The electrochemistry ncert notes are concise, well-organised study materials that summarise all the important concepts, formulas, reactions, and definitions from the Class 12 Chemistry syllabus. They help students revise topics quickly and understand complex concepts in a simplified way.
NCERT Class 12 Chemistry Chapter 3 Notes Electrochemistry - Download PDF
Have you ever wondered why metals corrode over time or how a battery powers your phone? What causes a chemical reaction to produce electricity? NCERT Notes for Class 12 Chemistry Chapter 2 Electrochemistry answers all these questions that deal with the relationship between electrical energy and chemical reactions. It forms the basis that is going to help students understand complex topics. In our daily lives, we often use batteries in Smartphones and electric vehicles for their charging. This phenomenon is based on Electrochemistry. Many industries depend on Electrochemistry for the refining of metals, wastewater treatment, and impurity removal.
This Story also Contains
- NCERT Notes for Class 12 Chemistry Chapter 2 Electrochemistry: Download PDF
- NCERT Notes for Class 12 Chapter 2 Electrochemistry
- NCERT Chapter 2 Electrochemistry Previous Year Questions and Answers
- How to Master Class 12 Chemistry Chapter 2 Electrochemistry
- Advantages of Class 12 Chemistry Chapter 2 Electrochemistry Notes
- NCERT Class 12 Notes Chapter-Wise
- NCERT Solutions for Class 12 Chemistry
- NCERT Exemplar Solutions Class 12 Subject-Wise

The NCERT Notes for Class 12 will be helpful for a quick revision of topics. These electrochemistry class 12 ncert notes are designed by our subject experts, which ensures the credibility of the content provided. It contains all the important formulas of electrochemistry. The galvanic cell converts the chemical energy of the spontaneous reaction into electrical energy. Electrochemical principles are widely used in energy storage systems like lead-acid batteries in vehicles, fuel cells, lithium-ion batteries in smartphones, etc. It becomes difficult and time-consuming for students to read the NCERT books point-to-point. So, to solve this problem, we are providing these NCERT notes that cover all the topics and concepts provided in the chapter in a very clear and comprehensive way. These Electrochemistry class 12 notes are also valuable resources for various competitive exams.
NCERT Notes for Class 12 Chemistry Chapter 2 Electrochemistry: Download PDF
Students can download the class 12 chemistry chapter 2 electrochemistry notes pdf from the icon given below to make your learning simple and effective. These NCERT Notes for Class 12 Chemistry cover important topics like electrochemical cells, Nernst equation, and batteries, helping in quick revision and exam preparation.
Also read
NCERT Notes for Class 12 Chapter 2 Electrochemistry
These electrochemistry class 12 ncert notes cover a brief outline of topics such as electrochemical cells, Nernst equation, the Gibbs energy of cell reaction, conductivity, Kohlrausch law and its applications, electrolysis, etc. These notes are the best resource for quick revision, also they help build a clear understanding of fundamental principles and their real-life applications, such as batteries and corrosion. Detailed notes of this chapter are given below.
Conductance of Electrolytic Solutions;
Conductance:
Conductance is the measure of the ease with which current flows through a conductor.
The inverse of resistance, R, is called conductance, G:
$G=1 / R$
where ($R=\rho l / A$)
$\mathrm{G}=\mathrm{A} / \mathrm{\rho l}$
G = KA/l
Where R is resistance, l is length, A is an area of cross-section, ρ (rho) is resistivity, and κ(kappa) is conductivity.
Conductivity:
The inverse of resistivity is called conductivity. The SI units of conductivity are $\mathrm{S} \mathrm{m}^{-1}$, but quite often, κ is expressed in $\mathrm{S} \mathrm{cm}^{-1}$.
κ = 1/ρ
The conductivity of a material in $\mathrm{S} \mathrm{m}^{-1}$ is its conductance when it is 1 m long and its area of cross-section is $1 \mathrm{~m}^2$. It may be noted that 1 $\mathrm{S} \mathrm{cm}^{-1}$.
Molar conductivity:
Molar Conductivity is described as the conducting power produced by the ions by dissolving one mole of electrolyte in a solvent.
Molar conductivity =$\Lambda _{m}=\frac{\kappa }{C}$
Conductivity and molar conductivity-
m= κ×1000/M
Equivalent conductivity-
Equivalent conductivity is defined as the conductivity power of combining ions formed by the dissolution of an electrolyte of one gram equivalent in a solution.
e= κ×1000/Ceq
Measurement of the Conductivity of Ionic Solutions:
We know that accurate measurement of an unknown resistance can be performed on a Wheatstone bridge.
Wheatstone bridge:
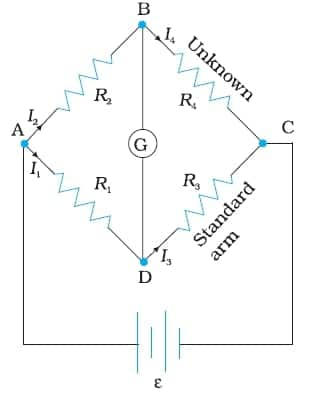
It consists of two resistors, R1 and R3, a variable resistor, R2, and the unknown resistor, R4. The Wheatstone bridge is fed by an oscillator $\varepsilon$ (a source of a.c. power). G is a suitable detector (an electronic device, such as a galvanometer), and the bridge is balanced when no current passes through the detector. Under these conditions:
Unknwon resistance $R_{4}=\frac{R_{2}R_{3}}{R_{1}}$
Once the cell constant and the resistance of the solution in the cell are determined, the conductivity of the solution is given by the equation:
$\kappa$ = cell constant/R =G*/R
Variation of conductivity and molar conductivity with concentration:
Electrolytic conductance decreases with an increase in concentration or increases with an increase in dilution.
Molar conductivity increases with dilution:
The equation of Debye-Huckel-Onsager shows the variation of molar conductivity along with concentration for strong electrolytes.
For strong electrolytes: For strong electrolytes, $\Lambda _{m}$ increases slowly with dilution and can be represented by the equation:
$\Lambda _{m}=\Lambda ^{^{\circ}}_{m} - Ac^{1/2}$
Variation of molar conductivity with concentration for weak electrolytes:
Molar conductivity of weak electrolytes cannot be found for weak electrolytes because the dissociation of weak electrolytes is much lower compared to strong electrolytes.
For weak electrolytes, molar conductivity at infinite dilution can be found using Kohlrausch law.
At any concentration c, if α is the degree of dissociation, then it can be approximated by the ratio of molar conductivity $\Lambda _{m}$ at the concentration c to the limiting molar conductivity $\Lambda ^{^{\circ}}_{m}$. Thus we have:
α = $\Lambda _{m}$/ $\Lambda ^{^{\circ}}_{m}$
Kohlrausch’s Law:
At infinite dilution when ions are completely dissociated, every ion makes its unique contribution to the molar conductivity of the electrolyte, irrespective of the nature of the other ion with which it is associated. Students can also refer to ncert class 12 chemistry chapter 2 electrochemistry notes for practicing questions related to these topics.
Applications of Kohlrausch's Law -
1. Calculation of molar conductance at infinite dilution for weak electrolytes
2. The degree of dissociation of weak electrolytes is calculated as
α = $\Lambda _{m}$/ $\Lambda ^{^{\circ}}_{m}$
3. Calculation of the dissociation constant of weak electrolytes.
$K_{a}=\frac{C\alpha^2}{1-\alpha }$
4. Calculation of the solubility of sparingly soluble salts.
solubility= κ×1000
Electrochemical Cells:
Electrochemical cells are devices that convert chemical energy into electrical energy or vice versa through redox reactions. Students can also refer to NCERT Solutions for Class 12 Chapter 2 Electrochemistry to practise and solve questions from these topics effectively.
Galvanic cells:
Converts the chemical energy of a spontaneous reaction into electrical energy.
Two half cells -
$\mathrm{Cu}^{+2}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}_{(\mathrm{s})}($ reduction half cell $)$
$\mathrm{Zn}_{(\mathrm{s})} \rightarrow \mathrm{Zn}^{2+}+2 \mathrm{e}^{-}$(oxidation half cell)
Overall cell reaction-
$\mathrm{Zn}_{(\mathrm{s})}+\mathrm{Cu}^{+2}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}{ }_{(\mathrm{aq})}+\mathrm{Cu}_{(\mathrm{s})}$
Cell potential-
The Potential difference between two electrodes of a Galvanic Cell is called cell potential.
Measurement of Electrode Potential: EMF (Electromotive Force):
Emf Of Cell is the potential difference between the anode and the cathode when no current is drawn through the cell.
$E_{\text {cell }}=E_{\text {right }}-E_{\text {left }}$
Feasibility of a reaction-
$E_{\text {cell }}=E_{\text {right }}-E_{\text {left }}$
Reduction Half-$2 \mathrm{Ag}^{+}(a q)+2 e^{-} \rightarrow 2 \mathrm{Ag}(s)$
Oxidation Half-$\mathrm{Cu}(s) \rightarrow \mathrm{Cu}^{2+}(a q)+2 e^{-}$
For the above reaction, the reaction is feasible if
Ecell =$\mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}$ - $\mathrm{E}_{\mathrm{Cu}^{+2} / \mathrm{Cu}}$ is positive.
SHE ( Standard Hydrogen Electrode) is assigned a zero potential to determine the potential of individual half-cells.
It is denoted by $\mathrm{Pt}_{(\mathrm{s})}\left|\mathrm{H}_{2(\mathrm{~g})}\right| \mathrm{H}^{+}{ }_{(\mathrm{aq})}$
Nernst equation:
For reaction-
$M^{n+}+n e^{-} \longrightarrow M$
$E_{M^{n+} / M}=E_{M^{n+} / M}^{\circ}-\frac{2.303 R T}{n F} \log \frac{1}{\left[M^{n+}\right]}$
$E_{M^{n+} / M}=E_{M^{n+} / M}^{\circ}-\frac{0.0591}{n} \log \left[\frac{1}{M^{n+}}\right]$
Applications of the Nernst equation-
1. Determining the cell potential using the Nernst equation-
Equilibrium Constant from Nernst Equation:
For a chemical reaction-
aA+bB→cC+dD
$E_{\text {cell }}=E_0$ cell $-(R T / n F) \ln (Q)$
$
E_{\text {cell }}=E_{\text {cell }}^{\circ}-\frac{0.0591}{n} \log \left(\frac{[C]^c[D]^d}{[A]^a[B]^b}\right)
$
This is the Nernst equation at 298 K, where:
$E_{\text {cell }}$ is the cell potential under non-standard conditions
$E_{\text {cell }}^{\circ}$ is the standard cell potential
$n$ is the number of moles of electrons transferred
$[A],[B],[C],[D]$ are concentrations of the respective species
$a, b, c, d$ are the stoichiometric coefficients in the balanced redox equation.
2. Determination of the concentration of a solution of a half-cell
Using the Nernst Equation, the concentration of the unknown species can be found.
3. To find the equilibrium constant using the Nernst equation-
At equilibrium, the Nernst equation takes the form of –
$\mathrm{E}^{\circ}$ cell=2.303 (RT/nF) $\log (\mathrm{K})$
Electrochemical Cell and Gibbs Energy of the Reaction:
$\Delta_{\mathrm{r}} \mathrm{G}=-\mathrm{nFEcell}$
This equation can help to predict the feasibility of the reaction.
Electrolysis- The process in which chemical changes take place due to the passage of current.
Faraday’s Law of electrolysis:
There are two Faraday’s Laws Of Electrolysis, Faraday’s first law of electrolysis says that the quantity of substance deposited at the electrode is in direct proportion to the amount the electricity passed through the solution.
$w \propto Z Q$
where w is the gram of substance deposited on passing Q coulombs of electricity if a current of 1 ampere is passed for t seconds.
Faraday’s second law of electrolysis- It says that when an equal amount of electricity is passed through different solutions lined up in series, the mass of the substance deposited at the electrodes is in direct proportion to the equivalent weight. Students can also download these electrochemistry class 12 ncert notes pdf to study offline anytime and anywhere.
Weight of Cu deposited = Weight of Ag deposited
= Eq. wt. of Cu = Eq. wt. of Ag
Batteries:
Primary batteries
1. Dry cells- Found in torches, flashlights, calculators, tape recorders, and many other devices.
Reactions occurring at the electrode are-
Anode
$\mathrm{Zn} \rightarrow \mathrm{Zn}^{+2}+2 \mathrm{e}^{-}$
Cathode
$2 \mathrm{NH}_4^{+}(\mathrm{aq})+2 \mathrm{MnO}_2+2 \mathrm{e}^{-} \rightarrow \mathrm{Zn}^{+2}+2 \mathrm{MnOOH}+2 \mathrm{NH}_3$
Overall-
$\mathrm{Zn}+2 \mathrm{NH}_4^{+}(\mathrm{aq})+2 \mathrm{MnO}_2+\mathrm{Zn}^{+2}+2 \mathrm{MnOOH}+2 \mathrm{NH}_3$
2. Mercury cell-
Found in electrical circuits.
Reactions occurring at the electrodes are-
Anode-
$\mathrm{ZnHg}+2 \mathrm{OH}^{-} \rightarrow \mathrm{ZnO}+\mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-}$
Cathode-
$\mathrm{HgO}_{(\mathrm{s})}+\mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg}+2 \mathrm{OH}^{-}$
Overall-
$\mathrm{ZnHg}+\mathrm{HgO} \rightarrow \mathrm{ZnO}+2 \mathrm{OH}^{-}$
Secondary batteries:
1. Lead storage batteries-
Battery used in automobiles.
Reactions taking place at electrodes-
Anode-
$\mathrm{PbS}+\mathrm{SO}_4{ }^{2-} \rightarrow \mathrm{PbSO}_4+2 \mathrm{e}^{-}$
Cathode-
$\mathrm{PbO}_2(\mathrm{~s})+\mathrm{SO}_4^{2-}+4 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{PbSO}_4+2 \mathrm{H}_2 \mathrm{O}$
Overall-
$\mathrm{Pb}+\mathrm{PbO}_2+2 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow 2 \mathrm{PbSO}_4+2 \mathrm{H}_2 \mathrm{O}$
2. Nickel-cadmium storage cell-
Has a longer life than the lead storage battery.
Reactions occurring at electrodes-
Anode-
$\mathrm{Cd}+2 \mathrm{OH}^{-} \rightarrow \mathrm{CdO}+\mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-}$
Cathode−
$2 \mathrm{Ni}(\mathrm{OH})_3+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Ni}(\mathrm{OH})_2+2 \mathrm{OH}^{-}$
Overall-
$\mathrm{Cd}+2 \mathrm{Ni}(\mathrm{OH})_3 \rightarrow \mathrm{CdO}+2 \mathrm{Ni}(\mathrm{OH})_2+\mathrm{H}_2 \mathrm{O}$
Fuel Cells-
- Features
- Reactants are supplied continuously.
- The energy of Combustion of fuels such as H2, CO, CH4, etc. is converted to electrical energy.
- Reactions taking place at electrodes-
Anode-
$2\left[\mathrm{H}_2+2 \mathrm{OH}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{e}^{-}\right]$
Cathode-
$\mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O}+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-}(\mathrm{aq})$
Overall-
$2 \mathrm{H}_{2(\mathrm{~g})}+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}$
Corrosion:
Deterioration of metal over time due to its reaction with air and water.
Except gold, platinum, and palladium all other metals undergo corrosion.
Rusting of iron-
At anode-
$\left[\mathrm{Fe} \rightarrow \mathrm{Fe}^{2+}{ }_{(\mathrm{aq})}+2 \mathrm{e}^{-}\right] \times 2$
At cathode-
$4 \mathrm{H}^{+}+\mathrm{O}_2+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_2 \mathrm{O}$
Overall reaction-
$2 \mathrm{Fe}+4 \mathrm{H}^{+}+\mathrm{O}_2 \rightarrow 2 \mathrm{Fe}^{+2}{ }_{(\mathrm{aq})}+2 \mathrm{H}_2 \mathrm{O}$
Prevention of corrosion-
- Barrier protection
- Sacrificial protection
- electrical protection
NCERT Chapter 2 Electrochemistry Previous Year Questions and Answers
Given below previous years to help you understand important concepts and exam trends. Practising these will improve your accuracy. To understand these concepts better students can refer class 12 chemistry chapter 2 electrochemistry notes.
Question 1. The molar conductance of an infinitely dilute solution of ammonium chloride was found to be $185 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$ and the ionic conductance of hydroxyl and chloride ions are 170 and $70 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^1$, respectively. If molar conductance of 0.02 M solution of ammonium hydroxide is $85.5 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$, its degree of dissociation is given by $\mathrm{x} \times 10^{-1}$. The value of $x$ is _______ (Nearest integer)
(1) 3
(2) 9
(3) 4
(4) 10
Answer:
$\begin{aligned}
& \lambda_{\mathrm{m}}^{\prime \prime} \text { of } \mathrm{NH}_4 \mathrm{Cl}=185 \\
& \left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4}+\left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{Cl}^{-}}=185 \\
& \left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4}=185-70=115 \mathrm{Scm}^2 \mathrm{~mol}^{-1} \\
& \left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4 \mathrm{OH}}=\left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4^{\circ}}+\left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{OH}^{-}} \\
& =115+170 \\
& \left(\lambda_{\mathrm{m}}^0\right)_{\mathrm{NH}_4 \mathrm{OH}}=285 \mathrm{Scm}^2 \mathrm{~mol}^{-1} \\
& \text { degree of dissociation }=\frac{\left(\lambda_{\mathrm{m}}\right)_{\mathrm{NH}_4 \mathrm{OH}}}{\left(\lambda_{\mathrm{m}}^{\circ}\right)_{\mathrm{NH}_4 \mathrm{OH}}} \\
& =\frac{85.5}{285} \\
& =0.3 \\
& =3 \times 10^{-1}
\end{aligned}$
Hence, the answer is option (1).
Question 2: Given below are two statements :
1 M aqueous solution of each of $\mathrm{Cu}\left(\mathrm{NO}_3\right)_2$, $\mathrm{AgNO}_3, \mathrm{Hg}_2\left(\mathrm{NO}_3\right)_2 ; \mathrm{Mg}\left(\mathrm{NO}_3\right)_2$ are electrolysed using inert electrodes,
Given : $\mathrm{E}_{\mathrm{Ag}^* / \mathrm{Ag}}^0=0.80 \mathrm{~V}, \mathrm{E}_{\mathrm{Hg}_2^{2+} / \mathrm{Hg}}^0=0.79 \mathrm{~V}$, $\mathrm{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^0=0.24 \mathrm{~V}$ and $\mathrm{E}_{\mathrm{Mg}^{2+} / \mathrm{Mg}}^0=-2.37 \mathrm{~V}$
Statement (I) : With increasing voltage, the sequence of deposition of metals on the cathode will be $\mathrm{Ag}, \mathrm{Hg}$ and Cu
Statement (II) : Magnesium will not be deposited at cathode instead oxygen gas will be evolved at the cathode.
In the light of the above statement, choose the most appropriate answer from the options given below
(1) Both statement I and statement II are incorrect
(2) Statement I is correct but statement II is incorrect
(3) Both statement I and statement II are correct
(4) Statement I is incorrect but statement II is correct
Answer:
We are electrolyzing 1 M aqueous solutions of the following nitrates using inert electrodes:
-
$\mathrm{Cu}\left(\mathrm{NO}_3\right)_2$
-
$\mathrm{AgNO}_3$
-
$\mathrm{Hg}_2\left(\mathrm{NO}_3\right)_2$
-
$\mathrm{Mg}\left(\mathrm{NO}_3\right)_2$
At the cathode, reduction occurs. So we must look at the standard reduction potentials (E°) for the metal cations.
From the electrochemical series, we can see that,
| Reaction | E° (V) |
|---|
| Ag⁺ + e⁻ → Ag | +0.80 V |
| Hg₂²⁺ + 2e⁻ → 2Hg | +0.79 V |
| Cu²⁺ + 2e⁻ → Cu | +0.24 V |
| Mg²⁺ + 2e⁻ → Mg | –2.37 V |
-
In electrolysis, the ion with the highest E° gets reduced (i.e., deposited) first at the cathode.
-
So as the voltage increases, the cations get deposited in order of decreasing E°.
Thus, the expected order of deposition at the cathode is:
Ag>Hg>Cu
So, Statement I: "With increasing voltage, the sequence of deposition of metals on the cathode will be Ag, Hg, and Cu" is correct.
Let’s analyze the possibility of Mg²⁺ getting reduced:
-
E° for Mg²⁺/Mg = –2.37 V (very negative).
-
Water can also be reduced at the cathode:
$2 \mathrm{H}_2 \mathrm{O}+2 e^{-}->\mathrm{H}_2+2 \mathrm{OH}^{-} \quad \mathrm{E}^{\circ}=-0.83 \mathrm{~V}$
-
Since water has a less negative E° than Mg²⁺, water gets reduced before Mg²⁺.
So, Mg²⁺ is not deposited — instead, hydrogen gas is evolved at the cathode.
But Statement II says:
"Magnesium will not be deposited at the cathode instead oxygen gas will be evolved at the cathode", is incorrect because oxygen is evolved at the anode, not at the cathode.
At the cathode, hydrogen gas evolves if Mg is not deposited.
Hence, the correct answer is option (2).
Question 3. Given below are two statements :
Statement I : Mohr's salt is composed of only three types of ions-ferrous, ammonium, and sulphate.
Statement II : If the molar conductance at infinite dilution of ferrous, ammonium and sulphate ions are $\mathrm{x}_1, \mathrm{x}_2$ and $\mathrm{x}_3 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$, respectively then the molar conductance for Mohr's salt solution at infinite dilution would be given by $\mathrm{x}_1+\mathrm{x}_2+2 \mathrm{x}_3$
In the light of the given statements, choose the correct answer from the options given below :
(1) Both statements I and Statement II are false
(2) Statement I is false but Statement II is true
(3) Statement I is true but Statement II are false
(4) Both statements I and Statement II are true
Answer:
Mohr's salt : $\mathrm{FeSO}_4 \cdot\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \cdot 6 \mathrm{H}_2 \mathrm{O}$
Mohr's salt dissociates into one Fe ion, two ammonium ions and two sulphate ions. Therefore, using Kohlrausch's law we get
$\lambda_{\mathrm{m}}^*(\text { Mohr's salt })=\mathrm{x}_1+2 \mathrm{x}_2+2 \mathrm{x}_3$
Hence, the correct answer is option (3).
Question 4. The correct order of limiting molar conductivity for cations in water at 298 K is
(1) $\mathrm{H}^{+}>\mathrm{Na}^{+}>\mathrm{K}^{+}>\mathrm{Ca}^{2+}>\mathrm{Mg}^{2+}$
(2) $\mathrm{H}^{+}>\mathrm{Ca}^{2+}>\mathrm{Mg}^{2+}>\mathrm{K}^{+}>\mathrm{Na}^{+}$
(3) $\mathrm{Mg}^{2+}>\mathrm{H}^{+}>\mathrm{Ca}^{2+}>\mathrm{K}^{+}>\mathrm{Na}^{+}$
(4) $\mathrm{H}^{+}>\mathrm{Na}^{+}>\mathrm{Ca}^{2+}>\mathrm{Mg}^{2+}>\mathrm{K}^{+}$
Answer:
The limiting molar conductivities of ions are important in determining how well they conduct electricity in solution. Here are the values for some common cations at 298 K :
$\mathrm{H}^{+}: 349.8 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$
$\mathrm{Na}^{+}: 50.11 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$
$\mathbf{K}^{+}: 73.52 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$
$\mathbf{C a}^{\mathbf{2 +}}: 119 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-\mathbf{1}}$
$\mathbf{M g}^{\mathbf{2 +}}: \mathbf{1 0 6 . 1 2} \mathrm{S} \mathrm{cm}^{\mathbf{2}} \mathrm{mol}^{-\mathbf{1}}$
Therefore correct order of limiting molar conductivity of cations will be -$\{\mathrm{H}^{+}>\mathrm{Ca}^{+2}>\mathrm{Mg}^{+2}>\mathrm{K}^{+}>\mathrm{Na}^{+}$}
Hence, the correct answer is option (2).
Question 5. $\mathrm{O}_2$ gas will be evolved as a product of electrolysis of:
(A) an aqueous solution of $\mathrm{AgNO}_3$ using silver electrodes.
(B) an aqueous solution of $\mathrm{AgNO}_3$ using platinum electrodes.
(C) a dilute solution of $\mathrm{H}_2 \mathrm{SO}_4$ using platinum electrodes.
(D) a high concentration solution of $\mathrm{H}_2 \mathrm{SO}_4$ using platinum electrodes.
Choose the correct answer from the options given below :
(1) (B) and (C) only
(2) (A) and (D) only
(3) (B) and (D) only
(4) (A) and (C) only
Answer:
(A) An aqueous solution of $\mathrm{AgNO}_3$ using silver electrodes.
Cathode - Reduction - $A g_{(a q)}^{+}+e^{-} \rightarrow A g(s)$
Anode - Oxidation - $A g(s) \rightarrow A g_{(a q)}^{+}+e^{-}$
Solid silver will be deposited at the cathode. Solid anode (silver) will dissolve, releasing silver ions into the solution.
So, there is no formation of $\mathrm{O}_2$ gas in this electrolysis.
(B) An aqueous solution of $\mathrm{AgNO}_3$ using platinum electrodes.
Cathode - Reduction - $A g_{(a q)}^{+}+e^{-} \rightarrow A g(s)$
Anode - Oxidation - $2 \mathrm{H}_2 \mathrm{O} \rightarrow 4 \mathrm{H}^{+}+\mathrm{O}_2+4 e^{-}$
When platinum electrodes are used, $\mathrm{Ag}^{+}$from solution is reduced and deposited at cathode whereas $\mathrm{O}_2$ is produced at the anode.
(C) A dilute soution of $\mathrm{H}_2 \mathrm{SO}_4$ using platinum electrodes.
Cathode - Reduction - $2 \mathrm{H}^{+}+2 e^{-} \rightarrow \mathrm{H}_2$
Anode - Oxidation - $2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{O}_2+4 \mathrm{H}^{+}+4 e^{-}$
$\mathrm{H}_2$ gas is produded at the cathode and $\mathrm{O}_2$ gas is produced at the anode.
(D) a high concentration solution of $\mathrm{H}_2 \mathrm{SO}_4$ using platinum electrodes.
$\mathrm{O}_2$ gas is not formed in this case.
Cathode - Reduction - The substance formed is $\mathrm{H}_2$ gas.
Anode - Oxidation - The substance formed is not $\mathrm{O}_2$ gas.
So, statements (B) and (C) are correct.
Hence, the correct answer is option (1).
Also read
How to Master Class 12 Chemistry Chapter 2 Electrochemistry
These electrochemistry class 12 ncert notes help to understand the basic concepts from your NCERT book. Below are some points on how students master chapter 2
- In order to learn the basic concepts of electrochemistry students need to understand the basics like electrochemical cells, galvanic and electrolytic cells.
- Nernst equation to calculate electrode potential under different conditions plays an important role in this chapter. To understand this concept better students must refer to electrochemistry class 12 ncert notes.
- They must revise the concepts like standard electrode potentials, Faraday’s laws, and important constants regularly.
- To understand these chapters students must draw labelled diagrams of cells and flowcharts to simplify complex concepts.
- After that students can solve previous year questions from this chapter.
Advantages of Class 12 Chemistry Chapter 2 Electrochemistry Notes
The ncert class 12 chemistry chapter 2 electrochemistry notes provide a clear explanation of concepts that helps in mastering topics and scoring well in board and competitive exams. The advantages of using these notes are given below:
- These notes are designed to simplify the concepts like redox reactions, electrode potential, and electrochemical cells.
- Students can revise concepts using these before exams.
- These notes are prepared by experts in a very detailed manner and they also include diagrams and equations.
- Formulas for Nernst equation, EMF, and conductance are summarised in these ncert class 12 chemistry chapter 2 electrochemistry notes.
NCERT Class 12 Notes Chapter-Wise
Besides class 12 chemistry chapter 2 electrochemistry notes students can follow the links given below for NCERT notes for each chapter of class 12:
NCERT Solutions for Class 12 Chemistry
These solutions serve as a comprehensive guide to help students understand complex chemical concepts with ease. They provide step-by-step answers to all textbook questions, enabling students to build a strong foundation for board exams and competitive tests.
NCERT Exemplar Solutions Class 12 Subject-Wise
NCERT exemplar solutions for each subject are given below:
Frequently Asked Questions (FAQs)
Faraday's laws of electrolysis describe the relationship between the amount of substance altered during electrolysis and the quantity of electric charge passed through the circuit. The first law states that the mass of a substance produced at an electrode is directly proportional to the quantity of charge.
The Nernst equation relates the cell potential of an electrochemical cell to the concentrations of the reactants and products at any point in the reaction. It's important because it allows us to calculate the cell potential under non-standard conditions.
A galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through spontaneous redox reactions. It consists of two half-cells, each containing an electrode and an electrolyte, where oxidation occurs at the anode and reduction at the cathode.
Chapter 2 focuses on various important concepts such as electrochemical cells, types of electrochemical cells, standard electrode potential, Nernst equation, and Faraday's laws of electrolysis.
Electrochemistry is the study of chemical processes that cause the movement of electrons, resulting in the production or use of electrical energy. In Class 12 CBSE notes, it includes topics like electrochemical cells, redox reactions, electrode potentials, and the Nernst equation.
Electrochemistry NCERT notes are prepared by breaking down the chapter into simple sections, highlighting key concepts, formulas, and definitions. Important diagrams, solved examples, and shortcut tricks are added to make learning easier. They are designed to cover the entire NCERT syllabus while simplifying complex topics for quick revision.
You can find revision NCERT Notes for Class 12 Chemistry Chapter 2 Electrochemistry on trusted educational websites like Careers360 and popular study platforms. These notes are usually available as free downloadable PDFs for quick and easy revision.
Electrochemistry is crucial because it helps us understand how chemical energy can be converted into electrical energy and vice versa. This principle is fundamental in batteries, fuel cells, and electrolysis processes, which have practical applications in various industries and technologies.
To effectively study Chapter 2, you can create concise notes from the textbook, solve numerical problems, and review diagrams related to electrochemical cells.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
