NCERT Exemplar Class 12 Physics Solutions Chapter 13 Nuclei
The use of modern medical machines like MRI and PET scans enables the doctor to identify internal injuries and tumours without the need to utilise surgical procedures, all using the nuclear characteristics of atoms. Similarly, nuclear power plants produce power to serve the whole city, emitting energy in the form of atomic nuclei. These practical uses demonstrate that it is essential to know more about the atomic nucleus, the incredibly tiny but extremely heavy nucleus in the centre of every atom.
This Story also Contains
- NCERT Exemplar Class 12 Physics Solutions Chapter 13: MCQI
- NCERT Exemplar Class 12 Physics Solutions Chapter 13: MCQII
- NCERT Exemplar Class 12 Physics Solutions Chapter 13: Very Short Answer
- NCERT Exemplar Class 12 Physics Solutions Chapter 13: Short Answer
- NCERT Exemplar Class 12 Physics Solutions Chapter 13: Long Answer
- NCERT Exemplar Class 12 Physics Solutions Chapter 13 Nuclei: Important Concepts and Formulas
- Advantages of NCERT Exemplar Class 12 Physics Solutions Chapter 13 Nuclei
The nuclear structure, nuclear properties, nuclear forces, radioactivity, radioactive decay, nuclear fission, as well as nuclear fusion are some of the very important topics discussed in this chapter. These concepts are clear and systematic, as stated in the NCERT Exemplar Class 12 Physics Solutions Chapter 13 Nuclei. Every kind of question, such as MCQs, short answer, long answer, and very long answer questions, is tackled in NCERT Exemplar Class 12 Solutions Physics Chapter 13 Nuclei in a step-by-step manner in order to build up conceptual knowledge. These NCERT Exemplar solutions, prepared by experts, are highly beneficial in CBSE board exam preparation and also serve a great purpose in preparing students for competitive exams such as JEE and NEET.
Also see - NCERT Solutions for Class 12 Other Subjects
NCERT Exemplar Class 12 Physics Solutions Chapter 13: MCQI
NCERT Exemplar Class 12 Physics Chapter 13 MCQ I gives precise and clear answers to objective questions as per the NCERT Exemplar pattern. Such NCERT Exemplar Class 12 Physics Solutions Chapter 13 Nuclei can assist students in applying their understanding of concepts, achieving accuracy, and effectively training them to solve solution-oriented multiple-choice questions in subjects that are exam-oriented.
Question:13.1
Suppose we consider a large number of containers each containing initially 10000 atoms of a radioactive material with a half-life of 1 year. After 1 year
(a) all the containers will contain the same number of atoms of the material but that number will only be approximately 5000
(b) all the containers will have 5000 atoms of the material
(c) none of the containers can have more than 5000 atoms
(d) the containers will, in general, have different numbers of the atoms of the material but their average will be close to 5000
Answer:
The correct answer is option (d)
The containers will, in general, have different numbers of the atoms of the material, but their average will be close to 5000.
Question:13.2
The gravitational force between an H-atom and another particle of mass m will be given by Newton’s law: F = G M.m/r2, where r is in km and
(a) M = m proton + m electron
(b) M = m proton + m electron - (|V |/c2) (where |V| = magnitude of the potential energy of the electron in the H-atom)
(c) M is not related to the mass of the hydrogen atom
(d) M = m proton + m electron – (B/c2) (where, B = 13.6 eV)
Answer:
The correct answer is option (d)
Given,
$
\begin{aligned}
F & =\frac{G M m}{r^2} \\
M & =\text { effective mass of hydrogen atom } \\
& =\text { mass of electron }+ \text { mass of proton }-\frac{B^2}{C}
\end{aligned}
$
where $B$ is $B E$ of hydrogen atom $=13.6 \mathrm{eV}$.
Question:13.3
When a nucleus in an atom undergoes radioactive decay, the electronic energy levels of the atom
(a) change for
(b) change for
(c) do not change for any type of radioactivity
(d) change for α and
Answer:
The correct answer is option (d), change for
$\alpha-\beta$ particle carries one unit of negative charge, an $\alpha$-particle carries 2units of positive charge, and $\gamma$ (particle) carries no charge; therefore, electronic energy levels of the atom charges for $\alpha$ and $\beta$ decay, but not for $\gamma$-decay.
Question:13.4
Mx and My denote the atomic masses of the parent and the daughter nuclei respectively in a radioactive decay. The Q-value for a - decay is Q1 and that for a + decay is Q2. If me denotes the mass of an electron, then which of the following statements is correct?
(a) Q1 = (Mx – My)c2 and Q2 = (Mx – My )c2
(b) Q1 = (Mx – My)c2 and Q2 = (Mx – My – 2me )c2
(c) Q1 = (Mx – My– 2me)c2 and Q2 = (Mx – My +2me )c2
(d) Q1 = (Mx – My + 2me )c2 and Q2 = (Mx – My +2me )c2
Answer:
The correct answer is the option (b) Q1 = (Mx – My) c2 and Q2 = (Mx – My – 2me )c2
Let the nucleus is ${ }_z X^A \cdot \beta^{+}$decay is represented as
$
\begin{aligned}
&{ }_z X^A \rightarrow_{z-1} Y^A+{ }_{+1} e^0+v+Q_2 \\
& Q_2=\left[m_n\left({ }_z X^A\right)-m_n\left({ }_{z-1} Y^A\right)-m_e\right] c^2 \\
&=\left[m_n\left({ }_z X^A\right)+z m_e-m_n\left({ }_{z-1} Y^A\right)-(z-1) m_e-2 m_e\right] c^2 \\
&=\left[m\left({ }_z X^A\right)-m\left({ }_{z-1} Y^A\right)-2 m_e\right] c^2 \\
&=\left(M_x-M_y-2 m_e\right) c^2
\end{aligned}
$
$\beta^{-}$decay is represented as
$
\begin{aligned}
& { }_z X^A \rightarrow_{z+1} A^Y+{ }_{-1} e^0+\bar{v}+\alpha_1 \\
\alpha_1 & =\left[m_n\left({ }_z X^A\right)-m_n\left({ }_{z+1} Y^A\right)-m_e\right] c^2 \\
& =\left[m_n\left({ }_z X^A\right)+z m_e-m_n\left({ }_{z+1} Y^A\right)-(z+1) m e\right] c^2 \\
& =\left[m\left({ }_z X^A\right)-m\left({ }_{z-1} Y^A\right)\right] c^2 \\
& =\left(M_x-M_y\right) c^2
\end{aligned}
$
Question:13.5
Tritium is an isotope of hydrogen whose nucleus Triton contains 2 neutrons and 1 proton. Free neutrons decay into $p+\overline{\mathrm{e}}+\bar{v}$. If one of the neutrons in Triton decays, it would transform into $\mathrm{He}^3$ nucleus. This does not happen. This is because
(a) because free neutrons decay due to external perturbations which is absent in a triton nucleus
(b) the electron created in the beta decay process cannot remain in the nucleus
(c) both the neutrons in triton have to decay simultaneously resulting in a nucleus with 3 protons, which is not a He3 nucleus
(d) Triton energy is less than that of a He3 nucleus
Answer:
The correct answer is option (d) Triton energy is less than that of a He3 nucleus
Tritium $\left({ }_1 \mathrm{H}^3\right)$ contains 1 proton and 2 neutrons. A neutron decays as $n \longrightarrow p+\bar{e}+\bar{v}$, the nucleus may have 2 protons and one neutron, i.e., tritium will transform into $2 \mathrm{He}^3$ (2 protons and 1 neutron).
Triton energy is less than that of $2 \mathrm{He}^3$ nucleus, i.e., transformation is not allowed energetically.
Question:13.6
Heavy stable nuclei have more neutrons than protons. This is because of the fact that
(a) neutrons are heavier than protons
(b) electrostatic force between protons are repulsive
(c) neutrons decay into protons through beta decay
(d) nuclear forces between neutrons are weaker than that between protons
Answer:
The correct answer is option (b) electrostatic force between protons is repulsive
Stable heavy nuclei have more neutrons than protons. This is because the electrostatic force between protons is repulsive, which may reduce stability.
Question:13.7
In a nuclear reactor, moderators slow down the neutrons which come out in a fission process. The moderator used to have light nuclei. Heavy nuclei will not serve the purpose because
(a) they will break up
(b) elastic collision of neutrons with heavy nuclei will not slow them down
(c) the net weight of the reactor would be unbearably high
(d) substances with heavy nuclei do not occur in a liquid or gaseous state at room temperature
Answer:
The correct answer is option (b) elastic collision of neutrons with heavy nuclei will not slow them down
According to the question, the moderator used had light nuclei (like protons). When protons undergo a perfectly elastic collision with the neutron emitted, their velocities are exchanged, i.e., neutrons come to rest and protons move with the velocity of neutrons. Heavy nuclei will not serve the purpose because elastic collisions of neutrons with heavy nuclei will not slow them down.
NCERT Exemplar Class 12 Physics Solutions Chapter 13: MCQII
NCERT Exemplar Class 12 Physics Chapter 13: MCQII is aimed at assisting students to analyse their in-depth knowledge of the nuclear physics concepts by asking carefully formulated questions that are structured as objectives. These NCERT Exemplar Class 12 Physics Solutions Chapter 13 focus on applications and critical thinking of the concepts to make the learners think carefully and correct the most common mistakes on competitive and board exams.
Question:13.8
Fusion processes, like combining two deuterons to form a He nucleus are impossible at ordinary temperatures and pressure. The reasons for this can be traced to the fact:
(a) nuclear forces have short-range
(b) nuclei are positively charged
(c) the original nuclei must be completely ionized before fusion can take place
(d) the original nuclei must first break up before combining with each other
Answer:
The correct answers are the options,
(a) nuclear forces have short-range
(b) nuclei are positively charged
Fusion processes are impossible at ordinary temperatures and pressures. The reason is that nuclei are positively charged, and nuclear forces are the strongest short-range forces.
Question:13.9
Samples of two radioactive nuclides A and B are taken.
(a) Initial rate of decay of B is the same as the rate of decay of A at t = 2h and λB < λA
(b) Initial rate of decay of A is twice the initial rate of decay of B and
(c) Initial rate of decay of A is twice the initial rate of decay of B and
(d) Initial rate of decay of B is twice the initial rate of decay of A and
Answer:
The correct answers are the options,
(a) Initial rate of decay of B is the same as the rate of decay of A at t = 2h and λB < λA
(b) Initial rate of decay of A is twice the initial rate of decay of B, and λA > λB
The two samples of the two radioactive nuclides $A$ and $B$ can simultaneously have the same decay rate at any time if the initial rate of decay of $A$ is twice the initial rate of decay of $B$ and $\lambda_A>\lambda_B$. Also, when the initial rate of decay of $B$ is the same as the rate of decay of $A$ at $t=2$ and $\lambda_B<\lambda_A$.
Question:13.10
The variation of the decay rate of two radioactive samples A and B with time is shown in the figure. Which of the following statements are true?

(a) The decay constant of A is greater than that of B, hence A always decays faster than B
(b) The decay constant of B is greater than that of A but its decay rate is always smaller than that of A
(c) The decay constant of A is greater than that of B but it does not always decay faster than B
(d) The decay constant of B is smaller than that of A but still, its decay rate becomes equal to that of A at a later instant
Answer:
The correct answers are the options,
(c) The decay constant of A is greater than that of B, but it does not always decay faster than B
(d) The decay constant of B is smaller than that of A, but still, its decay rate becomes equal to that of A at a later instant
From the given figure, it is clear that the slope of curve $A$ is greater than that of curve $B$. So the rate of decay is faster for $A$ than that of $B$.
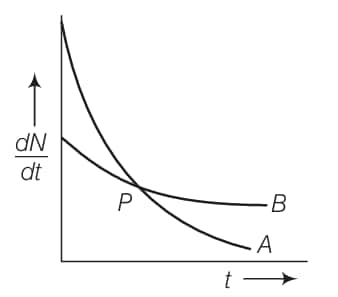
We know that $\left(\frac{d N}{d t}\right) \propto, \lambda$, at any instant of time, hence we can say that $\lambda_A>\lambda_B$. At point $P$ shown in the diagram, the two curves intersect.
Hence, at point $P$, the rate of decay for both $A$ and $B$ is the same.
NCERT Exemplar Class 12 Physics Solutions Chapter 13: Very Short Answer
NCERT Exemplar Class 12 Physics Chapter 13: Very Short Answer provides some brief, but clear explanations that give attention to essential concepts and terminologies of nuclear physics. These solutions enable students to strengthen basics, improve memory skills, and get ready for those questions which require clarity with minimum words.
Question:13.11
Answer:
Both inQuestion:13.12
Draw a graph showing the variation of decay rate with the number of active nuclei.
Answer:

From the Rutherford and Soddy law, we get, the radioactive decay can be expressed as
Question:13.13
Which sample, A or B shown in the figure has shorter mean-life?

Answer:
$
\begin{aligned}
&\text { From the given figure, we can say that }\\
&\begin{aligned}
& \text { at } t=0,\left(\frac{d N}{d t}\right)_A=\left(\frac{d N}{d t}\right)_B \\
& \Rightarrow \quad\left(N_0\right)_A=\left(N_0\right)_B
\end{aligned}
\end{aligned}
$
Considering any instant $t$ by drawing a line perpendicular to the time axis, we find that $\left(\frac{d N}{d t}\right)_A>\left(\frac{d N}{d t}\right)_B$
$\begin{array}{lrl}\Rightarrow & \lambda_A N_A>\lambda_B N_B & \\ \because & N_A>N_B & \text { (rate of decay of } B \text { is slower) } \\ \therefore & \lambda_B>\lambda_A & \\ \Rightarrow & \tau_A>\tau_B & {\left[\because \text { Average life } \tau=\frac{1}{\lambda}\right]}\end{array}$
Question:13.14
Which one of the following cannot emit radiation and why? Excited nucleus, excited electron.
Answer:
An excited electron cannot emit radiation because the energy of electronic energy levels is in the range of eV and not MeV ( mega electron volt ).Question:13.15
Answer:
An electron and a positron destroy each other to produce gamma radiation in the case of pair annihilation. During this process, they used to go in opposite directions in order to conserve their momentum.NCERT Exemplar Class 12 Physics Solutions Chapter 13: Short Answer
Nuclei Class 12 NCERT Exemplar: Short Answer provides clear and structured explanations for questions that require concise reasoning and formula-based solutions. These answers help students strengthen conceptual understanding, present solutions effectively, and perform confidently in exams.
Question:13.16
Why do stable nuclei never have more protons than neutrons?
Answer:
In stable nuclei, the number of protons is less than the number of neutrons. This is because protons repel each other as they have a charge. Due to this high repulsion, the excess neutrons produce attractive forces. As a result, the stability is maintained.Question:13.17
Answer:

Here, in the above graph:
At t=0, NA = N0
NA falls off exponentially with the increase in time.
As a result, the number of atoms of B increases, it will be maximum once and then decays to zero at
Question:13.18
Answer:
As per the information given in the question, the 14C activity of a piece of wood from the ruins is R = 12 dis/min per gram14C activity of a living wood = Ro = 16 dis/min per gram
The half-life of 14C = 5760 years
According to the radioactive decay law,
$
R=R_0 e^{-\lambda t} \text { or } \frac{R}{R_0}=e^{-\lambda t} \text { or } e^{\lambda t}=\frac{R_0}{R}
$
Taking the log on both sides
$\begin{array}{rlr}\lambda t \log _e e & =\log _e \frac{R_0}{R} \Rightarrow \lambda t=\left(\log _{10} \frac{16}{12}\right) \times 2.303 & \\ t & =\frac{2.303(\log 4-\log 3)}{\lambda} & \\ & =\frac{2.303(0.6020-4.771) \times 5760}{0.6931} & \left(\because \lambda=\frac{0.6931}{T_{1 / 2}}\right) \\ & =2391.20 \mathrm{yr} & \end{array}$
Question:13.19
Answer:
We know that,E = hc/
Kinetic energy of an electron
KE = PE = hc/
Question:13.20
A nuclide 1 is said to be the mirror isobar of nuclide 2 if Z1 =N2 and Z2 =N1.
(a) What nuclide is a mirror isobar of ${ }_{11}^{23} \mathrm{Na}$?
(b) Which nuclide out of the two mirror isobars has greater binding energy and why?
Answer:
(a) According to the question, a nuclide 1 is said to be a mirror isobar of nuclide 2, if $Z_1=N_2$ and $Z_2=N_1$.
Now in ${ }_{11} \mathrm{Na}^{23}, Z_1=11, N_1=23-11=12$
$\therefore$ Mirror isobar of ${ }_{11} \mathrm{Na}^{23}$ is ${ }_{12}, \mathrm{Mg}^{23}$, for which $Z_2=12=N_1$ and $N_2=23-12=11=Z_1$
(b) As ${ }_{12}^{23} \mathrm{Mg}$ contains even number of protons (12) against ${ }_{11}^{23} \mathrm{Na}$ which has odd number of protons (11), therefore ${ }_{11}^{23} \mathrm{Mg}$ has greater binding energy than ${ }_{11} \mathrm{Na}^{23}$.
NCERT Exemplar Class 12 Physics Solutions Chapter 13: Long Answer
Nuclei Class 12 NCERT Exemplar: Long Answer presents detailed, step-by-step solutions for descriptive questions that require in-depth reasoning and proper application of nuclear physics concepts. These NCERT Exemplar Class 12 Physics Chapter 13 Solutions help students develop a systematic problem-solving approach and confidently tackle high-mark questions in exams.
Question:13.21
Answer:
Consider the chain of two decays
$
{ }^{38} \mathrm{~S} \xrightarrow[2.48 \mathrm{~h}]{ }{ }^{38} \mathrm{Cl} \xrightarrow[0.62 \mathrm{~h}]{ }{ }^{38} \mathrm{Ar}
$
At time $t$, Let ${ }^{38} \mathrm{~S}$ have $N_1(t)$ active nuclei and ${ }^{38} \mathrm{Cl}$ have $N_2(t)$ active nuclei.
$\begin{aligned}
\frac{d N_1}{d t} =-\lambda_1 N_1=\text { rate of formation of } \mathrm{Cl}^{38} \\
\begin{aligned}
\text { Also, }\frac{d N_2}{d t} =-\lambda_1 N_2+\lambda_1 N_1 \\
\text { But }N_1 =N_0 e^{-\lambda_1 t} \\
\frac{d N_2}{d t}=\lambda_1 N_0 e^{-\lambda_1 t}-\lambda_2 N_2--------(I)
\end{aligned}
\end{aligned}$
Multiplying by $e^{\lambda_2t} d t$ and rearranging
$
e^{\lambda_2 t} d N_2+\lambda_2 N_2 e^{\lambda_2 t} d t=\lambda_1 N_0 e^{\left(\lambda_2-\lambda_1\right) t} d t
$
$\begin{aligned}
&\text { Integrating both sides }\\
&\begin{aligned}
& N_2 e^{\lambda_2 t}=\frac{N_0 \lambda_1}{\lambda_2-\lambda_1} e^{\left(\lambda_2-\lambda_1\right) t}+C \\
& \text { Since, } \quad \text { at } t=0, N_2=0, C=-\frac{N_0 \lambda_1}{\lambda_2-\lambda_1} \\
& \therefore \quad N_2 e^{\lambda_2 t}=\frac{N_0 \lambda_1}{\lambda_2-\lambda_1}\left(e^{\left(\lambda_2-\lambda_1\right) t}-1\right) -----(ii)\\
& N_2=\frac{N_0 \lambda_1}{\lambda_2-\lambda_1}\left(e^{-\lambda_1 t}-e^{-\lambda_2 t}\right)
\end{aligned}
\end{aligned}$
$
\begin{aligned}
& \text { For maximum count, } \quad \frac{d N_2}{d t}=0 \\
& \lambda_1 N_0 e^{-\lambda_1 t}-\lambda_2 N_2=0 \ \ \ \ [From Eq. (i)]\\
& \Rightarrow \quad \frac{N_0}{N_2}=\frac{\lambda_2}{\lambda_1} e^{\lambda_1 t}\ \ \ \ [From Eq.(ii)]
\end{aligned}
$
$\begin{array}{r}e^{\lambda_2 t}-\frac{\lambda_2}{\lambda_1} \cdot \frac{\lambda_1}{\left(\lambda_1-\lambda_1\right)} e^{\lambda_1 t}\left[e^{\left(\lambda_2-\lambda_1\right) t}-1\right]=0 \\ e^{\lambda_2 t}-\frac{\lambda_2}{\left(\lambda_2-\lambda_1\right)} e^{\lambda_2 t}+\frac{\lambda_2}{\left(\lambda_2-\lambda_1\right)} e^{\lambda_1 t}=0\end{array}$
$\begin{aligned} 1-\frac{\lambda_2}{\left(\lambda_2-\lambda_1\right)}+\frac{\lambda_2}{\left(\lambda_2-\lambda_1\right)} e^{\left(\lambda_1-\lambda_2\right) t} & =0 \\ \frac{\lambda_2}{\left(\lambda_2-\lambda_1\right)} e^{\left(\lambda_1-\lambda_2\right) t} & =\frac{\lambda_2}{\left(\lambda_2-\lambda_1\right)}-1 \\ e^{\left(\lambda_1-\lambda_2\right) t} & =\frac{\lambda_1}{\lambda_2}\end{aligned}$
$\begin{aligned} t & =\left(\log _e \frac{\lambda_1}{\lambda_2}\right) /\left(\lambda_1-\lambda_2\right) \\ & =\frac{\log _e\left(\frac{2.48}{0.62}\right)}{2.48-0.62} \\ & =\frac{\log _e 4}{1.86}=\frac{2.303 \times 2 \times 0.3010}{1.86} \quad\left(\because \lambda=\frac{0.693}{T_{1 / 2}}\right) \\ & =0.745 \mathrm{~s}\end{aligned}$
Question:13.22
Given the binding energy of a deuteron, $\mathrm{B}=2.2 \mathrm{MeV}$
Let kinetic energy and momentum of neutron and proton be $K_n, K_P$ and $p_n, p_p$ respectively.
From the conservation of energy,
$
E-B=K_n+K_p=\frac{p_n^2}{2 m}+\frac{p_p^2}{2 m}
$
Now applying conservation of momentum,
$
p_n+p_p=\frac{E}{C}
$
As $\mathrm{E}=\mathrm{B}$, equation (i) $p_n^2+p_p^2=0$
It only happens if $p_n=p_p$.
So, the equation. (ii) cannot be satisfied, and the process cannot take place.
Let us take $\mathrm{E}=\mathrm{B}+\mathrm{x}$, where $\mathrm{x} \ll \mathrm{B}$ for the process to take place.
Putting the value of p, from equation (ii), in equation (i), we get
or $2 p_p^2-\left(\frac{2 E}{c}\right) p_p+\left(\frac{E^2}{c^2}-2 m x\right)=0$
Solving the quadratic equation, we get
$
p_p=\frac{2 E}{c}+\frac{\sqrt{\frac{4 E^2}{c^2}-8\left(\frac{E^2}{c^2}-2 m x\right)}}{4}
$
For the real value $p_p$ the discriminant is positive
$
\begin{aligned}
& \frac{4 E^2}{c^2}=8\left(\frac{E^2}{c^2}-2 m x\right) \\
& 16 m x=\frac{4 E^2}{c^2} \\
& \Rightarrow x=\frac{E^2}{4 m c^2}
\end{aligned}
$
But $\mathrm{x} \ll \mathrm{B}$, hence $\mathrm{E} \cong \mathrm{B}$
$
\Rightarrow x \approx \frac{B^2}{4 m c^2}
$
Question:13.23
$
F=\frac{1}{4 \pi \varepsilon_0} \frac{e^{\prime 2}}{r}
$
Answer:
The binding energy is the H-atom
$
E=\frac{m e^4}{\pi \varepsilon_0^2 h^2}=13.6 \mathrm{eV}
$
If proton and neutron had charge $e^{\prime}$ each and were governed by the same electrostatic force, then in the above equation we would need to replace electronic mass $m$ by the reduced mass $m^{\prime}$ of proton-neutron and the electronic charge e by e'.
$\begin{aligned} m^{\prime} & =\frac{M \times N}{M+N}=\frac{M}{2} \\ & =\frac{1836 \mathrm{~m}}{2}=918 \mathrm{~m}\end{aligned}$
Here, $M$ represents the mass of a neutron/proton
$$
\therefore \quad \text { Binding energy }=\frac{918 m\left(e^{\prime}\right)^4}{8 \varepsilon_0^2 h^2}=2.2 \mathrm{MeV}
$$
Dividing Eqs. (ii) and (i), we get
$$
\begin{aligned}
918\left(\frac{e^{\prime}}{e}\right)^4 & =\frac{2.2 \mathrm{MeV}}{13.6 \mathrm{eV}}=\frac{2.2 \times 10^6}{13.6} \\
\left(\frac{e^{\prime}}{e}\right)^4 & =\frac{2.2 \times 10^6}{13.6 \times 918}=176.21 \\
\frac{e^{\prime}}{e} & =(176.21)^{1 / 4}=3.64
\end{aligned}
$$
Question:13.24
Before the neutrino hypothesis, the beta decay process was thought to be the transition,
Answer:
Here, we have to observe the before and after cases of theAfter
pc= mnc2 – mpc2 = 938 MeV – 936 MeV = 2 MeV
Therefore, the Energy of the proton, Ep = 936 MeV
Therefore, the energy of the electron, Ee = 2.06 MeV
Question:13.25
|
t(h)
|
0
|
1
|
2
|
3
|
4
|
|
R(MBq)
|
100
|
35.36
|
12.51
|
4.42
|
1.56
|
(ii) Plot the graph of ln(R/R0) versus t and obtain the value of half-life from the graph.
Answer:

From the above graph, we can clearly understand that a 50% reduction of R has happened. Therefore, the value of the half-life is 40 mins.
In the graph, the value of t = OB, which is equal to 40 mins.
ii)

The above graph is ln(R/R0) versus t.
Here, the slope of the graph = –
Therefore, the value of
Now, Half-time(T1/2) = 0.693/
Question:13.26
Nuclei with magic no. of proton Z = 2, 8, 20, 28, 50, 52 and magic no. of neutrons N = 2, 8, 20, 28, 50, 82 and 126 are found to be very stable
(i) Verify this by calculating the proton separation energy Sp for Sn120(Z = 50) and Sb121 = (Z = 51). The proton separation energy for a nuclide is the minimum energy required to separate the least tightly bound proton from a nucleus of that nuclide. It is given by Sp = (MZ-1, N + MH – MZ,N)c2.
Answer:
(i) The proton separation energy is given by
$
\begin{aligned}
S_{p S n} & =\left(M_{119.70}+M_H-M_{120,70}\right) c^2 \\
& =(118.9058+1.0078252-119.902199) c^2 \\
& =0.0114362 c^2 \\
Similarly\ \ S_{p S p} & =\left(M_{120,70}+M_H-M_{121,70}\right) c^2 \\
& =(119.902199+1.0078252-120.903822) c^2 \\
& =0.0059912 c^2
\end{aligned}
$
Since $S_{p S n}>S_{p S b}, \mathrm{Sn}$ nucleus is more stable than the Sb nucleus.
(ii) The existence of magic numbers indicates that the shell structure of a nucleus is similar to the shell structure of an atom. This also explains the peaks in the binding energy/nucleon curve.
NCERT Exemplar Class 12 Physics Solutions Chapter 13 Nuclei: Important Concepts and Formulas
The atomic nucleus and its properties are the key to the mastery of nuclear physics and the phenomena associated with it. This chapter of NCERT Exemplar Class 12 Physics Chapter 13 - Nuclei is a collection of the key concepts and formulas, so that students can easily revise the critical concepts, like mass defect, binding energy, radioactivity, and nuclear reactions. With the help of these main concepts, the students will be able to enhance their problem-solving abilities and be able to solve the questions in the examinations (both numeric and theoretical ones) with much certainty.
1. Atomic Nucleus
- The dense central core of an atom contains protons and neutrons, holding almost all the mass of the atom while occupying a tiny fraction of its volume.
2. Mass Number (A)
- Total number of protons and neutrons in the nucleus.
- Formula: A = Z + N
Where Z = number of protons,
N = number of neutrons
3. Isotopes, Isobars, Isotones
- Isotopes: Same Z, different N
- Isobars: Same A, different Z
- Isotones: Same N, different Z
3. Binding Energy
- Energy required to disassemble a nucleus into its constituent protons and neutrons.
- Formula: $E_b=\Delta m c^2$
Where $\Delta m$ is the mass defect, $c$ is the speed of light
4. Mass Defect
- The difference between the mass of the constituent nucleons and the actual mass of the nucleus.
- Formula: $\Delta m=Z m_p+N m_n-m_{\text {nucleus }}$
5. Radioactivity
- Spontaneous decay of unstable nuclei with emission of α, β, or γ radiation.
Decay Law Formula: $N=N_0 e^{-\lambda t}$
Half-life: $T_{1 / 2}=\frac{0.693}{\lambda}$
Advantages of NCERT Exemplar Class 12 Physics Solutions Chapter 13 Nuclei
NCERT Exemplar Class 12 Physics Chapter 13 Solutions are meant to enable students to understand the basic aspects of nuclear physics in a format and systematic way. These will help in conceptual knowledge, solving numerical problems and good preparation for examinations in Class 12 Physics.
-
They explain complicated concepts such as binding energy, mass defect, radioactivity and nuclear reactions in steps and are hence easy to grasp.
-
The solutions are well updated to the latest NCERT and CBSE syllabus to give proper and relevant preparation of exams.
-
These solutions are practised to enable the students to know how to apply formulas properly and solve numerical problems without fear.
-
They enhance the clarity of concepts through connecting theoretical concepts to practical problems and questions.
-
The answers prepared by experts eliminate possible errors and enhance accuracy in both objective and descriptive questions.
-
These solutions can be used to revise quickly, strengthen formulas, definitions and significant ideas.
-
They are most helpful in both competitive exams, such as JEE and NEET, since nuclear physics is often a chapter of theory, as well as numerical questions.
NCERT Exemplar Class 12 Physics Solutions Chapter-Wise
NCERT Exemplar Class 12 Physics Solutions Chapter-Wise Links offer students quick and organised access to solutions for every chapter of the Class 12 Physics syllabus. These links make navigation easy, save study time, and support efficient revision and exam preparation.
|
Chapter 13 Nuclei |
NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class for 12 Physics
- NCERT Exemplar Class 12 Chemistry Solutions
- NCERT Exemplar Class 12 Mathematics Solutions
- NCERT Exemplar Class 12 Biology Solutions
Check Class 12 Physics Chapter-wise Solutions
Also, Read NCERT Solution subject-wise
- NCERT Solution for Class 12 Physics
- NCERT Solution for Class 12 Chemistry
- NCERT Solution for Class 12 Maths
- NCERT Solution for Class 12 Biology
Must read NCERT Notes subject-wise
- NCERT Notes for Class 12 Physics
- NCERT Notes for Class 12 Chemistry
- NCERT Notes for Class 12 Maths
- NCERT Notes for Class 12 Biology
Also, Check NCERT Books and NCERT Syllabus
Frequently Asked Questions (FAQs)
Absolutely. They also prove to be quite useful in exams such as JEE Main, JEE Advanced and NEET because nuclear physics subjects are quite often covered in theory and numerical sections.
One can use these NCERT exemplar Class 12 Physics solutions chapter 13 to understand the chapter in more detail so that it can be easy to solve questions in the exam.
Yes, the solutions offer stepwise ways of computing binding energy, decay rate, fission/fusion energy released and various other numbers, which simplifies and increases the accuracy of the problem-solving process.
Yes, the systematic structure, brief explanations, and worked-out examples are ideal to revise formulas, main concepts, and crucial definitions in a short time.
Yes, the nuclear power plants, medical imaging (MRI, PET), and radioactivity are examples that are mentioned in order to make students associate concepts with real-life examples.
This chapter will cover all the topics related to nucleus, its properties, nuclear energy, its mass, and radioactivity.
Questions related to CBSE Class 12th
On Question asked by student community
Dear Student,
Please go through the link to check 12th CBSE Chemistry question paper: https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers-class-12-chemistry
The Second Language English paper for the 2025-26 academic session (Summative Assessment 2 or SA-2 phase) focused on comprehension, grammar, and literature.
Exam Pattern & Marking Scheme (Class 8 English)
The second language English paper is divided into four main sections, totalling 80 Marks (for most state boards like Karnataka)
The Class 9th Social Science (SST) annual exam 2025-26 follows a standardised structure across CBSE and most State Boards. The exams for most of the boards are being held in February- March 2026. Check the marking scheme here for the SST exams
|
Section |
Type of Questions |
Number of Questions |
Marks |
Dear Student,
You can check Class 12 Physics CBSE Hindi medium PYQs here:
CBSE Class 12 Previous Year Question Papers With Solutions PDF Download
I am assuming the CBSE Board Physics Exam 2026 question paper. Download it here .
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
