NCERT Exemplar Class 11 Chemistry Solutions Chapter 13 Hydrocarbons
Do you know why petrol, diesel, LPG and natural gas belong to the same family of compounds but serve different purposes? The answer to this question lies in NCERT Exemplar Class 11 Chemistry Chapter 13 Hydrocarbons, the fundamental compounds of organic chemistry that are taking the world forward because they are the key ingredients of fuels. They are building blocks of fuels, plastics, and many essential chemicals. They consist of carbon and hydrogen atoms, which are further classified into alkanes, alkenes, and alkynes.
This Story also Contains
- NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: MCQ (Type 1)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: MCQ (Type 2)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: Short Answer Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: Matching Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: Assertion and Reason Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: Long Answer Type
- Class 11 Chemistry NCERT Chapter Hydrocarbons: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 13 Hydrocarbons
- Major Topics Of NCERT Exemplar Class 11 Chemistry Chapter Hydrocarbons
- Advantages of Using Chemistry Class 11 NCERT Exemplar Chapter 13 Hydrocarbons Solutions
- NCERT Exemplar Class 11 Chemistry Solutions Chapter-Wise
- NCERT Solutions for Class 11 Chemistry Chapter-wise
- NCERT Exemplar Class 11 Solutions
- NCERT Solution subject-wise
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus

In this article, students will learn about the nomenclature, preparation methods, properties, and reactions of hydrocarbons. They will also understand and learn the concepts of aromaticity, hydrogenation, nitration, and sulfonation, as well as all the reactions happening in aromatic hydrocarbons. To help students, our subject experts have designed comprehensive NCERT Exemplar Class 11 Chemistry Solutions offering clear explanations and conceptual clarity. In this article, higher order thinking skills are also added to enhance your understanding of the concepts. These NCERT exemplar solutions are beneficial for competitive exams like JEE and NEET.
NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: MCQ (Type 1)
The NCERT Exemplar Solutions Class 11 Chemistry Chapter 13 Hydrocarbons MCQ questions are given below. This chapter is very important from exam point of view. Students can also check NCERT Solutions to all questions chapter-wise.
Question 1. Arrange the following in decreasing order of their boiling points.
(A) n–butane
(B) 2–methylbutane
(C) n-pentane
(D) 2,2–dimethylpropane
(i) A > B > C > D
(ii) B > C > D > A
(iii) D > C > B > A
(iv) C > B > D > A
Answer:
The answer is the option (iv) C > B > D > A
Explanation: We know that Boiling point α molar mass & Boiling point is α surface area.
It means that the boiling point will decrease on branching (surface area decreases on branching). Therefore, the highest boiling point is that of n-pentane and the lowest is of n-butane. The other two options have branches; therefore, 2-methyl butane has a higher boiling point than 2,2-dimethyl propane.
Question 2. Arrange the halogens $F_{2}, Cl_{2}, Br_{2}, I_{2}$, in order of their increasing reactivity with alkanes.
$(i) I_{2}< Br_{2} < Cl_{2} < F_{2}$
$(ii) Br_{2} < Cl_{2} < F_{2} < I_{2}$
$(iii) F_{2} < Cl_{2} < Br_{2} < I_{2}$
$(iv) Br_{2} < I_{2} < Cl_{2} < F_{2}$
Answer:
The answer is the option $(i) I_{2}< Br_{2} < Cl_{2} < F_{2}$
Explanation: Since electronegativity of halogens decreases down the group, Fluorine is the most electronegative. As electronegativity of Fluorine decreases their reactivity with alkanes also decreases. Thus, $F_{2}$ is highly reactive, whereas $I_{2}$ is the least reactive.
Question 3. The increasing order of reduction of alkyl halides with zinc and dilute HCl is
(i) R–Cl < R–I < R–Br
(ii) R–Cl < R–Br < R–I
(iii) R–I < R–Br < R–Cl
(iv) R–Br < R–I < R–Cl
Answer:
The answer is the option (ii) R-Cl < R-Br < R-I
Explanation: We know that reactivity of halogen decreases down the group; therefore, reduction of alkyl halides with Zn/HCl follow reverse order.
Reactivity of reduction $\alpha$ 1bond strength of C-X
$Reactivity of reduction \propto \frac{1}{bond strength C-X}$
It also depends on the size of the halogen. Therefore, we can say that to increase the reactivity; the bond strength must be reduced.
Question 4. The correct IUPAC name of the following alkane is
(i) 3,6 – Diethyl – 2 – methyloctane
(ii) 5 – Isopropyl – 3 – ethyloctane
(iii) 3 – Ethyl – 5 – isopropyloctane
(iv) 3 – Isopropyl – 6 – ethyloctane
Answer:
The answer is the option (i) 3,6-Diethyl-2-methyloctane
Explanation: Since the alkane has 8 carbon atoms, it is octane, viz., the longest chain. There is a methyl group on carbon 2, ethyl groups on carbon 3 & 6. Since 2 ethyl groups are present, it will be diethyl, and alphabetically it comes before methyl. The lowest sum rule is followed by side chains present on the carbon atoms 2, 3 & 6. Hence, the option (i).
Question 5. The addition of HBr to 1-butene gives a mixture of products A, B and C

(C)
The mixture consists of
(i) A and B as major and C as minor products
(ii) B as major, A and C as minor products
(iii) B as minor, A and C as major products
(iv) A and B as minor and C as major products
Answer:
The answer is option (i) A and B as major and C as minor products.
Explanation: According to Markovnikov’s rule, the major product is 2-Bromobutane, and the minor product is I-Bromobutane. Butane-1 is unsymmetrical. 2-Bromobutane has chiral carbon and hence exists in two enantiomers.
Thus, it is clear that the major products are A & B while the minor product is C.
Question 6. Which of the following will not show geometrical isomerism ?
Answer:
The answer is the option
Explanation: Alkenes show geometrical isomerism where the double-bonded carbons must have different atoms or groups.
Therefore, the structure in option (iv) does not show geometrical isomerism since the double-bonded carbons have 3 same groups and one different group.
Question7. Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
(i) HCl > HBr > HI
(ii) HBr > HI > HCl
(iii) HI > HBr > HCl
(iv) HCl > HI > HBr
Answer:
The answer is the option (iii) HI > HBr > HCl
Explanation: The factors that affect the reactivity of hydrogen halides are bond strength and bond dissociation energy. We know that, in halogen halides the size of the halogen atom increases, the bond dissociation energy, as well as bond strength, decreases.
Hence, the option (iii) is the correct order.
Question 8. Arrange the following carbanions in order of their decreasing stability.
(i) A > B > C
(ii) B > A > C
(iii) C > B > A
(iv) C > A > B
Answer:
The answer is option (ii) B > A > C
Explanation: CH3 group has +I effect, which decreases the stability of the carbon anion and in C this effect direct to the negatively charged carbon. +I effect is also present in A, but there is more distant from the negatively charged carbon, and in B there is no +I effect
Question 9. Arrange the following alkyl halides in decreasing order of the rate of $\beta$ – elimination reaction with alcoholic KOH.

(i) A > B > C
(ii) C > B > A
(iii) B > C > A
(iv) A > C > B
Answer:
The answer is the option (iv) A > C > B
Explanation: When alkyl halides are heated with alc. KOH, an alkene is formed by eliminating one molecule of halogen acid. Hydrogen is eliminated from the beta carbon atom. Order of reactivity is $3^{\circ} > 2^{\circ} > 1^{\circ}$, hence option (iv). The rate of reaction is determined by the nature of alkyl groups.
Question10. Which of the following reactions of methane is incomplete combustion:
$(i)2CH_{4}+O_{2}\xrightarrow[Cu/523]{K/100atm}2CH_{3}OH$
$(ii)CH_{4}+O_{2}\overset{Mo_{2}O_{3}}{\rightarrow}HCHO+H_{2}O$
$(iii)CH_{4}+O_{2}\rightarrow C(s)+2H_{2}O(l)$
$(iv)CH_{4}+2O_{2}\rightarrow CO_{2}(g)+2H_{2}O(l)$
Answer:
The answer is the option $(iii)CH_{4}+O_{2}\rightarrow C(s)+2H_{2}O(l)$
Explanation: Insufficient supply of air or oxygen leads to incomplete combustion and carbon black is formed.
Hence, $CH_{4}+O_{2}\rightarrow C(s)+2H_{2}O(l)$
NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: MCQ (Type 2)
The Chapter 13 Hydrocarbons NCERT Exemplar MCQ questions are given below. The chapter is vast yet easy to learn. These solutions provide thorough explanations of the problems, helping students to apply theoretical concepts effectively.
Question 11. Some oxidation reactions of methane are given below. Which of them is/are controlled oxidation reactions?
$(i)CH_{4}+2O_{2}\rightarrow CO_{2}(g)+2H_{2}O(l)$
$(ii)CH_{4}+O_{2}\rightarrow C(s)+2H_{2}O(l)$
$(iii)CH_{4}+O_{2}\overset{M_{2}O_{3}}{\rightarrow}HCHO+H_{2}O$
$(iv)2CH_{4}+O_{2}\xrightarrow[Cu/523]{K/100atm}2CH_{3}OH$
Answer:
The answer is the option $(iii)CH_{4}+O_{2}\overset{M_{2}O_{3}}{\rightarrow}HCHO+H_{2}O$ and
$(iv)2CH_{4}+O_{2}\xrightarrow[Cu/523]{K/100atm}2CH_{3}OH$
Explanation: When combustion takes place in an insufficient supply of oxygen or air, water and carbon black are formed. Therefore, alkanes are heated with a regular supply of oxygen or in a controlled way to give HCHO and $CH_{3}OH$.
Question 12. Which of the following alkenes on ozonolysis give a mixture of ketones only?
Answer:
The answer is the option (iii) and (iv)
Explanation: Depending on groups or atoms attached, alkenes give two molecules of carbonyl compounds on ozonolysis. Ketones are formed if the double-bonded carbon atoms have alkyl groups.
Question 13. Which are the correct IUPAC names of the following compound?
(i) 5– Butyl – 4– isopropyldecane
(ii) 5– Ethyl – 4– propyldecane
(iii) 5– sec-Butyl – 4– iso-propyldecane
(iv) 4–(1-methylethyl)– 5 – (1-methylpropyl)-decane
Answer:
The answer is the option (iii) 5-sec-Butyl-4-iso-propyldecane & (iv) 4-(1-methylethyl)-5-(1-mrthylpropyl)-decane
Explanation: The longest carbon chain has 10 atoms, hence ‘decane’. According to the lowest sum rule, side chains are on 4th(isopropyl group) & 5th(sec-butyl group) carbon atoms. Isopropyl is called 1-Methylethyl group and sec-butyl is called 1-Methylpropyl group in IUPAC. Hence the correct names are in option (iii) & (iv).
Question14. Which are the correct IUPAC names of the following compound?
(i) 5 – (2′, 2′–Dimethylpropyl)-decane
(ii) 4 – Butyl – 2,2– dimethylnonane
(iii) 2,2– Dimethyl – 4– pentyloctane
(iv) 5 – neo-Pentyl decane
Answer:
The answer is the option (i) 5-(2’,2’-Dimethylpropyl)-decane & (iv) 5-neo-Pentyldecane
Explanation: The longest carbon chain has 10 carbon atoms, hence ‘decane’. Sidechain is present on 5th carbon atom, viz., neo-Pentyl or 2’,2’-Dimethylpropyl according to IUPAC. Hence option (i) & (iv).
Question15. For an electrophilic substitution reaction, the presence of a halogen atom in the benzene ring _______.
(i) deactivates the ring by inductive effect
(ii) deactivates the ring by resonance
(iii) increases the charge density at ortho and para position relative to meta position by resonance
(iv) directs the incoming electrophile to meta position by increasing the charge density relative to ortho and para position.
Answer:
The answer is the option (i) deactivates the ring by inducive effect & (iii) increases the charge density at ortho and para position relative to meta position by resonance.
Explanation: Presence of halogen atom on benzene ring direct electrophile on ortho and para positions and increases electron density on ortho and para positions. It also shows the +R effect. Due to -I effect halogen pulls an electron from benzene since they are more electronegative and decreases electron density. The electron density on ortho and para position is greater than meta-position due to resonance.
Question 16. In an electrophilic substitution reaction of nitrobenzene, the presence of nitro group ________.
(i) deactivates the ring by inductive effect.
(ii) activates the ring by inductive effect.
(iii) decreases the charge density at ortho and para position of the ring relative to meta position by resonance.
(iv) increases the charge density at meta position relative to the ortho and para positions of the ring by resonance.
Answer:
The answer is the option (i) deactivates the ring by inducive effect & (iii) decreases the charge density at ortho and para position of the ring relative to meta position by resonance.
Explanation: Meta position is attacked by an electrophile. Due to the -I effect nitro group deactivates the benzene ring and decreases electron density at ortho and para positions than meta position.
Question 17. Which of the following are correct?
(i) $CH_{3}-O-CH_{2}^{+}$ is more stable than $CH_{3}-CH_{2}^{+}$
(ii) $\left (CH_{3} \right )_{2}CH^{+}$ less table than $CH_{3}-CH_{2}-CH_{2}^{+}$
(iii) $CH_{2}=CH-CH_{2}^{+}$ is more stable than $CH_{3}-CH_{2}-CH_{2}^{+}$
(iv) $CH_{2}=CH^{+}$ is more stable than $CH_{3}-CH_{2}^{+}$
Answer:
The answer is the option $(i)CH_{3}-O-CH_{2}^{+}$ is more stable than $CH_{3}-CH_{2}^{+}$ & (iii) $CH_{2}=CH-CH_{2}^{+}$ is more stable than $CH_{3}-CH_{2}-CH_{2}^{+}$.
Explanation: $(i)CH_{3}-O-CH_{2}^{+}$is more stable than $CH_{3}-CH_{2}^{+}$ because +R effect of $CH_{3}-O$ is greater than $-CH_{3}$ (stability of carbocation increases due to the +R effect).
(iii) We know that the resonance effect is stronger than +I effect. Here, $CH_{2}=CH-CH_{2}^{+} \leftrightarrow ^+CH_{3}-CH=CH_{2}$ is stabilised by resonance effect; whereas $CH_{3}-CH_{2}-CH_{2}^{+}$ is stabilised by +I effect only . Hence, $CH_{2}=CH-CH_{2}^{+}$ is more stable than $CH_{3}-CH_{2}-CH_{2}^{+}$.
Question 18. Four structures are given in options (i) to (iv). Examine them and select the aromatic structures.
(i)
(ii)
(iii)
(iv)
Answer:
The answer is the option (i) & (iii)
Explanation: The following conditions should be fulfilled by a compound in order to become aromatic-
-
$\pi$ electrons in the ring should be completely delocalised.
-
Planarity
-
Huckel Rule should be followed, viz., presence of $(4n + 2) \pi$ electrons in the ring.
Now, analysing the given options:
(i) 
$\pi$ electrons = 2
(ii) 
n = not an integer
(iii) 
$\pi$electrons = (4n + 2) = 6 in each ring
(iv) 
n = not an integer
Hence, option (i) and (iii) are aromatic structure.
Question 19. The molecules having dipole moment are __________.
(i) 2,2-Dimethylpropane
(ii) trans-Pent-2-ene
(iii) cis-Hex-3-ene
(iv) 2, 2, 3, 3 – Tetramethylbutane
Answer:
The answer is the option (ii) trans-Pent-2-ene & (iii) cis-Hex-3-ene
Explanation: (ii) Because of different groups attached, trans-Pent-2-ene shows net dipole moment.
(iii) Here, both $C_2H_5$ groups are inclined to each other at an angle of $60^{\circ}$ and hence has a resultant dipole moment. Thus, cis-Hex-3-ene shows a dipole moment.
NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: Short Answer Type
The Class 11 NCERT Exemplar Chemistry Chapter 13 Hydrocarbons short answer type questions are given below. This chapter is vast yet easy to learn chapter.
Question 20. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain.
Answer:
Both Alkenes and arenes are unsaturated and electron rich. In order to give a highly stable and saturated product, Alkenes undergo addition reaction to give more stable saturated product. Under this reaction hybridization transforms from sp2 to sp3.
Arenes are stabilized by resonance with delocalization of π electrons. On addition reaction to the double bond of arene, a product is obtained which is not resonance stabilized whereas on substitution the resonance stability of arene is maintained. Thus, arenes prefer to undergo substitution reaction while alkenes prefer to undergo addition reaction.
Question 21. Alkynes on reduction with sodium in liquid ammonia form trans alkenes. Will the butene thus formed on reduction of 2-butyne show the geometrical isomerism?
Answer:
Butene-2, where either both the methyl groups are on the same side or opposite side to show geometrical isomers, is formed on the reduction of 2-Butyne.
$H_{3}C-C\equiv C-CH_{3}\overset{Na/liq.NH_{3}}{\rightarrow}$
Question 22. Rotation around carbon-carbon single bond of ethane is not completely free. Justify the statement.
Answer:
(i)Alkanes have a carbon-carbon sigma bond where the distribution of electron of sigma molecular orbit is symmetrical around the internuclear axis of C-C bond, viz., not distributed due to rotation about its axis.
(ii) Hence, C-C single bond is permitted for free rotation which results in different spatial arrangements of atoms in space which can change into one another.
Such spatial arrangements of atoms are called conformations or rotamers or conformers.
(iii) Due to rotation around C-C bonds alkenes have infinite no. of conformations. However, rotation around C-C single bond is not completely free and is hindered by small energy barrier of1-20 kJ mol-1 because of weak repulsive interaction between adjacent bonds. It is called a torsional strain.
Question 23. Draw Newman and Sawhorse projections for the eclipsed and staggered conformations of ethane. Which of these conformations is more stable and why?
Answer:
(i) Sawhorse projections of ethane
(ii) Newman projections of ethane
Stability of conformation:
(i) There are minimum repulsive forces, minimum energy and maximum stability in the staggered form of ethane. Here, the electrons clouds of carbon-hydrogen bonds are as far as possible.
(ii) the electron clouds come closer when the staggered form changes to the eclipsed form. It results in increased electron cloud repulsions. Here, molecules will have more energy and lesser stability.
Therefore, the staggered form is more stable.
Reactivity of hydrogen halides depends on the dissociation enthalpy of H-X. The order of reactivity of hydrogen halides is HI > HBr > HCl. They add up to alkanes to form alkyl halides.
Bond enthalpy of HI<HBr<HCl, hence reactivity in reverse order is:
HCl < HBr < HI
Question 25. What will be the product obtained as a result of the following reaction and why?

Answer:
When alkylation of Friedel-Crafts takes place with a higher alkyl halide in which primary carbocation is formed first and then converted into secondary carbocation, which is more stable. It is formed by rearrangement; therefore, the product is isopropyl benzene.
Primary carbocation- $CH_{3}-CH_{2}-CH_{2}Cl+AlCl_{3}\rightarrow AlCl_{4}^{+}+CH_{3}-CH_{2}-CH_{2}^{+}$
Secondary carbocation- $CH_{3}-CH_{2}-CH_{2}^{+}\rightarrow CH_{3}-CH^{+}-CH_{3}$

Question 26. How will you convert benzene into
(i) p – nitrobromobenzene
(ii) m – nitrobromobenzene
Answer:
(i) Converting benzene into p-nitro bromobenzene:
$C_{6}H_{6} + Br_{2}$ (In presence of anhyd. $FeBr_{3}$) → $C_{6}H_{5} Br_{2}+HBr$
$C_{6}H_{5}Br + Conc. HNO_{3} + Conc. H_{2}SO_{4}$ → p-nitrobromobenzene
(ii) Converting benzene into m-nitrobromobenzene:
$C_{6}H_{6} + Conc. HNO_{3} + Conc. H_{2}SO_{4}$ → $C_{6}H_{5}NO_{2}$
$C_{6}H_{5}NO_{2} + Br$ (In presence of anhyd. $FeBr_{3}$) → m-nitrobromobenzene
Question 27. Arrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile. Give reason.
Answer:
The methoxy group$(-OCH_{3})$makes anisole more reactive than benzene because it is an electron-releasing group and increases the electron density of the benzene ring due to the +R effect.
Cl group is less reactive than methoxybenzene as it gives +R and -I effect. And, Chlorobenzene is more reactive than $-NO_{2}$ group since the $-NO_{2}$ gives -I and -R effect.
Question 28. Despite their – I effect, halogens are o- and p-directing in haloarenes. Explain.
Answer:
Halogens are ortho and para-directing because they have +R and -I effect. Now, halogens present on the benzene ring has these effects in which the -I effect deactivates the ring and +R effect increases the electron density on ortho and para positions.
Answer:
The benzene ring deactivated and the electron density decreases on the ortho and para positions as compared to the meta positions due to the presence of the nitro group which has -I and -R effects.
Question 30. Suggest a route for the preparation of nitrobenzene starting from acetylene?
Answer:
Ethylene undergoes cyclic polymerisation when passed through red hot iron tube at 873 K and gives benzene from which
nitrobenzene can be obtained through nitration.
Question 31. Predict the major product (s) of the following reactions and explain their formation.
$H_{3}C-CH=CH_{2}\xrightarrow[HBr]{(Pb-CO-O)_{2}}$
$H_{3}C-CH=CH_{2}\overset{HBr}{\rightarrow}$
Answer:
(i) $H_{3}C-CH=CH_{2}\xrightarrow[HBr]{(Pb-CO-O)_{2}}$ $H_{3}C-CH_{2}-CH_{2}-Br$
Step 1: Homolysis of peroxide for forming free radicals
Step 2: Formation of Bromine free radical
$C_6H_5 + H-Br \rightarrow ^\cdot C_6H_6 + Br^\cdot$
Step 3: Hydrogen bromide reaction with an alkyl radical
(ii)
Electrophiles can be defined as the electron seeking species or electron-deficient species. They can be either positively charged or neutral.
Therefore, the following species are electrophiles-
(iii) $Cl^{.}$
$(iv)Cl_{2}C:$
$(v)(H_{3}C)_{3}C^{+}$
electron rich species are called nucleophiles; they can be either negatively charged or neutral.
The following species are nucleophiles-
$(i)H_{3}CO^{-}$
(ii)
$(vi)Br^{-}$
$(vii)H_{3}COH$
$(viii)R-NH-R$
Answer:
There are 9 primary, 2 secondary and 1 tertiary hydrogen atoms in 2-methylbutane and the relative reactivity of $1^{\circ}, 2^{\circ}, 3^{\circ}$hydrogen atoms towards chlorination is 1:3:8:5.
The relative amount of product after chlorination = no. of hydrogen atom X relative reactivity.
|
Relative |
Amount |
|
$1^{\circ}$ halide |
9 × 1 = 9 |
|
$2^{\circ}$halide |
2 × 3.8 = 7.6 |
|
$3^{\circ}$ halide |
1 × 5 = 5 |
Therefore, the total amount of monochloro product will be :
9 + 7.6 + 5 = 21.6
Now, $1^{\circ}$ monochloro product% = $9 \times \frac{100}{21.6} =41.7$ %
$2^{\circ}$ monochloro product % = $7.6\times \frac{100}{21.6} = 35.2$%
$3^{\circ}$ monochloro product % = $5\times \frac{100}{21.6}=23.1$%
Question 34. Write the structures and names of products obtained in the reactions of sodium with a mixture of 1-iodo-2-methylpropane and 2-iodopropane.
Answer:
Alkanes having double bonds are obtained when n-alkyl halide is treated with name talin in the presence of ether, products obtained are based on Wurtz reaction.
$RX + 2Na +XR \rightarrow R-R + 2NaX$
$CH_{3}-CH(CH_{3})-CH_{2}I + 2Na + ICH_{2}-CH(CH_{3})-CH_{3}\overset{Dry ether}{\rightarrow} 2NaI + CH_{3}-CH(CH_{3})-CH_{2}-CH_{2}-CH(CH_{3})-CH_{3}$
(2,5-dimethylHexane)
$CH_{3}-CH(CH_{3})I + 2Na + I(CH_{3})CH-CH_{3} \rightarrow 2NaI + CH_{3}-CH(CH_{3})-CH(CH_{3})-CH_{3}$
(2,4-dimethylbutane)
$CH_{3}-CH(CH_{3})-CH_{2}I + 2Na + I-CH(CH_{3})-CH_{3} \rightarrow 2NaICH_{3}-CH(CH_{3})-CH-CH_{3}-CH_{3}$
(2,4-dimethylpentane)
Question 35. Write hydrocarbon radicals that can be formed as intermediates during monochlorination of 2-methylpropane? Which of them is more stable? Give reasons.
Answer:
Due to hyperconjugation and 9 $\alpha$ hydrogen tertiary $3^{\circ}$ free radical is more stable and sit stabilised; whereas, $1^{\circ}$ free radical has 1 $\alpha$ hydrogen and one hyper conjugative structure and hence is less stable.
Answer:
|
1 |
|
It is aromatic since the ring is planar, has complete delocalisation of $\pi$ electrons, also 6$\pi$-electrons is there. |
|
2 |
|
It is non-aromatic because the ring is non-planar, has incomplete delocalisation of $\pi$ electrons, 6$\pi$ electrons is present there. |
|
3 |
|
It is aromatic since the ring is planar, has complete delocalisation of $\pi$ electrons, also 6$\pi$-electrons is there. |
|
4 |
|
It is non-aromatic because the ring is non-planar, has incomplete delocalisation of $\pi$ electrons, 4$\pi$ electrons is present there. |
|
5 |
|
It is aromatic since the ring is planar, has complete delocalisation of $\pi$ electrons. Juckel rule is obeyed here |
|
6 |
|
It is aromatic since the ring is planar, has complete delocalisation of $\pi$ electrons. Huckel rule is obeyed here |
|
7 |
|
It is non-aromatic because the ring is planar, has incomplete delocalisation of $\pi$ electrons, 8$\pi$ electrons is present there |
Question 38. Which of the following compounds are aromatic according to Huckel’s rule?
Answer:
|
|
It is non-aromatic and has 8$\pi$ electrons. |
|
|
It is aromatic since it has delocalised 6$\pi$ electrons and follows Huckel rule. |
|
|
It is nonaromatic since delocalised 6π electrons are not present in this compound. |
|
|
It is aromatic since it has 10 delocalised $\pi$ electrons and obeys Huckel’s rule |
|
|
It is aromatic since it obeys Huckel’s rule and has 8$\pi$ electrons out of which 6$\pi$ electrons are delocalised |
|
|
It is aromatic since it follows Huckel’s rule, is planar and has 14$\pi$ electrons which are in conjugation. |
Question 39. Suggest a route to prepare ethyl hydrogen sulphate $(CH_{3}-CH_{2}-OSO_{2}-OH)$ starting from ethanol $(C_{2}H_{5}OH)$
Answer:
Preparing ethyl hydrogen sulphate starting from ethanol.
Step I-
Protonation of alcohol and formation of a carbocation.
$H_{2}SO_{4} \rightarrow H^{+} + ^{-}OSO_{2}OH$
$CH_{3}- CH_{2} - O - H + H^{+} \rightarrow CH_{3} - CH_{2} - ^{+}OH2$
$CH_{3} - CH_{2} - ^{+}OH2 \rightarrow CH_{3}- ^{+}CH_2 + H_{2}O$
Step II-
Attack of nucleophile
$HO - SO_{2} - O^{-} + ^{+}CH_{2} - CH_{3} \rightarrow CH_{3} - CH_{2} - O - SO_{2}- OH$
NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: Matching Type
NCERT Exemplar Class 11 Chemistry Chapter 13 Hydrocarbons important questions are discussed below. These are generally asked in exams to test your knowledge. These exemplar solutions is quite helpful for competitive exams.
|
C olumn I |
Column II |
Answer:
(i) → (d); (ii) → (a); (iii) → (e); (iv) → (c); (v) → (b)
Explanation:
(i)$O_{3}/Zn + H_{2}O$: Two carbonyl compounds are obtained on ozonolysis of alkene
(ii) $KMnO_{4}/H^{+}$: Propene gives $CH_{3}COOH$ and $CO_{2}$ on reacting with $KMnO_{4}$ in an acidic medium.
$CH_{3} - CH \equiv CH^{2} + KMnO_{4}/H^{+} \rightarrow CH_{3}COOH + CO_{2}$
(iii) $KMnO_{4}/OH^{-}$-: Alkaline $KMnO_{4}$is decolourised by propene
(iv) $H_{2}O/H^{+}$: Propanol-2 is obtained when $H_{2}O$ is added to propene
$CH_{3}- CH = CH_{2} + H^{+}/^{-}OH \rightarrow CH_{3}-CH(OH)-CH_{3}$
(v) $B_{2}H_{6}/NaOH and H_{2}O_{2}$: Propanol-1 is obtained as a product
Question 41. Match the hydrocarbons in Column I with the boiling points given in Column II.
|
Column I |
Column II |
|
(i) n–Pentane |
(a) 282.5 K |
|
(ii) iso-Pentane |
(b) 309 K |
|
(iii) neo-Pentane |
(c) 301 K |
Answer:
$(i) \rightarrow (b); (ii) \rightarrow (c); (iii) \rightarrow (a)$
Explanation:
(i) There are more van der Waal’s forces in n-pentane, and its boiling point is also high since there is no branching and surface area.
(ii) The boiling point of iso-pentane is less because the molar mass is the same except there’s one brach which reduces the surface area.
(iii) The boiling point of neo-pentane is the lowest because it has two side chains which have the same molar mass.
Question 42. Match the following reactants in Column I with the corresponding reaction products in Column II.
|
Column I |
Column II |
|
(i) Benzene + $Cl_{2}\overset{AlCl_{3}}{\rightarrow}$ |
(a) Benzoic acid |
|
(ii) Benzene + $CH_{3}Cl\overset{AlCl_{3}}{\rightarrow}$ |
(b) Methyl phenyl ketone |
|
(iii) Benzene + $CH_{3}COCl\overset{AlCl_{3}}{\rightarrow}$ |
(c) Toluene |
|
(iv) Toluene $\overset{KMnO_{4}/NaOH}{\rightarrow}$ |
(d) Chlorobenzene |
|
|
(e) Benzene hexachloride |
Answer:
$(i) \rightarrow(d); (ii) \rightarrow (c); (iii) \rightarrow (b); (iv) \rightarrow (a)$
Explanation:
Question 43. Match the reactions given in Column I with the reaction types in Column II.
|
Column I |
Column II |
|
(i) $CH_{2}=CH_{2}+H_{2}O\overset{H^{+}}{\rightarrow}CH_{3}CH_{2}OH$ |
(a) Hydrogenation |
|
(ii) $CH_{2}=CH_{2}+H_{2}\overset{Pd}{\rightarrow}CH_{3}-CH_{3}$ |
(b) Halogenation |
|
(iii) $CH_{2}=CH_{2}+Cl_{2}\rightarrow Cl-CH_{2}-CH_{2}-Cl$ |
(c) Polymerization |
|
(iv) $3CH\equiv CH\xrightarrow[Heat]{Cu\; tube}C_{6}H_{6}$ |
(d) Hydration |
|
|
(e) Condensation |
Answer:
$(i) \rightarrow (d); (ii) \rightarrow (a); (iii) \rightarrow (b); (iv) \rightarrow (c)$
Explanation:
(i) Hydration refers to the addition of water molecule
$CH_{2}=CH_{2}+H_{2}O\overset{H^{+}}{\rightarrow}CH_{3}CH_{2}OH$
(ii) Addition of H2 in the presence of a catalyst is called as Hydrogenation
$CH_{2}=CH_{2}+H_{2}\overset{Pd}{\rightarrow}CH_{3}-CH_{3}$
(iii) Addition of halogens means halogenation
$CH_{2}=CH_{2}+Cl_{2}\rightarrow Cl-CH_{2}-CH_{2}-Cl$
(iv) When a no. of smaller molecules come together to give one bigger molecule, it is known as polymerisation.
NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: Assertion and Reason Type
The Assertion and Reason type questions of NCERT Exemplar Class 11 Chemistry Chapter 13 Hydrocarbons test students conceptual clarity and logical reasoning skills. These Solutions help you understand the basic concepts.
Question 44. In the following questions a statement of assertion (A) followed by a statement of reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A) : The compound cyclooctane has the following structural formula :
It is cyclic and has conjugated 8$\pi$-electron system but it is not an aromatic compound.
Reason (R) : $(4n + 2) \pi$ electrons rule does not hold good and ring is not planar.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
The answer is the option (i) Both A and R are correct, and R is the correct explanation of A.
Explanation:
There are some characteristics possessed by compounds which show aromaticity:
-
Complete delocalisation of $\pi$ electrons in the ring
-
Planarity of the compound
-
Huckel Rule, viz., presence of $(4n + 2) \pi$ electrons where n is an integer.
Question 45. In the following questions a statement of assertion (A) followed by a statement of reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A) : Toluene on Friedel Crafts methylation gives o– and p–xylene.
Reason (R) : $CH_3$ group bonded to benzene ring increases electron density at o– and p– position.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
The answer is the option (i) Both A and R are correct, and R is the correct explanation of A.
Explanation:$CH_3$ group is an electron-withdrawing group. It activates the benzene ring due to hyperconjugation effect. Toluene has -$CH_3$ group attached to the benzene ring. This group also increases the electron density at ortho and para positions for electrophile attacks.
Question 46. In the following questions a statement of assertion (A) followed by a statement of reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A) : Nitration of benzene with nitric acid requires the use of concentrated sulphuric acid.
Reason (R) : The mixture of concentrated sulphuric acid and concentrated nitric acid produces the electrophile, $NO_{2}^{+}$ .
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
(i) Both A and R are correct, and R is the correct explanation of A.
Explanation: During nitration, benzene is treated with the nitrating mixture, viz., Conc. $HNO_{3}$ and $H_{2}SO_{4}$. $H_{2}SO_{4}$ helps in furnishing the electrophile, i.e., $NO_{2}^{+}$.
$H_{2}SO_{4}+HNO_{3}\underset{H_{2}O}{\rightarrow}NO_{2}^{+}+HSO_{3}^{-}$
Question 47. In the following questions a statement of assertion (A) followed by a statement of reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A) : Among isomeric pentanes, 2, 2-dimethylpentane has highest boiling point.
Reason (R) : Branching does not affect the boiling point.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
The answer is the option (iii) Both A and R are not correct.
Explanation: 2,2-dimethylpentane has the lowest boiling point among isomeric pentanes, and its boiling point decreases further on branching.
NCERT Exemplar Class 11 Chemistry Solutions Chapter Hydrocarbons: Long Answer Type
Chemistry Class 11 NCERT Exemplar Chapter 13 Hydrocarbons Long Answer Type questions test a students conceptual clarity and logical reasoning skills.
To identify A, B, C and D, the reactions involved are-
$C_{5}H_{11}�Br(A) + alc. KOH \rightarrow C_{5}H_{10}(B)$
$C_{5}H_{10}(B) + Br/CS_{2} \rightarrow C_{5}H_{10}Br_{2}(C)$
$C_{5}H_{10}Br_{2}(C) + alc. KOH \rightarrow C_{5}H_{8}(D) Alkyne$
$2C_{5}H_{8 }+ 2Na \rightarrow 2C_{5}H_{7}Na + H_{2}$
All the compounds above must be straight-chain as hydrogenation of alkyne (D) gives straight-chain alkane. It is clear that D is terminal alkyne as alkyne gives sodium alkenyde.
To calculate molar mass of hydrocarbon A it is given that 896 ml of Hydrocarbon ACxHy weighs 3.28 g at STP.
22400 ml of has ACxHy mass = $\frac{3.28 \times 22400 ml}{896 ml}$
= 83.1 g/mol.
Thus, the molar mass of A = 83.1 g/mol
|
Element |
C |
H |
|
Percentage |
87.8% |
12.19% |
|
Atomic mass |
12 |
1 |
|
Relative ratio |
7.31 |
12.19 |
|
Relative no. of atoms |
1 |
1.66 |
|
Simplest ratio |
3 |
4.98 = 5 |
We know that empirical formula = $C_{3}H_{5}$
Empirical formula with weight = $3 \times12 = 36 + 5 = 41$
Molecular formula = (empirical formula) n, where n = mol mass/empirical mass = $\frac{831}{41}=2.02$
Molecular formula = $[C_{3}H_{5}]_{2} = C_{6}H_{10}$
Now, determining the structure of (A) and (B)-
$C_{6}H_{12}$ is obtained when hydrogenation of $C_{5}H_{10}$ happens with two moles of H2. The structure is methylpentane.
$C_{6}H_{12}O$ (which gives positive iodoform test) is obtained when hydration of (A) takes place in the presence of dil. H=$H_{2}SO_{4}$ and $HgSO_{4}$.
Thus, the structure of $A = (CH_{3})_{2}CH-CH-C\equiv CH$(4-methyl pent-yne) and (B) is 4-methyl pentanone-2.
Two molecules of hydrogen add on ‘A’ this shows that ‘A’ is either an alkadiene or an alkyne.
On ozonolysis compound A gives
$CH_{3}CHO + O = CH - CH_{2}- CH_{2}- CHO + O = C(CH_{3})_{2}$
Hence, the structure of A(Its IUPAC name will be 2-methyl octa 2,6 diene) is,
Answer:
(i)
(ii) $^.C_{6}H_{5}+H-Br\overset{homolysis}{\rightarrow}C_{6}H_{6}+Br^.$
(iii)
(iv) $CH_{3}+\dot{C}H-CH_{2}Br+H-Br\overset{Homolysis}{\rightarrow}CH_{3}-CH_{2}-CH_{2}Br+\dot{B}r$
Since the H-Cl bond is stronger than H-Br bond, peroxide effect is observed only in the case of HBr. It is not observed in the case of HI as well.
H-Br has lesser bond energy than H-Cl; thus, H-Cl bond is not cleaved by the free radical, whereas the H-I bond is weaker and iodine free radicals combine to form dimer iodine molecules.
Class 11 Chemistry NCERT Chapter Hydrocarbons: Higher Order Thinking Skills (HOTS) Questions
Some NCERT Exemplar Class 11 Chemistry Chapter 13 Hydrocarbons questions and answers are given below that will help you tackle complex problems. The questions below will help you evaluate your understanding of the concepts.
Question 1. The number of optically active products obtained from the complete ozonolysis of the given compound is:

- 2
- 0
- 1
- 4
Answer:
Ozonolysis cleaves every C=C double bond into two carbonyl groups (aldehydes or ketones). The double bonds break completely, replacing the alkene carbons with $\mathrm{C}=\mathrm{O}$ groups.
Here, in this molecule,
$
C H 3-C H=C H-C^*(C H 3)(H)-C H=C H-C^*(C H 3)(H)-C H=C H-C H 3
$
It contains three $\mathrm{C}=\mathrm{C}$ double bonds.
The two starred carbon atoms are chiral centers.
From ozonolysis, the central part forms carbonyl compounds with chiral centers. From the cleavage of the entire molecule, two optically active products are formed, with each retaining a chiral center in the carbonyl-containing fragment.
Hence, the correct answer is (1).
Question 2. In the following series of reactions identify the major products A & B respectively.

Answer:

Hence, the correct answer is option (2).
Question 3. Given below are two statements :
Statement I : Ozonolysis followed by treatment with $\mathrm{Zn}, \mathrm{H}_2 \mathrm{O}$ of cis-2-butene gives ethanal.
Statement II : The production obtained by ozonolysis followed by treatment with $\mathrm{Zn}, \mathrm{H}_2 \mathrm{O}$ of 3, 6-dimethyloct-4-ene has no chiral carbon atom.
In the light of the above statements, choose the correct answer from the options given below :
(1) Both Statement I and Statement II are true
(2) Statement I is false but Statement II are true
(3) Statement I is true but Statement II is false
(4) Both Statement I and Statement II are false
Answer:
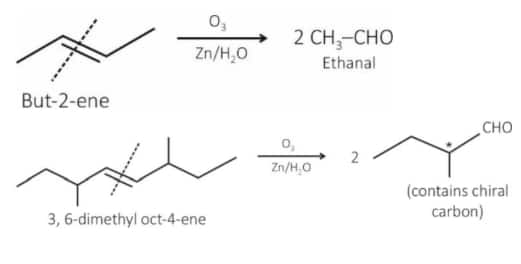
St-I : Correct statement
St-II : Incorrect statement because the product has a chiral center.
Hence, the correct answer is option (3).
Question 4:

The reagents ( X ) and ( Y ) required for steps (ii) and (iii) are respectively.
(1) $\quad \mathrm{CH}_3 \mathrm{OH}$ and $\mathrm{H}_3 \mathrm{O}^{+}$
(2)$\quad \mathrm{CH}_3 \mathrm{I}$ and NaOH
(3) $\quad \mathrm{CH}_3 \mathrm{MgBr}$ and $\mathrm{H}_3 \mathrm{O}^{+}$
(4) $\quad \mathrm{NaOH}$ and $\mathrm{CH}_3 \mathrm{Cl}$
Answer:

Hence, the correct answer is option (3).
Question 5: Choose the correct set of reagents for the following conversion.
(1) $\mathrm{Br}_2 / \mathrm{Fe} ; \mathrm{Cl}_2, \Delta$; alc. KOH
(2) $\mathrm{Cl}_2 / \mathrm{Fe}$; $\mathrm{Br}_2 /$ anhy. $\mathrm{AlCl}_3$; aq. KOH
(3) $\mathrm{Br}_2 /$ anhy. $\mathrm{AlCl}_3 ; \mathrm{Cl}_2, \Delta$; aq. KOH
(4) $\mathrm{Cl}_2 /$ anhy. $\mathrm{AlCl}_3 ; \mathrm{Br}_2 / \mathrm{Fe}$; alc. KOH
Answer:
Step 1 is Electrophilic aromatic substitution, in which bromination of ethylbenzene is done in the presence of iron. Step 2 is the free radical halogenation of the above obtained product, followed by an elimination reaction in the presence of alcoholic KOH, and the obtained product is 4-bromostyrene.

Hence, the correct answer is option (1).
Question 6: Designate whether each of the following compounds is aromatic or not aromatic.

(1) e, g aromatic and a, b, c, d, f, h not aromatic
(2) b, e, f, g aromatic and a, c, d, h not aromatic
(3) a, b, c, d aromatic and e, f, g, h not aromatic
(4) a, c, d, e, h aromatic and b, f, g not aromatic
Answer:
Aromatic compounds

b, f, g, are not aromatic, these compounds do not follow Huckel’s rule
Hence, the correct answer is option (4).
Question 7: The product B formed in the following reaction sequence is :

(1)

(2)

(3)

(4)

Answer:

Hence, the correct answer is option (4).
Approach to Solve Questions of Chapter 13 Hydrocarbons
A structured approach that works well for both theory-based and numerical problems is given below. It is advisable to follow the NCERT Exemplar Class 11 Chemistry Solutions Chapter 13 Hydrocarbons for deep learning. This chapter builds a strong foundation for advanced organic chemistry and frequently appears in Boards, NEET, and JEE, especially in reaction-based and mechanism questions. Here is a concise and structured approach to follow for hydracarbon:
1. Students can start by identifying the key topics covered like Types of hydrocarbons- alkanes, alkenes, alkynes, aromatic hydrocarbons, nomenclature rules, Isomerism, preparation and reactions of hydrocarbons, and their physical and chemical properties. You can also follow NCERT class 11 hydrocarbons notes available on our website.
2. Questions related to important reactions are frequently asked in exams. So it is very important to learn about reactions and their mechanisms in detail. Given below some important reaction
- Halogenation via a free radical mechanism
- Electrophilic addition reactions
- Markovnikov’s Rule and Anti-Markovnikov’s addition
- Oxidation: Baeyer’s test, ozonolysis
- Hydrogenation: Addition of H2 using Ni/Pd catalyst
- Electrophilic substitution: Nitration, halogenation, sulphonation, Friedel Crafts alkylation/acylation
- Directive influence of substituents (activating vs deactivating)
3. Learn to categorise questions into conceptual/theoretical questions, structural drawing, reaction-based questions, IUPAC nomenclature or numerical questions. This will help you prepare for them accordingly.
4. While doing Hydrocarbons Class 11 NCERT Exemplar Chemistry Chapter 13 Solutions it is very important to read the question carefully and identify exactly what is being asked. Relate it to the concept learned. The hydrocarbons class 11 questions and answers are well explained in our exemplar solutions.
5. While solving questions of organic chemistry, it is very important to note down the information given and you can also use bullet points for clarity when writing answers. Students can refer to the solved examples in the textbook and then solve the in-text questions. Students can also access the NCERT solutions for class 11 chemistry for quick revision of the concepts.
Major Topics Of NCERT Exemplar Class 11 Chemistry Chapter Hydrocarbons
Following are the important topics from the chapter s-block elements. Practice more at NCERT Class 11 chemistry chapter 13 exemplar solutions.
A. Classification
B. Alkanes
i. Nomenclature And Isomerism
ii. Preparation
iii. Properties
iv. Conformations
C. Alkenes
i. Structure Of Double Bond
ii. Nomenclature
iii. Isomerism
iv. Preparation
v. Properties
D. Alkynes
i. Nomenclature And Isomerism
ii. Structure Of Triple Bond
iii. Preparation
iv. Properties
E. Aromatic Hydrocarbon
i. Nomenclature And Isomerism
ii. Structure Of Benzene
iii. Aromaticity
iv. Preparation Of Benzene
v. Properties
vi. Directive Influence Of A Functional Group In Monosubstituted Benzene
F. Carcinogenicity And Toxicity
Advantages of Using Chemistry Class 11 NCERT Exemplar Chapter 13 Hydrocarbons Solutions
NCERT Exemplar Solutions Class 11 Chemistry Chapter 13 Hydrocarbons covers all important concepts from the NCERT book in a simple and organised manner. Advantages of using these NCERT Exemplar Class 11 solutions are given below:
- Students can use these Hydrocarbons Class 11 NCERT Exemplar Chemistry Chapter 13 Solutions to understand the concepts like alkanes, alkenes, alkynes, aromatic hydrocarbons, methods of preparation, physical and chemical properties using solved questions.
- These hydrocarbons class 11 questions and answers are written in a systematic manner that provide clear concepts to help students understand how hydrocarbons behave and react.
- Science students can use these solutions for clear and comprehensive explanations of the concepts covered in NCERT.
- Hydrocarbon solutions are prepared by subject experts in an organised manner to help students understand the concepts.
NCERT Exemplar Class 11 Chemistry Solutions Chapter-Wise
Besides NCERT Exemplar Solutions Class 11 Chemistry Chapter 13 Hydrocarbons students can refer to the links given below for Class 11 NCERT exemplar chapter-wise solutions. These solutions are designed to help students understand concepts clearly and practise effectively for exams.
NCERT Solutions for Class 11 Chemistry Chapter-wise
Class 11 NCERT chemistry chapter-wise solutions are given below. These solutions provide step-by-step solutions for all questions, helping students understand concepts easily. They are good for revision, and preparation for both school and competitive exams.
NCERT Exemplar Class 11 Solutions
Follow the links provided in the table below to get hands-on exemplar solutions of other subjects as well:
NCERT Solution subject-wise
The NCERT subject-wise solutions will help you broaden your concepts and will also help in revision. Learn more from Class 11 NCERT notes.
NCERT Notes subject-wise
You can follow the links given in the table below to get access to the Class 11 NCERT notes.
NCERT Books and NCERT Syllabus
You can find links to the Class 11 NCERT chemistry book and syllabus for the respective subjects.
| NCERT Books Class 11 Chemistry |
| NCERT Syllabus Class 11 Chemistry |
| NCERT Books Class 11 |
| NCERT Syllabus Class 11 |
Frequently Asked Questions (FAQs)
Hydrocarbons are organic compounds that are composed solely of carbon and hydrogen atoms. They are the fundamental building blocks of organic chemistry.
Hydrocarbons are crucial because: They serve as the primary source of fuels (e.g., natural gas, gasoline). They are the raw materials for the production of numerous synthetic materials like plastics, polymers, and various organic chemicals. They are essential components of biological molecules (e.g., fats, oils).
Hydrocarbons are mainly classified into two major categories:
- Aliphatic Hydrocarbons: These are open-chain or branched-chain compounds (e.g., alkanes, alkenes, alkynes).
- Saturated Hydrocarbons (Alkanes): Contain only single bonds between carbon atoms.
- Unsaturated Hydrocarbons (Alkenes & Alkynes): Contain at least one double (alkenes) or triple (alkynes) bonds between carbon atoms.
- Aromatic Hydrocarbons: These contain at least one benzene ring structure.
Alkanes are saturated hydrocarbons containing only single bonds. Examples include methane, ethane, propane, butane, etc.
Isomerism refers to the existence of two or more compounds with the same molecular formula but different structural formulas (different arrangements of atoms). In alkanes, this is called structural isomerism (specifically, chain isomerism).
NCERT Exemplar Class 11 Chemistry Solutions Chapter 13 Hydrocarbons provides advanced, application-based questions on alkanes, alkenes, alkynes, and aromatic compounds to help students strengthen conceptual understanding and improve problem-solving skills.
Alkenes commonly undergo addition reactions, where substances like water, hydrogen, or halogens add across the double bond. Alkynes can also participate in similar addition reactions, but they can further undergo polymerization or reduction to form alkenes or alkanes.
Aromatic hydrocarbons contain conjugated pi electron systems within rings, leading to their unique stability due to resonance. This stability influences their reactivity and properties, distinguishing them from aliphatic hydrocarbons, which lack this structure. Additionally, aromatic hydrocarbons often participate in electrophilic substitution reactions, while aliphatic hydrocarbons generally undergo addition reactions.
The properties of hydrocarbons can be modified through various methods such as functional group addition, isomerization, and polymerization. For example, adding functional groups can enhance solubility or reactivity, while changing the structure or using catalysts can lead to the production of different isomers with varying characteristics.
The study of hydrocarbons is essential in environmental chemistry because many hydrocarbons are natural components of fossil fuels, which release pollutants when burned. Understanding their behavior and reactivity helps in addressing issues like air quality, energy production, and the development of cleaner alternative fuels.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters



















































