In NCERT Exemplar Class 11 Chemistry Solutions Chapter 11 P-Block Elements students study Group 13 and Group 14 elements, including their properties, trends, important compounds.
NCERT Exemplar Class 11 Chemistry solutions Chapter 11 P-Block Elements
From life-saving medicines to rocket and space science, p-block elements have been a game changer. The Helium that makes our voice squeaky clean, nitrogen present in the air, and fluorine the most reactive element, all of them are p-block elements. By p-block element, we mean those atoms whose last electron is in the p-orbital. From groups 13 to 18, the p-block consists of metal, non-metal, and metalloids, all playing crucial roles in the biological, industrial, and environmental processes.
This Story also Contains
- NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: MCQ (Type 1)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: MCQ (Type 2)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: Short Answer Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: Matching Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: Assertion and Reason Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: Long Answer Type
- Class 12 Chemistry NCERT Chapter 11: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 11 The P-Block Elements
- Topics Of NCERT Exemplar Class 11 Chemistry Chapter: The p-block Elements
- Advantages of Using Class 11 Chemistry NCERT Exemplar Solutions Chapter 11The p-Block Elements
- NCERT Exemplar Class 11 Chemistry Solutions Chapter-wise
- NCERT Solutions for Class 11 Chemistry Chapter-wise
- NCERT Solution subject-wise
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus
The NCERT Exemplar Solutions is designed by our subject matter experts which ensures that the students understand key concepts by providing detailed explanations and easy-to-follow solutions. NCERT Exemplar Solutions for Class 11 Chemistry includes topics like periodic properties of p-block elements, chemical and physical properties, and a detailed study of all the families, viz. Boron, carbon, nitrogen, oxygen, fluorine, helium. By following the NCERT Solutions, students will learn the basics of the chapter and gain confidence to excel in the CBSE Board exams and in competitive exams as well.
NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: MCQ (Type 1)
At first, the MCQ questions are covered in the Class 11 Chemistry NCERT Exemplar Solutions Chapter 11 P-Block Elements to enhance your knowledge. The concepts are explained in detail in class 11 chemistry chapter 11 notes available on our website.
Question 1. The element which exists in a liquid state for a wide range of temperature and can be used for measuring high temperature is
(i) B
(ii) Al
(iii) Ga
(iv) In
Answer:
The answer is the option (iii) Ga
Galium has different structural properties and can remain in the liquid state for a wide range of temperature since it is melting point is 30°C and boiling point is 224°C.
Question 2. Which of the following is a Lewis acid?
$(i) AlCl_{3}$
$(ii) MgCl_{2}$
$(iii) CaCl_{2}$
$(iv) BaCl_{2}$
Answer:
The answer is the option $(i) AlCl_{3}$
AlCl3 is electron-deficient and ready to accept electrons and thus acts as a Lewis acid. It is an electron acceptor and a covalent compound.
Question 3. The geometry of a complex species can be understood from the knowledge of type of hybridisation of orbitals of central atom. The hybridisation of orbitals of central atom in $[Be(OH)_{4}]^{-}$ and the geometry of the complex are respectively
(i)$sp^{3}$ , tetrahedral
(ii)$sp^{3}$, square planar
(iii) $sp^{3}d^{2}$, octahedral
(iv) $dsp^{2}$ , square planar
Answer:
The answer is the option (i)$sp^{3}$ , tetrahedral
Boron in the excited state has 2s orbitals which are not paired and one electron which is in the p-orbital. In hybridization, the $sp^{3}$ hybridization is formed, and tetrahedral geometry is observed.
Electronic configuration of boron; $1s^{2}2s^{2}2px^{1}2p^{0}y2p^{^{\circ}}z$
Question 4. Which of the following oxides is acidic in nature?
$(i) B_{2}O_{3}$
$(ii) Al_{2}O_{3}$
$(iii) Ga_{2}O_{3}$
$(iv) In_{2}O_{3}$
Answer:
The answer is the option $(i) B_{2}O_{3}$ because it is acidic and forms metal borates on reaction with basic oxides.
Question 5. The exhibition of highest co-ordination number depends on the availability of vacant orbitals in the central atom. Which of the following elements is not likely to act as central atom in $MF^{3-}_{6}$?
(i) B
(ii) Al
(iii) Ga
(iv) In
Answer:
The answer is the option (i) B
The element M in the complex ion $MF^{3-}_{6}$ has 6 as the coordination number and the B has no d orbitals. Therefore the maximum coordination number it can show is 4 and cannot form the $MF^{3-}_{6}$.
Question 6. Boric acid is an acid because its molecule
(i) contains replaceable $H^{+}$ ion
(ii) gives up a proton
(iii) accepts $OH^{-}$ from water releasing proton
(iv) combines with proton from water molecule
Answer:
The answer is the option (iii) accepts $OH^{-}$from water releasing proton.
Boron has a small atomic size and only 6 electrons in the valence shell, $B(OH)_{3}$ releases a proton, when it accepts electrons from $OH^{-}$ ion of$H_{2}O$.
Question 7. Catenation i.e., linking of similar atoms depends on size and electronic configuration of atoms. The tendency of catenation in Group 14 elements follows the order:
$(i) C > Si > Ge > Sn$
$(ii) C >> Si > Ge \approx Sn$
$(iii) Si > C > Sn > Ge$
$(iv) Ge > Sn > Si > C$
Answer:
The answer is the option $(ii) C >> Si > Ge \approx Sn$
The atomic size decreases when there is movement along down the group and the bond energy, as well as the catenation property, also decreases. Therefore carbon shows maximum catenation in group 14.
Question 8. Silicon has a strong tendency to form polymers like silicones. The chain length of silicone polymer can be controlled by adding
$(i) MeSiCl_{3}$
$(ii) Me_{2}SiCl_{2}$
$(iii) Me_{3}SiCl$
$(iv) Me_{4}Si$
Answer:
The answer is the option $(iii) Me_{3}SiCl$
By adding $(CH_{3})_{3} SiCl$ the length of the chain can be controlled, and the ends are blocked.
Question 9. Ionisation enthalpy $(\Delta i H_1 kJ mol^{-1})$ for the elements of Group 13 follows the order.
$(i) B > Al > Ga > In > Tl$
$(ii) B < Al < Ga < In < Tl$
$(iii) B < Al > Ga < In > Tl$
$(iv) B > Al < Ga > In < Tl$
Answer:
The answer is the option l$(iv) B > Al < Ga > In < Tl$
The ionization energy decreases on moving down a group, and the atomic size increases. Till Ga, the ionization energy increases slightly but soon starts decreasing due to the shielding effect of d-electrons and increases once the shielding effect is less. Thus the nuclear energy increase when the ionization energy rises.
Question 10. In the structure of diborane
(i) All hydrogen atoms lie in one plane and boron atoms lie in a plane perpendicular to this plane.
(ii) 2 boron atoms and 4 terminal hydrogen atoms lie in the same plane and 2 bridging hydrogen atoms lie in the perpendicular plane.
(iii) 4 bridging hydrogen atoms and boron atoms lie in one plane and two terminal hydrogen atoms lie in a plane perpendicular to this plane.
(iv) All the atoms are in the same plane.
Answer:
The answer is the option (ii) 2 boron atoms and 4 terminal hydrogen atoms lie in the same plane and 2 bridging hydrogen atoms lie in the perpendicular plane.
Question 11. A compound X, of boron reacts with $NH_{3}$ on heating to give another compound Y which is called inorganic benzene. The compound X can be prepared by treating $BF_{3}$ with Lithium aluminium hydride. The compounds X and Y are represented by the formulas.
$(i) B_{2}H_{6} , B_{3}N_{3}H_{6}$
$(ii) B_{2}O_{3}, B_{3} N_{3} H_{6}$
$(iii) BF_{3}, B_{3}N_{3} H_{6}$
$(iv) B_{3}N_{3}H_{6} , B_{2}H_{6}$
Answer:
The answer is the option $(i) B_{2}H_{6} , B_{3}N_{3}H_{6}$
$3 \mathrm{~B}_2 \mathrm{H}_6+6 \mathrm{NH}_3 \rightarrow 3\left[\mathrm{BH}_2\left(\mathrm{NH}_3\right)_2\right]^{+}\left[\mathrm{BH}_4\right]^{-}$
Intermediate $\xrightarrow{\Delta} 2 \mathrm{~B}_3 \mathrm{~N}_3 \mathrm{H}_6+12 \mathrm{H}_2$
$4 B F_3+3 L i A l H_4 \rightarrow 2 B_2 H_6+3 L i F+3 A l F_3$
Question 12. Quartz is extensively used as a piezoelectric material, it contains ___________.
(i) Pb
(ii) Si
(iii) Ti
(iv) Sn
Answer:
The answer is the option (ii) Si because silica is the element that crystallizes to form Quartz.
Question 13. The most commonly used reducing agent is
$(i) AlCl_3$
$(ii) PbCl_2$
$(iii) SnCl_4$
$(iv) SnCl_{2}$
Answer:
The answer is the option $(iv) SnCl_{2}$
Sn has the oxidation state of +4 and is more stable than the other oxidation states. Thus it is easily oxidized to $Sn^{4+}$ and becomes a reducing agent.
$SnCl_{2} + 2Cl \rightarrow SnCl_{4} + 2e^{-}$
Question 14. Dry ice is
(i) Solid $NH_{3}$
(ii) Solid $SO_{2}$
(iii) Solid $CO_{2}$
(iv) Solid $N_{2}$
Answer:
The answer is the option (iii) Solid $CO_{2}$
Dry ice is solid $CO_{2}$.
Question 15. Cement, the important building material is a mixture of oxides of several elements. Besides calcium, iron and sulphur, oxides of elements of which of the group (s) are present in the mixture?
(i) group 2
(ii) groups 2, 13 and 14
(iii) groups 2 and 13
(iv) groups 2 and 14
Answer:
The answer is the option (ii) Groups 2,13 and 14
Elements of group 2- calcium and group 13- aluminium and group 14- silicon, constitute Cement.
NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: MCQ (Type 2)
The class 11 chemistry Chapter 11 P-Block Elements questions are provided here with simple explanations. Learn more through these advanced MCQs.
Question 16. The reason for the small radius of Ga compared to Al is _______.
(i) poor screening effect of d and f orbitals
(ii) increase in nuclear charge
(iii) presence of higher orbitals
(iv) higher atomic number
Answer:
The answer is the option (i) poor screening effect of d and f orbitals and (ii) increase in nuclear charge.
The poor screening effect of the electrons and additional 10d electrons increase charge in Ga, and thus the radius of Ga is less than Al.
Question 17. The linear shape of $CO_{2 }$ is due to _________.
(i) $sp^{3}$hybridisation of carbon
(ii) sp hybridisation of carbon
(iii) $p\pi - p\pi$ bonding between carbon and oxygen
(iv) $sp^{2}$ hybridisation of carbon
Answer:
The answer is the option (ii) sp hybridization of carbon and (iii) $p\pi - p\pi$ bonding between carbon and oxygen
$CO_{2 }$ has a structure which is linear, and the bonding of $p\pi - p\pi$structure leads is the reason.
Question 18. $Me_{3}SiCl$ is used during polymerisation of organic silicones because
(i) the chain length of organic silicone polymers can be controlled by adding $Me_{3}SiCl$
(ii) $Me_{3}SiCl$ blocks the end terminal of a silicone polymer
(iii) $Me_{3}SiCl$ improves the quality and yield of the polymer
(iv) $Me_{3}SiCl$ acts as a catalyst during polymerisation
Answer:
The answer is the option (i) The chain length of organic silicone polymers can be controlled by adding Me3 and (ii) $Me_{3}SiCl$ blocks the end terminal of a silicone polymer.
The length of the chain can be in control by increasing the amount of $Me_{3}SiCl$, which helps in blocking of the polymer ends.
Question 19. Which of the following statements are correct?
(i) Fullerenes have dangling bonds
(ii) Fullerenes are cage-like molecules
(iii) Graphite is a thermodynamically most stable allotrope of carbon
(iv) Graphite is slippery and hard and therefore used as a dry lubricant in Machines
Answer:
The answer is the option (ii)Fullerenes are cage-like molecules and (iii) Graphite is a thermodynamically most stable allotrope of carbon.
The cage-like molecules are known as Fullerenes and are known to be the most stable carbon allotropes.
Question 20. Which of the following statements are correct. Answer based on the given figure,
(i) The two bridged hydrogen atoms and the two boron atoms lie in one plane;
(ii) Out of six B–H bonds two bonds can be described in terms of 3 centre 2-electron bonds.
(iii) Out of six B-H bonds, four B-H bonds can be described in terms of 3 centres 2 electron bonds;
(iv) The four-terminal B-H bonds are two centre-two electron regular bonds.
Answer:
The answer is the option: -
(i) The two bridged hydrogen atoms and the two boron atoms lie in one plane.
(ii) Out of six B – H, bonds two bonds can be described in terms of 3 centre 2-electron bonds.
(iv) The four-terminal B – H bonds are two centre-two electron regular bonds.
The two boron atoms have a $sp^{3}$-hybrid state. Only three atoms have one electron each while the fourth is empty. They lie in one plane and two bridging hydrogen atoms.
Question 21. Identify the correct resonance structures of carbon dioxide from the ones given below :
(i) $\mathrm{O}-\mathrm{C} \equiv \mathrm{O}$
(ii) $\mathrm{O}=\mathrm{C}=\mathrm{O}$
(iii) ${ }^{-} \mathrm{O} \equiv \mathrm{C}-\mathrm{O}^{+}$
(iv) ${ }^{-} \mathrm{O}-\mathrm{C} \equiv \mathrm{O}^{+}$
Answer:
The answer is the option (ii) $\mathrm{O}=\mathrm{C}=\mathrm{O}$ and (iv) ${ }^{-} \mathrm{O}-\mathrm{C} \equiv \mathrm{O}^{+}$
NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: Short Answer Type
Here some short answer type questions from NCERT Exemplar Solutions for Class 11 Chemistry Chapter 11 P-Block Elements are given for practice. This section contains important questions that are asked in the exams.Practice short answer types from the questions below.
Question 22. Draw the structures of $BCl_{3}.NH_{3}$ and $AlCl_{3}$ (dimer)
Answer:
$BCl_{3}$ is the covalent compound which has 6 electrons in the valence shell. Thus it is an electron acceptor and needs 2 electrons to become an octet. It is a Lewis acid and can react with $NH_{3}$ as it donates electrons easily and is a Lewis Base. They combine as shown
In $AlCl_{3}$ , Al has 6 electrons in the valance shell and functions similarly as B and thus combines with Chlorine to form an octet.
Question 23. Explain the nature of boric acid as a Lewis acid in water.
Answer:
Boric acid is a weak monobasic acid that acts as a Lewis acid as it accepts electrons from a hydroxyl ion. Boric acid accepts $OH^{-}$ and then the formation of hydroxyl ion.
$B(OH)_{3} + 2H_{2}O\rightarrow [B(OH)_{4}]^{-} + H_{3}O^{+}$
Answer:
$B(OH)_{3} + 2H_{2}O \rightarrow [B(OH)_{4}]^{-} + H_{3}O^{+}$
The$H_{3}BO_{4}$ forms hexagonal rings through hydrogen bonds and is a weak acid. The hybridization is $sp^{3}$. Acting as an electron-deficient, it is a Lewis acid.
Question 25. Explain why the following compounds behave as Lewis acids?
$(i) BCl_{3}$
$(ii) AlCl_{3}$
Answer:
$(i) BCl_{3}$ – It is an electron-deficient compound due to the presence of 6 electrons in its outermost orbital along with a vacant p orbital. Therefore, it behaves as Lewis acid and accepts a lone pair of electrons.
$(ii) AlCl_{3}$ – It forms a covalent bond with chlorine through formation of three single bonds of chlorine. There are three electrons in the valence shell of Aluminium and thus it acts as an electron-deficient compound, therefore behaving as a Lewis acid.
Answer:
(i) $CCl_{4}$ is a compound that is unable to form H bonds with water as it is a polar compound. It is insoluble, and carbon does not have d orbital and cannot take in any extra electrons from the oxygen molecule. If the central atom cannot take in lone pair of electrons, then it cannot be hydrolyzed. $SiCl_{4}$ can undergo hydrolysis as it can accept a lone pair of electrons.
(ii) As the atomic size increases, the bond dissociation energy decreases, and since carbon has a size much smaller than silicon, then the dissociation energy of carbon is higher. Thus the carbon bonds are stronger and higher tendency for catenation than silicon.
Answer:
(i) In $CO_{2}$ , sp hybridization occurs, and the carbon atoms overlap to form two sigma bonds while $p\pi -p\pi$ bonding occurs with oxygen. This brings out the linear shape and no dipole energy.
Silicon dioxide is covalent and forms a tetrahedral structure, and each corner is covalent and shares with another tetrahedron.
(ii) The 3d orbitals in silicon are all in the valence shell, and thus the octet expands to give a $sp^{3}d^{2}$ hybridization. But carbon does not have d-orbitals present in the valence shell. It can only acquire $sp^{3}$ hybridisation. Thus, carbon is unable to form $CF_{6}^{2-}$ anion.
Answer:
The group 13 and 14, on going down a group, the energy to form bonds decreases. This is given the weak shielding of the s-electrons and the interference by the d-electrons. The inert pair effect arises, and thus the s-electrons, especially of groups 13 and 14, don’t indulge in bonding and the oxidation states of group 13 and 14 become stable due to an increase in atomic number.
Question 29. Carbon and silicon both belong to the group 14, but despite the stoichiometric similarity, the dioxides, (i.e., carbon dioxide and silicon dioxide), differ in their structures. Comment.
Answer:
Carbon in group 14 has the ability to form stable p-p bonds with itself and other elements of the first row.
With $CO_{2}$ the link formed between the atoms is a double bond.
The silicon does not form p-p bonds easily and instead link by single covalent bonds due to its large nuclear size.
Question 30. If a trivalent atom replaces a few silicon atoms in a three-dimensional network of silicon dioxide, what would be the type of charge on an overall structure?
Answer:
The Si atoms are in 3-dimensional structure in $SiO_{2}$, and the tetrahedral atoms are responsible for this. These atoms are replaced by trivalent atoms, and the valence electron is freely available, and thus there is one negative charge in the structure. This makes the entire compound negatively charged.
Answer:
$BCl_{3} + 3H_{2}O\rightarrow B(OH)_{3} + 3HCl$
$B(OH)_{3} + H_{2}O\rightarrow [B(OH)_{4}]^{-} + H^{+}$
$B(OH)_{3}$ in order to complete its octet, accepts an electron pair as $( OH^{-})$ to give $[B(OH)_{4}]^{-}$. Boron here has one 2s orbital and three 2p orbitals. Thus, hybridization of B is $sp^{3}$ in $[B(OH)_{4}]^{-}$
$AlCl_{3} + 6H_{2}O \rightarrow [Al(H_{2}O)_{6}]^{3+} + 3Cl^{-}$
The 6 H2O molecules get attached with Al i.e. they donate 6 electron pairs to the 3s,3p and 3d orbital of $Al^{3+}$ ion. Therefore, the hybridization of the Al atom in $[Al(H2O)_{6}]^{3+}$+ is $sp^{3}d^{2}$
Answer:
Al when dissolved in acids and alkalis and thus is amphoteric and thus gives out H2 gas which can be tested as it burns with a pop sound.
$2Al + 6HCl \rightarrow 2AlCl_{3} + 3H_{2}$
$2Al + 2NaOH + 2H_{2}O \rightarrow 2NaAlO_{2} + 3H_{2}$
When the nitric acid becomes passive, the reaction stops, and the thin layer of aluminium oxide ceases the reaction.
$2Al + 6HNO_3 \rightarrow Al_{2}O_{3} + 6NO_{2} + 3H_{2}O$
(i) The ionization energy of Al is lower than that of Ga and Ga has less d and f orbital electrons and thus has an increases nuclear charge in order to balance the screening effect.
(ii) The atomic size of Boron is small, and the energy of hydrogen is immensely high. Therefore Boron cannot ionize to form B3+ but instead forms covalent compounds.
(iii) The vacant orbitals in Al are the d orbitals which can expand, and the coordination number can rise to 6; thus Al undergoes sp3d2 hybridization to form [AlF6]3- ion, which is octahedral. But only 4 as the coordination number is possible and thus forms [BF4]– not [BF6]3-.
(iv) The +4 oxidation state of Pb is less stable than its +2 oxidation state given the inert pair effect. PbX4 has the oxidation state of Pb is +4 and is less stable than PbX2, which has the oxidation state of Pb is +2.
(v) Pb has a more dominant inert pair effect than Sn. Pb4+ can gain 2 electrons to become Pb 2+ which is more stable and then Sn2+ when it loses 2 electrons. Thus Pb is an oxidizing agent, and Sn is a reducing one.
(vi) F has a small atomic size, and the repulsion between the electrons is quite strong, and any extra electron is not accepted and as the Cl atom where the repulsion is weak. Thus the energy given out by Cl through gaining an electron is much lesser and negative as compared to F.
(vii)The +3 oxidation state of Tl is less stable than its +1 oxidation state due to the inert pair, and it is a strong effect. In Tl(NO3)3, Tl’s oxidation state is +3; therefore, it can accept two electrons to form TlNO3. This will make the oxidation state of Tl is +1. Thus it is an oxidizing agent.
(viii)The strength of element-element bond determines the strength and properties of catenation. This also depends on the atomic size and thus if carbon has a small atomic size in comparison to Pb, then the strength between the carbon bonds is much higher than that of Pb-Pb bonds which explains carbon’s higher tendency for catenation.
(ix)$BF_{3} + 3H_{2}O \rightarrow H_{3} BO_{3} + 3HF (x4)$
$H_{3} BO_{3} + 4HF \rightarrow H^{+} + BF_{4} ^{-} + 3H_{2}O (x3)$
$4 BF_{3} + 3H_{2}O \rightarrow H_{3} BO_{3} + 3[ BF_{4} ^{-}] + 3H^{+}$
$BF_{3}$ does not completely hydrolyze, and it forms boric and fluoroboric acid instead.
(x) Carbon is $sp^{2}$-hybridised to form graphite, and it forms hexagonal rings and links 3 other atoms. This leaves one p orbital which is unhybridized and forms double bonds instead. Thus this forms a layered structure and rings are fused together. Silicon is not formed out of carbon as it does not undergo the same hybridization or form p-p bonds but has three-dimensional network due to $sp^{3}$-hybridisation.
Question 34. Identify the compounds A, X and Z in the following reactions :
$A +2HCl+5H_{2}O\rightarrow 2NaCl+X$
$X\xrightarrow[370K]{\Delta }HBO_{2}\xrightarrow[>370K]{\Delta }Z$
Answer:
In the given reaction A is Borax which reacts with HCl in the presence of water to give Orthoboric acid i.e. X.
When the Orthoboric acid is heated, it gives metaboric and on further heating gives Boron trioxide i.e. the compound Z.
$BF_{3} + 3 LiAiH_{4} \rightarrow 2B_{2}H_{6} + 3 LiF + 3AlF_{3}$
$B_{2}H_{6} + 6H_{2}O \rightarrow 2H_{3}BO_{3} + 6H_{2}$
$3B_{2}H_{6} + 3O_{2} \rightarrow B_{2} O_{3} + 3 H_{2}O$
NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: Matching Type
Class 11 chemistry Chapter 11 P-Block Elements important questions are discussed below. These are generally asked in exams to test your knowledge. These exemplar solutions is quite helpful for competitive exams.
Question 36. Match the species given in Column I with the properties mentioned in Column II.
|
Column I |
Column II |
|
(i) $BF_4^{-}$ |
(a) Oxidation state of central atom is +4 |
|
(ii) $AlCl_{3}$ |
(b) Strong oxidising agent |
|
(iii) $ |
(c) Lewis acid |
|
(iv) $PbO_{2}$ |
(d) Can be further oxidised |
|
|
(e) Tetrahedral shape |
Answer:
(i→e);
$BF_4^{-}$: Tetrahedral shape
Due to the $sp^3$ hybridization, it has regular geometry with no lone pairs but 4 pairs that have bonded.
(ii → c);
$Al_{3}Cl_{3}$: Lewis acid
The octet of is incomplete and thus is an electron acceptor making it a Lewis acid.
(iii —> d);
$
\text { SnO }
$: Can be further oxidized
$Sn^{2+}$ can lose 2 more electrons to form a +4 oxidation state.
(iv →a, b)
$PbO_{2}$ : Oxidation state of the central atom is +4, Strong oxidizing agent
Question 37. Match the species given in Column I with properties given in Column II.
|
Column I |
Column II |
|
(i) Diborane |
(a) Used as a flux for soldering metals |
|
(ii) Gallium |
(b) Crystalline form of silica |
|
(iii) Borax |
(c) Banana bonds |
|
(iv) Aluminosilicate |
(d) Low melting, high boiling, useful for measuring high temperatures |
|
(v) Quartz |
(e) Used as catalyst in petrochemical industries |
Answer:
(i → c);
-
$\mathrm{BH}_3$ is not stable, has $sp^{3}$ hybridization and shows banana bonds, and forms diborane $B_{2}H_{6}$ by 3 centres -2 electron bonds
(ii → d)
-
Gallium has a different structure as it can exist in the liquid state for high ranges of temperature and the low melting point and high boiling point make it flexible to be used in fluctuating temperature.
(iii → a);
-
Borax is used as a flux for soldering metals and coating as it has a high melting point and can sustain heat.
(iv → e);
-
Aluminosilicates also contain zeolites and widely used as a catalyst in petrochemical industries.
(v → b)
-
Silica crystallizes to form Quartz.
Question 38. Match the species given in Column I with the hybridisation given in Column II.
|
Column I |
Column II |
|
(i) Boron in $[B(OH)_{4}]^-$ |
(a) $sp^2$ |
|
(ii) Aluminium in $[Al(H_{2}O)_{6}]^{3+}$ |
(b) $sp^{3}$ |
|
(iii) Boron in $B_{2}H_{6}$ |
(c) $sp^{3}d^{2}$ |
|
(iv) Carbon in Buckminsterfullerene |
|
|
(v) Silicon in $SiO_{4}^{4-}$ |
|
|
(vi) Germanium in $[GeCl_{6}]^{2-}$ |
|
Answer:
(i →b)
-
Boron in $[B(OH)_{4}]^{-}$ is $sp^{3}$ and is the central atom.
(ii→ c);
-
Aluminium in $[Al(H_{2}O)_{6}]^{3+}$ is $sp^{3}d^{2}$ hybridized and has octahedral geometry.
(iii → b);
-
Boron in $B_{2}H_{6}$ is $sp^{3}$, and the 4 hybrids have the same geometric configuration except one.
(iv→ a);
-
Carbon in Buckminsterfullerene $sp^2$ is hybridized. Each atom forms a sigma bond with 3 others.
(v →b);
-
Silicon in $SiO_{4}^{4-}$ is $sp^{3}$ and has tetrahedral geometry.
(vi→ c)
-
Germanium in $[GeCl_{6}]^{2-}$ is $sp^{3}d^{2}$ and has octahedral geometry.
NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: Assertion and Reason Type
This is one of the most important sections covered in the NCERT Exemplar Solutions for Class 11 Chemistry Chapter 11 P-Block Elements. These questions will improve your critical thinking.The most typical and important section for exams
Question 39. Assertion (A): If aluminium atoms replace a few silicon atoms in three dimensional network of silicon dioxide, the overall structure acquires a negative charge.
Reason (R) : Aluminium is trivalent while silicon is tetravalent.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct
(iv) A is not correct but R is correct.
Answer:
The answer is the option (i) Both A and R are true, and R is the correct explanation of A.
The aluminium atoms are trivalent while silicon’s tetravalency gives negatively charged ion.
Question 40. Assertion (A): Silicons are water-repelling in nature.
Reason (R): Silicons are organosilicon polymers, which have $(-R_{2}SiO-)$ as repeating unit.
(i) A and R both are correct, and R is the correct explanation of A.
(ii) Both A and R are correct, but R is not the correct explanation of A.
(iii) A and R both are not true.
(iv) A is not true but R is true.
Answer:
The answer is the option (ii) Both A and R are true, but R is not the correct explanation of A.
Silicones are hydrophobic and thus, are organosilicon polymers. So they neither react nor absorb water molecules.
NCERT Exemplar Class 11 Chemistry Solutions Chapter The p-block Elements: Long Answer Type
The following are the long-answer type questions that needs more practice. These are the chemistry Chapter 11 P-Block Elements important questions that are asked in the exams.
Answer:
(i) Group 13 has a greater atomic size as when going down a group, the atomic size increases, so an extra shell with electrons is added, and the poor shielding effect is observed.
Group14- These have a covalent radius, and a small radius is observed, and the presence of the shielding effect is high as there are completely filled d and f orbitals.
(ii) The ionization enthalpy is unaffected by the group, and it decreases with increase in size. Group 13 has erratic ionization enthalpy as the nuclear charge is complemented by the screening effect.
Group 14 experiences a high ionization enthalpy, and it subsequently decreases and due to the poor shielding effect.
(iii) All elements in group 13 are non-metallic except Boron, and the shielding effect is responsible for the low metallic character.
Group 14 has Sn and Pb as metals and the metallic character increases on going down the group, so the carbon is classified as a non-metal and all others are metalloids
(iv) Group 13 has prevalent +3 oxidation states due to the lone pairs but slowly change to +1 as the group order persist
Group14 has + 4, and +2 oxidation states and the enthalpies are high, and they are covalent in nature.
(v)Group 13 forms trihalides and some electron acceptors act as Lewis Acids.
Group 14 forms Halides by accepting electrons or sharing, making them covalent compounds.
(i) To complete the octet, it accepts electrons and thus acts as a Lewis Acid.
(ii) Boron’s vacant p orbital is not utilized and thus forms back bonding which is strong in F. The increasing atomic size decreases the strength of this bond, and thus Boron is completed using this bond
(iii)The inert pair effect is responsible, which increases in a group and Pb experiences this effect strongly and is an oxidizing agent.
(iv)The inert pair effect causes the strengthening of the oxidation state.
Question 43. When aqueous solution of borax is acidified with hydrochloric acid, a white crystalline solid is formed which is soapy to touch. Is this solid acidic or basic in nature? Explain.
Answer:
HCl acidifies the borax to give boric acid, which monobasic and weak. It needs to accept electrons in order to complete its octet and thus acts as a Lewis Acid.
$Na_{2}B_{4}O_{7} + HCl + H_{2}O\rightarrow 2NaCl + 4H_{3}BO_{3}$
$B(OH)_{3} + H_{2}O\rightarrow [B(OH)_{4}]^{-} +H^{+}$
i)
$\mathrm{TICl}$ is more stable as the inert pair effect strengthens the oxidation state.
ii)
$AlCl_3$ has no inert pair effect but instead is a covalent compound and thus accept electrons to become stable, acting as a Lewis acid.
iii)
Due to inert pair effect, Indium exists in both +1 and +3 oxidation states out of which +3 oxidation state is more stable is more stable than +1 oxidation state. In other words, $\mathrm{InCl}_3$ is more stable than $
\text { InCl. }
$
Question 45. $BCl_{3 }$ exists as monomer whereas $AlCl_{3}$ is dimerised through halogen bridging. Give reason. Explain the structure of the dimer of $AlCl_{3}$ also.
Answer:
$BCl_{3 }$ has 6 electrons in its valence shell and is in trivalent state. It requires two additional electrons to complete the octet. $BCl_{3 }$ act as a Lewis acid and $NH_{3}$ can donate its electron. Therefore, $BCl_{3 }$ readily accepts the lone pair of an electron from $NH_{3}$ and forms $BCl_{3}.NH_{3}.$
$BCl_{3} + NH_{3} \rightarrow BCl_{3}.NH_{3}$
$AlCl_{3}$ makes a stable molecule by forming a Dimer. Aluminium’s valence shell has 6 electrons and it requires 2 additional electrons for completing the octet. While chlorine has 3 lone pairs of electrons. Therefore, Aluminium takes up 1 lone pair and forms Dimer with chlorine.
Answer:
The lone pair of electrons in Boron fluoride form a pπ- pπ bonds and accept electrons to reduce the deficiency of them. Thereby reducing the ability of Boron to accept electrons and not becoming a Lewis Acid but instead increasing stability.
In $BH_3$ hydrogen has a lone pair. It therefore, cannot fulfil the deficiency of boron to dimerise to form $B_{2}H_{6}$ which is in the shape of a banana.
Answer:
(i) Silicones are organosilicon polymers and have a repeating unit. The alkyl groups form silicones and the methyl chloride in the presence of the copper as a catalyst with silicon form $Me_{4}Si$. The straight-chain polymers are formed by condensation and polymerization.
Uses: - They are used in industries as greasing agents and sealants as well as insulators. They are also expensively employed in the cosmetic industry for surgical implants.
(ii) The binary forms of boron and hydrogen form alkanes and the covalent hybrids are called diborane.
$4BF_{3} + 3LiAlH_{4} \rightarrow 2B_{2}H_6 + 3LiF + 3AlF_{3}$
Answer:
Compound A reacts with Boron and gives [B], and thus A is a Lewis acid as it accepts electrons. [B] reacts with [C] and liberates hydrogen and thus [A] is$B_{2}H_{6}$. B is thus $2BH_{3}NMe_{3}$ and C is boric acid.
A = $B_{2}H_{6}$ (DIBORANE)
B = $2BH_{3}NMe_{3}$ (ADDUCT)
C = $2B_{3}N_{3}H_{6}$ (INORGANIC BORAZINE)
$B_{2}H_{6} + 2NMe_{3}\rightarrow 2BH_{3}NMe_{3}$
$3 B_{2}H_{6} + 6NH_{3} \rightarrow 3[BH_{3}(NH_{3})_{2}]+[BH_{4}]^{+} \rightarrow 2B_{3}N_{3}H_{6} + 12H_{2}$
The element being discussed is Boron. It has an extremely high melting point and is black in colour along with being extremely hard.
Reaction of Boron Trifluoride with ammonia: - $BF_{3} + NH_{3} \rightarrow BF_{3}\leftarrow NH_{3}$
Monomeric trihalides are deficient in the electrons, thus they act as strong Lewis acid. In order to complete the octet of boron, trifluoride of boron reacts conveniently with Lewis bases like $NH_{3}$. Due to the absence of d electrons in boron, it is not able to exceed the above mentioned covalence.
The element being discussed is Carbon which is tetravalent. It can thus produce carbon monoxide and dioxide on reaction with oxygen.
-
$2C + O_{2} \rightarrow 2CO$ (As a result of the direct oxidation of carbon)
-
$HCOOH\rightarrow H_{2}O + CO$ (Dehydration of formic acid with concentrated $H_{2}SO_{4}$ at 373k)
-
$C + H_{2}O \rightarrow CO + H_{2}$ (The mixture of CO and $H_2$ is known as water gas)
-
$2C + O_{2} + 4N_{2} \rightarrow 2CO + 4N_{2}$ (The mixture of CO and$N_2$ is known as the producer gas)
Reduction Of Ferric Oxide With Monoxide
$Fe_{2}O_{3} + 3CO \rightarrow \rightarrow 2Fe + 3CO_2$
Class 12 Chemistry NCERT Chapter 11: Higher Order Thinking Skills (HOTS) Questions
Some class 11 chemistry Chapter 11 P-Block Elements questions and answers are given below that will help you tackle complex problems. The questions below will help you evaluate your understanding of the concepts.
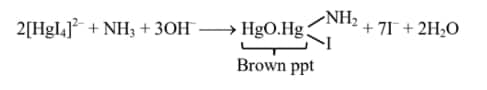
Question1: When a salt is treated with a sodium hydroxide solution, it gives gas X. On passing gas X through reagent Y, a brown-colored precipitate is formed. X and Y, respectively, are
Options
1) $\mathrm{X}=\mathrm{NH}_3$ and $\mathrm{Y}=\mathrm{HgO}$
2) $\mathrm{X}=\mathrm{NH}_3$ and $\mathrm{Y}=\mathrm{K}_2 \mathrm{HgI}_4+\mathrm{KOH}$
3) $\mathrm{X}=\mathrm{NH}_4 \mathrm{Cl}$ and $\mathrm{Y}=\mathrm{KOH}$
4) $\mathrm{X}=\mathrm{HCl}$ and $\mathrm{Y}=\mathrm{NH}_4 \mathrm{Cl}$
Answer:
$\mathrm{NH}_4^{+}+\mathrm{NaOH} \longrightarrow \mathrm{H}_2 \mathrm{O}+\mathrm{NH}_3 \uparrow$
$\mathrm{NH}_3$ is identify by $\mathrm{K}_2\left[\mathrm{HgI}_4\right]+\mathrm{KOH}$
Hence, the correct answer is option (2).
Question 2: A group 15 element forms $\mathrm{d} \pi-\mathrm{d} \pi$ bond with transition metals. It also forms hydride, which is a strongest base among the hydrides of other group members that form $\mathrm{d} \pi-\mathrm{d} \pi$ bond. The atomic number of the element is _______.
(1) 15
(2)16
(3)18
(4)14
Answer:
Phosphorus belongs to $15^{\text {th }}$ group and forms $\mathrm{d} \pi-\mathrm{d} \pi$ bond with transition metal and $\mathrm{PH}_3$ is strongest base among the other group members excepet $\mathrm{NH}_3$.
Atomic number of Phosphorous =15.
Hence, the answer is (15).
Question 3: Given below are two statements :
Statement I : $\mathrm{H}_2 \mathrm{Se}$ is more acidic than $\mathrm{H}_2 \mathrm{Te}$.
Statement II : $\mathrm{H}_2 \mathrm{Se}$ has higher bond enthalpy for dissociation than $\mathrm{H}_2 \mathrm{Te}$.
In the light of the above statements, choose the correct answer from the options given below.
(1) Both statement I and Statement II are false.
(2) Both statement I and Statement II are true.
(3) Statement I is true but Statement II is false.
(4) Statement I is false but Statement II is true.
Answer:
$\therefore \quad \mathrm{H}_2 \mathrm{Te}$ is more acidic than $\mathrm{H}_2 \mathrm{Se}$
because of lesser bond dissociation energy of $\mathrm{H}_2 \mathrm{Te}$
It can release $\mathrm{H}^{+}$more easily
$\mathrm{pK}_{\mathrm{a}_1}: \mathrm{H}_2 \mathrm{Se}(3.89)>\mathrm{H}_2 \mathrm{Te}(2.6)$
Hence, the correct answer is option (4).
Question 4: Which of the following occurs as a consequence of inert pair effect?
(A) $\mathrm{SnCl}_2$ acts as a reducing agent
(B) $\mathrm{SnCl}_4$ acts as an oxidising agent
(C) $\mathrm{SnO}_2$ is amphoteric
(D) $\mathrm{PbO}_2$ is an oxidant
(E) $\mathrm{CCl}_2$ is unstable but $\mathrm{PbCl}_2$ is stable
(1) $A, D, E$
(2) $D, E, C$
(3) $A, B, C, D, E$
(4) $A, B, C$
Answer:
The inert pair effect is the reluctance of the valence $n \mathrm{~s}^2$ electrons to participate in bonding in heavier p-block elements, so lower oxidation states (by 2 units) are increasingly stabilized down a group. In Group 14 this makes +2 more stable than +4 as we go from $\mathrm{C} \rightarrow \mathrm{Si} \rightarrow \mathrm{Ge} \rightarrow \mathrm{Sn} \rightarrow \mathrm{Pb}$. Consequently, Sn (II) tends to get oxidised to Sn (IV), so $\mathrm{SnCl}_2$ acts as a reducing agent (A true). $\mathrm{Pb}(\mathrm{IV})$ is comparatively unstable and readily reduces to $\mathrm{Pb}(\mathrm{II})$, so $\mathrm{PbO}_2$ is a strong oxidant (D true). Likewise, the +2 state is unstable for carbon but stable for lead, so $\mathrm{CCl}_2$ is unstable whereas $\mathrm{PbCl}_2$ is stable ( E true). By contrast, $\mathrm{SnCl}_4$ is not characteristically an oxidising agent; Sn (IV) in stannic chloride is fairly stable and behaves mainly as a Lewis acid rather than an oxidant (B false). $\mathrm{SnO}_2$ being amphoteric arises from borderline metallic/acid-base character, not from the inert pair effect specifically ( C not a consequence). Hence only A, D and E follow from the inert pair effect.
Hence, the correct answer is option (2).
Question 5: The correct statements from the following are :
(A) $\mathrm{Tl}^{3+}$ is a powerful oxidising agent
(B) $\mathrm{Al}^{3+}$ does not get reduced easily
(C) Both $\mathrm{Al}^{3+}$ and $\mathrm{Tl}^{3+}$ are very stable in solution
(D) $\mathrm{Tl}^{+}$is more stable than $\mathrm{Tl}^{3+}$
(E) $\mathrm{Al}^{3+}$ and $\mathrm{Tl}^{+}$are highly stable
Choose the correct answer from the options given below :
(1) (A), (B), (C), (D) and (E)
(2) (A), (B), (D) and (E) only
(3) (B), (D) and (E) only
(4) (A), (C) and (D) only
Answer:
$\mathrm{Tl}^{3+}$ is a powerful oxidizing agent as $\mathrm{Tl}^{+}$is more stable and $\mathrm{Tl}^{3+}$ is less stable in solution due to the inert pair effect. Thallium(III) is an unstable oxidation state, particularly in aqueous solutions.
Aluminum readily loses three electrons to achieve a noble gas configuration that makes the +3 oxidation state the most stable for aluminum.
Hence, the correct answer is option (2).
Question 6: The elements of Group 13 with the highest and lowest first ionization enthalpies are, respectively:
(1) B & Ga
(2) B & Tl
(3) Tl & B
(4) B & In
Answer:
Ionization enthalpy is the energy required to remove the outermost electron
1. General periodic trend:
- Increases across a period (left to right)
- Decreases down a group (top to bottom)
2. Exception- Group 13 shows anomalies due to poor shielding by d- and f-electrons in Ga, In, and Tl.
-
Boron (B): Smallest size, highest nuclear attraction → highest first ionization enthalpy
-
Thallium (Tl): Larger size, but relativistic effects and poor shielding by 4f electrons slightly increase ionization energy compared to In
-
Aluminium (Al): Has lower ionization enthalpy than B
-
Indium (In): Lowest first ionization enthalpy in the group due to maximum shielding effect
So, IE order
$\mathrm{B}>\mathrm{T} \ell>\mathrm{Ga}>\mathrm{Al}>\mathrm{In}$
Hence, the correct answer is option (4).
Question 7: The group 14 elements A and B have the first ionisation enthalpy values of 708 and $715 \mathrm{~kJ} \mathrm{~mol}^{-1}$ respectively. The above values are lowest among their group members. The nature of their ions $\mathrm{A}^{2+}$ $\mathrm{B}^{4+}$ respectively is
(1) both reducing
(2) both oxidising
(3) reducing and oxidising
(4) oxidising and reducing
Answer:
As per given information of ionisation energy
$\mathrm{A}=\mathrm{Sn} \& \mathrm{~B}=\mathrm{Pb}$
$\mathrm{A}^{+2}=\mathrm{Sn}^{2+}=$ Reducing agent (as can be oxidized to +4 oxidation state).
$\mathrm{B}^{+4}=\mathrm{Pb}^{+4}=$ Oxidizing agent (can be reduced to +2 oxidation state readily as +2 is more stable).
Hence, the correct answer is option (3).
Approach to Solve Questions of Chapter 11 The P-Block Elements
Approach to Solve Questions of Chapter 11 The P-Block Elements For better understanding of questions and ability to solve the questions in less time, following steps are useful:
1. While solving P-Block Elements Class 11 Chemistry NCERT Exemplar Solutions, students are guided to first understand the basic concepts about group 13 to 18, General valence shell configuration of p-Block elements, and recall all the basic concepts like atomic size, ionization energy, electronegativity, and oxidation states
2. Compare properties like oxidation states, electronegativity, ionization energy, atomic radius down the group and across the period.
3. Use inert pair effect for heavier elements, check which oxidation states are stable.
4. Acidic/basic/ amphoteric behavior of oxides and hydroxides, reducing vs oxidizing nature.
5. Key points for Group
Group 13
- Behavior of Boron is anomalous.
- Important Compounds of boron are Borax ($\mathrm{Na}_2 \mathrm{~B}_4 \mathrm{O}_7 \cdot 10 \mathrm{H}_2 \mathrm{O}$), Boric acid ($\mathrm{H}_3 \mathrm{BO}_3$), Diborane ($B_2 H_6$)
Group 14
- Allotropes of Carbon are Diamond, Graphite, Fullerenes.
- Catenation is the ability of Carbon to form long chains.
- Silicon Compounds like Silica ($\mathrm{SiO}_2$), Silicones ($\mathrm{R}_2 \mathrm{SiO}$)
Group 15
- Nitrogen is Inert due to strong $N \equiv N$ triple bond.
- Ammonia ($\mathrm{NH}_3$) is prepared by Haber’s process.
- Oxyacids of Nitrogen are $\mathrm{HNO}_3$(Nitric Acid), $\mathrm{HNO}_2$ (Nitrous Acid)
Group 16
- Ozone $\left(O_3\right)$ is an oxidizing agent.
- Allotropes of sulphur are Rhombic, Monoclinic,$\mathrm{H}_2 \mathrm{SO}_4$.
- Oxoacids of Sulphur are $\mathrm{H}_2 \mathrm{SO}_3, \mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_7$.
Group 17
- Reactivity decreases down the group $\left(\mathrm{F}_2>\mathrm{Cl}_2>\mathrm{Br}_2>\mathrm{I}_2\right)$.
- Oxoacids of Chlorine are $\mathrm{HClO}_4$ (Perchloric Acid), $ \mathrm{HClO}$ (Hypochlorous Acid).
- Interhalogens ae$\mathrm{ClF}_3, \mathrm{IF}_7$.
Group 18
- Xenon Compounds like $\mathrm{XeF}_2, \mathrm{XeF}_4, \mathrm{XeO}_3$ (sp ${ }^3 \mathrm{~d}$ hybridization).
- Structure Drawing Example: Practice the structure of $\mathrm{XeF}_2, \mathrm{XeF}_4, \mathrm{XeO}_3$ (sp ${ }^3 \mathrm{~d}$ hybridization).
6. Practice questions from NCERT, as these questions are asked directly in boards and other competitive exams. For revision students can follow Class 11 P-Block Elements Notes. While solving questions, students must take care of a few basic points like, converting the units where required, and significant figures must be thoroughly checked.
Topics Of NCERT Exemplar Class 11 Chemistry Chapter: The p-block Elements
Class 11 Chemistry NCERT Exemplar solutions chapter 11 P-Block Elements cover the following topics. The chapter covers key concepts and reactions that are frequently asked in examinations.
A. Group 13 Elements: The Boron Family
i. Electronic Configuration
ii. Atomic Radii
iii. Ionization Enthalpy
iv. Electronegativity
v. Physical Properties
vi. Chemical Properties
B. Important Trends And Anomalous Properties Of Boron
C. Some Important Compounds Of Boron
i. Borax
ii. Orthoboric Acid
iii. Diborane, B2h6
D. Uses Of Boron And Aluminium And Their Compounds
E. Group 14 Elements: The Carbon Family
i. Electronic Configuration
ii. Covalent Radius
iii. Ionization Enthalpy
iv. Electronegativity
v. Physical Properties
vi. Chemical Properties
F. Important Trends And Anomalous Behaviour Of Carbon
G. Allotropes Of Carbon
i. Diamond
ii. Graphite
iii. Fullerenes
iv. Uses Of Carbon
H. Some Important Compounds Of Carbon And Silicon
i. Carbon Monoxide
ii. Carbon Dioxide
iii. Silicon Dioxide, Si02
iv. Silicones
v. Silicates
vi. Zeolites
Advantages of Using Class 11 Chemistry NCERT Exemplar Solutions Chapter 11The p-Block Elements
NCERT Exemplar Solutions for Class 11 Chemistry Chapter 11 P-Block Elements helps students understand the periodic trends and the behaviour of group 13 to 18 elements. Given below some poi ts on advantages of using these solutions:
- These P-Block Elements Class 11 Chemistry NCERT Exemplar Solutions help students to understand group 13 to 18 elements, their properties, trends, and important compounds in a clear and simple way.
- How concepts are applied in questions that help students to attempt questions easily are well explained in these NCERT Exemplar Solutions for Class 11.
- Class 11 Chemistry NCERT Exemplar Solutions Chapter 11 P-Block Elements are prepared by subject experts in a very clear and comprehensive manner.
- Students get step-by-step solutions to every question that will help them in CBSE boards and competitive exams.
NCERT Exemplar Class 11 Chemistry Solutions Chapter-wise
NCERT Exemplar problems are designed to strengthen conceptual understanding and analytical thinking. Students can follow the links given below to access all NCERT Exemplar Solutions for Class 11.
NCERT Solutions for Class 11 Chemistry Chapter-wise
Click on the links below to access the NCERT Class 11 solutions for all the chapters to make your preparations better. These chapter-wise solutions provide clear explanations and step-by-step answers for effective revision.
NCERT Solution subject-wise
The NCERT subject-wise solutions will help you broaden your concepts and will also help in revision. Learn more from Class 11 NCERT notes.
NCERT Notes subject-wise
You can follow the links given in the table below to get access to the Class 11 NCERT notes.
NCERT Books and NCERT Syllabus
You can find links to the Class 11 NCERT chemistry book and syllabus for the respective subjects.
Frequently Asked Questions (FAQs)
P-block elements are those found in groups 13 to 18 of the periodic table. They include elements such as boron, carbon, nitrogen, oxygen, fluorine, and noble gases. These elements are characterized by the filling of the p orbital, which influences their chemical properties and reactivity.
P-block elements play crucial roles in everyday life. For example, carbon is the foundation of organic chemistry and thus life itself. Oxygen is essential for respiration, while nitrogen is a key component of proteins and DNA. Other p-block elements are used in various household products, fertilizers, and even in technological applications.
The properties of P-block elements vary across the groups due to differences in their electron configurations. For instance, as you move from top to bottom within a group, atomic size increases, and the ionization energy typically decreases.
P-block elements can form a wide variety of compounds. For example, phosphorus can form phosphine, while oxygen typically forms water. Carbon forms carbon dioxide and a plethora of organic compounds.
Noble gases, such as helium, neon, and argon, are unique because they have a complete valence shell, making them very stable and largely unreactive under standard conditions. This low reactivity allows them to be used in a variety of applications, like lighting and inert atmospheres in chemical processes.
The NCERT Exemplar PDF for class 11 chemistry Chapter 11 P-Block Elements questions provides advanced, concept-based questions with solutions that help students understand the properties and reactions of group 13 and 14 elements in depth.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 11 P-Block Elements refer to elements whose last electron enters the p-subshell. This chapter mainly covers Group 13 and Group 14 elements, including their properties, trends, compounds, and anomalous behaviour.
To prepare well, focus on understanding NCERT concepts thoroughly, make clear notes, and revise regularly. Practise exemplar questions, sample papers, and previous years’ questions to strengthen problem-solving skills and improve exam confidence.
Studying 2–3 focused hours daily with proper revision and practice of NCERT and exemplar questions is usually enough to stay consistent and well-prepared for Class 11 exams.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters