NCERT Exemplar Class 12 Physics Solutions Chapter 11 Dual Nature of Radiation and Matter
Have you ever thought about how light can behave both like a wave and a particle? Or how tiny particles like electrons can show wave-like behavior? That is what Chapter 11: Dual Nature of Radiation and Matter in Class 12 Physics is all about. These ideas are the basics of modern physics. These NCERT exemplar solutions are prepared by expert faculty as per the latest syllabus
The NCERT Exemplar Solutions for Class 12 Physics Chapter 11 by Careers360 offer step-by-step answers to all exemplar problems. These include multiple-choice questions(MCQs), short answers and long form problems all aligned with the latest CBSE syllabus. Solving these NCERT exemplar for class 12 physics questions will help you understand important ideas better, boost your analytical thinking and prepare effectively for exams like CBSE Boards, JEE, and NEET.
This Story also Contains
- NCERT Exemplar Class 12 Physics Solutions Chapter 11: MCQI
- NCERT Exemplar Class 12 Physics Solutions Chapter 11: MCQII
- NCERT Exemplar Class 12 Physics Solutions Chapter 11: Very Short Answer
- NCERT Exemplar Class 12 Physics Solutions Chapter 11: Short Answer
- NCERT Exemplar Class 12 Physics Solutions Chapter 11: Long Answer
- Main Subtopics Covered in NCERT Exemplar Class 12 Physics Solutions Chapter 11 Dual Nature of Radiation and Matter
- NCERT Exemplar Class 12 Physics Solutions Chapter-Wise

NCERT Exemplar Class 12 Physics Solutions Chapter 11: MCQI
Question:1
Answer:
The answer is the option (d)Velocity of freely falling body after falling from a height H is given b
y $v= \sqrt{2gH}$
de-Broglie wavelength $\lambda$ is given by
$\lambda =\frac{h}{p}=\frac{h}{mv}\: or\: \frac{h}{m\sqrt{2gH}}$
where h, m and g are constant
So,
$\lambda \propto \frac{1}{\sqrt{H}}\Rightarrow \lambda \propto H^{-\frac{1}{2}}$
Question:2
The wavelength of a photon needed to remove a proton from a nucleus which is bound to the nucleus with 1 MeV energy is nearly
(a) 1.2 nm
(b) 1.2 x 10-3 nm
(c) 1.2 x 10-6 nm
(d). 1.2 x 10 nm
Answer:
The answer is the option (b)Energy of the photon should be equal to the binding energy of the proton
So, the energy of photon=
$1MeV=10^{6}\ast 1.6\ast 10^{-19}$
$\lambda=\frac{hc}{E}=6.63 \ast 10^{-34} \ast 3 \ast\frac{10^{8}}{1.6 \ast 10^{-13}}\\ =1.24 \ast 10^{-3}nm$
Question:3
Consider a beam of electrons (each electron with energy E0) incident on a metal surface kept in an evacuated chamber. Then
(a) no electrons will be emitted as only photons can emit electrons
(b) electrons can be emitted but all with an energy, E0
(c) electrons can be emitted with any energy, with a maximum of E0 – $\phi$ ($\phi$ is the work function)
(d) electrons can be emitted with any energy, with a maximum of E0
Answer:
The answer is the option (d) When a beam of electrons (each electron with energy E0 ) incident on a metal surface is kept in an evacuated chamber, energy of incident electrons will be transferred to the emitted electrons due to elastic collisions. A part of E0 of incident electrons is consumed against work function to emit the electrons. Thus, the maximum energy of emitted electrons is E0Question:4
Consider Fig. 11.7 in the NCERT textbook of physics for Class XII. Suppose the voltage applied to A is increased. The diffracted beam will have the maximum at a value of θ that
(a) will be larger than the earlier value
(b) will be the same as the earlier value
(c) will be less than the earlier value
(d) will depend on the target
Answer:
The answer is the option (c)The de-Broglie wavelength of diffracted beam of electrons is given by
$\lambda_{d}=\frac{12.27}{\sqrt{V}}A^{\circ}..............(i)$
If there is a maxima of the diffracted electrons at an angle $\theta$, then $2d \sin \theta =\lambda$
From equation (i), we get that on increasing the voltage, $\lambda_{d}$ decreases which in turn
decreases the value of $\theta$
Question:5
A proton, a neutron, an electron and an α-particle have the same energy. Then their de Broglie wavelengths compare as
$(a) \lambda _{p} = \lambda _{n} > \lambda _{e} > \lambda _{\alpha} $
$ (b) \lambda _{\alpha} < \lambda _{p} = \lambda _{n} < \lambda _{e}$
$ (c) \lambda _{e} < \lambda _{p} = \lambda _{n} > \lambda _{\alpha} $
$ (d) \lambda _{e} = \lambda _{p} = \lambda _{n} = \lambda _{\alpha}$
Answer:
The answer is the option (b)de-Broglie wavelength $\lambda$ is given by
$\begin{aligned} & \lambda=\frac{h}{p}=\frac{h}{m v} \\ & \quad 2 K=m v^2 \\ & 2 K m=m^2 v^2 \\ & 2 m K=p^2 \\ & p=\sqrt{2 m K} \\ & y_d=\frac{h}{p} \\ & y_d=\frac{h}{\sqrt{2 m K}} \\ & \text { Since } \lambda \propto \frac{1}{\sqrt{m}} \\ & M_a>m_p=m_n>m_e \\ & \lambda_a>\lambda_p=\lambda_n>\lambda_e\end{aligned}$
Question:6
An electron is moving with an initial velocity v = v0i and is in a magnetic field B = B0j. Then, its de-Broglie wavelength
(a) remains constant
(b) increases with time
(c) decreases with time
(d) increases and decreases periodically
Answer:
The answer is the option (a)$\vec{v}=v_{0}\hat{i}\: \: \: \: \: B=B_{0}\hat{j}$
Force on the elctron in perpendicular magnetic field B is
$F=-e\left (\vec{v} \times \vec{B} \right )$
$=-ev_{0}B_{0}k$
Therefore, the force is perpendicular to v and B both. The de-Broglie wavelength will not change.
Question:7
An electron (mass m) with an initial velocity v = v0i(v0 > 0) is in an electric field $E=E_{0}\hat{i}$ (E0 = constant > 0). Its de-Broglie wavelength at time t is given by
$(a) \frac{\lambda_{0}}{\left [ 1+\frac{eE_{0}}{m}\frac{t}{v_{0}} \right ]}$
$ (b)\lambda_{0}\left [ 1+\frac{eE_{0}t}{mv_{0}} \right ]$
$(c)\lambda_{0}$
$ (d)\lambda_{0}t$
Answer:
The answer is the option (a)Initial de-Broglie wavelength $\lambda$is given by
$\lambda_{0}=\frac{h}{p}=\frac{h}{mv_{0}}$
Force on electron=F=qE
$ma=eE_{0}\hat{i}$
$ a=\frac{eE}{m}\hat{i}$
Velocity of electron after time t is v=v0+at
$v=v_{0}i+\frac{eE}{m}i.t\\ v=\left [ v_{0}+\frac{eEt}{m} \right ]\hat{i}\\$
New de-Broglie wavelength
$\lambda=\frac{h}{mv}$
$\lambda=\frac{h}{m\left [ v_{0}+\frac{eE_{0}t}{m} \right ]}$
$ \lambda=\frac{\lambda_{0}}{\left [ 1+\frac{eE_{0}t}{mv_{0}} \right ]}\; \; \; \left ( Since \frac{h}{mv_{0}}=\lambda_{0} \right )$
Question:8
An electron (mass m) with an initial velocity $v=v_{0}\hat{i}$ is in an electric field $E=E_{0}\hat{i}$ IF $\lambda_{0}=\frac{h}{mv_{0}}$ its de-broglie wavelength at time t is given by
$(a)\lambda_{0}$
$(b)\lambda_{0}\sqrt{1+\frac{e^{2}E_{0}^{2}t^{0}}{m^{2}v_{0}^{2}}}$
$(c)\frac{\lambda_{0}}{\sqrt{1+\frac{e^{2}E_{0}^{2}t^{0}}{m^{2}v_{0}^{2}}}}$
$(d)\frac{\lambda_{0}}{\left (1+\frac{e^{2}E_{0}^{2}t^{0}}{m^{2}v_{0}^{2}} \right )}$
Answer:
The correct answer is option (c)$\frac{\lambda_{0}}{\sqrt{1+\frac{e^{2}E_{0}^{2}t^{0}}{m^{2}v_{0}^{2}}}}$
NCERT Exemplar Class 12 Physics Solutions Chapter 11: MCQII
Question:9
Relativistic corrections become necessary when the expression for the kinetic energy 1/2 mv2 , becomes comparable with mc2, where m is the mass of the particle. At what de Broglie wavelength will relativistic corrections become important for an electron?
$(a)\lambda=10nm$
$ (b)\lambda=10^{-1}$
$ (c)\lambda=10^{-4}$
$ (d)\lambda=10^{-6}\\$
Answer:
The answer is the option (c, d)The wavelength of de-Broglie wave is given by
$\lambda$ = h/p = h/mv
Here, h=6.6*10-34
and for electron, m=9*10-31 Kg
$\begin{aligned} & \quad v=\frac{h}{m \lambda} \\ & =6.6 * \frac{10^{-34}}{9 * 10^{-31} \lambda} \\ & =6.6 * \frac{10^{-34+31}}{9 \lambda}=0.73 * \frac{10^{-3}}{\lambda} \ldots \ldots(i) \\ & (a) \lambda=10 \mathrm{~nm}=10^{-8} \mathrm{~m} \\ & \text { using the formula }(i) v=7.3 * 10^4<3 * 10^8 \\ & (b) \lambda=10^{-1} \mathrm{~nm}=10^{-10} \mathrm{~m} \\ & v=7.3 * 10^6<10^8 \\ & (c) \lambda=10^{-4} \mathrm{~nm}=10^{-13} \mathrm{~m} \\ & v=7.3 * 10^9>10^8 \\ & (d) \lambda=10^{-6} \mathrm{~nm}=10^{-15} \mathrm{~m} \\ & v=7.3 * 10^{11}>10^8\end{aligned}$
Question:10
Two particles $A_{1}$ and $A_{2}$ of masses $m_{1}$, $m_{2}$ ($m_{1}$ >$ m_{2}$) have the same de Broglie wavelength. Then
(a) their momenta are the same
(b) their energies are the same
(c) energy of $A_1$ is less than the energy of $A_2$
(d) energy of $A_1$ is more than the energy of $A_2$
Answer:
The answer is the option (a. c)$\begin{aligned} & \quad \lambda=\frac{h}{p} \\ & p=\frac{h}{\lambda} \text { or } p \propto \frac{1}{\lambda} \\ & \frac{p_1}{p_2}=\frac{\lambda_2}{\lambda_1} \\ & \lambda_1=\lambda_2=\lambda(\text { given }) p_1=p_2 \text { vertifies }(\text { a }) \\ & E_a=\frac{1}{2} m v^2=\frac{\frac{1}{2} m^2 v^2}{m}=\frac{p^2}{2 m} \\ & E \propto \frac{1}{m}\left[\text { as } p_1=p_2\right] \\ & \frac{E_1}{E_2}=\frac{m_2}{m_1} \\ & \frac{E_1}{E_2}<1\left[m_1>m_2\right] E_2>E_1\end{aligned}$
Thus c is correct.
Question:11
The de Broglie wavelength of a photon is twice the de Broglie wavelength of an electron. The speed of the electron is ve = c/100. Then
(a) Ee/Ep = 10-4
(b) Ee/Ep = 10-2
(c) Pe/mec = 10-2
(d) Pe/mec = 10-4
Answer:
The answer is the option (b,c)The energy of a charged particle when accelerated through a potential difference V is:
$E=\frac{1}{2}mv^{2}=qV$
De-Broglie wavelength
$\begin{gathered}
\lambda=\frac{h}{p}=\frac{h}{\sqrt{2 m E}}=\frac{h}{\sqrt{2 m q V}} \\
\lambda_{\text {Neutron }}=\frac{0.286 * 10^{-10}}{\sqrt{E}} m=\frac{0.286 * 10^{-10}}{\sqrt{E}} A^{\circ}
\end{gathered}$
At ordinary temperature, the energy of thermal neutrons
$\begin{aligned}
& \quad E=k T \Rightarrow \lambda=\frac{h}{\sqrt{2 m k T}} \\
& \lambda_{\text {Thermal } \text { Neutron }}=\frac{30.83}{\sqrt{T}} A^{\circ} \\
& \text { Mass of electron }=m_e \\
& \text { Mass of photon }=m_p
\end{aligned}$
de Broglie wavelength of the electron
$\begin{aligned}
& \quad \lambda_e=\frac{h}{m_e v_e}=\frac{100 h}{m_e c} \\
& K E_e=\frac{1}{2} m_e v_e^2 \\
& m_e v_e^2=\sqrt{2 E_e m_e} \\
& \lambda_e=\frac{h}{m_e v_e}=\frac{h}{\sqrt{2 E_e m_e}} \\
& E_e=\frac{h^2}{2 \lambda_e^2 v_e}
\end{aligned}$
de Broglie wavelength of the proton is $\lambda_p$
$\begin{aligned}
& E_p=\frac{h c}{\lambda_p}=\frac{h c}{2 \lambda_e} \\
& \frac{E_p}{E_e}=\left(\frac{h c}{\lambda_e}\right) *\left(\frac{2 \lambda_e^2 m_e}{h}\right)=100 \\
& p_e=m_e v_e=m_e * \frac{c}{100} \\
& \frac{p_e}{m_e c}=10^{-2}
\end{aligned}$
Question:12
Photons absorbed in the matter are converted to heat. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice at 0°C to water at 0°C. Then, the time T taken for the conversion
(a) decreases with increasing n, with ν fixed
(b) decreases with n fixed, ν increasing
(c) remains constant with n and ν changing such that nν = constant
(d) increases when the product nν increases
Answer:
The answer is the option (a, b. c).Heat energy required to convert 1Kg of ice at $0^{\circ}$ C to water at $0^{\circ}$ C is E=mL
If n is the number of photons incident per second and time t is taken by radiation to
melt the ice at $0^{\circ}$C.
Then E=n hv t
$t=\frac{mL}{nhv}\\$
Or $t\propto \frac{1}{nv}\\$
[m, L and h are constants ] Hence, (a), (b) and (c) are correct.
If nv increases then T decreases ;so (d) isn't correct.
Question:13
A particle moves in a closed orbit around the origin, due to a force which is directed towards the origin. The de-BrOglie wavelength of the particle varies cyclically between two values $\lambda_{1},\lambda_{2}$ with $\lambda_{1}>\lambda_{2}$ Which of the following statements are true?
(a) The particle could be moving in a circular orbit with origin as centre
(b) The particle could be moving in an elliptic orbit with origin as its focus
(c) When the de-Broglie wavetength is $\lambda_{1}$ the particle is nearer the origin than when its value is $\lambda_{2}$
(d) When the de-Broglie wavelength is $\lambda_{2}$ the particle is nearer the origin than when its value is $\lambda_{1}$
Answer:
The answer is the option (b,d)A particle moving in an elliptic orbit with Origin as the centre can have its de-Broglie wavelength varying between $\lambda_{1}$ and $\lambda_{2}$ cyclically. When the de-Broglie wavelength is lower, the particle is closer to the origin.
$\lambda_{1}=\frac{h}{mv_{1}}\;\text{ and }\; \lambda_{2}=\frac{h}{mv_{2}}$
As,$\lambda_{1}>\lambda_{2} \implies v_{2}>v_{1}$
The particle will have a higher velocity closer to focus.
NCERT Exemplar Class 12 Physics Solutions Chapter 11: Very Short Answer
Question:14
Answer:
It is given that the proton and $\alpha$-particle are accelerated at thesame potential difference so their kinetic energies will be equal.
$\begin{aligned}
K_1&=K_2=K=q V \\
\lambda & =\frac{h}{\sqrt{2 m K}}=\frac{h}{\sqrt{2 m q V}} \\
\frac{\lambda_p}{\lambda_a} & =\sqrt{\frac{m_a q_a V_a}{m_p q_p V_p}} \\
m_a & =4 m_p ; q_a=2 e ; q_p=e ; V_p=V_a=V \\
\frac{\lambda_p}{\lambda_a} & =\sqrt{\frac{4 m_p 2 e V}{m_p e V}}=\sqrt{8} \\
\lambda_p & =\sqrt{8} \lambda_\alpha
\end{aligned}$
Question:15
(i) In the explanation of photoeletric effect, we assume one photon of frequency v collides with an electron and transfers its energy. This leads . to the equation for the maximum energy Emax of the emitted electron as $E_{max} = hv - \phi_{0}$
where $\phi_{0}$ is the work function of the metal. If an electron absorbs 2 photons (each of frequency v), what will be the maximum energy for the emitted electron?
(ii) Why is this fact (two photon absorption) not taken into consideration in our discussion of the stopping potential?
Answer:
(i) In the question, 2 photons transfer its energy to one electron as E=hvEe=Ep
hve=2hv
ve=2v
v is the frequency
The maximum energy of the emitted electron is Emax=hve - $\phi_{0}$
=h(2v) - $\phi_{0}$
=2hv - $\phi_{0}$
(ii) Due to the mass difference, the probability that the electron absorbs 2 photons is extremely low. Therefore the chances of such emission of electrons is close to negligible.
Question:16
Answer:
With an increase in the wavelength of the photon, there is a decrease in the frequency or increase in the energy of the photon. Let us consider 2 possible cases:- The photons having a smaller wavelength emit photons of smaller energy wherein some of it is consumed against work function. The law of conservation of energy is upheld in this case.
- Photons having a longer wavelength always emit photons with shorter wavelength. Photons having smaller energy can never emit photons of larger energy as some part of energy is consumed in work function of metal. So, it isn’t possible in stable materials.
Question:17
Do all the electrons that absorb a photon comes out as photo electrons?
Answer:
A common observation in the photoelectric effect is that most of the electrons knocked by photons are scattered into the metal by absorbing photons. In order to escape from the surface, the electron must absorb enough energy to overcome the positive ion attraction in the material of the surface.Therefore, not all the electrons that absorb a photon comes out from the metal surface.
Question:18
Answer:
Let EX and EV be the energies given by one photon in X-rays andvisible rays respectively.
Then EX=hvX and EV=hvV
Let nX and nV be the number of photons from x-rays and visible
light are of equal energies and they emit 100 W power.
$\begin{aligned} & \quad n_X h v_X=n_V h v_V \\ & \frac{n_X}{n_V}=\frac{v_V}{v_X}=\frac{\lambda_X}{\lambda_V} \\ & \frac{n_X}{n_V}=\frac{1}{500} \\ &n_X: n_V=1: 500\end{aligned}$
NCERT Exemplar Class 12 Physics Solutions Chapter 11: Short Answer
Question:19
Answer:
There is a transfer of momentum to the atoms on the metal surface when the photons strike the surface. This also results in a decrease in speed of the photons. Thus, the momentum of photons is transferred to the nucleus and electrons of the metal.Although the direction of emission of electrons is opposite to that of photons, the total momentum transferred by them is equal.
Question:20
Answer:
Let the maximum energies of emitted electrons be K1 and K2 when 600 nm and 400 nm visible light are used according to question$\begin{aligned} & \quad K_2=2 K_1 \\ & K_{\max }=h v-\phi=\frac{h c}{\lambda}-\phi \\ & K_1=\frac{h c}{\lambda_1}-\phi \\ & K_2=\frac{h c}{\lambda_2}-\phi=2 K_1 \\ & \frac{h c}{\lambda_2}-\phi=2\left[\frac{h c}{\lambda_1}-\phi\right]=\frac{2 h c}{\lambda_1}-2 \phi \\ & \phi=h c\left[\frac{2}{\lambda_1}-\frac{1}{\lambda_2}\right] \\ & =1240\left[\frac{2}{600}-\frac{1}{400}\right] e V \\ & =\frac{1240}{200}\left[\frac{2}{3}-\frac{1}{2}\right]=6.2\left(\frac{4-3}{6}\right) \\ & \text { Work function } \phi=\frac{6.2}{6}=1.03 \mathrm{eV}\end{aligned}$
Question:21
Answer:
As the electrons rotate in a circular path,$\Delta r=1nm=10^{-9}m$
$\begin{gathered}\Delta p=\frac{h}{\Delta X} \\ \Delta p=\left(\frac{331}{314}\right) * 10^{25} \\ E=\frac{1}{2} m v^2=\frac{\Delta p^2}{2 m} \\ E=3.8 * 10^{-2} \mathrm{eV}\end{gathered}$
Question:22
Answer:
$\begin{aligned} & I_A=I_B(\text { Given in the question }) \\ & n_A h v_A=n_B h v_B \\ & n_A=2 n_B[\text { Given }] \\ & 2 n_B v_A=n_B v_B \\ & 2 v_A=v_B\end{aligned}$So, the frequency of source B is twice the frequency of source A.
Question:23
Answer:
The de-Broglie wavelength is given by$\lambda=\frac{h}{p} \; or\; p=\frac{h}{\lambda}$
$p_{1}=\frac{h}{\lambda_{1}}, p_{2}=\frac{h}{\lambda_{2}}, p_{3}=\frac{h}{\lambda_{3}}$
By the law of conservation of momentum
$p_{1}+p_{2}= p_{3}$
$\frac{h}{\lambda_{1}}+\frac{h}{\lambda_{2}}=\frac{h}{\lambda_{3}}$ ($\lambda_{3}$ is the wavelength of particle C)
$\frac{h}{\lambda_{1}}+\frac{h}{\lambda_{2}}=\frac{h}{\lambda_{3}}\\ \frac{\lambda_{2}+\lambda_{1}}{\lambda_{1}\lambda_{2}}=\frac{h}{\lambda_{3}}$
Case I:When p1 and p2 are positive then $\lambda_{3}=\frac{\lambda_{1}\lambda_{2}}{\lambda_{1}+\lambda_{2}}$
Case II:When p1 and p2 both are negative $\lambda_{3}=\left |-\frac{\lambda_{1}\lambda_{2}}{\lambda_{1}+\lambda_{2}} \right |=\frac{\lambda_{1}\lambda_{2}}{\lambda_{1}+\lambda_{2}}$
Case III: $p_{A}>0,p_{B} <0$
$\frac{h}{\lambda_{3}}=\frac{h}{\lambda_{1}}-\frac{h}{\lambda_{2}}\; or\; \frac{h}{\lambda_{3}}=\frac{(\lambda_{2}-\lambda_{1})h}{\lambda_{1}\lambda_{2}}$
Case IV: $p_{A}<0,p_{B} >0$
$\frac{h}{\lambda_{3}}=\frac{h}{\lambda_{1}}-\frac{h}{\lambda_{2}}=\frac{\left (\lambda_{1}-\lambda_{2} \right )h}{\lambda_{1}\lambda_{2}}\\ \lambda_{3}=\frac{\lambda_{1}\lambda_{2}}{\lambda_{1}-\lambda_{2} }$
Question:24
Answer:
According to the Bragg’s law of diffraction, condition for the nth maxima is given by$\begin{aligned} & \quad 2 d \sin \theta=n \lambda \\ & n=1 \\ & \text { so } \lambda=2 d \sin \theta\left[\theta=30^{\circ}\right] \\ & =2 * 0.1 * 10^{-9} \sin 30^{\circ} \\ & p=\frac{h}{\lambda}=6.6 * \frac{10^{-34}}{10^{-10}}=6.6 * 10^{-24} \mathrm{~kg} \frac{\mathrm{~m}}{\mathrm{~s}} \\ & E=\frac{1}{2} m v^2=\frac{\frac{1}{2} m^2 v^2}{m} \\ & =\frac{p^2}{2 m}=\frac{6.6 * 6.6 * 10^{-48}}{2 * 1.6 * 10^{-27} * 1.6 * 10^{-19}} \mathrm{eV} \\ & =\frac{66 * 66 * 10^{-48+46}}{2 * 16 * 16}=33 * \frac{33}{126} * 10^{-2} \\ & =8.5 * 10^{-2}=0.085 \mathrm{eV}\end{aligned}$
NCERT Exemplar Class 12 Physics Solutions Chapter 11: Long Answer
Question:25
Answer:
Area (A)=10-2 cm2=10-4m2thickness (t)=10-3m
photo current (i)=100*10-6=10-4A
Intensity (I)=100 W/m2
wavelength ($\lambda$)=660 nm=660*10-9m
density (ρNa)=0.97 kg/m3
Avogadro's Number=6*1026Kg atom
Volume of target (V)=A*d=10-4*10-3=10-7m3
$M_{Na}$ (6*1026 atoms of Na)=23 kg
$V_{Na}$ (6*1026 atoms of Na) =$\frac{23}{0.97}m^{3}$
NNa=$\frac{10^{-7}}{\frac{\frac{23}{0.97}}{6*10^{26}}}=2.53*10^{18}$
Energy of photon=$\frac{hc}{\lambda}$
Total Energy=$\frac{nhc}{\lambda}=IA$
$\begin{aligned} & n=\frac{n A \lambda}{h c}=3.3 * 10^{16} \\ & N=n * N_{N a} * p \\ & i=N e=n * N_{N a} * p * e \\ & 10^{-4}=33 * 10^{16} * 2.53 * 10^{18} * 1.6 * 10^{-19} * p \\ & p=7.48 * 10^{-21}\end{aligned}$
Question:26
Answer:

Let us consider the above-given figure wherein an electron is displaced slowly by a distance x by the means of an external force which is given by
$F=\frac{q^{2}}{4*4\pi\varepsilon_{0}d^{2}}\; where \;d=0.1nm=10^{-10}m$
Now work done by an external agency in taking the electron from distance d to infinity is
$\begin{aligned} W&=\int_d^{\infty} F_x d x=\int_d^{\infty} \frac{q^2 d x}{4 * 4 \pi \varepsilon_0} * \frac{1}{x^2} \\ &= \frac{q^2 d x}{4 * 4 \pi \varepsilon_0}\left(\frac{1}{d}\right)=\frac{\left(1.6 * 10^{-19}\right)^2}{1.6 * 10^{-19}} * \frac{\left(9 * 10^9\right)}{4 * 10^{-10}}=3.6 \mathrm{eV}\end{aligned}$
Question:27
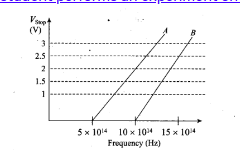
(i) Which material A or B has a higher work function?
(ii)Given the electric charge of an electron = 1.6 x 10-19C, find the value of h obtained from the experiment for both A and B. Comment on whether it is consistent with Einstein's theory.
Answer:
We know that $\phi_{0}=hv_{0}$ where v0=Threshold frequency$\frac{\phi_{0A}}{\phi_{0B}}=\frac{hv_{0A}}{hv_{0B}}=\frac{5*10^{14}}{10*10^{14}}=\frac{1}{2}$
So, $\phi_{0B}=2\phi_{0A}$
(ii) By the differentiation of potential
$V=\frac{W}{Q}$
hv=eV
Differentiating both the sides we get h.dv=edV
$h=\frac{edV}{dv}$
Using this formula
For metal A, h=6*10-34Js….(i)
For metal B, h=0.8*10-33…(ii)
Since the value of plank'sconstant is not equal for both the experimental graphs, the experiment inconsistent with Einstein's theory. But the values are very close to 6.6*10-34Js due to experimental limitation and hence can be considered consistent with Einstein theory.
Question:28
Answer:
When the collision is elastic, law of conservation of momentum is followed.$\begin{aligned} & m_A v+m_B 0=m_A v_1+m_B v_2 \\ & m_A\left(v-v_1\right)=m_B v_2 \\ & \frac{1}{2} m_A v_2=\frac{1}{2} m_A v_1^2+\frac{1}{2} m_B v_2^2 \\ & v_1=\frac{m_A-m_B}{\left(m_A-m_B\right)} v \quad v_2=\frac{2 m_A}{\left(m_A-m_B\right)} v \\ & \lambda_{\text {initial }}=\frac{h}{m_A v} \\ & \lambda_{\text {final }}=\frac{h}{m_A v_1} \\ & \Delta \lambda=\lambda_{\text {final }}-\lambda_{\text {initial }}=\frac{h}{m_A v}\left[\frac{m_A+m_B}{m_A-m_B}-1\right]\end{aligned}$
Question:29
Consider a 20 W bulb emitting light of wavelength 5000 $A^{\circ}$ and shining on a metal surface kept at a distance 2m. Assume that the metal surface has work function of 2 eV and that each atom on the metal surface can be treated as a circular disk of radius 1.5 $A^{\circ}$ .
(i) Estimate no. of photons emitted by the bulb per second. [Assume no other losses]
(ii) Will there be photoelectric emission?
(iii) How much time would be required by the atomic disk to receive energy equal to work function (2 eV)?
(iv) How many photons would atomic disk receive within time duration calculated in (iii) above?
(v) Can you explain how photoelectric effect was observed instantaneously?
Answer:
Given that $\mathrm{P}=20 \mathrm{~W}$
$\begin{aligned}
& \lambda=5000 A^{\circ}=5000 * 10^{-10} \mathrm{~m} \\
& d=2 \mathrm{~m} \\
& \phi=2 \mathrm{ev} \\
& r=1.5 * 10^{10} \mathrm{~m}
\end{aligned}$
i) Number of photon emitted by bulb per second= $\frac{p \lambda}{h c}=5 * 10^{19} \mathrm{sec}$
ii Energy of the incident photon= $\frac{h c}{\lambda}=2.48 \mathrm{eV}$
iii Time required by the atomic disk to receive energy is=28.4 sec
Let the time spent be $\Delta T$
$E=P * A * \Delta t=P * \pi r^2 \Delta t$
Energy transferred by the bulb in full solid angle to atoms= $ 4 \pi d^2 \phi$
$\begin{aligned}
& p * \pi r^2 \Delta t=4 \pi d^2 \phi \\
&\Delta t=\frac{4 d^2 \phi}{P r^2}=\frac{4 * 2 * 2 * 2 * 1.6 * 10^{-10}}{20 * 1.5 * 1.5 * 10^{-10} * 10^{-10}} \sec =\frac{12.8 * 10^{-19+20}}{5 * 2.25}
\end{aligned}$
(iv) Number of photons received by the atomic disk $=\mathrm{N}$
$\begin{aligned}
\frac{n_1 \pi r^2 \Delta t}{4 \pi d^2}&=\frac{n_1 r^2 \Delta t}{4 d^2} \\
&= \frac{5 * 10^{19} * 1.5 * 1.5 * 10^{-20} * 11.4}{4 * 2 * 2} \\
&\approx 0.80 \cong 1 \text { photon per atom }
\end{aligned}$
(iv) We say that photoelectric emission is instantaneous as it involves a collision between the incident photon and free electron lasting for a very short span of time, say $\leq 10^{-9} \mathrm{sec}$
Main Subtopics Covered in NCERT Exemplar Class 12 Physics Solutions Chapter 11 Dual Nature of Radiation and Matter
- Introduction
- Electron emission
- Photoelectric effect
- Hertz’s observations
- Hallwachs’ and Lenard’s observations
- Experimental study of photoelectric effect
- Effect of intensity of light on Photo current
- Effect of potential on photoelectric current
- Effect of frequency of incident radiation on stopping potential
- Photoelectric effect and wave theory of light
- Einstein’s photoelectric equation: Energy quantum of radiation
- Particle nature of light: The photon
- Wave nature of matter
- Davisson and Germer Experiment
NCERT Exemplar Class 12 Physics Solutions Chapter-Wise
|
Chapter 11 Dual Nature of Radiation and Matter |
NCERT Exemplar Class 12 Solutions
- NCERT Exemplar Class for 12 Physics
- NCERT Exemplar Class 12 Chemistry Solutions
- NCERT Exemplar Class 12 Mathematics Solutions
- NCERT Exemplar Class 12 Biology Solutions
Check Class 12 Physics Chapter-wise Solutions
Also, Read NCERT Solution subject-wise
- NCERT Solution for Class 12 Physics
- NCERT Solution for Class 12 Chemistry
- NCERT Solution for Class 12 Maths
- NCERT Solution for Class 12 Biology
Must read NCERT Notes subject-wise
- NCERT Notes for Class 12 Physics
- NCERT Notes for Class 12 Chemistry
- NCERT Notes for Class 12 Maths
- NCERT Notes for Class 12 Biology
Also, Check NCERT Books and NCERT Syllabus
Frequently Asked Questions (FAQs)
These questions are solved keeping in mind the CBSE pattern and how the marks are divided as per the steps.
Yes, one can download these questions so that one uses the solutions offline.
Try to understand the NCERT exemplar solutions for Class 12 Physics chapter 11 and connect them to the topics that one has studied in the chapter.
One will learn in detail about wave theory of light, particle nature of light, photoelectric effect, Davisson and Germer experiment, etc.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Hello,
Here is your Final Date Sheet Class 12 CBSE Board 2026 . I am providing you the link. Kindly open and check it out.
https://school.careers360.com/boards/cbse/cbse-class-12-date-sheet-2026
I hope it will help you. For any further query please let me know.
Thank you.
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
