groups
2) Preparation methods are quite important from the exam point of view. Some of them include the reduction of nitriles, amides, and nitro compounds, Hoffmann Bromamide degradation, Reduction of Nitrobenzene. Students can refer to the amines ncert notes for better understanding.
3) Carefully studying the physical properties of compounds like boiling point, melting point, solubility trends, physical properties and the effect of Hydrogen bonding on them is important.
4) Students can make use of short notes or charts to remember the concepts, like Basicity of Amines and the reactions involving amines. Also, the test to distinguish the amines is crucial, such as the Carbylamine test, Hinsberg's test, and the Nitrous acid reaction.
5) Solve the problems involving molecular weight, percentage yield, and reaction stoichiometry. Also, solve the in text and exercise problems from the class 12 chemistry chapter 9 amines solutions.
Topics of Chapter 9 In NCERT Book Class 12 Chemistry
The topics covered in amines ncert solutions are given below. They include key concepts, reactions, and properties that help in understanding the structure and behaviour of amines in detail.
9.1 Structure of Amine
9.2 Classification
9.3 Nomenclature
9.5 Physical Properties
9.6 Chemical Properties
9.7 Method of Preparation of Diazonium Salts
9.8 Physical Properties
9.9 Chemical Reactions
9.10 Importance of Diazonium Salts in the Synthesis of Aromatic Compounds
What Extra Should Students Study Beyond the NCERT for JEE/NEET?
Beyond the class 12 chemistry chapter 9 amines question answer, students should focus on the important concepts at a deeper level, understanding how and why they work. They should practise complex questions, learn shortcut methods, and regularly revise to build strong problem-solving skills for the exams.
What Students Learn from NCERT Solutions for Class 12 Chemistry Chapter 9 Amines
These amines class 12 question answer help students understand the structure, classification, properties, and reactions of amines in a clear and systematic way. Given below some points on what students will learn using these solutions of NCERT:
- These solutions help students to understand the nomenclature, classification and types of amines.
- The molecular structure and basic character of amines are explained in these solutions.
- These class 12 chemistry chapter 9 amines solutions also explains industrial methods of synthesis of amines are
- Here physical and chemical properties of amines are discussed.
- They also cover comparison of basicity of amines and factors affecting it and reactions like alkylation, acylation, diazotisation, and coupling
Importance of Class 12 Chemistry Chapter 9 Amines Solutions
The amines class 12 question answer are important as they are nitrogen containing organic compounds, which are essential in both biological systems and industries.
- This chapter explains how amines are used in the production of drugs, dyes, polymers, and pesticides.
- The class 12 chemistry amines question answer are important for both board exams and competitive exams like JEE and NEET.
- These solutions help students understand classification, basicity, and reactions
NCERT Solutions for Class 12 Chemistry
Apart from the NCERT Solutions for Class 12 Chemistry Chapter 9 Amines, students can also explore the links provided below for solutions to other chapters.
NCERT Solutions for Class 12 Subject-wise
Excel your preparation by solving the NCERT solutions for other subjects as well.
NCERT Exemplar subject-wise
Follow the links below to get your hands on the NCERT exemplar for other subjects for better learning.
NCERT Books and NCERT Syllabus
Get the syllabus and books from the links provided in the table below
| NCERT Books Class 12 Chemistry |
| NCERT Syllabus Class 12 Chemistry |
| NCERT Books Class 12 |
| NCERT Syllabus Class 12 |
Frequently Asked Questions (FAQs)
Amines behave as weak bases because the nitrogen atom has a lone pair of electrons that can accept protons. This makes them fundamental to understanding acid-base reactions in organic chemistry, as they can neutralize acids to form ammonium salts.
The amines class 12 question answer discusses various methods for preparing amines, including the reduction of nitriles or amides, the alkylation of ammonia, and the reduction of nitro compounds. Each method provides insights into how amines can be synthesized in the lab.
Amines are organic compounds derived from ammonia by replacing one or more hydrogen atoms with alkyl or aryl groups. They are important because they form the basis of many biologically active compounds, including amino acids, proteins, vitamins and neurotransmitters.
Ammonolysis yields a mixture of primary, secondary and tertiary amines and also quaternary ammonium salts. This is because the amine formed in the first step can further react with the alkyl halide. This issue is addressed to some extent by using a large excess of ammonia which favors the formation of the primary amine.
The Gabriel synthesis produces pure primary amines without contamination by secondary or tertiary amines.
The carbylamine test is a chemical test used to detect primary amines both aliphatic and aromatic. When heated with chloroform and alcoholic KOH , primary amines produce a foul-smelling isocyanide. Secondary and tertiary amines do not give this test.
Amines can be classified into three categories based on the number of carbon-containing groups attached to the nitrogen atom: primary amines, secondary amines, and tertiary amines. Each type has distinct properties and reactivity.
Questions related to CBSE Class 12th
On Question asked by student community
Hello,
You can get the Class 11 English Syllabus 2025-26 from the Careers360 website. This resource also provides details about exam dates, previous year papers, exam paper analysis, exam patterns, preparation tips and many more. you search in this site or you can ask question we will provide you the direct link to your query.
LINK: https://school.careers360.com/boards/cbse/cbse-class-11-english-syllabus
Hello,
No, it’s not true that GSEB (Gujarat Board) students get first preference in college admissions.
Your daughter can continue with CBSE, as all recognized boards CBSE, ICSE, and State Boards (like GSEB) which are equally accepted for college admissions across India.
However, state quota seats in Gujarat colleges (like medical or engineering) may give slight preference to GSEB students for state-level counselling, not for all courses.
So, keep her in CBSE unless she plans to apply only under Gujarat state quota. For national-level exams like JEE or NEET, CBSE is equally valid and widely preferred.
Hope it helps.
Hello,
The Central Board of Secondary Education (CBSE) releases the previous year's question papers for Class 12.
You can download these CBSE Class 12 previous year question papers from this link : CBSE Class 12 previous year question papers (http://CBSE%20Class%2012%20previous%20year%20question%20papers)
Hope it helps !
Hi dear candidate,
On our official website, you can download the class 12th practice question paper for all the commerce subjects (accountancy, economics, business studies and English) in PDF format with solutions as well.
Kindly refer to the link attached below to download:
CBSE Class 12 Accountancy Question Paper 2025
CBSE Class 12 Economics Sample Paper 2025-26 Out! Download 12th Economics SQP and MS PDF
CBSE Class 12 Business Studies Question Paper 2025
CBSE Class 12 English Sample Papers 2025-26 Out – Download PDF, Marking Scheme
BEST REGARDS
Hello,
Since you have passed 10th and 12th from Delhi and your residency is Delhi, but your domicile is UP, here’s how NEET counselling works:
1. Counselling Eligibility: For UP NEET counselling, your UP domicile makes you eligible, regardless of where your schooling was. You can participate in UP state counselling according to your NEET rank.
2. Delhi Counselling: For Delhi state quota, usually 10th/12th + residency matters. Since your school and residency are in Delhi, you might also be eligible for Delhi state quota, but it depends on specific state rules.
So, having a Delhi Aadhaar will not automatically reject you in UP counselling as long as you have a UP domicile certificate.
Hope you understand.
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
.PNG)

