NCERT Exemplar Solutions of Class 11 Chemistry provide detailed, step-by-step answers to advanced and application-based questions, helping students strengthen concepts.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic Chemistry Some Basic Principles and Technique
Do you know what makes carbon so special that it makes an entire new branch of chemistry, why Carbon forms millions of compounds and how it is widely useful in everything from medicines or fuel? The answer to all these questions lies in NCERT exemplar Class 11 chemistry chapter 12 Some Basic Principles and Technique. This chapter is quite engaging as it deals with carbon-containing compounds along with their structure, composition, reactions and preparations. Electron movement in organic reactions, electron displacement effects in covalent bonds, inductive effect, resonance structure, resonance effect, etc., which are complex concepts, are well explained in this chapter.
This Story also Contains
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: MCQ (Type 1)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: MCQ (Type 2)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: Short Answer Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: Matching Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: Assertion and Reason Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: Long Answer Type
- Class 12 Chemistry NCERT Chapter 12: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
- Topic Of NCERT Class 11 Chemistry Organic Chemistry Some Basic Principles and Techniques
- Advantages of Using Class 11 Chemistry Chapter 8 Organic Chemistry: Some Basic Principles and Techniques NCERT Exemplar Solutions
- NCERT Exemplar Class 11 Chemistry Solutions
- NCERT Solutions for Class 11 Chemistry Chapter-wise
- NCERT Exemplar Solutions Class 11 Subject-Wise
- NCERT Solution subject-wise
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus

These NCERT Exemplar Solutions of Class 11 Chemistry are designed by our subject experts to ensure a deep understanding of the concept of organic chemistry aligned with the syllabus of CBSE and are structured to help students effectively. These NCERT Exemplar Solutions also include higher-order thinking skills questions that are beyond memorization and promote conceptual understanding, improve analytical thinking, enhance application skills, and build confidence in chemistry. Students can also check NCERT Solutions to all questions chapter-wise.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: MCQ (Type 1)
At first, the MCQ questions are covered in the Class 11 Chemistry NCERT Exemplar Solutions Chapter 12 Organic Chemistry Some Basic Principles and Technique to enhance your knowledge. The concepts are explained in detail in class 11 chemistry chapter 12 Organic Chemistry Some Basic Principles and Technique notes available on our website.
Question 1. Which of the following is the correct IUPAC name?
(i) 3-Ethyl-4, 4-dimethylheptane
(ii) 4,4-Dimethyl-3-ethylheptane
(iii) 5-Ethyl-4, 4-dimethylheptane
(iv) 4,4-Bis(methyl)-3-ethylheptane
Answer:
The answer is the option (i) 3-Ethyl-4, 4-dimethylheptane
Explanation: According to the IUPAC naming, in case of presence of more than one different types of alkyl groups, they are written in alphabetical order and hence ethyl is wrritten first and then methyl.
Question 2. The IUPAC name for is _______:
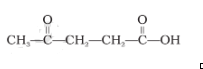
(i) 1-hydroxypentane-1,4-dione
(ii) 1,4-dioxopentanol
(iii) 1-carboxybutan-3-one
(iv) 4-oxopentanoic acid
Answer:
The answer is the option (iv) 4-oxopentanoic acid
Explanation: According to the IUPAC naming, in case of presence of more than one different types of functional groups, one functional group will be taken as the main functional group on a priority basis and is mentioned as a suffix, while the other functional group is written as a prefix.
Question 3. The IUPAC name for
(i) 1-Chloro-2-nitro-4-methylbenzene
(ii) 1-Chloro-4-methyl-2-nitrobenzene
(iii) 2-Chloro-1-nitro-5-methylbenzene
(iv) m-Nitro-p-chlorotoluene
Answer:
The answer is the option (ii) 1-Chloro-4-methyl-2-nitrobenzene
Explanation: The substituent of the base compound is considered to be number 1, and then the direction of numbering is chosen such that the next substituent group is getting the lowest number. The substituents appear in the name, arranged in alphabetical order.
Question 4. Electronegativity of carbon atoms depends upon their state of hybridisation. In which of the following compounds, the carbon marked with asterisk is most electronegative?
$(i) CH_3 - CH_{2}-^{\ast }CH_{2} -CH_{3}$
$(ii) CH_{}3 - ^{\ast}CH = CH - CH_{3}$
$(iii) CH_{3} - CH_{2} - C \equiv ^{\ast } CH$
$(iv) CH_{3} - CH_{2} - CH = ^{\ast}CH_{2}$
Answer:
The answer is the option $(iii) CH_{3} - CH_{2} - C \equiv ^{\ast }CH$
Explanation: The electronegativity of the carbon atom depends on its hybridisation state and the percentage of ‘s’ character. It is directly proportional to the ‘s’ character. Here we have sp hybridised carbon having 50 percent s character.
Question 5. In which of the following, functional group isomerism is not possible?
(i) Alcohols
(ii) Aldehydes
(iii) Alkyl halides
(iv) Cyanides
Answer:
The answer is the option (iii) Alkyl halides
Explanation: Isomerism is a phenomenon in which two compounds have the exact same molecular formula, but the different structural formula and such compounds are called isomers, and Functional isomers have same molecular formula but a different functional group. Alcohols are functional isomers of ethers. Aldehydes are functional isomers of ketones. Cyanides are functional isomers of isocyanides. Only alkyl halides do not show functional isomerism.
Question 6. The fragrance of flowers is due to the presence of some steam volatile organic compounds called essential oils. These are generally insoluble in water at room temperature but are miscible with water vapour in vapour phase. A suitable method for the extraction of these oils from the flowers is:
(i) Distillation
(ii) Crystallisation
(iii) Distillation under reduced pressure
(iv) Steam distillation
Answer:
The answer is the option (iv) Steam distillation
Explanation: This is a technique which is applied to separate substances which are steam volatile and are immiscible with water, differently. In case of steam distillation, steam, from a steam generator is passed through a heated flask containing the liquid to be distilled. The mixture of steam and the volatile organic compound is then condensed and collected together. The compound is later separated from water using a separating funnel.
Question 7. During hearing of a court case, the judge suspected that some changes in the documents had been carried out. He asked the forensic department to check the ink used at two different places. According to you which technique can give the best results?
(i) Column chromatography
(ii) Solvent extraction
(iii) Distillation
(iv) Thin layer chromatography
Answer:
The answer is the option (iv) Thin layer chromatography
Explanation: Thin-layer chromatography (TLC) is a method for analysing mixtures by separating the components of the mixture. This method can be used to determine the number of components in the mixture, to identity the compounds and the purity of compounds by observing the appearance of a product or the disappearance of a reactant, whichever is the case.
Question 8. The principle involved in paper chromatography is
(i) Adsorption
(ii) Partition
(iii) Solubility
(iv) Volatility
Answer:
The answer is the option (ii) Partition
Explanation: Partition chromatography is based on the concept of continuous differential partitioning of components of a mixture between stationary and mobile phases.
Question 9. What is the correct order of decreasing stability of the following cations?
(i) II > I > III
(ii) II > III > I
(iii) III > I > II
(iv) I > II > III
Answer:
(i) II > I > III
Explanation: In the case of (I) +vely charged C is attached to two alkyl groups and so +I effect stabilises the carbocation. In the case of (II) +R effect of $OCH_3$ group stabilises the carbocation. In the case of (III), - I effect of $-OCH_3$ gp, destabilises the carbocation; hence, the order of stability will be such: II > I >III.
Question 10. Correct IUPAC name for 
(i) 2- ethyl-3-methylpentane
(ii) 3,4- dimethyl hexane
(iii) 2-sec-butylbutane
(iv) 2, 3-dimethylbutane
Answer:
(ii) 3,4- dimethyl hexane
Explanation: In the longest continuous chain, both ethyl groups are included, and 3rd and 4th carbon have methyl groups present as the substituent.
Question 11. In which of the following compounds the carbon marked with asterisk is expected to have greatest positive charge?
$(i) ^{\ast}CH_{3} -CH_{2}-Cl$
$(ii) ^{\ast}CH_{3} -CH_{2}-Mg^{+}Cl^{-}$
$(iii) ^{\ast}CH_{3} -CH_{2}-Br$
$(iv) ^{\ast}CH_{3} -CH_{2}-CH_{3}$
Answer:
The answer is the option $(i) ^{\ast}CH_{3} -CH_{2}-Cl$
Explanation: Order of electronegativity is as follows: Cl > Br > C > Mg. The more electronegative group attached to the C will give a more positive charge. Therefore, in (i) case, asterisk C will have the greatest positive charge.
Question 12. Ionic species are stabilised by the dispersal of charge. Which of the following carboxylate ion is the most stable?
Answer:
The answer is the option
Explanation: The dispersal of the negative charge depends on the stabilisation of the carboxylate ion. The negative charge which is shown is dispersed by two factors i.e +R effect of carboxylate ion and -I effect of halogens. In the above cases, +R effect is common, but halogen atoms are different and hence dispersal of the negative charge depends upon halogen atoms, where F is most electronegative.
Question 13. Electrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction.
$H_{3}C-HC = CH_{2} + H^{+} \rightarrow ?$
$(i) 2^{\circ} Carbanion$
$(ii) 1^{\circ} Carbocation$
$(iii) 2^{\circ} Carbocation$
$(iv) 1^{\circ} Carbanion$
Answer:
The answer is the option $(iii) 2^{\circ} Carbocation$
Explanation: When H+ attacks on propene delocalisation of electrons can take place in two possible ways:
Question 14. Covalent bond can undergo fission in two different ways. The correct representation involving a heterolytic fission of $CH_{3}-Br$ is
Answer:
The answer is the option (ii).
Explanation: Br is more electronegative than C, hence heterolytic fission takes place in that case and so electrons displace from carbon to Br. Therefore,$CH_{3}$ gets the positive charge and Br gets the negative charge.
Question 15. The addition of HCl to an alkene proceeds in two steps. The first step is the attack of H+ ion to$> C= C<$ portion which can be shown as
(iv) All these are possible
Answer:
The answer is the option (ii) because the double bond is an electron source. The charge flows from higher electron density; thus, the proton is attacked by n electrons of the double bond.
It can be shown as
NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: MCQ (Type 2)
The class 11 chemistry Chapter 12 Organic Chemistry Some Basic Principles and Technique questions are provided here with simple explanations. Learn more through these advanced MCQs. All the MCQ (type 2) questions with solutions are given below:
Question 16. Which of the following compounds contain all the carbon atoms in the same hybridisation state?
$(i) H-C \equiv C-C\equiv C-H$
$(ii) CH_{3}-C \equiv C-CH_{3}$
$(iii) CH_{2} = C = CH_{2}$
$(iv) CH_{2} = CH-CH = CH_{2}$
Answer:
The answer is the option $(i) H-C \equiv C-C\equiv C-H$ and $(iv) CH_{2} = CH-CH = CH_{2}$
Explanation: In these two compounds only all carbon atoms are in the same hybridisation state, which is sp and sp2 hybridised, respectively.
Question 17. In which of the following representations given below spatial arrangement of group/ atom different from that given in structure ‘A’?
Answer:
The answer is the option (i), (iii) and (iv)
Explanation: The spatial arrangement of groups of atoms can be checked by interchanging and bringing the H below the plane of the paper and then finding out the sequence of the remaining groups in a particular order whether clockwise or anticlockwise where it is started from the atom with the highest atomic number to the atom with the lowest atomic numbers.
Question 18. Electrophiles are electron seeking species. Which of the following groups contain only electrophiles?
$(i)BF_{3}, NH_{3},H_{2}O$
$(ii)AlCl_{3}, SO_{3},NO_{2}^{+}$
$(iii)NO_{2}^{+},CH_{3}^{+},CH_{3}-C^{+}=O$
$(iv)C_{2}H_{5}^{-},C^{\cdot }_{2}H_{5},C_{2}H_{5}^{+}$
Answer:
The answer is the option (ii) and (iii)
Explanation: Electrophiles are the +vely charged species or the electron-deficient species. They are Lewis acids. $AlCl_{3}, SO_{3}$ and are Lewis acids, $(iii)NO_{2}^{+},CH_{3}^{+},CH_{3}-C^{+}=O$ are +vely charged species
Question 19. Which of the following pairs are position isomers?
(i) I and II
(ii) II and III
(iii) II and IV
(iv) III and IV
Answer:
The answer is the option (ii) II and III
Explanation: When two or more compounds have functional groups or substituent atoms attached at different positions on the carbon skeleton, they are termed as position isomers, and this phenomenon is termed as position isomerism.
Question 20. Which of the following pairs are not functional group isomers?
(i) II and III
(ii) II and IV
(iii) I and IV
(iv) I and II
Answer:
The answer is the option (i) and (iii)
Explanation: Isomerism is a phenomenon in which two compounds have the exact same molecular formula, but the different structural formula and such compounds are called isomers, and Functional isomers have same molecular formula but a different functional group.
Question 21. Nucleophile is a species that should have
(i) a pair of electrons to donate
(ii) positive charge
(iii) negative charge
(iv) electron deficient species
Answer:
The answer is the option (i) and (iii)
Explanation: Nucleophiles are -vely charged or electron rich (lone pair of electrons) species.
Question 22. Hyperconjugation involves delocalisation of ______.
(i) electrons of carbon-hydrogen $\sigma$ bond of an alkyl group directly attached to an atom of unsaturated system.
(ii) electrons of carbon-hydrogen $\sigma$ bond of alkyl group directly attached to the positively charged carbon atom.
(iii) $\pi$-electrons of carbon-carbon bond
(iv) lone pair of electrons
Answer:
The answer is the option (i) and (ii)
Explanation: Hyperconjugation means the delocalisation of electrons of the C-H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p orbital. Electrons of C-H bond of the alkyl group enter into the partial conjugation with the attached unsaturated system or with the unshared p orbital. Thus, Hyperconjugation is a permanent effect.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: Short Answer Type
Here some short answer type questions from NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic Chemistry Some Basic Principles and Technique are given for practice. This section contains important questions that are asked in the exams.Practice short answer types from the questions below.
Question 17. The reaction
Question 23. Which of the above compounds form pairs of metameres?
Answer:
Compounds with same molecular formula but with different alkyl groups on either side of the functional groups are called metameres. In compounds above, structures V and VI or VI and VII or V and VII form a pair of metameres.
Question 24. Identify the pairs of compounds which are functional group isomers.
Answer:
Isomerism is a phenomenon in which two compounds have the exact same molecular formula, but the different structural formula and such compounds are called isomers, and Functional isomers have same molecular formula but a different functional group. Hence, I and V, I and VI, I and VII; II and V, II and VI, II and VII; III and V, III and VI; III and VII; IV and V, IV and VI, IV and VI are functional group isomers.
Question 25. Identify the pairs of compounds that represents position isomerism.
Answer:
When two or more compounds have functional groups or substituent atoms attached at different positions on the carbon skeleton, they are termed as position isomers, and this phenomenon is termed as position isomerism. In the given structures, I and II, III and IV, and VI and VII are position isomers.
Question 26. Identify the pairs of compounds that represents chain isomerism.
Answer:
compounds I and III, I and IV, II and III and II and IV represent chain isomerism.
Elements like nitrogen, halogens, sulphur and phosphorous present in organic compounds converted into ions by fusing with sodium metal which is then plucked in distilled water getting sodium extract. On adding diluted $H_{2}SO_{4}$ in place of diluted HNO3 for testing halogens by $AgNO_{3}$, a white precipitate of $Ag_2SO_{4}$ is formed. This will lead to the wrong result of chloride. Hence, only $HNO_{3}$ is used instead of diluted $H_{2}SO_{4}$.
Question 28. What is the hybridisation of each carbon in $H_{2}C = C = CH_{2}$.
Answer:
All three carbon atoms are linked to each other by double bonds only. Carbon at 1 and 3 are $sp^{2}$ hybridised because it has $3\sigma$ and $1\pi$ bonds, whereas carbon at 2 has 2 $\sigma$ bonds and $2\pi$ bonds, so it is sp hybridised.
Question 29. Explain, how is the electronegativity of carbon atoms related to their state of hybridisation in an organic compound?
Answer:
Electronegativity of carbon is directly proportional to the ‘s’ character. If C is $sp^{3}$hybridised then V character is 25%, $sp^{2}$ hybridised then V character is 33% and if sp hybridised then V character is 50%. Hence, sp hybridised carbon has strong hybridisation, s electrons are more strongly attracted by nucleus than p-electrons thus electronegativity of carbon increases with increase in ‘s’ character.
Question 30. Show the polarisation of carbon-magnesium bond in the following structure.
$CH_{3}-CH_{2}-CH_{2}-CH_{2}-Mg-X$
Answer:
Carbon is more electronegative than magnesium; hence Mg has a partial positive charge, and C has a partial negative charge because the bonded pair of electrons are attracted towards carbon.
$CH_{3}-CH_{2}-CH_{2} -CH_{2}^{-\delta} -Mg^{+\delta}- X$
Question 31. Compounds with same molecular formula but differing in their structures are said to be structural isomers. What type of structural isomerism is shown by
Answer:
These are Metamers. When two or more compounds have differnet arrangement of atoms attached at different positions on the carbon skeleton, they are termed as metamers, and this phenomenon is termed as Metamarism.
Question 32. Which of the following selected chains is correct to name the given compound according to IUPAC system.
Answer:
IUPAC says that the selected longest carbon chain must have maximum functional groups present in the compound. Hence 4 carbon chain is correct to name the given compound according to IUPAC system
Question 33. In DNA and RNA, nitrogen atom is present in the ring system. Can Kjeldahl method be used for the estimation of nitrogen present in these? Give reasons.
Answer:
In the case of DNA and RNA, nitrogen is present in the heterocyclic base and in the ring not as a substituent. Therefore, nitrogen present in the ring cannot be converted into$(NH_{4} )_{2}SO_{4}$. Hence, it cannot be estimated by the Kjeldahl method.
Steam distillation can be used for its purification for a compound when the compound decomposes at its boiling point but is steam volatile and insoluble in water and is also stable at low pressures
Question 35. Draw the possible resonance structures for
and predict which of the structures is more stable. Give a reason for your answer.
Answer:
C has a more stable structure than A because. This is because in C, the octet of all the atoms is complete, but, in A, C-atom with a positive charge does not have 8 electrons in the valence shell.
Question 36. Which of the following ions is more stable? Use resonance to explain your answer.
Answer:
Structure A is more stable. Carbocation A is more Planar, and $\pi$ electrons from the ring shift to side group and are stabilised by resonance. Moreover, the double bond is more stable within the ring as compared to the side chain.
Question 37. The structure of triphenylmethyl cation is given below. This is very stable, and some of its salts can be stored for months. Explain the cause of high stability of this cation.
Answer:
Triphenylmethyl cation is very stable because the +ve charge of methyl carbon is delocalised in three phenyl rings. In each phenyl ring, +ve charge is developed on 2 ortho position and para position, i.e. three resonating structures. Thus, the total resonating structures given by triphenylmethyl cation are nine. Hence, it is very stable.
Question 38. Write structures of various carbocation’s that can be obtained from 2- methylbutane. Arrange these carbocation’s in order of increasing stability.
Answer:
The stability order according to increasing stability is (III) > (II) > (IV) > (I)
because (I) and (IV) are primary, (II) is secondary carbocation, and (III) is tertiary carbocation.
If an organic compound contains N and S both, then on fusion with Na metal, the compound gives NaSCN or NaCN and $Na_{2} S$ depending on what is the quantity of Na metal. If Na metal is less, then only NaSCN is formed. In that case L.E on treating with $FeSO_{4}$ and $H_{2}SO_{4}$ gives red colour due to the formation of ferric thiocyanide $Fe (SCN)_{3}$ . In case of NaCN, L.E on treating with $FeSO_{4}$ and $H_{2}SO_{4}$ gives a total Prussian blue colour.
Chemical reactions:
$NaCNS+2Na\rightarrow NaCN+Na_{2}S$
$3NaCNS+FeCl_{3}\rightarrow Fe(CNS)_{3}+NaCl$
Ferric sulphocynanide
(Blood red in colour)
Question 40. Name the compounds whose line formulae are given below:
Answer:
(i) 3-Ethyl-4-methylheptan-5-en-2-one (because the longest chain of carbon atoms is selected in such a way that the functional group > C = O gets lowest possible locant number)
(ii) 3-Nitrocyclohex-1-ene. (Carbon atoms of the ring are numbered in such a way that double bonded carbon gets the lowest number followed by the nitro group –$NO_{2}$)
Question 41. Write structural formulae for compounds named as- (a) 1-Bromoheptane (b) 5-Bromoheptanoic acid
Answer:
(a) $CH_{3}-CH_{2}-CH_{2}-CH_{2}-CH_{2}-CH_{2}-CH_{2}-Br$
7 6 5 4 3 2 1
(b)
Question 43. Identify the most stable species in the following set of ions giving reasons:
Answer:
(i) $CH_{3}^{+}$ will be more stable. This is because bromine atom destabilises the positive charge on a carbon atom. Bromine atom has a lone pair of electrons and is from the electron-withdrawing group.
(ii) $^-CCl_{3}$ will be most stable. This is because chlorine is more electron-withdrawing atom. The negative charge on carbon will be stabilised by the chlorine atom. As the number of chlorine atoms that are attached to carbocation increase, its stability also enhances.
Question 44. Give three points of differences between inductive effect and resonance effect.
Answer:
|
S.No |
Inductive effect |
Resonance effect |
|
1 |
This is due to displacement of $\sigma$ electrons in saturated compounds. |
This is due to s displacement of the $\pi$ electrons or the lone pair of electrons in unsaturated and conjugated compounds. |
|
2. |
Partial +ve or –ve charge is developed. |
Complete +ve or –ve charge is developed. |
|
3 |
Inductive effect is effective only upto 3 to 4 carbons. |
Movement of electrons takes place along the length of the conjugated system. |
Question 45. Which of the following compounds will not exist as resonance hybrid. Give reason for your answer:
$(i) CH_{3} OH$
$(ii) R-CONH_{2}$
$(iii) CH_{3}CH = CHCH_{2}NH_{2}$
Answer:
$(i) CH_{3} OH$: It does not show resonance because there are no electrons.
$(ii) R-CONH_{2}$ : It shows resonance due to the presence of lone pair of electrons on N atom and electrons on C = O bond. Hence it can be represented by three resonating structures.
$(iii) CH_{3}CH = CHCH_{2}NH_{2}$ : Lone pair of electrons on N atom, is not conjugated with the $\pi$ electrons of double bond, so no resonance.
Question 46. Why does $SO_{3}$ act as an electrophile?
Answer:
$SO_{3}$ has total three highly electronegative oxygen atoms, that are attracted to the sulphur atom in $SO_{3}$ thus making sulphur electron deficient, that is it gets +ve and thus acts as an electrophile.
Question 47. Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
Answer:
Structure I, propenal, is more stable than structure II because structure I has more covalent bonds in its resonating structures and moreover in structure II has only six electrons on its terminal carbon making it less stable.
Because both the components above have a large difference in their boiling points, so simple distillation method is used to separate. The temperature when it rises to the low boiling point the hydrocarbon vapours are only formed without any contamination of alcohol.
Question 49. Which of the two structures (A) and (B) given below is more stabilised by resonance? Explain.
(A) $CH_{3}COOH$ and
Answer:
Structure ‘B’ is more stabilised as it does not involve charge separation.. Hence $CH_{3}COO^{-}$ is more stable.
(A)
$CH_{3}COOH$
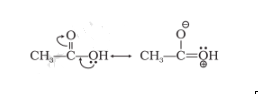
NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: Matching Type
Organic Chemistry Some Basic Principles and Technique Class 11 chemistry Chapter 12 important questions are discussed below. These are generally asked in exams to test your knowledge. These exemplar solutions is quite helpful for competitive exams.
|
Column I |
Column II |
|
(i) Two solids which have different solubilities in a solvent, and which do not undergo reaction when dissolved in it. |
(a) Steam distillation |
|
(ii) Liquid that decomposes at its boiling point |
(b) Fractional distillation |
|
(iii) Steam volatile liquid |
(c) Simple distillation |
|
(iv) Two liquids which have boiling points close to each other |
(d) Distillation under reduced pressure |
|
(v) Two liquids with a large difference in boiling points. |
(e) Crystallisation |
Answer:
(i) → (e); (ii) → (d); (iii) → (a);(iv) → (b); (v) → (c)
Question 51. Match the terms mentioned in Column I with the terms in Column II.
|
Column I |
Column II |
|
(i) Carbocation |
(a) Cyclohexane and 1-hexene |
|
(ii) Nucleophile |
(b) Conjugation of electrons of C – H $\sigma$ bond with empty p-orbital present at adjacent positively charged carbon. |
|
(iii) Hyperconjugation |
(c) sp2 hybridised carbon with empty p-orbital |
|
(iv) Isomers |
(d) Ethyne |
|
(v) sp hybridisation |
(e) Species that can receive a pair of electrons |
|
(vi) Electrophile |
(f) Species that can supply a pair of electrons |
Answer:
(i) →(c);(ii) → (f); (iii) → (b); (iv) → (a); (v) → (d); (vi) → (e)
Question 52. Match Column I with Column II.
|
Column I |
Column II |
|
(i) Dumas method |
(a) $AgNO_{3}$ |
|
(ii) Kjeldahl method |
(b) Silica gel |
|
(iii) Carius method |
(c) Nitrogen gel |
|
(iv) Chromatography |
(d) Free radicals |
|
(v) Homolysis |
(e) Ammonium sulphate |
Answer:
(i) → (c); (ii) → (e); (iii) → (a); (iv) → (b);(v) → (d)
Question 53. Match the intermediates given in Column I with their probable structure in Column II.
|
Column I |
Column II |
|
(i) Free radical |
(a) Trigonal planar |
|
(ii) Carbocation |
(b) Pyramidal |
|
(iii) Carbanion |
(c) Linear |
Answer:
(i) → (a); (ii) → (a); (iii) → (b)
Question 54. Match the ions given in Column I with their nature-given in Column II.
|
Column I |
Column II |
|
(i) |
(a) Stable due to resonance |
|
(ii) |
(b) Destabilised due to inductive effect |
|
(iii) |
(c) Stabilised due to hyperconjugation |
|
(iv) |
(d) A secondary carbocation |
Answer:
(i) → (a), (b), (d) (ii) → (b) (iii) → (b) (iv) → (c), (d)
NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: Assertion and Reason Type
This is one of the most important sections covered in the NCERT exemplar solutions Class 11 chemistry chapter 12 Some Basic Principles and Technique. These questions will improve your critical thinking.The most typical and important section for exams
Question 55. In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Simple distillation can help in separating a mixture of propan-1-ol (boiling point $97{^\circ}C$) and propanone (boiling point $56{^\circ}C$).
Reason (R): Liquids with a difference of more than $20{^\circ}C$ in their boiling points can be separated by simple distillation.
(i) Both A and R are correct, and R is the correct explanation of A.
(ii) Both A and R are correct, but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
The answer is the option (i) Both A and R are correct, and R is the correct explanation of A.
Explanation: The liquids given above have quite a difference in their boiling points and liquids which have different boiling points also vaporise at different temperatures. Vapours gets condensed and is then collected separately.
Question 56. In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Energy of resonance hybrid is equal to the average of energies of all canonical forms.
Reason (R): Resonance hybrid cannot be presented by a single structure.
(i) Both A and R are correct, and R is the correct explanation of A.
(ii) Both A and R are correct, but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
The answer is the option (iv) A is not correct, but R is correct.
Explanation: Canonical structures always have more energy as compared to the resonance hybrid, and so the Resonance hybrids are always more stable than any of the canonical structures. The delocalisation of electrons lowers the orbitals energy in case of resonance hybrid and gives stability.
Question 57. In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Pent- 1- ene and pent- 2- ene are position isomers.
Reason (R): Position isomers differ in the position of functional group or a substituent.
(i) Both A and R are correct, and R is the correct explanation of A.
(ii) Both A and R are correct, but R is not the correct explanation of A
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct
Answer:
The answer is the option (i) Both A and R are correct, and R is the correct explanation of A.
Explanation: When two or more compounds have functional groups or substituent atoms attached at different positions on the carbon skeleton, they are termed as position isomers and this phenomenon is termed as position isomerism
Question 58. In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): All the carbon atoms in $H_{2}C=C=CH_{2}$ are $sp^{2}$ hybridised
Reason (R): In this molecule, all the carbon atoms are attached to each other by double bonds.
(i) Both A and R are correct, and R is the correct explanation of A.
(ii) Both A and R are correct, but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
The answer is the option (iv) A is not correct, but R is correct.
Explanation: Hybridisation of C can be found out by counting the number of $\sigma$ bonds and $\pi$ bonds present on the C atom. If C has 3$\sigma$ bonds, it is $sp^2$ hybridised. If C has 2$\sigma$ bonds, it is sp hybridised.
Question 59. In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Sulphur present in an organic compound can be estimated quantitatively by Carious method.
Reason (R): Sulphur is separated easily from other atoms in the molecule and gets precipitated as light yellow solid.
(i) Both A and R are correct, and R is the correct explanation of A.
(ii) Both A and R are correct, but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
The answer is the option (iii) Both A and R are not correct.
Explanation: Sulphur is estimated by the Carius method in the form of white precipitate of $BaSO_{4}$ on heating with fuming and $BaCl_{2}$. If light yellow solid is obtained it means that impurities are present. It is then filtered, washed, and then dried to get pure $BaSO_{4}$.
The answer is the option (i) Both A and R are correct, and R is the correct explanation of A.
Explanation: In paper chromatography what is used, is a special quality paper known as chromatography paper, which contains water trapped in it, and which acts as the stationary phase. A strip of chromatography paper which is spotted at its base is suspended with the solution of the mixture in a suitable solvent or a mixture of solvents. This solvent acts as the mobile phase. The solvent rises up the paper by the capillary action and flows all over to the spot. The paper now selectively retains different components according to their differing partition in the two phases. The paper strip so developed is now known as a chromatogram.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 12: Long Answer Type
These are NCERT exemplar solutions Class 11 chemistry chapter 12 Some Basic Principles and Technique long-answer type questions that needs more practice.
Answer:
Atomic orbitals combine together to form a new set of orbitals which are termed as the hybrid orbitals and different from pure orbitals, these hybrid orbitals are used in the bond formation and this particular phenomenon is known as hybridisation, which can be defined as the process of intermixing of orbitals of different energy levels so as to redistribute their energies, which results in the formation of a new set of orbitals of equivalent energies and shape.
As the two-terminal Carbon atoms are forming three sigma bonds, therefore the hybridisation will be $sp^{2}$. Whereas the central carbon atom is forming only two sigma bonds so, therefore the hybridisation will be sp.
It is known to us that the hybridisation of atoms is predicted by the total numbers of the sigma bonds formed by that particular atom and the lone pair of electrons present at the atom.
The p-orbitals in one plane overlap with one of the p-orbital of left terminal carbon atom and the p-orbital in other plane overlaps with p-orbital of right side terminal carbon atom. This fixes the position of two terminal carbon atoms and the hydrogen atoms attached to them in planes perpendicular to each other. Due to this the pair of hydrogen atoms attached to terminal carbon atoms are present in different planes.
Hence, the molecule $CH_{2} = C = CH_{2}$ is an not a planar molecule.
As per the principle of crystallisation, it is known to us that the solute must be more soluble in hot water and less soluble in the cold water or any other similar solvent.
Now, the solubility of Benzoic acid in water is lesser as it is an organic compound. Moreover, it is less polar, whereas, in comparison, the water molecule is highly polar. But when the temperature of the water is increased, we observed that the solubility of benzoic acid is better in hot water in comparison to the cold water. The other reason being that the impurities are not insoluble in the water. Therefore, they can be filtered from the benzoic acid solution (if not soluble) or they would remain dissolved even after cooling the solution(if completely soluble in cold water). They will not be interfering with recrystallisation of the benzoic acid, as the extra solution can be discarded once the process of crystallisation is over.
The properties of benzoic acid and the impurity which makes this process of purification suitable are: -
-
Impurities which are present in benzoic acid are either insoluble in water or soluble in water to such an extent that they remain in solution.
-
The solubility of benzoic acid is slightly higher in hot water as compared to cold water.
The two liquids which we are considering above does not have much difference in their boiling points, and so simple distillation cannot just be used for the separation process. So the technique of fractional distillation is used in very such cases, which is a technique where vapours of a liquid mixture are passed through a fractionating column before condensation and the column used for fractionating is fitted over the mouth of the round bottom flask and the liquid with low boiling point distils first.
When vapours of a liquid mixture are passed through a fractionating column, the capours of the low boiling liquid (A) will move up while those of the high boiling liquid will condense and fall back into the flask. Therefore, liquid (A) with low boiling point will distill first.
As per the information available in the question, liquid A boils at a higher temperature than B and C and boiling point of B is lower than C.
Therefore, the correct order of boiling point for all three compounds is:
A > C > B
As it has been provided in the question that there is a significant difference in the boiling points of A and B and C. So, it is possible to separate liquid A from the mixture through the process of simple distillation. The setup for simple distillation is: -
As the remaining two liquids B and C have close boiling points; thus the process of fractional distillation will be used for the separation of the two. The setup for fractional distillation is
Answer:
The two liquids which we are considering above does not have much difference in their boiling points and so dimple distillation cannot just be used for the separation process. So, the technique of fractional distillation is used in such very cases, which is a technique where vapours of a liquid mixture are passed through a fractionating column before condensation and the column used for fractionating is fitted over the mouth of the round bottom flask and the liquid with low boiling point distills first.
Question 66. A liquid with high boiling point decomposes on simple distillation but it can be steam distilled for its purification. Explain how is it possible?
Answer:
Steam distillation is a different type of separation process for temperature sensitive materials like natural organic compounds. What happens with some organic compounds is that they decompose at higher temperatures and thus normal distillation does not suit the purpose. So, steam or water is added to the apparatus and the temperature of the compounds are depressed as a reason of that, evaporation happens at lower temperatures. Steam distillation is useful for separation if substances that are volatile, insoluble in water and have high vapour pressure at the boiling point of water i.e. $100^{\circ}C$. Then after distillation is over vapours are condensed and hence constituents separate at ease.
Class 12 Chemistry NCERT Chapter 12: Higher Order Thinking Skills (HOTS) Questions
These Class 11 Chemistry NCERT Exemplar Solutions Chapter 12 Organic Chemistry Some Basic Principles and Technique are designed to test deep conceptual understanding and application of concepts. They require analytical thinking, problem-solving skills, and logical reasoning.
Question 1: A Mixture of 1 g each of chlorobenzene, aniline and benzoic acid is dissolved in 50 mL ethyl acetate and placed in a separating funnel, 5 M NaOH ( 30 mL ) was added in the same funnel. The funnel was shaken vigorously and then kept aside. The ethyl acetate layer in the funnel contains:
1) benzoic acid
2) benzoic acid and aniline
3) benzoic acid and chlorobenzene
4) chlorobenzene and aniline
Solution:
NaOH and benzoic acid will react to form a salt. Aniline and chlorobenzene, being organic compounds, will remain in the ethyl acetate layer as they will not react with NaOH.
Hence, the correct answer is option (4).
Question 2: Given below are two statements :
Statement I : Hyperconjugation is not a permanent effect.
Statement II : In general, greater the number of alkyl groups attached to a positively charged C -atom, greater is the hyperconjugation interaction and stabilization of the cation.
In the light of the above statements, choose the correct answer from the options given below
1) Statement I is true but Statement II is false
2) Both Statement I and Statement II are false
3) Statement I is false but Statement II is true
4) Both Statement I and Statement II are true
Solution:
Hyper conjugation is a permanent effect because an external reagent is not required, so Statement I is false and Statement II is true. because more alkyl group, more $\alpha-\mathrm{H}$, so more hyperconjugation which results more stability of carbocation.
Hence, the correct answer is option (3).
Question 3: Total number of sigma $(\sigma)$ ______ and $\mathrm{pi}(\pi)$ _______ bonds respectively present in hex-1-en-4-yne are :
(1) 13 and 3
(2) 11 and 3
(3) 3 and 13
(4) 14 and 3
Solution:
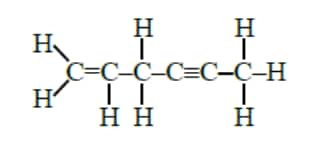
$\sigma$ bonds $=13$
$\pi$ bonds $=3$
Hence, the correct answer is option (1).
Question 4: If total number of plane of symmetry in a molecule of terephthalic acid be ' $x$ ' and total number of elements of symmetry (excluding axis and alternate axis of symmetry) in a molecule of meso-2,3-dichlorobutane be ' $y$ ' and total number of stereocentres in pentan-2-ol be ' $z$ ', then the value of $(x+y+z)$ will be $\_\_\_\_$ .
Answer:
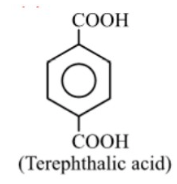
It has 3 planes of symmetry, i.e., x = 3
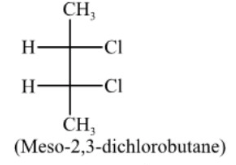
It has one plane of symmetry and one centre of symmetry, i.e., y = 2
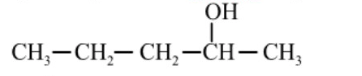
It has one plane of symmetry, i.e., z = 1
then the value of $(x+y+z)$ is 6 .
Hence, the answer is 6.
Question 5: Given below are two statements :
Statement I: Steam distillation technique is applied to separate substances which are steam volatile and are immiscible with water.
Statement II: In steam distillation, the liquid boils when the sum of vapour pressures due to the organic liquid $\left(p_1\right)$ and that due to water $\left(p_2\right)$ becomes equal to the atmospheric pressure (p), i.e. $p=p_1+p_2$.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Statement I is correct but Statement II is incorrect.
(2) Statement I is incorrect but Statement II is correct.
(3) Both Statement I and Statement II are correct.
(4) Both Statement I and Statement II are incorrect.
Answer:
Statement I: Steam distillation technique is applied to separate substances which are steam volatile and are immiscible with water.
Statement II: In steam distillation, the liquid boils when the sum of vapour pressures due to the organic liquid $\left(p_1\right)$ and that due to water $\left(p_2\right)$ becomes equal to the atmospheric pressure (p), i.e. $p=p_1+p_2$. Hence state 1 is incorrect and statement II is correct.
Hence, the correct answer is option (3).
Question 6: Given below are two statements :
Statement (I) : and
and 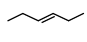 are isomeric compounds.
are isomeric compounds.
Statement (II) :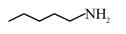 and
and 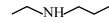 are functional group isomers.
are functional group isomers.
In the light of the above statements, choose the correct answer from the options given below :
(1) Both Statement I and Statement II are false
(2) Both Statement I and Statement II are true
(3) Statement I is true but Statement II is false
(4) Statement I is false but Statement II is true
Answer:
Statement-I $\rightarrow$ True
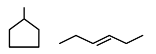
Both are ring chain isomers
Statement-II $\rightarrow$ True
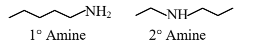
$1^{\circ}$ Amine and $2^{\circ}$ Amine are different functional groups, hence both are functional group isomers.
Hence, the correct answer is option (2).
Question 7: The correct order of stability of following carbocations is :
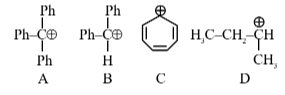
(1) $\mathrm{A}>\mathrm{B}>\mathrm{C}>\mathrm{D}$
(2) $\mathrm{B}>\mathrm{C}>\mathrm{A}>\mathrm{D}$
(3) $\mathrm{C}>\mathrm{B}>\mathrm{A}>\mathrm{D}$
(4) $\mathrm{C}>\mathrm{A}>\mathrm{B}>\mathrm{D}$
Answer:
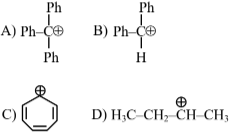
C is aromatic due to $\oplus \mathrm{ve}$ charge hence it is most stable
A have more resonance structure
B have less resonance structure
D have only hyper conjugation
Consider First Aromaticity > Resonance > Hyper conjugation
Ans. $\mathrm{D}<\mathrm{B}<\mathrm{A}<\mathrm{C}$
Hence, the correct answer is option (4).
Approach to Solve Questions of Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
The following are the points that can help you build a good approach to solve the questions of NCERT exemplar Class 11 chemistry chapter 12 Some Basic Principles and Technique as sometimes, problems related to organic chemistry seem difficult, but once we understand the basic rules and strategy, it becomes very easy to solve all the questions related to organic chemistry. We can follow the steps given below to these questions.
1) Before solving questions, a basic understanding of the topics is a must, like IUPAC Nomenclature, electronic effect and the types of organic reactions, classification of organic compounds, stability and reactivity of intermediates like carbocations, carbanions and free radicals.
2) Learn the type of organic compounds:
- Acyclic compounds
- Cyclic compounds
- Aromatic compounds
- Aliphatic compounds
- Saturated and unsaturated compounds
3) Read the question carefully and identify exactly what is being asked. Students also pay attention to conditions and reagents.
4) While solving questions it is important to identify which functional group is present and understand whether the reagent is acting as a nucleophile or an electrophile as these questions related to these topics are frequently asked in exams.
5) While solving Organic Chemistry Some Basic Principles and Technique Class 11 chemistry Chapter 12 related to molecular formulas or empirical formulas, make sure that you are using the given data and formulas accurately. Students can follow class 11 chemistry chapter 12 Organic Chemistry Some Basic Principles and Technique notes
6) Regularly revise topics and practice questions from NCERT books also practice previous year questions.
Topic Of NCERT Class 11 Chemistry Organic Chemistry Some Basic Principles and Techniques
All the topics and subtopics covered in the Class 11 Chemistry NCERT Exemplar Solutions Chapter 12 Organic Chemistry Some Basic Principles and Technique are listed below:
- Tetravalence Of Carbon: Shapes Of Organic Compound
- Structural Representations Of Organic Compounds
- Classification Of Organic Compounds
- Nomenclature Of Organic Compounds
- Isomerism
- Fundamental Concepts In Organic Reaction Mechanism
- Methods Of Purification Of Organic Compounds
- Qualitative Analysis Of Organic Compounds
- Quantitative Analysis
Advantages of Using Class 11 Chemistry Chapter 8 Organic Chemistry: Some Basic Principles and Techniques NCERT Exemplar Solutions
NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic Chemistry Some Basic Principles and Technique covers all important concepts from the NCERT book in a simple and organised manner. The advantages of using these solutions are given below:
- These NCERT exemplar solutions Class 11 chemistry chapter 12 Some Basic Principles and Technique cover topics like classification of organic compounds, nomenclature, and types of reactions, electronic displacements, purification and characterisation of organic compounds.
- Students can understand the reaction mechanisms and the basics of organic chemistry using these Class 11 Chemistry NCERT Exemplar Solutions Chapter 12 Organic Chemistry Some Basic Principles and Technique.
- These NCERT Exemplar Class 11 Solutions are prepared by subject experts in a very clear and comprehensive manner to help students understand the difficult concepts easily and perform well in exams.
NCERT Exemplar Class 11 Chemistry Solutions
The table below contains the link to the NCERT exemplar solutions for other chapters as well. Do check them out and give a push to your preparations. Referring to these solutions will ensure thorough revision of all important topics.
NCERT Solutions for Class 11 Chemistry Chapter-wise
Follow the links provided in the table below to get hands-on exemplar solutions of other subjects as well. These solutions will help you practise a variety of questions and help you strengthen conceptual understanding.
NCERT Exemplar Solutions Class 11 Subject-Wise
Follow the links provided in the table below to get hands-on exemplar solutions of other subjects as well:
NCERT Solution subject-wise
The NCERT subject-wise solutions will help you broaden your concepts and will also help in revision. Learn more from Class 11 NCERT notes.
NCERT Notes subject-wise
You can follow the links given in the table below to get access to the Class 11 NCERT notes.
NCERT Books and NCERT Syllabus
You can find links to the Class 11 NCERT chemistry book and syllabus for the respective subjects.
| NCERT Books Class 11 Chemistry |
| NCERT Syllabus Class 11 Chemistry |
| NCERT Books Class 11 |
| NCERT Syllabus Class 11 |
Frequently Asked Questions (FAQs)
The main objectives of studying organic chemistry in Class 11 include understanding the basic concepts of organic compounds, their classification, structure, and reactions. It helps students develop analytical and problem-solving skills essential for further studies in chemistry and related fields.
A homologous series is a group of organic compounds that possess the same functional group and similar chemical properties, while differing by a constant difference in molecular formula, usually by a CH₂ unit. For example, alkanes form a homologous series where each successive member has one more CH₂ group than the previous one.
Structural isomerism refers to compounds that have the same molecular formula but different structural formulas. This is significant as it showcases how the arrangement of atoms can lead to different physical and chemical properties. Understanding isomerism is crucial for grasping the diversity in organic compounds.
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. They are important because they determine the properties and reactivity of organic compounds. For example, the presence of a hydroxyl group (-OH) indicates that a compound is an alcohol.
NCERT exemplar Class 11 chemistry chapter 12 Some Basic Principles and Technique explain fundamental concepts like nomenclature, isomerism, and reaction mechanisms in a clear and structured manner, helping students build a strong base in organic chemistry.
You can download the Organic Chemistry Some Basic Principles and Technique Class 11 chemistry Chapter 12 from the official NCERT website or trusted educational platforms like Careers360 that offer free chapter-wise downloads.
NCERT exemplar Class 11 chemistry chapter 12 Some Basic Principles and Technique, which covers fundamental concepts such as nomenclature, isomerism, reaction mechanisms, and purification methods used in organic chemistry.
Chapter 12 explains the basics of organic chemistry, including nomenclature, isomerism, reaction mechanisms, and purification techniques.
It may seem challenging initially, but with practice, especially of nomenclature and mechanisms, it becomes easy to understand.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters






































