NCERT Exemplar Class 12 Chemistry Solutions Surface Chemistry provide detailed answers to all textbook questions, helping students understand concepts like adsorption, catalysis, and colloids.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 States of Matter
Do you know why steam burns skin more severely than water, why pressure cookers cooks food faster, and why we feel cold after spraying deodorants? The answer to all these questions lies in the NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 States of Matter. In this chapter, students will learn about fundamental topics of States of Matter, including intermolecular forces, kinetic molecular theory, Boyle’s law, Charles’ law, Avogadro’s law, Gay-Lussac’s law, and the ideal gas equation.
This Story also Contains
- NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: MCQ (Type 1)
- NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: MCQ (Type 2)
- NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: Short Answer Type
- NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: Matching Type
- NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: Assertion and Reason Type
- NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: Long Answer Type
- NCERT Exemplar Class 11 Chemistry Chapter 5: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Class 11 Chemistry Chapter 5
- Topics and Subtopics Covered in the NCERT Exemplar Class 11 Chemistry Chapter 5
- NCERT Exemplar Class 11 Chemistry Chapter 5: Important Formulas
- Advantages of Using Class 12 Chemistry NCERT Exemplar Solutions Chapter 5 Surface Chemistry
- NCERT Exemplar Solutions Class 11 Chemistry Chapter-Wise
- NCERT Solutions for Class 11 Chemistry Chapter-wise
- NCERT Solutions for Class 11 Subject-Wise
- NCERT Notes Subject-Wise
- NCERT Books and NCERT Syllabus
NCERT Exemplar Solutions are prepared by subject experts which provides detailed explanation to every question. These NCERT Exemplar Solutions Class 11 Chemistry explains several important concepts linked with the liquid and gaseous states of matter. In this article some higher-order thinking skills (HOTS) questions and approaches to solve questions are also included that helps in improving analytical thinking, enhancing application skills, and building confidence in chemistry.
NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: MCQ (Type 1)
MCQ-type questions are covered in the this chapter improves students conceptual thinking. Students can also follow States of matter Class 11 Chemistry notes , available on our website, to understand these concepts in detail. These NCERT Solutions provide a valuable resource to enhance performance in board exams as well as in the competitive exams.
Question 1. A person living in Shimla observed that cooking food without using pressure cooker takes more time. The reason for this observation is that at high altitude:
(i) pressure increases
(ii) temperature decreases
(iii) pressure decreases
(iv) temperature increases
Answer:
The answer is option (iii), pressure decreases.
Explanation: Due to low pressure at high altitudes, boiling takes place at low temperature.
Question 2. Which of the following property of water can be used to explain the spherical the shape of rain droplets?
(i) viscosity
(ii) surface tension
(iii) critical phenomena
(iv) pressure
Answer:
The answer is the option (ii) surface tension.
Explanation: We know that for a given volume, a sphere has a lower state of energy and minimum surface area. Hence, the raindrops are spherical.
Question 3. A plot of volume (V ) versus temperature (T ) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in Fig. 5.1. Which of the following order of pressure is correct for this gas?
(i) p1 > p2 > p3 > p4
(ii) p1 = p2 = p3 = p4
(iii) p1 < p2 < p3 < p4
(iv) p1 < p2 = p3 < p4
Answer:
The answer is the option (iii) $p_{1} < p_{2} < p_{3} < p_{4}$
Explanation: ……… (PV = constant, at constant temperature)
Hence, if $V_{1} = V_{2} = V_{3} = V_{4} ,$
Then, $P_{1} = P_{2} = P_{3} = P_{4} ,$
Question 4. The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon
(i) charge of interacting particles
(ii) mass of interacting particles
(iii) polarizability of interacting particles
(iv) strength of permanent dipoles in the particles.
Answer:
The answer is the option (iii) polarizability of interacting particles
Explanation: Because, if the magnitude of interaction energy will be greater, then the polarizability of the interacting particles will also be greater.
Question 5. Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of dipoles possess ‘partial charges’. The partial charge is
(i) more than unit electronic charge
(ii) equal to unit electronic charge
(iii) less than unit electronic charge
(iv) double the unit electronic charge
Answer:
The answer is the option (iii) less than unit electronic charge
Explanation: The unit charge is always greater than partial charges.
Question 6. The pressure of a 1:4 mixture of dihydrogen and dioxygen enclosed in a vessel is one atmosphere. What would be the partial pressure of dioxygen?
(i) $0.8 \times 10^{5} atm$
(ii) $0.008\; Nm^{-2}$
(iii) $8\times 10^{4}Nm^{-2}$
(iv) $0.25\; atm$
Answer:
We know that,
The partial pressure of O2 = Mole fraction of O2 × total pressure of the mixture
$=\frac{4}{5}\times 1\; atm=0.8\; atm$
$=0.8\times 10^{5}Nm^{-2}=8\times 10^{4}Nm^{-2}$
Hence opiton (iii) is correct
Question 7. As the temperature increases, average kinetic energy of molecules increases. What would be the effect of increase of temperature on pressure provided the volume is constant?
(i) increases
(ii) decreases
(iii) remains same
(iv) becomes half
Answer:
The answer is the option (i) increases
Explanation: Kinetic energy of molecules α temperature
Therefore, according to Gay Lussac's law, at constant volume, temperature as well as pressure both increases.
From the above data what would be the order of liquefaction of these gases? Start writing the order from the gas liquefying first
(i) $H_{2},He,O_{2},N_{2}$
(ii) $He,O_{2},H_{2},N_{2}$
(iii) $N_{2},O_{2},He,H_{2}$
(iv) $O_{2},N_{2},H_{2},He$
Answer:
Ans. The answer is the option (iv) $O_{2},N_{2},H_{2},He$
Explanation: Gas is easily liquified at a higher critical temperature.
Question 9. What is SI unit of viscosity coefficient $(\eta )$?
(i) Pascal
(ii) Nsm–2
(iii) Km–2 s
(iv) N m–2
Answer:
The answer is the option (ii) Nsm-2
Question 10. Atmospheric pressures recorded in different cities are as follows:
|
Cities |
Shimla |
Bengaluru |
Delhi |
Mumbai |
|
P in N/m2 |
1.01 x 105 |
1.2 x 105 |
1.02 x 105 |
1.21 x 105 |
Consider the above data and mark the place at which liquid will boil first.
(i) Shimla
(ii) Bangalore
(iii) Delhi
(iv) Mumbai
Answer:
The answer is the option (i) Shimla
Explanation: Boiling point of liquid α atmospheric pressure. Shimla has the lowest atmospheric pressure; hence, the liquid will boil first in Shimla.
Question 11. Which curve in Fig. 5.2 represents the curve of ideal gas?
(i) B only
(ii) C and D only
(iii) E and F only
(iv) A and B only
Answer:
The answer is the option (i) B only
Explanation: Since we know that PV is constant at all temperatures for an ideal gas, hence the curve B.
Question 12. Increase in kinetic energy can overcome intermolecular forces of attraction. How will the viscosity of liquid be affected by the increase in temperature?
(i) Increase
(ii) No effect
(iii) Decrease
(iv) No regular pattern will be followed
Answer:
The answer is the option (iii) Decrease
Explanation: Since kinetic energy increases with temperature, it can overcome intermolecular forces, and liquid starts flowing; hence the viscosity decreases.
Question 13. How does the surface tension of a liquid vary with increase in temperature?
(i) Remains same
(ii) Decreases
(iii) Increases
(iv) No regular pattern is followed
Answer:
The answer is the option (ii) Decreases
Explanation: We know that, Surface tension $\alpha$ 1/temperature & kinetic energy α temperature, hence the intermolecular attraction decreases.
NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: MCQ (Type 2)
The MCQ (Type 2) of States of Matter Class 11 NCERT Exemplar is designed to evaluate students ability to apply the concepts. These questions helps to test student understanding of concepts and helps them to strengthen their knowledge of the subject.
Question 14. With regard to the gaseous state of matter which of the following statements are correct?
(i) Complete order of molecules
(ii) Complete disorder of molecules
(iii) Random motion of molecules
(iv) Fixed position of molecules
Answer:
The answer is the option (ii) Complete disorder of molecules, (iii) Random motion of molecules
Explanation: Entropy of gases is very high; hence, molecules are in random motion and disorderly arranged.
Question 15. Which of the following figures does not represent 1 mole of dioxygen gas at STP?
(i) 16 grams of gas
(ii) 22.7 litres of gas
(iii) 6.022 × 1023 dioxygen molecules
(iv) 11.2 litres of gas
Answer:
The answer is the option (i) 16 grams of gas, (iv) 11.2 litres of gas
Explanation: 1 mole of O2 has 6.023 × 1023 molecules of O2, & at STP it occupies 22.4-litre volume and has a molar mass of 32g.
Question 16. Under which of the following two conditions applied together, a gas deviates most from the ideal behaviour?
(i) Low pressure
(ii) High pressure
(iii) Low temperature
(iv) High temperature
Answer:
The answer is the option (ii) High pressure, (iii) Low temperature
Explanation: =Gases deviate from PV=nRT under high pressure and low temperature, these are real gases, and it deviates from ideal behaviour.
Question 17. Which of the following changes decrease the vapour pressure of water kept in a sealed vessel?
(i) Decreasing the quantity of water
(ii) Adding salt to water
(iii) Decreasing the volume of the vessel to one-half
(iv) Decreasing the temperature of water
Answer:
The answer is the option (ii) Adding salt to the water, (iv) Decreasing the temperature of the water
Explanation: Adding salt to water decreases the surface area for a water molecule to vaporize, and hence vapour pressure of water also decreases.
Now, vapour pressure α temperature, hence, option (iv).
NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: Short Answer Type
Given below the Solutions to the short answer type questions of NCERT Exemplar Solutions Class 11 Chemistry Chapter 5 States of Matter, they offer clear explanations and help students to understand the concepts of States of Matter.
Question 18. If 1 gram of each of the following gases are taken at STP, which of the gases will occupy (a) greatest volume and (b) smallest volume? $CO, H_{2}O, CH_{4}, NO.$
Answer:
According to Avogadro's law, we know that at STP, Volume of
1 mole gas = 22.4 L
1g CO = 22.4/28g L
1g $H_{2}O$ = 22.4/18g L
1g $CH_{4}$ = 22.4/16g L
1g NO = 22.4/30g L
Therefore,
(a) 1g $CH_{4}$ will occupy maximum volume &
(b) 1g NO will occupy the minimum volume
Question 19. Physical properties of ice, water and steam are very different. What is the chemical composition of water in all the three states.
Answer:
The molecular arrangement of water is different in the solid, liquid and gaseous state; however, the chemical composition is the same in all the states.
Question 20. The behaviour of matter in different states is governed by various physical laws. According to you what are the factors that determine the state of matter?
Answer:
The factors that determine the different states of matter are volume, mass, temperature and pressure.
Question 21. Use the information and data given below to answer the questions (a) to (c):
-
Stronger intermolecular forces result in higher boiling point.
-
Strength of London forces increases with the number of electrons in the molecule.
-
Boiling point of HF, HCl, HBr and HI are 293 K, 189 K, 206 K and 238 K respectively.
(a) Which type of intermolecular forces are present in the molecules HF, HCl, HBr and HI?
(b) Looking at the trend of boiling points of HCl, HBr and HI, explain out of dipole-dipole interaction and London interaction, which one is predominant here.
(c) Why is boiling point of hydrogen fluoride highest while that of hydrogen chloride lowest?
Answer:
(a) Dipole-dipole are present in HCl, HBr, HI and HF; but, in HF along with these Intermolecular hydrogen bonding is also present.
(b)Dipole moment decreases from HCl to HI due to decrease in electronegativity, whereas, from HCl to HI its boiling point increases, hence we can say that London forces are predominant here.
(c) HF has hydrogen bonding as well as the highest electronegativity; hence it has the highest dipole moment as well as the highest boiling point.
Question 22. What will be the molar volume of nitrogen and argon at 273.15K and 1 atm?
Answer:
The conditions given above are of STP where the volume occupies by q mole of gas is 22.4 L. Hence, the volume of N2 and argon, here, will be 22.4 L.
Question 23. A gas that follows Boyle’s law, Charles law and Avogadro’s law are called an ideal gas. Under what conditions a real gas would behave ideally?
Answer:
A real gas would behave ideal gas under low pressure and high temperature.
This is based on the phenomenon of critical temperature; here, Gas 'A' liquefies easily, hence, it is below the critical temperature, whereas Gas' B' doesn't liquefy even on applying high pressure; hence it is above the critical temperature.
Question 25. Value of universal gas constant (R) is the same for all gases. What is its physical significance?
Answer:
The eq. of universal gas constant (R) is R = PV/nRT. Therefore, the unit of R will depend on P, V & T viz, Pa m3 K-1 mol-1 or Jmol-1K-1. Since Joule is the unit of work; therefore, R is the work done by gas mole per kelvin.
Since there are no intermolecular forces of attraction between gas molecules of an ideal gas. Hence, the above statement is correct for ideal gases.
The molecules above are of water, alcohol, and hexane. Here water and alcohol are polar and have dipole-dipole interaction and intermolecular hydrogen bonding, whereas hexane is non-polar and has weak London dispersion forces.
H-bonding is stronger in water; hence, surface tension is also more in water than alcohol. Therefore, Hexane < Alcohol < Water.
Question 28. Pressure exerted by saturated water vapour is called aqueous tension. What correction term will you apply to the total pressure to obtain pressure of dry gas?
Answer:
Let us consider that at temperature 'T' pressure of moist gas is 'P'; then, the pressure of dry gas can be obtained by the equation:
$P_{Drygas} = P_{Total }$ - aqueous tension
Question 29. Name the energy which arises due to motion of atoms or molecules in a body. How is this energy affected when the temperature is increased?
Answer:
Thermal energy arises due to the motion of atoms or molecules in a body. It is a measure of kinetic energy. Thermal energy α temperature.
Question 30. Name two intermolecular forces that exist between HF molecules in liquid state.
Answer:
Hydrogen bond and dipole-dipole interactions are the two forces existing in HF molecules when they are in a liquid state.
The assumption is not applicable to real gases because they can be liquified by cooling and applying high pressure. Hence, the force of attraction exists between the molecules of real gases.
Question 32. Compressibility factor, Z, of a gas is given as Z = (pV/nRT)
(i) What is the value of Z for an ideal gas?
(ii) For real gas what will be the effect on value of Z above Boyle’s temperature?
Answer:
(i) Z is a compressibility factor. Its value for an ideal gas is Z = 1.
(ii) Real gases show positive deviation above Boyle's temperature, i.e., Z>1.
Question 33. The critical temperature (Tc) and critical pressure ( pc) of $CO_{2}$ are 30.98°C and 73 atm respectively. Can $CO_{2}$ (g) be liquefied at 32°C and 80 atm pressure?
Answer:
It does not matter that how high temperature or high-pressure is CO2 at; it cannot be liquified above 30.98o and 73 atm. Hence, it cannot be liquified at 32o and 80 atm pressure.
(i) We know that volume α size of the molecules. Hence, the increasing order of the value of 'b' will be:
$H_{2}<He<O_{2}<CO_{2}$
(ii) 'a' is the Van der Waal's constant which represents the magnitude of intermolecular attraction. An $\alpha$ size of the electron cloud. Hence, the greater the size of the electron cloud, the greater will be the dispersion forces and polarizability of the molecule. Hence, the gases in decreasing order of the magnitude of 'a' will be: $CH_{4} > O_{2} > H_{2}$
It is given that $P_{ideal}=P_{real}+an^{2}/V^{2}$
We know that the unit of p = Nm-2, unit of V = m3, number of moles(n) = mol
Unit of ‘a’ = Nm-2(m3)2/(mol)2 = Nm4mol-4
If we take the unit of p = atm, V = dm3 and n = mol
Then unit of ‘a’ = pV2/n2 = atm(dm3)2/(mol)2 = atm dm6mol-2.
Question 36. Name two phenomena that can be explained on the basis of surface tension.
Answer:
The two phenomena that can be explained on the basis of surface tension are-
(i) The spherical shape of liquid drops
(ii) Capillary action i.e. rise and dip of a liquid in the column of a capillary.
(a) Hexane has Vander Waal force of attraction present as the intermolecular force.
(b) Water has hydrogen bonding present in the form of intermolecular forces
(c) Glycerin has hydrogen bonding present as the intermolecular force
Hexane < water < glycerine
The reason behind this order is that in hexane, weak bonding forces exist; therefore, it has the least viscosity, whereas, in water and glycerine dipole-dipole interaction and extensive H-bonding are present. Glycerine has the strongest intermolecular forces. Therefore, the order of viscosity is-
When there is an increase in the temperature, the intermolecular force which operates between the particle reduces, subsequently the strength of the bond increases along with the kinetic energy. Thus, when there is an increase in the temperature, there is a reduction in the viscosity. This is a result of the fact that viscosity decreases when there is reduction in the intermolecular forces.
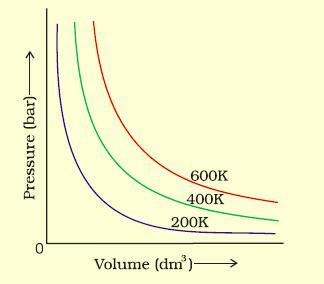
Answer:
(i) According to Boyle's law, The pressure of gas $\alpha$ 1/volume of gas, at a constant temperature.
Therefore, if volume decreases at a constant temperature, then the pressure increases and vice versa. For example, at 200K if pressure increases from p1 to p2, volume decreases from v1 to v2(v2 < v1)
(ii) According to Charles's law, The volume of gas α temperature, at constant pressure.Therefore, if temperature decreases from 200K to 400K, at a constant temperature, the volume of gas increases.
(i) Real gases show a very small deviation from ideal behaviour at low pressure because both the curves coincide with each other.
(ii) Real gases show large deviation at high pressure as the curves fall apart.
(iii)The point at which the curves intersect each other shows that the real gas behaves like an ideal gas.
NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: Matching Type
Class 11 States of Matter Questions and Answers are discussed below. These are generally asked in exams to test student knowledge. Notes of this chapter are quite helpful for preparing concepts discussed in this chapter.
Question 41. Match the graph between the following variables with their names.
|
Column I (Graphs) |
Column II (Names) |
|
(i) Pressure vs temperature graph at constant molar volume. |
(a) Isotherms |
|
(ii) Pressure vs volume graph at constant temperature. |
(b) Constant temperature curve |
|
(iii) Volume vs temperature graph at constant pressure. |
(c) Isochores |
|
|
(d) Isobars |
Answer:
(i)→(c); (ii) →(a); (iii) →(d)
Explanation:
(i) Constant molar volume graphs are called isochore.
(ii) Constant temperature graphs are called isotherm.
(iii) Constant pressure graphs are called isobar.
Question 42. Match the following gas laws with the equation representing them.
| Column I | Column II |
| (i) Boyle’s law | (a) V ∝ n at constant T and p |
| (ii) Charle’s law | (b) pTotal = p1 + p2 + p3 +…… at constant T, V |
| (iii) Dalton’s law | (c) PV/T = Constant |
| (iv) Avogadro law | (e) p ∝ 1/V at constant n and T |
| (e) p ∝ 1/V at constant n and T |
Answer:
(i) →(e); (ii) →(d); (iii) →(b); (iv) →(a)
Explanation:
(i) pressure of gas α 1/volume of gas (T constant) ….. (Boyle's law)
(ii) The volume of gas α temperature of the gas, at constant pressure …… (Charles's law)
(iii) At constant V and T, the total pressure of the gaseous mixture of two or more non-reacting gases is equal to the algebraic sum of their partial pressures ……. (Dalton's law)
(iv) Under same temperature and pressure conditions, equal volumes of all gases contain equal no. of moles. V α n. ……. (Avogadro's law)
Question 43. Match the following graphs of ideal gas with their co-ordinates :
Graphical representation x and y co-ordinates
Answer:
(i) →(b); (ii) →(c); (iii) →(a)
Explanation:
(i) pressure of gas $\alpha$ 1/volume of gas (T constant)
(ii) The pressure of gas $\alpha$ 1/V
(iii) Product of pressure and volume is constant. PV = constant
NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: Assertion and Reason Type
Assertion and Reason type questions is one of the most important sections covered in the NCERT Exemplar Class 11 Chemistry Chapter 5 Solutions. These questions help students to improve their critical thinking.
Question 44. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules.
Reason (R): Intermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A is false but R is true.
Answer:
The answer is the option (i) Both A and R are right, and R is the correct explanation of A.
Explanation: Balance is required in both intermolecular forces and thermal energy to decide the state of matter.
Question 45. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): At constant temperature, pV vs V plot for real gases is not a straight line.
Reason (R) : At high pressure all gases have Z > 1 but at intermediate pressure most gases have Z < 1.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A is false but R is true.
Answer:
The answer is the option (i). Both A and R are true and R is the correct explanation of A.
For a real gas pV vs V plot is not a straight line due to intermolecular forces and finite molecular volume.
At high pressures, repulsive forces dominate → gas occupies more volume than ideal → Z>1
At intermediate pressures, attractive forces dominate → gas compresses more → Z<1.
Question 46. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): The temperature at which vapour pressure of a liquid is equal to the external pressure is called boiling temperature.
Reason (R) : At high altitude atmospheric pressure is high.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A is false but R is true.
Answer:
The answer is the option (iii) A is true, but R is false.
Explanation: Boiling point of a liquid is the temperature at which the vapour pressure of the liquid is equal to the external pressure. As the altitude is high, the pressure is low.
Question 47. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Gases do not liquefy above their critical temperature, even on applying high pressure.
Reason (R) : Above critical temperature, the molecular speed is high and intermolecular attractions cannot hold the molecules together because they escape because of high speed.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A is false but R is true.
Answer:
The answer is the option (i)Both A and R are true, and R is the correct explanation of A.
Explanation: Gases do not liquefy even on applying high pressure if they are above the critical temperature as the Intermolecular forces of attraction cannot hold the molecules together, and the molecular speed is also high.
Question 48. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): At critical temperature liquid passes into gaseous state imperceptibly and continuously.
Reason (R) : The density of liquid and gaseous phase is equal to critical temperature.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A is false but R is true.
Answer:
The answer is the optio (i) Both A and R are true, and R is the correct explanation of A.
Explanation: Density of liquid becomes equal to its vapour phase at a critical temperature which causes the liquid to change into gaseous state imperceptibly and continuously.
Question 49. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Liquids tend to have maximum number of molecules at their surface.
Reason (R) : Small liquid drops have spherical shape.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A is false but R is true.
Answer:
The answer is the option (iv) A is false, but R is true.
Explanation: Liquid drops have a spherical shape as they try to reduce the no. of molecules and tend to reduce surface tension at their surface.
NCERT Exemplar Solutions Class 11 Chemistry Chapter 5: Long Answer Type
In this section some important long-answer questions from NCERT Exemplar Chemistry States of Matter are covered, as these questions are often asked in exams.
(i) Between the points, a and b, $CO_{2}$ will exist in a gaseous state at temperature T1.
(ii) At point b there is phase transition, and the plot becomes linear.
(iii) At Temperature T2, $CO_{2}$ will be completely liquefied at point g.
(iv) T3 > Tc. At T3 temperature, condensation will not take place.
(v) Liquid and gaseous $CO_{2}$ are in equilibrium between b and c.
(i) Approximately, BP of A is 315K, and that of B is 345K.
(ii) As pressure is kept on increasing in the closed vessel, liquid C will not boil.
(iii) 313K is the temperature corresponding to 60 mm Hg.
(iv) Since atmospheric pressure is low at high altitude, water boils at low temperature on hills.
As a liquid is heated in a closed vessel, its vapour pressure increases and the density of the vapour also increases, while the density of the liquid decreases. At a particular temperature known as the critical temperature, the densities of the liquid and vapour become equal. As a result, the clear boundary that separates the two phases disappears. At the critical temperature, the substance exists as a homogeneous single phase called a supercritical fluid. This supercritical state has unique properties like a gas, it can fill a container, and like a liquid, it can dissolve substances making it very useful in industrial applications.
Question 53. Why does sharp glass edge become smooth on heating it to its melting point in a flame? Explain which property of liquids is responsible for this phenomenon.
Answer:
When a sharp glass edge is heated to its melting point, the glass softens and behaves like a viscous liquid. In this state, the molecules of the glass have enough energy to move slightly and rearrange themselves. The property responsible for the smoothing of the sharp edges is surface tension. Surface tension tends to minimize the surface area of a liquid. As the glass melts, surface tension pulls the molecules into a more stable shape with the least surface area which is smooth and rounded. Thus, sharp edges become smooth when heated due to the action of surface tension in the softened glass.
Question 54. Explain the term ‘laminar flow’. Is the velocity of molecules the same in all the layers in laminar flow? Explain your answer.
Answer:
Laminar flow can also be defined as the flow in which there is a regular gradation of velocity in passing from one layer to the next.
When a liquid flows over a fixed surface, it comes in contact with it. The layer of molecules in the immediate contact of the surface is stationary. As the distance of layers increases from the fixed layer, the velocity of the upper layer also increases.
In the figure above, we can increase the temperature to move from A to F vertically and can reach G by compressing the gas at constant temperature along isotherm, thus, increasing the pressure. Then by lowering the temperature, we can move vertically downwards to D. We get liquid as soon as we cross the point H on isotherm. The substances will remain in one phase if we carry out this process at a critical temperature. This is called continuity of state between the gaseous and liquid states.
NCERT Exemplar Class 11 Chemistry Chapter 5: Higher Order Thinking Skills (HOTS) Questions
Class 11 States of Matter Questions and Answers are given below that will help students to solve complex problems. These solutions will help you evaluate your understanding of the concepts.
Question 1. How many times does the pressure of an ideal gas inside a cubic of side l change if the side is reduced to 1/2 and the temperature is kept constant?
(i) 1.9
(ii) 1.8
(iii) 2.4
(iv) 3.6
Answer:
$
\mathrm{P}=\frac{1}{3} \frac{\mathrm{mNu}^2}{\mathrm{v}}=\frac{2}{3 \mathrm{v}}\left(\frac{1}{2} \mathrm{mNu}^2\right)=\frac{2}{3 \mathrm{v}}(\mathrm{kE})
$
Temperature is constant, hence ( KE ) remains instant.
Volume initially of side $(I)=1^3$
Volume due to the change to $\left(\frac{1}{2}\right)^3=\frac{1^3}{8}$
Thus,
$
\mathrm{P}_1=\frac{2}{3} \frac{\mathrm{kE}}{\mathrm{l}^2}, \mathrm{P}_2=\frac{2}{3} \frac{\mathrm{kE}}{\mathrm{l}^3} \times \delta
$
therefore, $\frac{\mathrm{P}_2}{\mathrm{P}_1}=8$
Hence, the answer is option (ii).
Question 2: The diffusion coefficient of an ideal is proportional to its mean free path and mean speed. The absolute temperature of an ideal gas is increased 8 times, and its pressure 4 times. As a result, the diffusion coefficient of this gas increases A time. The value of A is -
(i) 1.4
(ii) 2.2
(iii) 3.8
(iv) 4.10
Answer:
Rate of diffusion $\alpha \lambda \propto \mathrm{U}_{\mathrm{Avg}}$
$
\begin{aligned}
& \alpha \frac{1}{\sqrt{2} \pi \sigma^2 \mathrm{~N}^*} \times \mathrm{U}_{\mathrm{Avg}} \\
& \alpha \frac{1}{\sqrt{2} \pi \sigma^2 \mathrm{~N}^*} \\
& \alpha \frac{\mathrm{U}_{\mathrm{Avg}}(\mathrm{KT})}{\sqrt{2} \mathrm{n} \sigma^2 \mathrm{P}}
\end{aligned}
$
Rate of diffuaion $\alpha \frac{\mathrm{T}^{3 / 2}}{\mathrm{p}}$
$
\frac{r_{\text {final }}}{r_{\text {inital }}}=\frac{(8)^{3 / 2}}{(4)}=2
$
Hence, the correct answer is option (ii).
Question 3. 0.4g of dihydrogen is made to react with 7.1g of dichlorine to form hydrogen chloride. Calculate the volume of hydrogen chloride formed at 273K and 1 bar pressure?
Answer:
The reaction occurs as follows:
$\mathrm{H}_2+\mathrm{Cl}_2 \rightarrow 2 \mathrm{HCl}$
Now, clearly 2g of hydrogen reacts with 71g of chlorine.
Thus, 0.4g of hydrogen will react with 14.2g of chlorine.
Thus, no reactant is the limiting reagent here. Both the reactants will consume completely.
Thus, 2g of hydrogen will produce 73g of HCl.
Thus, 0.4g of hydrogen will produce 14.6g of HCl.
Now, moles of HCl produced = 7.3/73 = 0.2 moles
Now, according to the ideal gas equation, we have:
PV = nRT
Thus, V = (nRT)/P
V = (0.2 x 0.0821 x 273)/1
V = 4.54L
Hence, the answer is 4.54 L.
Question 4: An air bubble of radius 0.1 cm lies at a depth of 20 cm below the free surface of a liquid of density $1000 \mathrm{~kg} / \mathrm{m}^3$. If the pressure inside the bubble is $2100 \mathrm{~N} / \mathrm{m}^2$ greater than the atmosphet ic pressure, then the surface tension of the liquid m . SI unit is (use $\mathrm{g}=10 \mathrm{~m} / \mathrm{s}^2$ )
(1) 0.02
(2) 0.1
(3) 0.25
(4) 0.05
Answer:
$P_{\text {bubble }}=P_{\text {atm }}+\rho g h+\frac{2 S}{r}$
Given
Given that the pressure inside the bubble is $P_{\text {atm }}+2100 \mathrm{~N} / \mathrm{m}^2$
The liquid pressure at a depth of 20 cm is
$
P_{\text {liquid }}=P_{\mathrm{atm}}+\rho g h=P_{\mathrm{atm}}+1000 \times 10 \times 0.20=P_{\mathrm{atm}}+2000 \mathrm{~N} / \mathrm{m}^2,
$
the pressure difference due solely to surface tension is
$
\Delta P=\left(P_{\text {bombubble }}-P_{\text {liquid }}\right)=\left(P_{\text {atm }}+2100\right)-\left(P_{\text {atm }}+2000\right)=100 \mathrm{~N} / \mathrm{m}
$
The Laplace pressure difference for a bubble (which has one interface) is given by $\Delta P=\frac{2 S}{r}$.
The given bubble radius is 0.1 cm , which in SI units is
$
r=0.1 \mathrm{~cm}=0.001 \mathrm{~m} .
$
Substituting the known values into the Laplace equation gives
$
\frac{2 S}{0.001}=100
$
Solving for $S$ :
$
\begin{aligned}
& 2 S=100 \times 0.001=0.1 \\
& S=\frac{0.1}{2}=0.05 \mathrm{~N} / \mathrm{m}
\end{aligned}
$
Hence, the correct answer is option (4).
Question 5: The purification method based on the following physical transformation is :
$\underset{(\mathrm{X})}{\mathrm{Solid}} \xrightarrow{\text { Heat }} \underset{(\mathrm{X})}{\text { Vapour }} \xrightarrow{\text { Cool }} \underset{(\mathrm{X})}{\text { Solid }}$
(1) Sublimation
(2) Distillation
(3) Crystallization
(4) Extraction
Answer:
$\underset{(X)}{\text { Solid }} \xrightarrow{\text { Heat }} \underset{(X)}{\text { Vapour }} \xrightarrow{\text { Cool }} \underset{(X)}{\text { Solid }}$
is known as Sublimation.
In sublimation, a solid substance directly transforms into its gaseous phase upon heating and reverts back to the solid phase upon cooling. This technique is commonly used to purify substances, particularly those that sublime more easily than others under the same conditions. Sublimation is effective for separating substances with different volatility, like separating ammonium chloride from a mixture with sodium chloride, as ammonium chloride sublimates while sodium chloride does not.
Hence, the correct answer is option (1).
Question 6: At a given temperature T , gases $\mathrm{Ne}, \mathrm{Ar}, \mathrm{Xe}$ and kr are found to deviate from ideal gas behaviour
$\mathrm{P}=\frac{\mathrm{RT}}{\mathrm{~V}-\mathrm{b}} \text { al } \mathrm{T}$
This equation of state is given as, $\mathrm{P}=\frac{\mathrm{R}}{\mathrm{V}-\mathrm{b}}$ al T . Which gas will exhibit steepest increase in the plot of ?
(1) $\mathrm{X}{\mathrm{e}}$
(2) Ar
(3) Kr
(4) Ne
Answer:
Noble gases such as Ne ,Ar ,Xe ,Kr found to deviate from ideal gas behaviour.
Xe gas will exhibit stepest increase in plot of 2 vsP.
$\begin{aligned} P=\frac{R T}{(V-b)} \Rightarrow & P(V-b)=R T \\ \Rightarrow & P V-P b=R T \\ \Rightarrow & \frac{P V}{R T}=1+\frac{P b}{R T} \\ \Rightarrow & z=\frac{P V}{R T} \\ & =1+\frac{\mathrm{Pb}}{\mathrm{RT}} \Rightarrow \mathrm{y}=\mathrm{c}+\mathrm{mx}\end{aligned}$
Hence the answer is the option (1).
Question 7: The density of a gas A is thrice that of a gas B at the same temperature .The molecular weight of gas B is twice that of A.What will the ratio of the pressure acting on A and B is _____
(1) $\frac{1}{4}$
(2) $\frac{7}{8}$
(3) $\frac{2}{5}$
(4) $\frac{1}{6}$
Answer:
$\frac{\mathrm{d}}{\mathrm{P}}=\frac{\mathrm{M}}{\mathrm{RT}}$
Let the density of gas B be d
of gas A =3d
and the molecular weight of A be M and the molecular of B will be 2 M,
Since,
R is gas constant and T is the same for both gases, so ____
$\begin{aligned} & P_A=\frac{d_A R T}{M_A} \therefore \frac{P_B}{P_A}=\frac{d_B}{d_A} \times \frac{M_A}{M_B}=\frac{d}{3 d} \times \frac{M}{2 M} \\ & P_B=\frac{d_B R T}{M_B}=\frac{1}{6}\end{aligned}$
Hence, the answer is the option (4).
Approach to Solve Questions of Class 11 Chemistry Chapter 5
NCERT Exemplar Solutions for Class 11 Chemistry Chapter 5 forms the basis of chemistry. The approaches given below are helpful and provide step-by-step approach to solve questions.
1. Before solving questions, of Chapter 5 it is important to have proper knowledge of basic concepts by clearly understanding the Intermolecular forces, Gas laws , Ideal gas equation, Deviation from ideal behavior, Real gases and the van der Waals equation, Liquefaction of gases and Properties of liquids.
2. Reading the NCERT textbook thoroughly is important as this chapter is full of laws, definitions, and derivations. Many questions in competitive and board exams come directly from the NCERT.
3. Questions related to Gas laws are frequently asked in exams. These laws describe the relationship between measurable properties of gases like pressure, volume, temperature, and number of moles. Gas laws discussed in this chapter are given below:
i. Boyle law
Ii. Charles law
Iii. Gay lussac law
4. To solve NCERT Exemplar Chemistry Chapter 5 States of Matter questions effectively first try to solve NCERT examples and in-text questions for a better understanding of the concept. Also, solve the textbook exercise questions as they are often directly asked in board exams.
Topics and Subtopics Covered in the NCERT Exemplar Class 11 Chemistry Chapter 5
NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 States of matter covers key concepts related to the States of matter. Understanding these concepts are essential for building a strong foundation in Chemistry. The following are the most important topics covered in this chapter.
- Intermolecular Forces
- Dispersion Forces or London Forces
- Dipole-dipole Forces
- Dipole-Induced Dipole Forces
- Hydrogen Bond
- Thermal Energy
- Intermolecular Forces Vs Thermal Interactions
- The Gaseous State Ex
- The Gas Laws Ex
- Boyle’s Law (Pressure-Volume Relationship)
- Charles’ Law (Temperature-volume Relationship)
- Gay Lussac’s Law (Pressure-Temperature Relationship)
- Avogadro's Law (Volume – Amount Relationship)
- Ideal Gas Equation
- Density and Molar Mass of a Gaseous Substance
- Dalton's Law Of Partial Pressure
- Kinetic Molecular Theory of Gases
- Behaviour Of Real Gases: Deviation from Ideal Gas Behaviour
- Liquefaction Of Gases
- Liquid State
- Vapour Pressure
- Surface Tension and Viscosity.
NCERT Exemplar Class 11 Chemistry Chapter 5: Important Formulas
Given below are Class 11 Chemistry chapter 5 States of matter important formulas that are essential for solving numerical problems. Go through these formulas carefully.
1. Gas Laws:
Boyle's law: Pressure–Volume relation at constant T.
$P\propto \frac{1}{V}$ or PV = Constant
Charle's law: Volume–Temperature relation at constant P.
$V\propto T$ or $\frac{V_{1}}{T_{1}}=\frac{V_{2}}{T_{2}}$
Gay-Lussac's law: Pressure–Temperature relation at constant V.
$P\propto T$ or $\frac{P_{1}}{T_{1}}=\frac{P_{2}}{T_{2}}$
Avogadro's law:
$V\propto n$ or $\frac{V_{1}}{n_{1}}=\frac{V_{2}}{n_{2}}$
2. Ideal Gas Equation:
PV = nRT
Where:
-
P= Pressure
-
V = Volume
-
n= Number of moles
-
R = Universal gas constant
-
T= Temperature (in Kelvin)
3. Density and Molar Mass
$d(density)=\frac{PM}{RT} $ , Where M = Molar Mass
4. Mole Fraction
$x_{i}=\frac{n_{i}}{n_{total}}$ and $p_{i}=x_{i}P_{total}$
5. Average Kinetic Energy of Gas Molecules
Average $KE=\frac{3}{2}RT$ (per mole)
6. Root Mean Square Speed ( rms speed)
$u_{rms}=\sqrt{\frac{3RT}{M}}$ , (M should be in kg/mol)
7. Real Gas - Van der Waals equation
$(P+\frac{an^{2}}{V^{2}})(V-nb)=nRT$
Where, a measures of intermolecular attraction and b measures of finite molecular volume.
8. Compressibility Factor (Z)
$Z=\frac{PV}{nRT}$ ,
-
For ideal gases: Z=1
-
For real gases: Z≠1
Advantages of Using Class 12 Chemistry NCERT Exemplar Solutions Chapter 5 Surface Chemistry
NCERT Exemplar Class 12 Chemistry Solutions Chapter 5 Surface Chemistry helps students to understand the key concepts of this chapter with the help of a variety of solved questions. Given below some points on advantages of these solutions:
-
These exemplar solutions help students to understand the topics like adsorption, catalysis, and colloids with the help of solved examples.
-
These answers are prepared to help students apply theoretical knowledge in numerical problems effectively.
-
Class 12 chemistry NCERT exemplar solutions chapter 5 surface chemistry are prepared by subject experts in a very clear and comprehensive manner.
-
Students can use these NCERT exemplar solutions for Class 11 to resolve common doubts and mistakes.
NCERT Exemplar Solutions Class 11 Chemistry Chapter-Wise
Class 11 NCERT exemplar chapter-wise solutions are given below:
NCERT Solutions for Class 11 Chemistry Chapter-wise
Class 11 NCERT chemistry chapter-wise solutions are given below:
NCERT Solutions for Class 11 Subject-Wise
Class 11 NCERT subject-wise solutions are given below:
NCERT Notes Subject-Wise
Class 11 NCERT subject-wise notes are given below:
NCERT Books and NCERT Syllabus
The NCERT books and syllabus links for class 11 are given below:
| NCERT Books Class 11 Chemistry |
| NCERT Syllabus Class 11 Chemistry |
| NCERT Books Class 11 |
| NCERT Syllabus Class 11 |
Frequently Asked Questions (FAQs)
Chapter 5 of the NCERT Exemplar discusses three primary states of matter: solids, liquids, and gases. Each state has distinct characteristics based on the arrangement and energy of its particles. Solids have tightly packed particle, liquids have particles that are close together but can move past one another, while gases have particles that are widely spaced and move freely.
The ideal gas law is a mathematical equation that describes the relationship between the pressure, volume, temperature, and number of moles of an ideal gas.
Ideal gas equation: PV = nRT
Where: P = Pressure, V = Volume, n = Number of moles, R = Ideal gas constant, T = Temperature
Real gases deviate from ideal gas behavior under certain conditions, as discussed in this chapter. Unlike ideal gases, which assume no intermolecular forces and that the volume of gas particles is negligible, real gases experience intermolecular attractions and have finite volumes. These deviations are more pronounced at high pressures and low temperatures, where the effects of intermolecular forces become significant.
Plasma is the fourth state of matter, where a gas is heated to extremely high temperatures, causing the atoms to become ionized. It consists of a mixture of ions and free electrons.
Phase transitions refer to the processes that occur when matter changes from one state to another, such as from a solid to a liquid, a liquid to a gas, or a gas to a liquid.
Class 12 chemistry NCERT exemplar solutions chapter 5 surface chemistry focus on understanding key concepts like adsorption, catalysis, and colloids through NCERT and exemplar questions.
Surface Chemistry is important as it explains vital processes like adsorption, catalysis, and colloidal behaviour, which have practical applications in industries and daily life.
Adsorption is the process by which molecules from a gas or liquid adhere to the surface of a solid or liquid, forming a thin film. In contrast, absorption involves the incorporation of a substance into the bulk of another material.
The two main types of adsorption are physisorption and chemisorption. Physisorption is a weak, reversible process involving van der Waals forces, whereas chemisorption involves a stronger chemical bond forming between the adsorbate and the surface, which is typically irreversible and more specific.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters









