Hydrogen is the lightest element with a 1s¹ configuration, showing both alkali metal and halogen-like behaviour. It forms H₂ molecules, creates covalent bonds easily, and is highly abundant in nature.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 9 Hydrogen
Why is the lightest element also one of the most powerful? How can a single proton play a crucial role in fuel technology? Why does hydrogen behave like alkali metals sometimes and halogens at other times? NCERT Exemplar Class 11 Chemistry Solutions Chapter 9 Hydrogen answer all these questions. Hydrogen is the most abundant element in the universe, and it plays an important role in both chemistry and in our daily life. NCERT Exemplar Class 11 Chemistry solutions provides a detailed explanation of the properties of Hydrogen, important applications of hydrogen, and the principles and theories that govern its behaviour. This chapter also includes hydrogen's role in acid-base chemistry as a proton donor and its importance in hydrogenation reactions and fuel cells, and preparation methods of hydrogen, such as steam reforming, electrolysis of water, and other industrial processes.
This Story also Contains
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: MCQ (Type 1)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: MCQ (Type 2)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: Short Answer Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: Matching Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: Assertion and Reason Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: Long Answer Type
- Class 11 Chemistry NCERT Chapter 9: Higher Order Thinking Skills (HOTS) Questions
- Approaches to Solve Questions of Chapter 9 Hydrogen
- Topics and Subtopics in NCERT Exemplar Class 11 Chemistry Chapter 9: Hydrogen
- Advantages of Using NCERT Exemplar Solutions for Class 11 Chemistry Chapter 9 Hydrogen
- NCERT Exemplar Class 11 Chemistry Solutions Chapter-Wise
- NCERT Solutions for Class 11 Chemistry
- NCERT Solution subject-wise
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus

These NCERT Exemplar Solutions provide a valuable resource to enhance performance in board exams as well as in competitive exams. In this article, we will discuss detailed NCERT Solutions to all the questions. This article includes some higher-order thinking skills questions that are beyond memorization and promote conceptual understanding, improve analytical thinking, enhance application skills, and build confidence in chemistry.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: MCQ (Type 1)
NCERT Exemplar solutions Chapter 9 Hydrogen Class 11 Chemistry for all the MCQ (type 1) questions are given below. The chemistry chapter 9 Hydrogen important questions cover key concepts, reactions, and applications frequently asked in exams.
Question:1 Hydrogen resembles halogens in many respects, for which several factors are responsible. Of the following factors, which one is most important in this respect?
(i) Its tendency to lose an electron to form a cation.
(ii) Its tendency to gain a single electron in its valence shell to attain a stable electronic configuration.
(iii) Its low negative electron gain enthalpy value.
(iv) Its small size.
Answer:
The answer is the option (ii). Its tendency to gain a single electron in its valence shell to attain a stable electronic configuration.
Hydrogen resembles halogens as halogens with the configuration of $ns^{2} np^5$in the seventeenth group, also harbouring the ability to accept one electron and configure as an inert gas. Similarly, Hydrogen can also accept an electron to configure itself like helium.
$H \rightarrow He$
$1s^1$ $1s^2$
Question:2 Why does $H^+$ ion always get associated with other atoms or molecules?
(i) The ionisation enthalpy of Hydrogen resembles that of alkali metals.
(ii) Its reactivity is similar to halogens.
(iii) It resembles both alkali metals and halogens.
(iv) Loss of an electron from a hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to its small size, it cannot exist freely.
Answer:
The answer is the option (iv) Loss of an electron from a hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to its small size, it cannot exist freely.
A positive hydrogen ion is extremely small in size and cannot exist as a single atom but instead only exists as an association with other elements.
$H \rightarrow H^{+} + e^{-}$
Question:3 Metal hydrides are ionic, covalent or molecular in nature. Among LiH, NaH, KH, RbH, CsH, the correct order of increasing ionic character is
$(i) LiH > NaH > CsH > KH>RbH$
$(ii) LiH < NaH < KH < RbH < CsH$
$(iii) RbH > CsH > NaH > KH > LiH$
$(iv) NaH > CsH > RbH > LiH > KH$
Answer:
The answer is the option $(ii) LiH < NaH < KH < RbH < CsH$
This happens due to the reason that as the atomic size increases, the ionic, electropositive character of the metal hybrides also increases.
Question:4 Which of the following hydrides is an electron-precise hydride?
$(i) B_{2}H_{6}$
$(ii) NH_{3}$
$(iii) H_{2}O$
$(iv) CH_{4}$
Answer:
The answer is the option $(iv) CH_{4}$
$CH_{4}$ has the precise number of electrons to form normal covalent bonds and is a precise electron donor, in order to figure out their own Lewis structures.
Question:5 Radioactive elements emit $\alpha, \beta \;and\; \gamma$ rays and are characterised by their half-lives. The radioactive isotope of Hydrogen is
(i) Protium
(ii) Deuterium
(iii) Tritium
(iv) Hydronium
Answer:
The answer is the option (iii) Tritium
Tritium is radioactive as the neutron-proton ratio is more than 1.5, and tritium has n=2 and p=1.
Question:6 Consider the reactions
(A) $H_{2}O_{2}+2HI\rightarrow I_{2}+2H_{2}O$ (B) $HOCl+H_{2}O_{2}\rightarrow H_{3}O^{+}+Cl^{-}+O_{2}$
Which of the following statements is correct about $H_{2}O_{2}$ with reference to these reactions? Hydrogen peroxide is ________.
(i) an oxidising agent in both (A) and (B)
(ii) an oxidising agent in (A) and a reducing agent in (B)
(iii) a reducing agent in (A) and an oxidising agent in (B)
(iv) a reducing agent in both (A) and (B)
Answer:
The answer is the option (ii) an oxidizing agent in (A) and a reducing agent in (B)
-
$H_{2}O_{2}+2HI\rightarrow I_{2}+2H_{2}O$
-
$HOCl+H_{2}O_{2}\rightarrow H_{3}O^{+}+Cl^{-}+O_{2}$
Hydrogen peroxide in (A) is an oxidising agent as the oxygen in equation (a) gets reduced from $H_{2}O_{2} \;to\; H_{2}O$
$H_{2}O_{2}+2HI\rightarrow I_{2}+2H_{2}O$
Hydrogen peroxide in (B) is a reducing agent as oxygen increases from $H_{2}O_{2} \;to\;O_{2}$
Question:7 The oxide that gives $H_{2}O_{2}$ on treatment with dilute $H_{2}SO_4$ is —
$(i) PbO_{2}$
$(ii) BaO_{2}. 8H_{2}O + O_{2}$
$(iii) MnO_{2}$
$(iv) TiO_{2}$
Answer:
The answer is the option $(ii) BaO_{2}. 8H_{2}O + O_{2}$
This oxide has peroxide linkage $(O^{2-}_{2})$ when reacted with dilute $H_{2}SO_4$, it produces $H_2O$, however, dioxides do not produce the same products and the metal atom doesn’t give out water on treatment with dilute $H_{2}SO_4$.
Question:8 Which of the following equations depicts the oxidising nature of $H_{2}O_{2}$?
$(i)2MnO_{4}^{-}+6H^{+}+5H_{2}O_{2}\rightarrow 2Mn^{2+}+8H_{2}O+5O_{2}$
$(ii)2Fe^{3+}+2H^{+}+H_2O_{2}\rightarrow 2Fe^{2+}+2H_{2}O+O_{2}$
$(iii)2I^{-}+2H^{+}+H_{2}O_{2}\rightarrow I_{2}+2H_{2}O$
$(iv)KIO_{4}+H_{2}O_{2}\rightarrow KIO_{3}+H_{2}O+O_{2}$
Answer:
The answer is the option $(iii)2I^{-}+2H^{+}+H_{2}O_{2}\rightarrow I_{2}+2H_{2}O$
Iodine ions have a negative charge and are oxidised to form $I_{2}$. Therefore, $H_{2}O_{2}$ is the oxidizing agent.
Question:9 Which of the following equation depicts reducing nature of $H_2O_2$ ?
$(i) \;2\left [ Fe(CN) _{6}\right ]^{4-}+2H^{+} + H_{2}O_{2}\rightarrow 2\left [Fe \left ( CN \right )_{ 6}\right ]^{3-}+2H_{2}O$
$(ii)I_{2}+H_{2}O_{2}+2OH^{-}\rightarrow 2I^{-}+2H_{2}O+O_{2}$
$(iii)Mn^{2+}+H_{2}O_{2}\rightarrow Mn^{4+}+2OH^{-}$
$(iv)PbS+4H_{2}O_{2}\rightarrow PbSO_{4}+4H_{2}O$
Answer:
The answer is the option $(ii)I_{2}+H_{2}O_{2}+2OH^{-}\rightarrow 2I^{-}+2H_{2}O+O_{2}$
Iodine in the form of $I_{2}$ is reduced to $I^{-}$ and thus $H_2O_2$ acts as the reducing agent.
Question:10 Hydrogen peroxide is _________.
(i) an oxidising agent
(ii) a reducing agent
(iii) both an oxidising and a reducing agent
(iv) neither oxidising nor reducing agent
Answer:
The answer is the option (iii) both an oxidizing and a reducing agent
We have seen examples of $H_2O_2$ acting as both the reducing and oxidising agent.
Question:11 Which of the following reactions increases the production of dihydrogen from synthesis gas?
$(i)CH_{4}(g)+H_{2}O (g)\xrightarrow[Ni]{1270K} CO(g)+3H_{2}(g)$
$(ii)C(s)+H_{2}O (g)\overset{1270K}{\rightarrow}CO(g)+H_{2}(g)$
$(iii)CO(g)+H_{2}O (g)\xrightarrow[catalyst]{673K}CO_{2}(g)+H_{2}(g)$
$(iv)C_{2}H_{6}+2H_{2}O \xrightarrow[Ni]{1270K}2CO+5H_{2}$
Answer:
The answer is the option $(iii)CO(g)+H_{2}O (g)\xrightarrow[catalyst]{673K}CO_{2}(g)+H_{2}(g)$
Carbon monoxide can be reacted with syngas mixtures using steam and iron chromate as enhancers to produce extra dihydrogen.
Question:12 When sodium peroxide is treated with dilute sulphuric acid, we get ______.
(i) sodium sulphate and water
(ii) sodium sulphate and oxygen
(iii) sodium sulphate, Hydrogen and oxygen
(iv) sodium sulphate and hydrogen peroxide
Answer:
The answer is option (iv), sodium sulphate and hydrogen peroxide.
$Na_{2}O_{2} + H_{2}SO_{4}\rightarrow Na_{2}SO_{4} + H_{2}O_{2}$
Question:13 Hydrogen peroxide is obtained by the electrolysis of ______.
(i) water
(ii) sulphuric acid
(iii) hydrochloric acid
(iv) fused sodium peroxide
Answer:
The answer is the option (ii), sulphuric acid
$H_{2}SO_{4}\rightarrow H^{+} + HSO_{4}^{-1}$
$2 HSO_{4}^{-1} \rightarrow HO_{3}SOOSO_{3}H + 2e^{-}$
$HO_{3}SOOSO_{3}H + 2H_2O (hydrolysis) \rightarrow 2 H_{2}SO_{4} + H_{2}O_{2}$
Question:14 Which of the following reactions is an example of the use of water gas in the synthesis of other compounds?
$(i)CH_{4}(g)+H_{2}O (g)\xrightarrow[Ni]{1270K} CO(g)+H_{2}(g)$
$(ii)CO(g)+H_{2}O (g)\xrightarrow[catalyst]{673K}CO_{2}(g)+H_{2}(g)$
$(iii)C_{n}H_{2n+n}+nH_{2}O(g)\xrightarrow[Ni]{1270K}nCO+(2n+1)H_{2}$
$(iv)CO(g)+2H_{2}(g)\xrightarrow[catalyst]{cobalt}CH_{3}OH(l)$
Answer:
The answer is the option $(iv)CO(g)+2H_{2}(g)\xrightarrow[catalyst]{cobalt}CH_{3}OH(l)$
Methanol used water and gas for the synthesis of other compounds.
Question:15 Which of the following ions will cause hardness in the water sample?
$(i) Ca^{2+}$
$(ii) Na^{+}$
$(iii) Cl^{-}$
$(iv) K^{+}$
Answer:
The answer is the option $(i) Ca^{2+}$
These ions, often in the common forms of $Ca (HCO_3)$ or $CaCl_2$ will have an effect on the water by making it harsh and hard as the salts of calcium are soluble in water, making it hard.
Question:16 Which of the following compounds is used for water softening?
$(i) Ca_3 (PO_4)_2$
$(ii) Na_{3}PO_{4}$
$(iii) Na_{6}P_{6}O_{18}$
$(iv) Na_{2}HPO_{4}$
Answer:
The answer is the option $(iii) Na_{6}P_{6}O_{18}$
Commercially known as Calgon, sodium hexametaphosphate is used to treat water and make it soft.
$2CaCl_{2} + Na_{2}[Na_{4}(P0_{3})_{6}] \rightarrow Na_{2}[Ca_{2}(P0_{3})_{6}] + 4NaCl$
Question:17 Elements of which of the following group(s) of periodic table do not form hydrides.
(i) Groups 7, 8, 9
(ii) Group 13
(iii) Groups 15, 16, 17
(iv) Group 14
Answer:
The answer is the option (i) Group 7, 8, 9
These elements are unable to form hydrides.
Question:18 Only one element of ________ forms hydride.
(i) group 6
(ii) group 7
(iii) group 8
(iv) group 9
Answer:
The answer is the option (i) Group 6
Only Chromium (Cr) is the element in group 6, capable of forming a hydride.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: MCQ (Type 2)
Class 11 Chemistry NCERT Exemplar solutions Chapter 9 Hydrogen for all the MCQ (type 2) questions are given below. These solutions are provided here with simple explanations. Learn more through these advanced MCQs.
Question:19 Which of the following statements are not true for Hydrogen?
(i) It exists as a diatomic molecule.
(ii) It has one electron in the outermost shell.
(iii) It can lose an electron to form a cation, which can freely exist
(iv) It forms a large number of ionic compounds by losing an electron.
Answer:
The answer is the option (iii) & (iv), i.e. it can lose an electron to form a cation which can freely exist, and it forms a large number of ionic compounds by losing an electron.
Hydrogen cannot form ionic compounds by giving an electron but instead forms many covalent bonds by the sharing of electrons.
Question:20 Dihydrogen can be prepared on a commercial scale by different methods. In its preparation by the action of steam on hydrocarbons, a mixture of CO and H2 gas is formed. It is known as ____________.
(i) Water gas
(ii) Syngas
(iii) Producer gas
(iv) Industrial gas
Answer:
The answer is the option (i) and (ii), i.e. water gas and syngas.
Synthesis gas is a combination of CO and H2, also known as water gas.
Question:21 Which of the following statement(s) is/are correct in the case of heavy water?
(i) Heavy water is used as a moderator in a nuclear reactor.
(ii) Heavy water is more effective as a solvent than ordinary water.
(iii) Heavy water is more associated than ordinary water.
(iv) Heavy water has a lower boiling point than ordinary water.
Answer:
The answer is option (i) and (iii), i.e. Heavy water is used as a moderator in a nuclear reactor, and Heavy water is more associated than ordinary water.
It is associated with water as it has a higher mass as well as facilitates as a moderator in exchange reactions.
Question:22 Which of the following statements about Hydrogen are correct?
(i) Hydrogen has three isotopes, of which protium is the most common.
(ii) Hydrogen never acts as a cation in ionic salts.
(iii) Hydrogen ion, $H^+$, exists freely in solution.
(iv) Dihydrogen does not act as a reducing agent.
Answer:
The answer is the option (i) and (ii), i.e. Hydrogen has three isotopes of which protium is the most common, and Hydrogen never acts as a cation in ionic salts.
Protium, one of Hydrogen’s 3 isotopes, is the most common as well and due to its small atomic size, it does not act as a cation but is associated with other molecules and compounds.
Question:23 Some of the properties of water are described below. Which of them is/are not correct?
(i) Water is known to be a universal solvent.
(ii) Hydrogen bonding is present to a large extent in liquid water.
(iii) There is no hydrogen bonding in the frozen state of water.
(iv) Frozen water is heavier than liquid water.
Answer:
The answer is the option (iii) and (iv). There is no hydrogen bonding in the frozen state of water, and Frozen water is heavier than liquid water.
Water exhibits different properties in different states due to hydrogen bonding, which exists in a water molecule. Ice is lighter than water due to the fact that there are empty spaces in the tetrahedra of the hydrogen bonds.
Question:24 Hardness of water may be temporary or permanent. Permanent hardness is due to the presence of
(i) Chlorides of Ca and Mg in water
(ii) Sulphates of Ca and Mg in water
(iii) Hydrogen carbonates of Ca and Mg in water
(iv) Carbonates of alkali metals in water
Answer:
The answer is the option (i) and (ii): Chlorides of Ca and Mg in water and sulphate of Ca and Mg in water.
Two salts of calcium and magnesium, when found as compounds of carbonate, chloride, and sulfate, dissolve in the water and make it hard.
Question:25 Which of the following statements is correct?
(i) Elements of group 15 form electron-deficient hydrides.
(ii) All elements of group 14 form electron-precise hydrides.
(iii) Electron-precise hydrides have tetrahedral geometries.
(iv) Electron-rich hydrides can act as Lewis acids.
Answer:
The answer is the option (ii) and (iii). All elements of group 14 form electron-pair hydrides, and electron-pair hydrides have tetrahedral geometries.
All group 14 elements are tetrahedral in terms of geometry and form their own Lewis structures. They are electron-precise hydrides that have enough electrons.
Question:26 Which of the following statements is correct?
(i) Hydrides of group 13 act as Lewis acids.
(ii) Hydrides of group 14 are electron deficient hydrides.
(iii) Hydrides of group 14 act as Lewis acids.
(iv) Hydrides of group 15 act as Lewis bases.
Answer:
The answer is the option (i) and (iv), i.e. Hydrides of group 13 act as Lewis acids and Hydrides of group 15 act as Lewis bases.
All the elements of group 13 are hydrides, which act as Lewis acids since they form electron-deficient compounds. The group 14 elements are known as electron-rich hydrides and thus have extra electrons, which are present in the form of lone pairs. Groups 15 to 17 have elements that form compounds that have 1 to 3 lone pairs and therefore act as Lewis bases.
(i) Metallic hydrides are deficient of Hydrogen.
(ii) Metallic hydrides conduct heat and electricity.
(iii) Ionic hydrides do not conduct electricity in solid state.
(iv) Ionic hydrides are very good conductors of electricity in solid state.
Answer:
The answer is the option (i), (ii), and (iii). Metallic hydrides are deficient of Hydrogen, Metallic hydrides conduct heat and electricity, and Ionic hydrides do not conduct electricity in the solid state.
Hydride and not volatile or conductive in the solid state, but are crystalline instead. While metallic hydrides are non-stoichiometric hydrides. They only conduct electricity in their molten state.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: Short Answer Type
Some short answer type questions from NCERT Exemplar Solutions for Class 11 Chemistry Hydrogen are given for practice. This section contains important questions that are asked in the exams.Practice short answer types from the questions below.
Question:28 How can the production of Hydrogen from water gas be increased by using a water gas shift reaction?
Answer:
The production of water gas is a chemical reaction. It is produced by superheated steam is passed over coal, nickel acts as the catalyst. The by-products are carbon dioxide and Hydrogen.
$CO + H_{2}O + H_{2} \xrightarrow[catalyst]{673K} CO_{2} + 2H_{2}$
Question:29 What are metallic/interstitial hydrides? How do they differ from molecular hydrides?
Answer:
Metallic hydrides are usually formed by d and f block elements. These hydrides are good conductors of heat and electricity; they lack Hydrogen, which makes them non-stoichiometric. These differ from molecular hydrides as they are formed by s, p block elements while the metals of group 7, 8, 9 do not form hydrides. Molecular hydrides are not good conductors of electricity or heat, unlike the metallic hydrides. They are also volatile compounds which have low melting and boiling points, but metallic hydrides, on the other hand, are hard in texture and have a certain metallic lustre.
Question:30 Name the classes of hydrides to which$H_2O$,$B_2H_6$ and $NaH$ belong.
Answer:
$H_2O$ – Is a molecular hydride which is covalent and electron-rich.
$B_2H_6$- Is a molecular hydride which is deficient in electrons.
$NaH$– Ionic hydride
Question:31 If the same mass of liquid water and a piece of ice is taken, then why is the density of ice less than that of liquid water?
Answer:
Ice and water have different structures and since water expands on freezing the volume of the same amount to ice is more than that of liquid water. Therefore, the density of water is much higher than that of ice and therefore, ice will float on water.
Question:32 Complete the following equations:
$(i)PbS(s)+H_{2}O_{2}(aq)\rightarrow$
$(ii)CO(g)+2H_{2} (g)\xrightarrow[catalyst]{cobalt}$
Answer:
$(i)PbS(s)+H_{2}O_{2}(aq)\rightarrow PbSO_{4}+2H_{2}O$
$(ii)CO(g)+2H_{2}(g)\xrightarrow[catalyst]{cobalt} CH_{3}OH$
Question:33 Give reasons:
(i) Lakes freeze from the top towards the bottom.
(ii) Ice floats on water.
Answer:
-
The lake freezes from top to bottom because the temperature during winter keeps on decreasing, and the movement of water happens in such a way that the cold water is heavier and so it sinks to the bottom. While warm water replaces it by coming on the surface. The process repeats until the temperature decreases below 4 degrees and the lake keeps on freezing from top to bottom.
-
The density of ice is less than water due to its structure, which forms empty spaces between the water molecules. 4 atoms of Hydrogen surround 1 of oxygen, and therefore make ice float on water
Question:34 What do you understand by the term ‘auto protolysis' of water? What is its significance?
Answer:
Autoprotolysis is the process when two similar molecules react with each other to produce products that are called ions with Proton transfer. Autoprotolysis of water means the transfer of one Proton from a certain molecule to another. This explains the ability of water act as both acid and base. Therefore, making it amphoteric in nature.
$H_2O (l) + H_2O (l) \overset{reversible}{\rightarrow} H_{3}O^{+} (aq) + OH^{-} (aq)$
Question:35 Discuss briefly de-mineralisation of water by ion exchange resin.
Answer:
Demineralization of water means that all the soluble salts present in water are removed through cation and anion exchange. In the cation exchange process, cations of sodium, calcium and magnesium replace those of Hydrogen. While the anion exchange process exchanges OH. These both combine to produce water.
$H^{+} + OH^{-} \rightarrow H_{2}O$
Question:36 Molecular hydrides are classified as electron-deficient, electron precise and electron-rich compounds. Explain each type with two examples.
Answer:
There are three major types of molecular hydrides.
Electron-precise hydrides are the ones that have just enough exact number of electrons to facilitate normal covalent bonds. These are the hydrides which are primarily comprised of group 14 elements: $CH_{4}$.
Electron-deficient hydrides are the ones which do not possess enough electrons to facilitate normal covalent bonds. Hydrides of group 13 a prime examples; $B_2H_6$.
Electron-rich hydrides are ones which have an excess number of electrons remaining after normal covalent bonds. Elements of group 15 16 17 are prime examples; $NH_3$.
Question:37 How is heavy water prepared? Compare its physical properties with those of ordinary water.
Answer:
Heavy water is prepared by the exhaustive electrolysis of water.
|
S.RNo. |
Property |
$H_{2}O$ |
$D_{2}O$ |
|
(i) |
Molecular mass (g/mol) |
18.015 |
20.027 |
|
(ii) |
Melting point (K) |
273.0 |
276.8 |
|
(iii) |
Boiling point (K) |
373.0 |
374.4 |
|
(iv) |
Density(298)g/cm |
1.0000 |
1.1059 |
|
(v) |
Enthalpy of vaporization(kJ/mol) |
40.6 |
41.61 |
Question:38 Write one chemical reaction for the preparation of $D_2O_2$.
Answer:
$D_{2}SO_{4}$ , when reacted in water over $BaO_{2}$ produces $D_{2}O_{2}$.
$BaO_{2} + D_{2}SO_{4}\rightarrow BaSO_{4} + D_{2}O_{2}$
Question:39 Calculate the strength of 5 volume $H_{2}O_{2}$ solution.
Answer:
5 volume $H_{2}O_{2}$solution signifies that 1 L of 5 volume $H_{2}O_{2}$ when decomposed, gives 5L of $O_2$ at NTP.
$H_{2}O_{2} \rightarrow 2H_{2}O + O_2$
$H_{2}O_{2}$- 68g
$2H_{2}O + O_2$– 22.4l at NTP
22.4L of $O_2$ at NTP is gotten from $H_{2}O_{2}$ is 68g
5L of $O_2$ at NTP is produced from $H_{2}O_{2}$ = $\frac{68}{22.4}\times5=15.18g$
But 5L of $O_2$ at NTP is from 5 volume of 1L $H_{2}O_{2}$
Strength of $H_{2}O_{2}$ in 5 volume $H_{2}O_{2}$ = 15.18L
% strength of $H_{2}O_{2}$ sol = $\frac{15.18}{1000}\times100=1.518$%
Question:40 (i) Draw the gas phase and solid phase structure of$H_2O_2$.
(ii) $H_2O_2$ is a better oxidising agent than water. Explain.
Answer:
(i) The structure of differ$H_2O_2$s according to its physical state, as explained below: -
ii) $H_2O_2$ is a better oxidising agent than water as it works in both acidic and alkaline media. Water as an oxidising agent is reduced to $H_{2}$ and reacts with only active metals with other conditions such as the electrode potential being less than -0.83V.
Question:41 Melting point, enthalpy of vapourisation and viscosity data of $H_{2}O$ and $D_{2}O$are given below:
|
Property | ||
|
Melting point / K |
373.0 |
374.4 |
|
Enthalpy of vapourisation at (373 K)/ kJ mol-1 |
40.66 |
41.61 |
|
Viscosity/centipoise |
0.8903 |
1.107 |
On the basis of this data, explain in which of these liquids intermolecular forces are stronger?
Answer:
The given properties, such as boiling point, enthalpy of vaporization, and viscosity,y are all dependent on the intermolecular forces of the liquids. Water has a lower intermolecular force of attraction as compared to $D_{2}O$ , and thus the values are lower for water.
Deuterium (D) is the isotope of Hydrogen that contains one proton and one neutron. Thus, on the reaction of water with deuterium, heavy water (deuterium oxide) is produced. Deuterium’s bond is stronger than the normal hydrogen bond, and thus $H_{2}$ reacts more with deuterium than oxygen.
$2D_{2} + O_{2} \rightarrow (heat) 2D_{2}O$
Question:43 Explain why HCl is a gas and HF is a liquid.
Answer:
F is a stronger electronegative than chlorine, and so forms a stronger bond with Hydrogen than chlorine. In order to break HF bonds, more energy is required as compared to break HCl bonds. This is the reason why HF has a higher boiling point than HCl and is a liquid at room temperature.
The first element is Hydrogen, and its molecular form is dihydrogen, so when it reacts with oxygen, it forms water, which is physical solid-state is ice and has a density lower than water, so it floats on water. Water has an amphoteric nature which acts as a base went around acids and vice versa.
$H_{2}O (as\;an\; acid) + NH_{3} \rightarrow NH4^{+} + OH^{-} (aq)$
$H_{2}O (as \;a\; base) + H_2S \rightarrow H_{3}O^{+} + HS^{-}$
Answer:
-
$H_{2}O_{2}$
-
The compound is used in many industries such as textile and paper, bleaching agent and is composed of light and dust particles. It is widely employed to curb pollution by reducing it through the oxidizing action of harmful cyanides and reducing the effluents. It can function as both an oxidising and reducing agent and thus is stored in the dark, away from dust, as these can cause harmful chemical reactions.
Question:46 Give reasons why Hydrogen resembles alkali metals?
Answer:
Hydrogen has a specific, unique electronic configuration which is similar to alkali metals but belongs to group 1 in the periodic table. Its strong nature,e like the alkali metals, due to its configuration, makes it give one electron to combine to form unipositive ions.
Question:47 Hydrogen generally forms covalent compounds. Give reason.
Answer:
Due to its configuration, Hydrogen also resembles certain halogens, given its ionization enthalpy. Therefore, when acting as a halogen, it forms hydrides by combining with the elements to form a diatomic molecule, along with covalent compounds.
Question:48 Why is the ionisation enthalpy of Hydrogen higher than that of sodium?
Answer:
Hydrogen has a higher ionization enthalpy than sodium because sodium has a different configuration that is in the last shell, the electron is in 3s, which, if lost, the configuration resembles neon, which is a noble gas. However, with Hydrogen, the case is different, and the electron is in the 1s orbital, which,h on losing, does not amount to attainment of noble gas configuration.
Hydrogen is an excellent source of energy. It can be alternate only used as fuel to empower automobiles and generate nuclear energy. It is looked at as a possible option and an economy run using Hydrogen as a fuel can be called as a hydrogen economy.
Hydrogen is freely abundant and replaceable, and so is regarded as a natural source since it also occurs as water, therefore can be largely utilised.
If Hydrogen is combusted, then it produces water and is not polluting for the environment.
A cell containing Hydrogen will give more power than the normal ones and as is looked at as the better alternative.
Hydrogen also acts as a great reducing agent and can be a substitute for the industries in place of carbon.
Question:50 What is the importance of heavy water?
Answer:
Heavy water is composed by electrolysis of water or through the fertilizer industries. It is a the great catalyst in nuclear reactors and can be used to study reactions.
Question:51 Write the Lewis structure of hydrogen peroxide.
Answer:
Question:52 An acidic solution of hydrogen peroxide behaves as an oxidising as well as the reducing agent. Illustrate it with the help of a chemical equation.
Answer:
1. Reactions which justify oxidising nature are-
H2O2 + 2KI $\rightarrow$ 2KOH + I2
2. Reactions which justify reducing nature are-
H2O2 + Cl2$\rightarrow$ 2HCl + O2
Question:53 With the help of suitable examples, explain the property of $H_{2}O_{2}$ that is responsible for its bleaching action?
Answer:
$H_{2}O_{2}$ serves as a great bleaching agent and disinfectant, commercially sold as perhydrol and disintegrates on exposure to direct light.
The bleaching action of H2O2 is due to labile oxygen, which it releases on decomposition.
H2O2 → H2O+[O]
The nascent oxygen combines with coloured material and makes it colourless. It can bleach feathers, silk, wool, paper, etc.
Question:54 Why is water molecule polar?
Answer:
The polar nature of the water molecule is due to the structure of the bent molecule and the bond length of 95.7pm and the angle of 104.5.
Question:55 Why does the water show a high boiling point as compared to hydrogen sulfide? Give reasons for your answer.
Answer:
Water has a higher boiling point than hydrogen sulfide because the hydrogen bonding in water is quite different, and hydrogen sulfide does not have hydrogen bonding at all.
Question:56 Why can dilute solutions of hydrogen peroxide not be concentrated by heating? How can a concentrated solution of hydrogen peroxide be obtained?
Answer:
Hydrogen peroxide causes burns when it is heated, so it should not be concentrated. You should use other methods other than heating to make it concentrated, such as distillation with water under pressure. Further distillation under pressure and careful extraction can give you pure hydrogen peroxide.
Question:57 Why is hydrogen peroxide stored in wax-lined bottles?
Answer:
Hydrogen peroxide is highly reactive, and that is stored in dark coloured bottles because it disintegrates on exposure to direct light.
$2 H_{2}O_{2} \rightarrow 2 H_{2}O + O_{2}$
Question:58 Why does hard water not form lather with soap?
Answer:
Hard water contains soluble salts which make it rough and form a precipitate when used along with soap. Due to the harsh chemicals dissolved in it, hard water is unsuitable for washing and laundry. The accumulation of salts makes it unsuitable for boilers.
Question:59 Phosphoric acid is preferred over sulphuric acid in preparing hydrogen peroxide from peroxides. Why?
Answer:
Phosphoric acid is preferred as it does not dissolve, but sulphuric acid can react with hydrogen peroxide to disintegrate and decompose.
Question:60 How will you account for $104.5^{\circ}$ bond angle in the water?
Answer:
$Sp^{3}$ hybridisation is present in oxygen along with a 109-degree angle, but the repulsive bond decreases the angle to a 104 degrees.
Question:61 Write redox reaction between fluorine and water?
Answer:
$2F_{2} (g) + 2H_{2}O \rightarrow 4H^{+} (aq) + 4F^{-} (aq) + O_{2}$
Question:62 Write two reactions to explain the amphoteric nature of water?
Answer:
$H_{2}O (l) + NH_{3} \rightarrow OH^{-} (aq) + NH_{4} (aq)$
$H_{2}O (l) + H_{2}S (aq) \rightarrow H_{2}O^{+} + HS^{-}$
NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: Matching Type
Hydrogen Class 11 Chemistry NCERT Exemplar solutions for all the matching type questions are given below. These are generally asked in exams to test your knowledge. These exemplar solutions is quite helpful for competitive exams.
|
Column I |
Column II |
|
(i) Synthesis gas |
(a) $Na_{2}\left [ Na_{4}(PO_{3})_{6} \right ]$ |
|
(ii) Dihydrogen |
(b) Oxidizing agent |
|
(iii) Heavy water |
(c) Softening agent |
|
(iv) Calgon |
(d) Reducing agent |
|
(v)Hydrogen Peroxide |
(e) Stochiometric compounds of s-block elements |
|
(vi) Salt-like hydrides |
(f) Prolonged electrolysis of water |
|
|
(g)Zn + NaOH |
|
|
(h) $Zn+dil. H_{2}SO_{4}$ |
|
|
(i) Synthesis of menthol |
|
|
(j) Mixture of CO and H2 |
Answer:
(i) → (i), (j)
(ii) → (d), (e), (g), (h), (i)
(iii) → (f)
(iv) → (a), (c)
(v) → (b), (d)
(vi) → (e)
Question:64 Match Column I with Column II for the given properties/applications mentioned therein.
|
Column I |
Column II |
|
(i) H |
(a) Used in the name perhydrol |
|
(ii) $H_{2}$ |
(b) Can be reduced to dihydrogen by NaH. |
|
(iii) $H_{2}O$ |
(c) Can be used in hydroformylation of olefin. |
|
(iv) $H_{2}O_{2}$ |
(d) Can be used in cutting and welding. |
Answer:
(i) → (d)
(ii) → (c)
(iii) → (b)
(iv) → (a)
Question:65 Match the terms in Column I with the relevant item in Column II.
|
Column I |
Column II |
|
(i) Electrolysis of water produces |
(a)atomic reactor |
|
(ii) Lithium aluminium hydride is used as |
(b) polar molecule |
|
(iii) Hydrogen chloride is a |
(c) recombines on the metal surface to generate high temperature |
|
(iv)Heavy water is used as |
(d) reducing agent |
|
(v) Atomic hydrogen |
(e) Hydrogen and oxygen |
Answer:
(i) → (e) (ii) → (d) (iii) → (b) (iv) → (a) (v) → (c)
Question:66 Match the items in Column I with the relevant item in Column II.
|
Column I |
Column II |
|
(i) Hydrogen peroxide is used as |
(a)zeolite |
|
(ii)Used in the Calgon method |
(b) perhydrol |
|
(iii) Permanent hardness of hard water is removed by |
(c) Sodium hexametaphosphate |
|
|
(d) propellant |
Answer:
i. → (b), (d)
ii. → (c)
iii. → (a), (c)
NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: Assertion and Reason Type
NCERT Class 11 Hydrogen Chemistry exemplar solutions for all the assertion and reason type questions are given below. This is one of the most important sections covered in the NCERT exemplar. These questions will improve your critical thinking.
Assertion (A): Permanent hardness of water is removed by treatment with washing soda.
Reason (R): Washing soda reacts with soluble magnesium and calcium sulphate to form insoluble carbonates.
(i) Statements A and R both are correct, and R is the correct explanation of A.
(ii) A is correct, but R is not correct.
(iii) A and R both are correct, but R is not the correct explanation of A.
(iv) A and R both are false.
Answer:
The answer is the option (i) Statements A and R both are correct, and R is the correct explanation of A.
Question:68 In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the options given below each question.
Assertion (A): Some metals like platinum and palladium can be used as storage media for Hydrogen.
Reason (R): Platinum and palladium can absorb large volumes of Hydrogen.
(i) Statements A and R both are correct, and R is the correct explanation of A.
(ii) A is correct, but R is not correct.
(iii) A and R both are correct, but R is not the correct explanation of A.
(iv) A and R both are false.
Answer:
The answer is the option (i) statements A and R both are correct, and R is the correct explanation of A.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 9: Long Answer Type
The following are the long-answer type questions that needs more practice. These are the chemistry chapter 9 Hydrogen important questions that are asked in the exams.
Question:69 Atomic Hydrogen combines with almost all elements, but molecular Hydrogen does not. Explain.
Answer:
Molecular Hydrogen is very stable, but atomic Hydrogen is very reactive, and the chemical behaviour of any molecule is determined by the bond dissociation enthalpy. In dihydrogen, the hydrogen bond dissociation enthalpy is very high, and therefore, it only reacts with a certain number of elements.
Answer:
Extended electrolysis of water can produce D2O; it differs from water as it has a high molecular mass.
|
|
|
$H_2O$ |
$D_2O$ |
|
1. |
Boiling Point |
373K |
373.4K |
|
2. |
Melting Point |
273K |
276.8K |
|
3. |
Molecular Mass |
18.016 |
20.3 |
Reactions showing the exchange of Hydrogen with deuterium
-
$NaOH + D_{2}O \rightarrow NaOD + HOD$
-
$HCl + D_{2}O \rightarrow DCl + HOD$
-
$NH_{4}Cl + D_{2}O \rightarrow NH_{3}DCl + HOD$
Question:71 How will you concentrate $H_{2}O_{2}$ ? Show differences between structures of $H_{2}O_{2}$ and $H_{2}O$ by drawing their spatial structures. Also mention three important uses of $H_{2}O_{2}$.
Answer:
To obtain hydrogen peroxide, you can use evaporation and barium peroxide by removing excess water. To make it more concentrated, use the process of distillation, and low pressure and then pure hydrogen peroxide can be obtained.
The spatial structures of $H_{2}O$ and $H_{2}O_{2}$ are as follows: -
Three important uses of $H_{2}O_{2}$ are as follows: -
-
Peroxide is used as a disinfectant and antiseptic in the market.
-
It is used to produce other chemicals in the industry and is also applied as a commercial bleaching product.
-
It finds great application in the textile industry.
Question:72 (i) Give a method for the manufacture of hydrogen peroxide and explain the reactions involved therein.
(ii) Illustrate oxidising, reducing and acidic properties of hydrogen peroxide with equations.
Answer:
Industrial preparation:$H_2O_2$ is prepared by the auto-oxidation of 2-alkylanthraquinols $H_2O (l) + H_2S (aq) \rightarrow$
$2Fe^{2+} + 2H^{+} + H_{2}O \rightarrow 2Fe^{3+} + 2 H_{2}O$
$PbS + H_{2}O_{2} \rightarrow PbSO_{4} + 4 H_{2}O$
(ii) (a) Reducing action in acidic medium
$2MnO^{-}_{4} + 6H^{+} + 5 H_{2}O_{2}\rightarrow 2Mn^{2+} + 8 H_{2}O + 5 O_{2}$
$HOCl + H_{2}O_{2} \rightarrow H_{3}O^{-} + Cl^{-} + O_{2}$
(b)Oxidising action in basic medium
$2Fe^{2+} + H_{2}O_{2} \rightarrow 2Fe^{3+} + 2OH^{-}$
(c)
$I_{2} + H_{2}O_{2} + OH^{-} \rightarrow 2I^{-} + 2 H_{2}O + O_{2}$
Answer:
We know that the molecular mass of $H_{2}O_2$ – 34
Mass of $H_{2}O_2$ present in 1 molar solution – 34 g
Therefore, the mass of $H_{2}O_2$ present in 2 litres of 1 molar solution – $2 \times 34 = 68g$
Hence, the mass of $H_{2}O_2$ that is present in 200ml solution of 1 molar $H_{2}O_2$ will be
$= \frac{34}{5}= 6.8g$
$2H_{2}O_{2} \rightarrow 2H_{2}O + O_{2}$
68g 32g
6.8g 3.2g
(i) A is Hydrogen Peroxide $(H_{2}O_{2})$ as it decomposes slowly on exposure to light and is stabilised by mixing urea to store in the presence of light.
Its possible structure is as follows: -
(ii) The chemical equations for its decomposition reaction in light are as follows-
$2H_{2}O_{2} (l)\rightarrow 2H_{2}O (l) + O_{2 }(g)$
Since the ionic hydride of alkali metal has a significant covalent character, therefore, it is LiH. Since LiH is very stable, therefore, it is almost unreactive towards O2?and Cl2. It reacts with Al2Cl6? form lithium aluminum hydride.
$8 \mathrm{LiH}+\mathrm{Al}_{2} \mathrm{Cl}_{6} \rightarrow 2 \mathrm{LiAlH}_{4}+6 \mathrm{LiCl}$
The ionic hydride of an alkali metal, which has a significant covalent character and is almost unreactive towards oxygen and chlorine at moderate temperatures, is lithium hydride is unreactive. That is why it is used for the synthesis of other useful hydrides.
Its reaction to $Al_{2}Cl_{6}$ can be explained as follows: -
$8LiH + Al_{2}Cl_{6} \rightarrow 2LiAlH_{4} + 6LiCl$
Saline hydrides produce dihydrogen gas during a violent reaction with water. The ionic solid crystal that is formed is non-conducting and non-volatile in nature, and reacts aggressively with water to produce dihydrogen gas.
The formula for this compound is NaH; its reaction with water is explained below: -
$NaH (s) + H_{2}O (aq) \rightarrow NaOH (aq) +H_{2} (g)$
Dihydrogen gas at the anode is liberated during electrolysis, which confirms the existence of H-ion
For students who wish to be able to reach out for queries and NCERT solutions from this chapter at any time so that they develop a strengthened understanding of the topic, there is a solution.
Class 11 Chemistry NCERT Chapter 9: Higher Order Thinking Skills (HOTS) Questions
Some higher-order thinking skills questions with solutions based on the NCERT Exemplar Class 11 Chemistry Solutions Chapter 9 Hydrogen are given below that will help you tackle complex problems. The questions below will help you evaluate your understanding of the concepts.
Question 1: $\mathrm{H}_2 \mathrm{O}_2$ acts as a reducing agent in
Options
1) $2 \mathrm{NaOCl}+\mathrm{H}_2 \mathrm{O}_2 \rightarrow 2 \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{O}_2$
2) $\mathrm{Na}_2 \mathrm{~S}+4 \mathrm{H}_2 \mathrm{O}_2 \rightarrow \mathrm{Na}_2 \mathrm{SO}_4+4 \mathrm{H}_2 \mathrm{O}$
3) $2 \mathrm{Fe}^{2+}+2 \mathrm{H}^{+}+\mathrm{H}_2 \mathrm{O}_2 \rightarrow 2 \mathrm{Fe}^{3+}+2 \mathrm{H}_2 \mathrm{O}$
4) $\mathrm{Mn}^{2+}+2 \mathrm{H}_2 \mathrm{O}_2 \rightarrow \mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O}$
Answer:
$2 \mathrm{NaO} \stackrel{+1}{\mathrm{Cl}}+\mathrm{H}_2 \mathrm{O}_2 \longrightarrow 2 \mathrm{Na} \stackrel{-1}{\mathrm{Cl}}+\mathrm{H}_2 \mathrm{O}+\mathrm{O}_2$
$\mathrm{H}_2 \mathrm{O}_2$ acts as a reducing agent.
Hence, the answer is option (1).
Question 2: The decreasing order of the hydrogen bonding in the following forms of water is correctly represented by
A.Liquid water
B. Ice
C. Impure water
Choose the correct answer from the options given below:
Options
1) $\mathrm{B}>\mathrm{A}>\mathrm{C}$
2) $\mathrm{A}>\mathrm{B}>\mathrm{C}$
3) $\mathrm{A}=\mathrm{B}>\mathrm{C}$
4) $\mathrm{C}>\mathrm{B}>\mathrm{A}$
Answer:
ice $>$ liquid water $>$ impure water
$\begin{aligned} & \text { ice }>\mathrm{H}_2 \mathrm{O} \text { liq. }>\text { impure } \mathrm{H}_2 \mathrm{O} \\ & \text { Hydrogen bond } \alpha \frac{1}{\text { Temp }}\end{aligned}$
Hence, the correct answer is option (1).
Question 3: Some of the properties of water are described below. Which of them is/are not correct?
(i) Water is known to be a universal solvent.
(ii) Hydrogen bonding is present to a large extent in liquid water.
(iii) There is no hydrogen bonding in the frozen state of water.
(iv) Frozen water is heavier than liquid water.
(1) (i) and (ii)
(2) (ii) and (iv)
(3) (iii) and (iv)
(4) None of the above
Answer:
There is no hydrogen bonding in the frozen state of water, and Frozen water is heavier than liquid water.
Water exhibits different properties in different states due to hydrogen bonding, which exists in a water molecule. Ice is lighter than water due to the fact that there are empty spaces in the tetrahedrons of the hydrogen bonds.
Hence, the answer is option (3).
Question 4: The starting material for the convenient preparation of deuterated hydrogen peroxide $\left(\mathrm{D}_2 \mathrm{O}_2\right)$ in the laboratory is:
(1) BaO
(2) $\mathrm{K}_2 \mathrm{~S}_2 \mathrm{O}_8$
(3) $\mathrm{BaO}_2$
(4) 2-ethylanthraquinone
Answer:
$2 \mathrm{HSO}_4^{-}(\mathrm{aq}) \xrightarrow{\text { Electrolysis }} \mathrm{HO}_3 \mathrm{SOOSO}_3 \mathrm{H}_{(\mathrm{aq})} \xrightarrow{\text { Hydrolysis }} 2 \mathrm{HSO}_4^{-}(\mathrm{aq})+2 \mathrm{H}^{+}$
This method is now used for laboratory preparation $\mathrm{D}_2 \mathrm{O}_2$
$\mathrm{K}_2 \mathrm{~S}_2 \mathrm{O}_8(\mathrm{~s})+2 \mathrm{D}_2 \mathrm{O}(\mathrm{l}) \longrightarrow 2 \mathrm{KDSO}_4(\mathrm{aq})+\mathrm{D}_2 \mathrm{O}_2(\mathrm{l})$
Hence, the answer is the option (2).
Question 5: Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason
Assertion A: Hydrogen is an environment friendly fuel.
Reason R: Atomic number of hydrogen is 1 and it is a very light element.
In the light of the above statements, choose the correct answer from the options given below
(1) A is true but R is false
(2) A is false but R is true
(3) Both and are true and A is the correct explanation of R
(4) Both and are true but is NOT the correct explanation of A
Answer:
No pollution occurs by combustion of hydrogen and very low density of hydrogen.
Hence, the answer is the option (4).
Question 6: $\mathrm{O}-\mathrm{O}$ bond length in $\mathrm{H}_2 \mathrm{O}_2$ is $\underline{\mathrm{X}}$ than the $\mathrm{O}-\mathrm{O}$ bond length in $\mathrm{F}_2 \mathrm{O}_2$. The $\mathrm{O}-\mathrm{H}$ bond length in $\mathrm{H}_2 \mathrm{O}_2$ is $\underline{Y}$ than that of the $\mathrm{O}-\mathrm{F}$ bond in $\mathrm{F}_2 \mathrm{O}_2$.
Choose the correct option for $X$ and $Y$ from those given below
(1) X-shorter, Y - longer
(2) X-shorter, Y-shorter
(3) X-longer, Y-shorter
(4) X-longer, Y-longer
Answer:
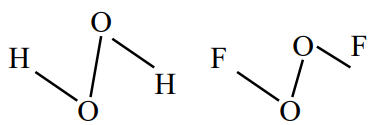
$\rightarrow(\mathrm{O}-\mathrm{O}) \mathrm{BL}$ in $\mathrm{H}_2 \mathrm{O}_2$ in longer then $(\mathrm{O}-\mathrm{O}) \mathrm{BL}$ in $\mathrm{O}_2 \mathrm{~F}_2$
$\rightarrow(\mathrm{O}-\mathrm{H}) \mathrm{BL}$ in $\mathrm{H}_2 \mathrm{O}_2$ in shorter than $(\mathrm{O}-\mathrm{F}) \mathrm{BL}$ in $\mathrm{O}_2 \mathrm{~F}_2$
Hence, the answer is the option (3).
Question 7: Given below are two statements :
Statement I : $\mathrm{H}_2 \mathrm{O}_2$ is used in the synthesis of Cephalosporin
Statement II : $\mathrm{H}_2 \mathrm{O}_2$ is used for the restoration of aerobic conditions to sewage wastes.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Both Statement I and Statement II are incorrect
(2) Statement I is incorrect but Statement II is correct
(3) Statement I is correct but Statement II is incorrect
(4) Both Statement I and Statement II are correct
Answer:
$\mathrm{H}_2 \mathrm{O}_2$ is used in the synthesis of hydroquinone, tartaric acid and certain food products and pharmaceuticals (cephalosporin). Nowadays it is also used in environmental (green) chemistry for example in pollution control treatment of domestic and industrial effluents, oxidation of cyanides restoration of aerobic conditions to sewage waste. Hence both statements are correct.
Hence, the answer is the option (4).
Approaches to Solve Questions of Chapter 9 Hydrogen
To solve class 11 chemistry Chapter 9 Hydrogen questions, it is important to follow a systematic approach. Given below the approaches to solve these questions effectively.
1. While solving questions from Hydrogen students are guided to first understand the basic concepts like Position of Hydrogen, Occurrence, Preparation of Dihydrogen, Properties of Dihydrogen, Electron Configuration.
2. It is very important to understand the position of hydrogen because many reasoning questions are asked on hydrogen dual resemblance with alkali metals and halogens. It is very important to understand the position of hydrogen because many reasoning questions are asked on hydrogen dual resemblance with alkali metals and halogens. Students can also refer to NCERT Class 11 Chapter 9 Hydrogen notes to understand these concepts in detail.
3. Questions from this topic are frequently asked in exams. Learn properties of protium, deuterium, tritium. Approach questions on isotopes by focusing on occurrence, stability, and uses.
4. Learn about the preparation methods from water, metals, and industrial processes, and then write the balanced equations with proper conditions like temperature or catalyst. For its properties, focus on physical features in short and emphasise chemical reactivity by writing reactions as these are commonly asked in exams.
5. Be thorough with the preparation, properties, and uses of compounds like water, heavy water, and hydrogen peroxide. Questions may also involve their structure, bonding, and behaviour in reactions. Understand the concept of hydrogen bonding in water and other compounds. Many reasoning questions test its role in anomalous properties of water, such as high boiling point and density anomali
6. Practice questions from NCERT Exemplar solutions Chapter 9 Hydrogen Class 11 Chemistry, as these questions are asked directly in boards and other competitive exams. For revision students can follow Class 11 Hydrogen Chemistry Notes. While solving questions, students must take care of a few basic points like, converting the units where required, and significant figures must be thoroughly checked.
Topics and Subtopics in NCERT Exemplar Class 11 Chemistry Chapter 9: Hydrogen
Class 11 Chemistry NCERT Exemplar solutions Chapter 9 includes the following topics. The chapter focuses on key concepts and reactions that are frequently asked in Class 11 exams.
- Position Of Hydrogen In The Periodic Table
- Dihydrogen, $\mathrm{H}_2$
- Occurrence
- Isotopes Of Hydrogen
- Preparation Of Dihydrogen, $\mathrm{H}_2$
- Laboratory Preparation Of Dihydrogen
- Commercial Production Of Dihydrogen
- Properties Of Dihydrogen
- Physical Properties
- Chemical Properties
- Uses Of Dihydrogen
- Hydrides
- Ionic Or Saline Hydrides
- Covalent Or Molecular Hydride
- Metallic Or Non-stoichiometric (Or Interstitial ) Hydrides
- Water Ex
- Physical Properties Of Water Ex
- Structure Of Water
- Structure Of Ice
- Chemical Properties Of Water
- Hard And Soft Water
- Temporary Hardness
- Permanent Hardness
- Hydrogen Peroxide ($\mathrm{H}_2 \mathrm{O}_2$)
- Preparation
- Physical Properties
- Structure
- Chemical Properties
- Storage
- Uses
- Heavy Water, $\mathrm{D}_2 \mathrm{O}$
- Dihydrogen as a Fuel
Advantages of Using NCERT Exemplar Solutions for Class 11 Chemistry Chapter 9 Hydrogen
Class 11 Chemistry NCERT Exemplar solutions Chapter 9 Hydrogen helps students understand the complete concepts of hydrogen. Give below some points on advantages of using these solutions:
- These solutions help students to understand the topics like isotopes, hydrides, and hydrogen properties with the help of solved questions.
- Using these NCERT Exemplar Solutions Class 11 questions help students to prepare effectively for boards and competitive exams.
- The solutions to class 11 chemistry Chapter 9 Hydrogen questions are prepared by subject experts in a very clear and comprehensive manner.
- Students can learn difficult concepts like position of hydrogen in the periodic table and its anomalous behaviour using these solutions of NCERT.
NCERT Exemplar Class 11 Chemistry Solutions Chapter-Wise
Besides NCERT Exemplar Class 11 Chemistry Solutions Chapter 9 Hydrogen, chapter-wise exemplar solutions are given below. These chapter-wise exemplar solutions are prepared to strengthen conceptual understanding and improve problem-solving skills for board and competitive exams.
NCERT Solutions for Class 11 Chemistry
NCERT Class 11 Chemistry chapter-wise solutions are given below. These solutions are prepared as per the latest NCERT syllabus to help students understand concepts clearly and prepare effectively for examinations.
NCERT Solution subject-wise
The NCERT subject-wise solutions will help you broaden your concepts and will also help in revision. Learn more from Class 11 NCERT notes.
NCERT Notes subject-wise
You can follow the links given in the table below to get access to the Class 11 NCERT notes.
NCERT Books and NCERT Syllabus
You can find links to the Class 11 NCERT chemistry book and syllabus for the respective subjects.
| NCERT Books Class 11 Chemistry |
| NCERT Syllabus Class 11 Chemistry |
| NCERT Books Class 11 |
| NCERT Syllabus Class 11 |
Frequently Asked Questions (FAQs)
Hydrogen is not a true alkali metal despite having 1 valence electron because it lacks metallic properties e.g., conductivity, lustre and forms covalent bonds, unlike alkali metals.
Hydrogen is found in vast quantities throughout the universe, primarily in stars like our Sun. On Earth, it is mostly found in compounds such as water (H₂O) and organic matter. Pure hydrogen gas is relatively rare in Earth's atmosphere.
The main focus of the NCERT Exemplar solutions Chapter 9 Hydrogen Class 11 Chemistry is to help students understand the occurrence, isotopes, preparation methods, physical and chemical properties, and important compounds of hydrogen.
Water is a universal solvent due to,
- Polarity (bent shape, δ⁺H and δ⁻O).
- Hydrogen bonding (dissolves polar/ionic compounds).
Fuel cells directly convert the chemical energy of a fuel (like hydrogen) into electricity, with water haet as byproducts. They are more efficient than internal combustion engines and also produce zero emissions
NCERT Exemplar Solutions for Class 11 Chemistry Hydrogen is a resource containing advanced, application-based questions designed to deepen understanding of hydrogen’s properties, isotopes, and compounds, helping students prepare for exams more effectively.
In, Hydrogen Class 11 Chemistry NCERT Exemplar solutions, hydrogen resembles halogens mainly because both need one electron to achieve a stable electronic configuration.
To prepare NCERT Exemplar solutions Chapter 9 Hydrogen Class 11 Chemistry, read the NCERT thoroughly, make short notes of key concepts like isotopes and hydrides, and practise NCERT Exemplar questions to strengthen understanding and improve exam readiness.
To prepare well, focus on understanding NCERT concepts, make concise notes, and revise regularly while solving exemplar questions and past papers to strengthen your exam readiness.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters






