NCERT Exemplar Class 11 Chemistry Chapter 4 focuses on how atoms combine to form molecules through different types of chemical bonds. It covers topics such as ionic, covalent, and coordinate bonds, the VSEPR theory, hybridisation, and molecular orbital theory.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure
Do you know how atoms are held together in molecules, why oxygen exists in a gas state while water exists in a liquid state under normal conditions? The answer to all these questions lies in NCERT Exemplar Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure. This chapter play an important role in understanding the structure, the bond formation, and the properties of the molecules. It explains how atoms combine to form molecules and the principles and theories that govern their behaviour. The NCERT Exemplar solutions for Class 11 Chemistry includes detailed solutions to every questions. Many key concepts such as the nature of chemical bonding, including covalent, ionic, and coordinate bonds, and the concept of hybridization are explained here through the series of solved examples.
This Story also Contains
- NCERT Exemplar Class 11 Chemistry solutions chapter 4: MCQ (Type 1)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: MCQ (Type 2)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: Short Answer Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: Matching Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: Assertion and Reason Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: Long Answer Type
- NCERT Class 11 Chemistry Chapter Chemical Bonding and Molecular Structure: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 4 Chemical Bonding and Molecular Structure
- Advantages of Using Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Solutions
- Topics Of NCERT Exemplar for Class 11 Chemistry Chapter 4
- NCERT Exemplar Class 11 Chemistry Solutions Chapter-Wise
- NCERT Solutions for Class 11 Chemistry
- NCERT Exemplar Class 11 Solutions
- NCERT Solutions subject-wise
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus
The NCERT Exemplar Solution are designed by our subject experts to offer a systematic and structured approach to these important concepts and help students to develop a clear understanding of critical concepts conceptual explanations, these NCERT Solutions provide a valuable resource to enhance performance in board exams as well as in the competitive exams like JEE Advanced, NEET, JEE Mains, etc. In this article, we will discuss detailed solutions to all the questions.
NCERT Exemplar Class 11 Chemistry solutions chapter 4: MCQ (Type 1)
The NCERT Exemplar Solutions Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure MCQ questions are given below. This chapter is very important from exam point of view.
Question 1. Isostructural species are those which have the same shape and hybridization. Among the given species identify the isostructural pairs.
(i) $\left [ NF_{3}\; and\; BF_{3} \right ]$
(ii) $\left [ BF{_{4}}^{-}\; and\; NH_{4}^{+} \right ]$
(iii) $\left [ BCl_{3}\; and\; BrCl_{3} \right ]$
(iv) $\left [ NH_{3}\; and\; NO{_{3}}^{-} \right ]$
Answer:
The answer is the option (ii) $\left [ BF{_{4}}^{-}\; and\; NH_{4}^{+} \right ]$
Explanation: The pair above is isostructural because both the molecules $\left [ BF{_{4}}^{-}\; and\; NH_{4}^{+} \right ]$ are sp3 hybridized and their shape is tetrahedral.
Question 2. Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
(i) $CO_{2}$
(ii) $HI$
(iii) $H_{2}O$
(iv) $SO_{2}$
Answer:
The answer is the option (iii) $H_{2}O$
Explanation:$H_{2}O$ has the highest dipole moment because it is highly electronegative and has two lone pair on it.
Question 3. The types of hybrid orbitals of nitrogen in $NO{_{2}}^{+},NO{_{3}}^{-}$ and $NH{_{4}}^{+}$respectively are expected to be
(i) $sp, sp^{3} \; and\; sp^{2}$
(ii) $sp, sp^{2} \; and\; sp^{3}$
(iii) $sp^{2}, sp \; and\; sp^{3}$
(iv) $sp^{2}, sp^{3} \; and\; sp$
Answer:
The answer is the option (ii) $sp, sp^{2} \; and\; sp^{3}$
Explanation:$NO{_{2}}^{+}$is sp hybridized while $NO{_{3}}^{-}$ is sp2 hybridised and $NH{_{4}}^{+}$ is $sp^{3}$ hybridized.
Question 4. Hydrogen bonds are formed in many compounds e.g., $H_{2}O, HF,NH_{3}.$ The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
(i) $HF>H_{2}O>NH_{3}$
(ii) $H_{2}O>HF>NH_{3}$
(iii)$NH_{3}>HF>H_{2}O$
(iv) $NH_{3}>H_{2}O>HF$
Answer:
The answer is the option (ii) $H_{2}O>HF>NH_{3}$
Explanation: The factors that affect the strength of hydrogen bond are electronegativity, size of atom and number of hydrogen bonds per mol. Usually, F is the most electronegative element, but, in H2O, there are more H-bonds, and their size is smaller than N.
Question 5. In $PO{_{4}}^{3-}$ ion the formal charge on the oxygen atom of $P-O$ bond is
(i) + 1
(ii) – 1
(iii) – 0.75
(iv) + 0.75
Answer:
The answer is the option (ii) -1
Explanation: Formal charge in an atom of a polyatomic molecule or ion can also be defined as the difference between the number of valence electrons of that atom in an isolated or free state and the number of electrons assigned to that atom in the Lewis structure.
Formula:
The formal charge of the atom in the molecule or ion = (Number of the valence electron in the free atom) – (Number of lone pair electrons + ½ number of bonding electrons)
Therefore, the formal charge on each O-atom
= 6 – (6+ 1/2 × 2) = 6 – 7 = -1
Question 6. In $NO{_{3}}^{-}$ ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
(i) 2, 2
(ii) 3, 1
(iii) 1, 3
(iv) 4, 0
Answer:
The answer is the option (iv) 4, 0
Explanation: N, viz., the central atom of the molecule is surrounded by 2 covalent bonds with 1 oxygen atom and 2 coordinate covalent bonds with 2 oxygen atoms. Therefore, there are 4 bond pairs and no lone pair on the nitrogen atom in $NO{_{3}}^{-}$
Question 7. Which of the following species has tetrahedral geometry?
(i) $BH{_{4}}^{-}$
(ii) $NH{_{2}}^{-}$
(iii) $CO{_{3}}^{2-}$
(iv) $H_{3}O^{+}$
Answer:
The answer is the option (i) $BH{_{4}}^{-}$
Explanation: 4 bond pairs surround Boron here.
In $BH{_{4}}^{-}$, there are 4 bond pair and no lone pair.
Its sp3 hybridized, and therefore has tetrahedral geometry
$CO{_{3}}^{-2}$ is triangular planar.
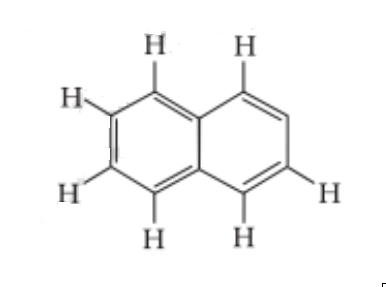
Question 8. Number of $\pi$ bonds and σ bonds in the following structure is–
(i) 6, 19
(ii) 4, 20
(iii) 5, 19
(iv) 5, 20
Answer:
The answer is the option (iii) 5, 19
Explanation: Here, each C atom is sp2 hybridized and surrounded by 3 sigma bonds and 1 pi bond between two C atoms.
8 C – H + 11 C – C bonds = 19 bonds.
There are $5\pi$ - bonds and 19 bonds.
Question 9. Which molecule/ion out of the following does not contain unpaired electrons?
(i) $N{_{2}}^{+}$
(ii) $O_{2}$
(iii) $O{_{2}}^{2-}$
(iv) $B_{2}$
Answer:
The answer is the option (iii) $O{_{2}}^{2-}$
Explanation:
The molecular orbital configuration of $O{_{2}}^{2-}$ ion is
$\sigma 1 s^2, \sigma^* 1 s^2, \sigma 2 s^2, \sigma^* 2 s^2, \sigma 2 p_x^2=\Pi 2 p_y^2, \Pi 2 p_z^2=\Pi^* 2 p_x^2, \Pi^* 2 p_y^2$
thus, $O{_{2}}^{2-}$ possesses no unpaired electrons.
Question 10. In which of the following molecule/ion, all the bonds are not equal?
(i) $XeF_{4}$
(ii) $BF{_{4}}^{-}$
(iii) $C_{2}H_{4}$
(iv) $SiF_{4}$
Answer:
The answer is the option (iii) $C_{2}H_{4}$
Explanation: In $C_{2}H_{4}$ all the bonds are not equal because here, each C atom is surrounded by 3 $\sigma$ bonds and $1\pi$ bond.
Question 11. In which of the following substances will hydrogen bond be strongest?
(i) $HCl$
(ii) $H_{2}O$
(iii) $HI$
(iv) $H_{2}S$
Answer:
The answer is the option (ii)$H_{2}O$
Explanation: The hydrogen bond will be the strongest in $H_{2}O$ because compared to the other elements Oxygen is more electronegative as well as it's small in size.
Question 12. If the electronic configuration of an element is $1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{2} 4s^{2},$ the four electrons involved in chemical bond formation will be_____.
(i) $3p^{6}$
(ii) $3p^{6},4s^{2}$
(iii) $3p^{6},3d^{2}$
(iv) $3d^{2},4s^{2}$
Answer:
The answer is the option (iv) $3d^{2},4s^{2}$
Explanation: In transition elements ns electrons and (n-1)d electrons participate, while in bonding, electrons and valence electrons from penultimate shell participate. Therefore, the bond formation will be $3d^{2},4s^{2}$
Question 13. Which of the following angle corresponds to sp2 hybridization?
(i) 90°
(ii) 120°
(iii) 180°
(iv) 109°
Answer:
The answer is the option (ii) 120°
Explanation: Triangular planar is forming an angle of 120°with each other as a result of the sp2 hybridization geometry.
Question 14. The electronic configurations of three elements A, B and C are given below.
Answer the questions from 14 to 17 on the basis of these configurations.
A $1s^{2} 2s^{2} 2p^{6}$
B $1s^{2}2s^{2}2p^{6}3s^{2}3p^{3}$
C $1 s^{2}2s^{2}2p^{6}3s^{2}3p^{5}$
Stable form of A may be represented by the formula
(i) A
(ii) A2
(iii) A3
(iv) A4
Answer:
The answer is the option (i) A
Explanation: The formula will be A only, as the octet of A is complete. It has an atomic number of 10.
Question 15. The electronic configurations of three elements A, B and C are given below.
Answer the questions from 14 to 17 on the basis of these configurations.
A $1s^{2} 2s^{2} 2p^{6}$
B $1s^{2}2s^{2}2p^{6}3s^{2}3p^{3}$
C $1 s^{2}2s^{2}2p^{6}3s^{2}3p^{5}$
Stable form of C may be represented by the formula
(i) C
(ii) C2
(iii) C3
(iv) C4
Answer:
The answer is the option (ii) C2
Explanation:
Bond order $=\frac{1}{2}[6-2]=2;$ hence stable form of c i.e. dichlorine (Cl2) may be represented as C2.
Question 16. The electronic configurations of three elements A, B and C are given below.
Answer the questions from 14 to 17 on the basis of these configurations.
A $1s^{2} 2s^{2} 2p^{6}$
B $1s^{2}2s^{2}2p^{6}3s^{2}3p^{3}$
C $1 s^{2}2s^{2}2p^{6}3s^{2}3p^{5}$
The molecular formula of the compound formed from B and C will be
(i) BC
(ii) B2C
(iii) BC2
(iv) BC3
Answer:
The answer is the option (iv) BC3
Explanation: Here, B represents phosphorus(P) and C represents Chlorine(Cl). Therefore, the compound formed will be PCl3, i.e., BC3.
Question 17. The electronic configurations of three elements A, B and C are given below.
Answer the questions from 14 to 17 on the basis of these configurations.
A $1s^{2} 2s^{2} 2p^{6}$
B $1s^{2}2s^{2}2p^{6}3s^{2}3p^{3}$
C $1 s^{2}2s^{2}2p^{6}3s^{2}3p^{5}$
The bond between B and C will be
(i) Ionic
(ii) Covalent
(iii) Hydrogen
(iv) Coordinate
Answer:
The answer is the option (ii) Covalent
Explanation: The compounds B and C are non-metals; hence, they will form a covalent bond between them.
Question 18. Which of the following order of energies of molecular orbitals of $N_{2}$ is correct?
(i) $(\pi 2py)<(\sigma 2pz)<(\pi ^{*}2px)\approx (\pi ^{*}2py)$
(ii) $(\pi 2py)>(\sigma 2pz)>(\pi ^{*}2px)\approx (\pi ^{*}2py)$
(iii) $(\pi 2py)<(\sigma 2pz)>(\pi ^{*}2px)\approx (\pi ^{*}2py)$
(iv) $(\pi 2py)>(\sigma 2pz)<(\pi ^{*}2px)\approx (\pi ^{*}2py)$
Answer:
The answer is the option (i) $(\pi 2py)<(\sigma 2pz)<(\pi ^{*}2px)\approx (\pi ^{*}2py)$
Explanation: Molecules like B2, C2 & N2 have 1 to 3 electrons in p orbital energy of $\sigma$ 2p molecular orbital are greater than that of $\pi2p_{x} \; and \; \pi 2p_{y}$ molecular orbitals.
Question 19. Which of the following statement is not correct from the view point of molecular orbital theory?
(i) Be2 is not a stable molecule.
(ii) He2 is not stable but $He{_{2}}^{+}$ is expected to exist.
(iii) Bond strength of N2 is maximum amongst the homonuclear diatomic molecules belonging to the second period.
(iv) The order of energies of molecular orbitals in N2 molecule is
$\sigma 2s<\sigma ^{*}2s<\sigma 2p_{z}<(\pi 2px=\pi 2pr)<(\pi ^{*}2px=\pi ^{*}2py)<\sigma ^{*}2pz$
Answer:
The answer is the option (iv) The order of the energies of molecular orbitals in N2 is
$\sigma 2s<\sigma ^{*}2s<\sigma 2p_{z}<(\pi 2px=\pi 2pr)<(\pi ^{*}2px=\pi ^{*}2py)<\sigma ^{*}2pz$
Question 20. Which of the following options represents the correct bond order :
(i) $O{_{2}}^{-}>O_{2}>O{_{2}}^{+}$
(ii) $O{_{2}}^{-}<O_{2}<O{_{2}}^{+}$
(iii) $O{_{2}}^{-}>O_{2}<O{_{2}}^{+}$
(iv) $O{_{2}}^{-}<O_{2}>O{_{2}}^{+}$
Answer:
The answer is the option (ii) $O{_{2}}^{-}<O_{2}<O{_{2}}^{+}$
Explanation: Nb electrons > Na electrons as well as, it depends on the bond order.
B.O. = ½(Nb – Na)
Question 21. The electronic configuration of the outer most shell of the most electronegative element is
(i) $2s^{2}2p^{5}$
(ii) $3s^{2}3p^{5}$
(iii) $4s^{2}4p^{5}$
(iv) $5s^{2}5p^{5}$
Answer:
The answer is the option (i) $2s^{2}2p^{5}$
Explanation: Elements of group 17 has $ns^{2}np^{5}$ electronic configuration. Electronegativity decreases down the group. The elements of group 17 are the most electronegative.
Question 22. Amongst the following elements whose electronic configurations are given below, the one having the highest ionization enthalpy is
(i) $[Ne]3s^{2}3p^{1}$
(ii) $[Ne]3s^{2}3p^{3}$
(iii) $[Ne]3s^{2}3p^{2}$
(iv) $[Ar]3d^{10}4s^{2}4p^{3}$
Answer:
The answer is the option (ii) $[Ne]3s^{2}3p^{3}$
Explanation: As compared to partially filled orbital, half-filled p-orbital is more stable. Hence opt(ii).
NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: MCQ (Type 2)
The Chapter 4 Chemical Bonding and Molecular Structure NCERT Exemplar Solutions MCQ questions are given below. The chapter is vast yet easy to learn.
Question 23. Which of the following have identical bond order?
(i) $CN^{-}$
(ii) $NO^{+}$
(iii) $O{_{2}}^{-}$
(iv) $O{_{2}}^{2-}$
Answer:
The answer is the option (i) $CN^{-}$and (ii) $NO^{+}$
Explanation: They have identical bond order because in both of the species number of electrons in Nb = number of electrons in Nab, i.e. 14. Whereas the number of electrons in $O{_{2}}^{-}$ is 17 and in $O{_{2}}^{2-}$ is 18.
Question 24. Which of the following attain the linear structure:
(i) $BeCl_{2}$
(ii) $NCO^{+}$
(iii) $NO_{2}$
(iv) $CS_{2}$
Answer:
The answer is the option (i) $BeCl_{2}$ and (iv) $CS_{2}$
Explanation: Both of the choices have the central atom sp hybridized; therefore both have a linear structure.
Question 25. CO is isoelectronic with
(i) $NO^{+}$
(ii) $N_{2}$
(iii) $SnCl_{2}$
(iv) $NO{_{2}}^{-}$
Answer:
The answer is the option (i) $NO^{+}$ and (ii) $N_{2}$
Explanation: In both the species have the same number of electrons but a different number of protons. Hence, they are isoelectronic with CO.
Question 26. Which of the following species have the same shape?
(i) $CO_{2}$
(ii) $CCl_{4}$
(iii) $O_{3}$
(iv) $NO{_{2}}^{-}$
Answer:
The answer is the option (iii) $O_{3}$ and (iv) $NO{_{2}}^{-}$
Explanation: In both the species the central atom has the same hybridized state and geometry. Hence, they have the same shape.
Question 27. Which of the following statements are correct about $CO{_{3}}^{2-}$?
(i) The hybridization of central atom is sp3.
(ii) Its resonance structure has one C–O single bond and two C=O double bonds.
(iii) The average formal charge on each oxygen atom is 0.67 units.
(iv) All C–O bond lengths are equal.
Answer:
The answer is option (iii) The average formal charge on each oxygen atom is 0.67 units and (iv) All C-O bond lengths are equal.
Explanation: In resonating structures, bonds are not fixed, and as a result, all bond lengths are equal.
Question 28. Dimagnetic species are those which contain no unpaired electrons. Which among the following are dimagnetic?
(i) $N_{2}$
(ii) $N{_{2}}^{2-}$
(iii) $O_{2}$
(iv) $O{_{2}}^{2-}$
Answer:
The answer is the option (i) $N_{2}$ and (iv) $O{_{2}}^{2-}$
Explanation: Both of them have no unpaired electrons; therefore, they are diamagnetic.
Question 29. Species having same bond order are :
(i) $N_{2}$
(ii) $N{_{2}}^{-}$
(iii) $F{_{2}}^{+}$
(iv) $O{_{2}}^{-}$
Answer:
The answer is the option (iii) $F{_{2}}^{+}$ and (iv) $O{_{2}}^{-}$
Explanation: They have the same order because, Both the species have the same number of electrons in bonding molecular orbital as well as in antibonding orbital.
Question 30. Which of the following statements are not correct?
(i) NaCl, being an ionic compound, is a good conductor of electricity in the solid state.
(ii) In canonical structure,s there is a difference in the arrangement of atoms.
(iii) Hybrid orbitals form stronger bonds than pure orbitals.
(iv) VSEPR Theory can explain the square planar geometry of $XeF_{4}.$
Answer:
The answer is the option (i) NaCl being an ionic compound is a good conductor of electricity in solid-state and (ii) In canonical structures, there is a difference in the arrangement of atoms
Explanation: In (i) ions are not free in solid-state but are strongly bonded through electrostatic forces, and in (ii) it does not show canonical structures being ionic compounds.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: Short Answer Type
The Class 11 NCERT Exemplar Chemistry chapter 4 short answer type questions are given below. Chemical Bonding and Molecular Structure is a vast yet easy to learn chapter.
Question 31. Explain the non-linear shape of $H_{2}S$ and the non-planar shape of $PCl_{3}$ using valence shell electron pair repulsion theory.
Answer:
In $H_{2}S$ as well as in $PCl_{3}$, lone pairs are present along with bond pairs around the central atom. Now, according to VSEPR, theory L.P-L.P > L.P-B.P > B.P-B.P. Therefore, $H_{2}S$ is non-linear, and $PCl_{3}$ is non-planar.
Question 32. Using molecular orbital theory, compare the bond energy and magnetic character of $O{_{2}}^{+}$ and $O{_{2}}^{-}$ species.
Answer:
The molecular orbital configuration of $O{_{2}}^{+}$ and $O{_{2}}^{-}$ has been specified as follows: -
$\mathrm{O}_2^{+}: \sigma 1 s^2, \sigma^* 1 s^2, \sigma 2 s^2, \sigma^* 2 s^2, \sigma 2 p_z^2, \Pi 2 p_y^2, \Pi 2 p_x^2, \Pi^* 2 p_x{ }^1$
$\mathrm{O}_2^{-}: \sigma 1 s^2, \sigma^* 1 s^2, \sigma 2 s^2, \sigma^* 2 s^2, \sigma 2 p_z^2, \Pi 2 p_y^2, \Pi 2 p_x^2, \Pi^* 2 p_x^2, \Pi^* 2 p_y^1$
Therefore, the bond order for $O{_{2}}^{+}= 10-5/2 = 2.5$
And the bond order for $O{_{2}}^{-}= 10-7/2 = 1.5$
We know that as per the molecular orbital theory, the greater is the bond order, the greater is the bond energy. Therefore, $O{_{2}}^{+}$ is more stable than $O{_{2}}^{-}$ .
Question 33. Explain the shape of $BrF_{5}.$
Answer:
The central atom in $BrF_{5}.$ is bromine. Its hybridization is $sp^{3}d^{2}$.
In total, there are 7 valence electrons in a Br atom out of which 5 valence electrons are used for making a pair with the F atoms, and two valence electrons are used for making a lone pair of electrons. We know that the bond pair and lone pair repel each other. Hence, the shape is square pyramidal.
(a) $NO_{2}$ and $OH$ groups are close together compared to the other compound; therefore, compound (I) will form an intramolecular hydrogen bonding, whereas compound (II) will show intermolecular bonding.
(b) Compound (II) forms intermolecular H-bonds, joining more and more molecules together through H-bonds; hence, it will have a higher melting point.
(c) Compound (I) will not be able to form hydrogen bonds with water due to intramolecular H-bonding, thus, will be less soluble in it while compound (II) will be able to form H-bond with water more easily and will be soluble in water.
Question 35. Why does the type of overlap given in the following figure not result in bond formation?
Answer:
No bond formation occurs because the same charges repel as well as there is zero overlap here.
Question 36. Both these compounds have different structures due to the different state of hybridisation of the central atom i.e., phosphous and iodine.
PCl5:sp3d
lF5:sp3d2
Answer:
In ,$PCl_{5}$ P is surrounded by 5 bond pairs and no lone pairs, whereas in $IF_{5}$ , iodine atom is surrounded by 5 bond pairs and one lone pair; therefore the one lone pair in $IF_{5}$ makes its shape different.
Because of more repulsion between bond pairs of $CH_{3}$ groups attached in ether than between bond pair of hydrogen atoms attached to oxygen in the water. Dimethyl ether will have a larger bond angle. The carbon of $CH_{3}$ in ether is attached to three hydrogen atoms through bonds, and electron pairs of these bonds add to the electronic charge density on the carbon atom. Hence, the repulsion between two $-CH_{3}$ groups will be more than that between two hydrogen atoms.
Question 38. Write Lewis structure of the following compounds and show formal charge on each atom.$HNO_{3},NO_{2},H_{2}SO_{4}$
Answer:
The formula for the calculation of the formal charge is as follows: -
Formal charge = ½ [total no: of bonding or shared electrons]
Oxygen with a single bond will have a formal charge of = 6-6-2/2 = -1
Oxygen with a double bond will have a formal charge of = 6-4-4/2 = 0
Therefore, nitrogen will have a formal charge of = 5-2-6/2 = 0
Oxygen 1 and 4 will have a formal charge = 6-4-4/2= 0
Oxygen 2 and 3 will have a formal charge = 6-4-4/2=0
Hydrogen 1 and 2 will have a formal charge = 1-0-2/2=0
Sulfur will have a formal charge =6-0-12/2 = 0
i) HNO3
ii) NO2
iii) H2SO4
We know that the general sequence of the energy levels of the molecular orbital can be written as,
$\sigma 1s<\sigma ^{*}1s<\sigma 2s<\sigma ^{*}2s<\pi 2px=\pi 2py<\sigma 2pz$
$N2\; \sigma 1s^{2}<\sigma ^{*}1s^{2}<\sigma 2s^{2}<\sigma ^{*}2s^{2}<\pi 2p^{2}x=\pi 2p^{2}y<\sigma 2p^{2}z$
$N2^{+}\; \sigma 1s^{2}<\sigma ^{*}1s^{2}<\sigma 2s^{2}<\sigma ^{*}2s^{2}<\pi 2p^{2}x=\pi 2p^{2}y<\sigma 2p^{1}z$
$N2^{-}\; \sigma 1s^{2}<\sigma ^{*}1s^{2}<\sigma 2s^{2}<\sigma ^{*}2s^{2}<\pi 2p^{2}x=\pi 2p^{2}y\sigma 2p^{2}z\sigma 2p^{2}x$
$N{_{2}}^{2+}\; \sigma 1s^{2}<\sigma ^{*}1s^{2}<\sigma 2s^{2}<\sigma ^{*}2s^{2}<\pi 2p^{2}x=\pi 2p^{2}y$
We are also aware that, Bond order = ½ [electrons in BMO – electrons in ABMO]
In case of $N_{2}=10-\frac{4}{2}=3$
Hence, the bond order for $N{_{2}}^{+}=9-\frac{4}{2}=2.5$
Hence, the bond order for $N{_{2}}^{-}=10-\frac{5}{2}=2.5$
Hence, the bond order for $N{_{2}}^{2+}=8-\frac{4}{2}=2$
Therefore, we can conclude that the order of stability is:
$N2>N{_{2}}^{-}>N{_{2}}^{+}>N{_{2}}^{2+}$
Question 40. What is the effect of the following processes on the bond order in $N_{2}$ and $O_{2}$?
(i) $N_{2}\rightarrow N{_{2}}^{+}+e^{-}$
(ii) $O_{2}\rightarrow O{_{2}}^{+}+e^{-}$
Answer:
(i) $N_{2}$ possesses a total of 14 electrons. When one electron is donated, these electrons are removed from the Bonding molecular orbital. Therefore, the BO for $N_{2}=3$
(ii) $O_{2}$ possesses a total of 16 electrons, out of which 8 electrons are in the molecular orbitals and 4 are in the antibonding molecular orbitals. Hence, the BO for $O_{2}=2$
(i) A covalent bond is formed by the overlapping of the atomic orbitals, therefore, it’s a directional bond. Ionic bond is formed by the transference of electrons, and the electrostatic field of an ion is there which is non-directional, hence it’s a non-directional bond. A positive ion is surrounded by a number of anions in any direction, depending upon the size and similarly, a negative ion is surrounded by no. of cations in any direction, depending upon the size.
(ii) In $H_{2}O$, the oxygen atom is sp3 hybridized and we already know that it is surrounded by two lone pairs and two bonded pairs. There are four sp3 hybrid orbitals which give a distorted tetrahedral geometry in which the two corners are occupied by hydrogen atoms and the other two by lone pairs. The bond angle is reduced to 104.5° and 109.5°due to greater repulsive forces between 1p-1p, and molecules acquire a bent or V-shaped structure. Carbon atom is sp hybridized in $CO_{2}$ and the two sp-hybrid orbitals form a linear shape, i.e., an angle of 180°, and hence are oriented in opposite directions.
(iii) Both the carbon atoms are sp-hybridized in the ethyne molecule, and there are two unhybridized orbitals. The hybridized orbitals of both carbon atoms are directed in opposite directions, forming an angle of 180°, and hence, a linear structure is formed.
Question 42. What is an ionic bond? With two suitable examples explain the difference between an ionic and a covalent bond?
Answer:
Ionic bond: The bond which is formed by the transferring electrons from one atom to other atom completely, and as a result, positive and negative ions are formed in this bond. Here, the ions are held together through electrostatic force of attraction. e.g.,
The formation of calcium fluoride,
$Ca\rightarrow Ca^{2+}+2e^{-}$
$[Ar]4s^{2}[Ar]$
$F+e^{-}\rightarrow F^{-}$
$[He]2s^{2}2p^{5}[He]2s^{2}2p^{6}\; or[Ne]$
$Ca^{2+}+2F^{-}\rightarrow CaF_{2}\; or\; Ca^{2+}(F^{-})_{2}$
The information of $NaCl$
$Na\rightarrow Na^{+}+e^{-}$
$[Ne]3s^{2}3p^{5}[Ne]3s^{2}3p^{6}\; or[Ar]$
$Na^{+}+Cl^{-}\rightarrow NaCl\; or\; Na^{+}Cl^{-}$
Covalent bond: The bond which is formed between the two atoms of non-metals by mutual sharing of electrons between them is called covalent bond, e.g., the formation ofthe chlorine molecule can be explained as
Also formation of HCl:
Question 43. Arrange the following bonds in order of increasing ionic character giving a reason
$N-H, F-H, C-H \; and\; O-H$
Answer:
Ionic character of the bond depends on the electronegativity.
Ionic character $\alpha$ to the difference of electronegativity.
In the following bonds, hydrogen is in all but has different electronegativity.
$N-H, F-H, C-H \; and\; O-H$
Increasing order of electronegativity is:
$C<N<O<F$
Therefore, ionic character of the bond in increasing order will be-
$C-H < N-H < O-H < F-H$
Question 44. Explain why $CO{_{3}}^{2-}$ ion cannot be represented by a single Lewis structure. How can it be best represented?
Answer:
In ion, $CO{_{3}}^{2-}$ there are three C-O bonds, and all have the same bond length.
The reason is that they show resonating structures not only one structure. All the properties are explained below by the Lewis structure.
Question 45. Predict the hybridization of each carbon in the molecule of the organic compound given below. Also indicate the total number of sigma and pi bonds in this molecule.
Answer:
In the given structure, two carbon atoms are sp hybridized and linked through triple bond, two carbon atoms are sp2 hybridized and linked through double bonds to O atoms and one is sp3 hybridized and that is linked to two C atoms through single bonds and to two H atoms through single bonds, hence, there are total 5 carbon atoms.
Therefore it is clear that in the molecule, there are 11$\sigma$ bonds and 4$\pi$ bonds.
Question 46. Group the following as linear and non-linear molecules :
$H_{2}O, HOCl, BeCl_{2}, Cl_{2}O$
Answer:
Due to lp-lp repulsion, all the molecules except $BeCl_{2}$ are non-linear. It is because in all other molecules, central atom O is surrounded by two lone pairs.
The structure of $BeCl_{2}$ is-
Cl-Be-Cl
(i) The molecular formula of the compounds formed by X, Y and Z with hydrogen atom are XH4, YH3 and H-Z.
(ii) X, Y and Z have 4, 5 and 7 electrons respectively. These elements belong to the second period and 14th, 15th and 17th groups. The electronegativity of elements increases across the period from group 1 to group 17. Hence, H-Z will have the highest dipole moment.
Question 48. Draw the resonating structure of
(i) Ozone molecule
(ii) Nitrate ion
Answer:
(i) Resonating structure of Ozone $(O)_{3}:$:
(ii) Resonating structure of $NO{_{3}}^{-}:$
Question 49. Predict the shapes of the following molecules based on hybridization.
$BCl_{3}, CH_{4}, CO_{2}, NH_{3}$
Answer:
(i) In $BCl_{3}$, Boron is sp2 hybridized and thus, the shape of $BCl_{3}$ is trigonal planar.
(ii) The shape of $CH_{4}$ is tetrahedral, carbon is sp3 hybridized.
(iii) The shape of $NH_{3}$ molecule is pyramidal, nitrogen atom is sp3 hybridized nut lone pair on N gives pyramidal shape.
(iv) $CO_{2}$ is linear because carbon is sp hybridized.
Question 50. All the C—O bonds in the carbonate ion $\mathrm{CO}_3{ }^{2-}$ are equal in length. Explain
Answer:
In $\mathrm{CO}_3{ }^{2-}$ ion, carbon is bonded to 3 oxygen atoms. It is bonded to 2 oxygen atoms by a double bond. Here, the bonds are not fixed and show resonance; therefore, all C-O bonds are equal in length.
Question 51. What is meant by the term average bond enthalpy? Why is there a difference in bond enthalpy of O—H bond in ethanol $(C_{2}H_{5}OH)$ and water?
Answer:
(i) Average bond enthalpy is obtained by dividing total bond dissociation enthalpy by the number of bonds broken.
(ii) All the identical bonds in a molecule do not have the same bond enthalpies, e.g., in water $(H_{2}O)$, there are two O-H bonds but breaking of first O-H bond, the second O-H bond undergoes some change because of the charge.
Therefore, in polyatomic molecules, the average bond enthalpy is used and calculated by dividing the total bond dissociation enthalpy by the number of bonds broken.
$H_{2}O\; (g)\; \rightarrow H(g)+OH(g);\Delta _{a}H{_{1}}^{o}=502\; kJ\; mol^{-1}$
$OH\; (g)\; \rightarrow H(g)+O(g);\Delta _{b}H{_{2}}^{o}=427\; kJ\; mol^{-1}$
Therefore, Average O-H bond enthalpy $= 502+\frac{427}{2} = 464.5\; kJ\; mol-1$
The bond enthalpies of O-H in $C_{2}H{_{5}}^{2}OH.H_{2}O$ are different because of the different electronic environment around the oxygen atom.
$CH_{3}CH_{2}OH\; H-O-H$
In ethanol, -OH is attached to carbon and in water O-H is attached to a hydrogen atom.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: Matching Type
NCERT Exemplar Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure important questions are discussed below. These are generally asked in exams to test your knowledge. These exemplar solutions is quite helpful for competitive exams.
Question 52. Match the species in Column I with the type of hybrid orbitals in Column II.
|
Column I |
Column II |
|
(i) $SF_{4}$ |
(a) sp3d2 |
|
(ii) $IF_{5}$ |
(b) d2sp3 |
|
(iii) $NO{_{2}}^{+}$ |
(c) sp3 d |
|
(iv) $NH_{4}^{+}$ |
(d) sp3 |
|
|
(e) sp |
Answer:
(i)→ (c); (ii) →(a); (iii) →(e); (iv) →(d)
Explanation:
|
Column I |
Column II |
|
S is surrounded by 4 bond pairs and 1 lone pair (sp3d hybridization) |
|
I is surrounded by 5 bond pairs and 1 lone pair (sp3d2 hybridization) |
|
N has 2 bond pairs and no lone pair (sp hybridization) |
|
N has 4 bond pairs and no lone pair (sp3 hybridization) |
Question 53. Match the species in Column I with the geometry/shape in Column II.
|
Column I |
Column II |
|
(i) $H_{3}O^{+}$ |
(a) Linear |
|
(ii) $HC\equiv CH$ |
(b) Angular |
|
(iii) $ClO{_{2}}^{-}$ |
(c) Tetrahedral |
|
(iv) $NH{_{4}}^{+}$ |
(d) Trigonal bipyramidal |
|
– |
(e) Pyramidal |
Answer:
(i) →(e); (ii) →(a); (iii) →(b); (iv) →(c)
Explanation :
|
Column I |
Column II |
|
1. $H_{3}O^{+}$ |
Oxygen has 3 bond pairs and 1 lone pair (pyramidal shape) |
|
2.$HC\equiv CH$ |
Linear sp hybridization |
|
Cl has 2 bond pairs and two lone pairs (Angular shape) |
|
N has 4 bond pairs and no lone pair (tetrahedral) |
Question 54. Match the species in Column I with the bond order in Column II.
|
Column I |
Column II |
|
(i) NO |
(a) 1.5 |
|
(ii) CO |
(b) 2.0 |
|
(iii) $O{_{2}}^{-}$ |
(c) 2.5 |
|
(iv) $O_{2}$ |
(d) 3.0 |
Answer:
(i) →(c); (ii) →(d); (iii) →(a); (iv) →(b)
Explanation:
|
Column I |
Column II |
|
$NO(7+8=15\; electrons)$ |
|
$CO (6+8 = 14\; electrons)$ |
|
$(8+8+1=17\; electrons)$ |
|
$(8+8=16\; electrons)$ |
Question 55. Match the items given in Column I with examples given in Column II.
|
Column I |
Column II |
|
(i) Hydrogen bond |
(a) C |
|
(ii) Resonance |
(b) LiF |
|
(iii) Ionic solid |
(c) H2 |
|
(iv) Covalent solid |
(d) HF |
|
|
(e) O3 |
Answer:
(i) →(d); (ii) →(e); (iii) →(b); (iv) →(a)
Question 56. Match the shape of molecules in Column I with the type of hybridization in Column II.
|
Column I |
Column II |
|
(i) Tetrahedral |
(a) sp2 |
Answer:
(i) →(c);(ii) →(a); (iii) →(b)
NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: Assertion and Reason Type
The Assertion and Reason type questions of Chapter 4 test students conceptual clarity and logical reasoning skills. These Chapter 4 Chemical Bonding and Molecular Structure NCERT Exemplar Solutions help you understand the reason behind periodic trends and element classification.
Question 57. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Sodium chloride formed by the action of chlorine gas on sodium metal is a stable compound.
Reason (R): This is because sodium and chloride ions acquire octet in sodium chloride formation.
(i) A and R both are correct, and R is the correct explanation of A.
(ii) A and R both are correct, but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A and R both are false.
Answer:
The answer is option (i) A and R both are correct, and R is the correct explanation of A
Explanation:
$Na+Cl\rightarrow NaCl$
2,8,1 2,8,7 (2,8 X 2,8,8)
In NaCl, Sodium and Chlorine ions have a complete octet. Therefore, NaCl is a stable compound.
Question 58. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A) : Though the central atom of both NH3 and H2O molecules are sp3 hybridized, yet H–N–H bond angle is greater than that of H–O–H.
Reason (R) : This is because nitrogen atom has one lone pair and oxygen atom has two lone pairs.
(i) A and R both are correct, and R is the correct explanation of A.
(ii) A and R both are correct, but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A and R both are false.
Answer:
The answer is option (i) A and R both are correct, and R is the correct explanation of A.
Explanation:
$H_{2}O$ has two lone pairs but NH3 has one lone pair. Hence, $H_{2}O$.
Question 59. In the following questions, a statement of Assertion (A) followed by a statement of the reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Among the two O–H bonds in H2O molecule, the energy required to break the first O–H bond and the other O–H bond is the same.
Reason (R) : This is because the electronic environment around oxygen is the same even after the breakage of one O–H bond.
(i) A and R both are correct, and R is the correct explanation of A.
(ii) A and R both are correct, but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) A and R both are false
Answer:
The answer is option (iv) A and R both are false.
Explanation:
Correct assertion: The bond enthalpy in H-O-H is not the same for both the O-H bonds.
Correct reason: Because the electronic charge on the oxygen atom is different after breaking of one O-H bond.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 4: Long Answer Type
Long Answer Type questions of this chapter test students conceptual clarity and logical reasoning skills. These Chemical Bonding and Molecular Structure Class 11 NCERT Exemplar solutions help you understand the reason behind periodic trends and element classification.
Question 60. i) Discuss the significance/ applications of dipole moment.
(ii) Represent diagrammatically the bond moments and the resultant dipole moment in $CO_{2}, NF_{3}$ and $CHCl_{3}$.
Answer:
(i) The formula of calculating dipole moment is,
Dipole moment $(\mu )$ = charge(Q) × distance of separation(r)
Dipole moment is expressed in Debye units (D).
The significance of the Dipole moment is:
-
Helps predict the polar and non-polar nature of compounds
-
The percentage of ionic character can be calculated by the formula:
% of ionic character = $(\mu )$ observed × 100/ $\mu$, ionic
-
It helps to know the symmetry of the molecule.
Symmetrical molecules have zero dipole moment, although they have two or more polar bonds. For example,
The dipole moment in the case of $BeF_{2}$ is zero. This is because the two equal bond dipoles point in opposite directions and cancel the effect of each other.
(ii)
Question 61. Use the molecular orbital energy level diagram to show that $N_{2}$ would be expected to have a triple bond, $F_{2}$ a single bond and $Ne_{2}$ no bond.
Answer:
The general sequence of the energy level of molecular orbitals for nitrogen is:
$\sigma 1s<\sigma ^{*}1s<\sigma 2s<\sigma ^{*}2s<\pi 2p_{x}=\pi 2p_{y}<\sigma 2p_{z}$
The molecular orbitals in the sequence of their energy levels for the given molecules have been described below:
$N_{2}\sigma 1s^{2}\; \sigma^{*} 1s^{2}\; \sigma 2s^{2}\sigma^{*} 2s^{2}\pi 2p{_{x}}^{2}=\pi 2p{_{y}}^{2}\sigma 2p{_{z}}^{2}$
Now, we know that the,
Bond order for $N_{2}=\frac{8-2}{2}=3$
Now, as the bond order is 3, it means that $N_{2}$ will have a triple bond.
The molecular orbital of Fluorine has been described below: -
$F_{2}= \sigma 1s^{2}, \sigma ^{*} 1s^{2}, \sigma 2s^{2}, \sigma ^{*} 2s^{2}, \sigma \; 2px^{2}, \pi 2px^{2} = \pi 2py^{2}$
The bond order of $F_{2}=\frac{10-8}{2}=1$
Thus, we can imply that when the bond order is 1, the number of bonds must also be 1.
$Ne_{2}= \sigma 1s^{2}, \sigma ^{*} 1s^{2}, \sigma 2s^{2}, \sigma ^{*} 2s^{2}, \sigma \; 2px^{2}, \pi 2px^{2} = \pi 2py^{2},\pi ^{*}2p{_{x}}^{2}=\pi ^{*}2p{_{y}}^{2}$
Now, we can calculate the bond order of $Ne_{2}=\frac{10-10}{2}=0$
Thus, $Ne_{2}$ has no bond.
Question 62. Briefly describe the valence bond theory of covalent bond formation by taking the example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
Answer:
Valence bond theory was introduced by Heitler and London in 1927. It was developed by Pauling. It is based on knowledge of atomic orbitals, electronic configuration of elements, overlapping of atomic orbitals, and hybridization of orbitals.
Let us consider two hydrogen atoms A and B. Let’s assume that they are approaching each other and let their nuclei be NA and NB and electrons present in them be eA and eB.
Now let us imagine that the two atoms are at a large distance from each other, hence, there is no interaction between them. Now, think that they are coming close to each other, and new attractive and repulsive forces begin to operate.
Now we know that attractive forces arise between:
-
Nucleus of one atom and its own electron that is $N_{A}-e_{A}$ and $N_{B}-e_{b}$.
-
Nucleus of one atom and electron of another atom, i.e., $N_{A}-e_{B},N_{B}-e_{A}$, NB-eA. Similarly, repulsive forces arise between
(a) Electrons of two atoms like $e_{A}-e_{B}$
(b) Nuclei of two atoms $N_{A}-N_{B}$.
It has been found that the magnitude of the new attractive force is more than the new repulsive forces. As a result, two atoms approach each other and the potential energy decreases. Eventually, the net force of attraction balances the force of repulsion andthe system acquires minimum energy. At this point, two hydrogen atoms are said to be bonded together to form a stable molecule having a bond length 74pm.
Since the energy is released when the bond is formed between two hydrogen atoms, the hydrogen molecule is more stable than of the isolated hydrogen atoms. The energy so released is called the bond enthalpy, which corresponds to a minimum in the curve depicted in the figure. Conversely, 435.8kJ of energy is required to dissociate one mole of H2 molecules.
$H_{2}(g)+435.8 \; kJ\; mol^{-1}\rightarrow H(g)+H(g).$
The potential energy curve for the formation of H2 molecule as a function of the internuclear distance of the H atoms. The minimum in the curve corresponds to the most stable state of H2.
The hybridization of the S in $SF_{6}$ is $sp^{3}d^{2}$ and the hybridization of the P in $PCl_{5}$ is $sp^{3}d.$
We know that $PCl_{5}$ has trigonal bipyramidal geometry. As such, the axial bonds are slightly longer as compared to the equatorial bonds. This is a result of the axial bonds experiencing greater repulsion from other bonds as compared to the equatorial bonds.
We know that $SF_{6}$ has an octahedral structure. As such, all the bonds possess the same length, because all bonds experience similar repulsion from other bonds.
Question 64. (i)Discuss the concept of hybridization. What are its different types in carbon atoms?
(ii) What is the type of hybridization of carbon atoms marked with star.
Answer:
(i) The process of hybridization can be defined as the process that involves the intermixing of the orbitals with slightly different or similar energy levels in order to form new orbitals that have similar shapes and energy levels. These orbitals are known as the hybrid orbitals.
The valence shell of an atom is always hybridized. Additionally, the merging orbitals are of nearly similar energy or the same energy. These orbitals do not form pie bonds.
(a) sp hybridization: Carbon compounds with a triple bond i.e.$C\equiv C$ have this type of hybridization.
(b) sp2 hybridization: Carbon compounds with a double bond i.e. $C= C$ have this type of hybridization.
(c) sp3 hybridization: Carbon compounds with single bonds i.e. $C - C$ have this type of hybridization.
(ii)
(a)
(b)
(c)
(d)
(e)
(i) In the formation of dioxygen from oxygen atoms, 10 molecular orbitals will be formed.
The above statement is the correct answer.
There are unpaired electrons in two molecular orbitals in dioxygen. Hence, the statement (ii) is not correct.
The total number of antibonding orbitals in dioxygen is equal to the total number of bonding molecular orbitals. Therefore, statement (iii) is not correct.
The number of filled antibonding orbitals is not always going to be the same as the number of filled bonding orbitals. Hence, the statement (iv) is not correct.
Question 66. Which of the following molecular orbitals has the maximum number of nodal planes?
(i) $\sigma ^{*}1s$
(ii) $\sigma ^{*}2p_{z}$
(iii) $\pi 2p_{x}$
(iv) $\pi^{*} 2p_{y}$
Answer:
The answer is option (ii) The molecular orbital that has the maximum no. of nodal planes is $\sigma ^{*}2p_{z}$
Question 67. Which of the following pair is expected to have the same bond order?
(i) $O_{2},N_{2}$
(ii) $O{_{2}}^{+},N{_{2}}^{-}$
(iii) $O{_{2}}^{-},N{_{2}}^{+}$
(iv) $O{_{2}}^{-},N{_{2}}^{-}$
Answer:
The answer is the option (ii) $O{_{2}}^{+},N{_{2}}^{-}$; because the Isoelectronic species have a similar bond order.
Option $I-O_{2}$ has 16 electrons and N2 has 14 electrons.
Option II - Both $O_{2}^{+}$ and $N_{2}^{-}$ has 15 electrons.
Option III - $O_{2}^{-}$ has 17 electrons and $N_{2}^{+}$ has 13 electrons.
Option IV - $O_{2}^{-}$has 17 electrons and $N_{2}^{-}$ has 15 electrons
Question 68. In which of the following molecules, $\sigma 2p_{z}$ molecular orbital is filled after $\pi 2p_{x}$ and $\pi 2p_{y}$ molecular orbitals?
(i) $O_{2}$
(ii) $Ne_{2}$
(iii) $N_{2}$
(iv) $F_{2}$
Answer:
The answer is option (iii) N2
The first atomic orbitals on two atoms form two molecular orbitals designated as
$\sigma 1s\; and\; \sigma ^{*}1s$
Similarly, the 2s and 2p atomic orbitals give rise to the eight molecular orbitals given below
Antibonding $MOs\; \sigma^{*}2s\; \sigma^{*}2p_{z}\pi ^{*}2p_{x}\; \pi ^{*}2p_{y}$
Bonding $Mos\; \sigma 2s\; \sigma2p_{z}\; \pi 2p_{x}\; \pi 2p_{y}$
For molecules such as B2, C2, N2, etc. it is observed that the increasing order of energies of various molecular orbitals is -
Here, the important characteristic feature is that energy of $\sigma 2p_{z}$ molecular orbital is higher than that of $\pi 2p_{x}$ and $\pi 2p_{y}$ molecular orbitals
NCERT Class 11 Chemistry Chapter Chemical Bonding and Molecular Structure: Higher Order Thinking Skills (HOTS) Questions
HOTS questions of Chapter 4 Chemical Bonding and Molecular Structure NCERT Exemplar are designed to enhance analytical thinking and application-based understanding.
Question 1: A molecule with the formula AX4Y has all it’s elements from the p-block. Element A is rarest,
monoatomic, non-radioactive from its group and has the lowest ionization enthalpy value among A, X and Y. Elements X and Y have first and second highest electronegativity values respectively among all the known elements. The shape of the molecule is :
(1). Square pyramidal
(2). Octahedral
(3). Pentagonal planar
(4). Trigonal bipyramidal
Solution: Given A is rarest, monoatomic, non-radioactive p-block element and form $\mathrm{AX}_4 \mathrm{Y}$ type of molecule.
$\therefore$ It is concluded that it is Xe
It is given the electronegativity of A is less than X & Y
It is given the electronegativity of $\mathrm{X} \& \mathrm{Y}$ is highest and second highest respectively among all element.
$\therefore \mathrm{X} \& \mathrm{Y}$ are $\mathrm{F} \& \mathrm{O}$
$\therefore$ Compound is consider as $\mathrm{XeOF}_4$ with square pyramidal shape.

Hence, the correct answer is option (1).
Question 2: Which of the following statement is true with respect to $\mathrm{H}_2 \mathrm{O}, \mathrm{NH}_3$ and $\mathrm{CH}_4$ ?
A. The central atoms of all the molecules are $\mathrm{sp}^3$ hybridized.
B. The $\mathrm{H}-\mathrm{O}-\mathrm{H}, \mathrm{H}-\mathrm{N}-\mathrm{H}$ and $\mathrm{H}-\mathrm{C}-\mathrm{H}$ angles in the above molecules are $104.5^{\circ}, 107.5^{\circ}$ and $109.5^{\circ}$ respectively.
C. The increasing order of dipole moment is $\mathrm{CH}_4<\mathrm{NH}_3<\mathrm{H}_2 \mathrm{O}$.
D. Both $\mathrm{H}_2 \mathrm{O}$ and $\mathrm{NH}_3$ are Lewis acids and $\mathrm{CH}_4$ is a Lewis base
E. A solution of $\mathrm{NH}_3$ in $\mathrm{H}_2 \mathrm{O}$ is basic. In this solution $\mathrm{NH}_3$ and $\mathrm{H}_2 \mathrm{O}$ act as Lowry-Bronsted acid and base respectively.
Choose the correct answer from the options given below :
(1). A, B and C only
(2). C, D and E only
(3). A, D and E only
(4). A, B, C and E only
Solution: 
Dipole moment
$$
\mathrm{H}_2 \mathrm{O}>\mathrm{NH}_3>\mathrm{CH}_4
$$
$\mathrm{H}_2 \mathrm{O}$ & $\mathrm{NH}_3$ are Lewis Bases
$\mathrm{NH}_3$ act as Lowry- Bronsted base
Hence, the correct answer is option (1)
Question 3. Which of the following molecules(s) show/s paramagnetic behavior ?
(A) $\mathrm{O}_2$
(B) $\mathrm{N}_2$
(C) $\mathrm{F}_2$
(D) $\mathrm{S}_2$
(E) $\mathrm{Cl}_2$
Choose the correct answer from the options given below :
(1) B only
(2) A & C only
(3) A & E only
(4) A & D only
Solution:
| No. of unpaired $e^{-}$ | ||
| (A) | $\mathrm{O}_2$ | 2 |
| (B) | $\mathrm{~N}_2$ | 0 |
| (C) | $\mathrm{~F}_2$ | 0 |
| (D) | $\mathrm{~S}_2$ | 2 |
| (E) | $\mathrm{Cl}_2$ | 0 |
If species contain unpaired electron than it is paramagnetic.
So A & D are paramagnetic.
Hence, the correct answer is option (4).
Question 4:
Given below are two statements:
Statement (I) : for $C \ell \mathrm{F}_3$, all three possible structures may be drawn as follows.
Statement (II) : Structure III is most stable, as the orbitals having the lone pairs are axial, where the $\ell \mathrm{p}-\mathrm{bp}$ repulsion is minimum.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Statement I is incorrect but statement II is correct.
(2) Statement I is correct but statement II is incorrect.
(3) Both Statement I and statement II are correct.
(4) Both Statement I and statement II are incorrect.
Answer:
Statement 1 is correct.
Statement 2 is incorrect since in $\mathrm{sp}^3 \mathrm{~d}$ hybridization, a lone pair cannot occupy an axial position due to lone pair-bond pair repulsion. The lone pairs will be at equatorial position for maximum stability.
Hence, the correct answer is option (2).
Question 5: In $\mathrm{SO}_2, \mathrm{NO}_2^{-}$and $\mathrm{N}_3^{-}$the hybridizations at the central atom are respectively :$\mathrm{sp}^2, \mathrm{sp}^2$ and $\mathrm{sp}^2$
(1) $\mathrm{sp}^2, \mathrm{sp}^2$ and sp
(2) $\mathrm{sp}^2, \mathrm{sp}$ and sp
(3) $\mathrm{sp}^2, \mathrm{sp}^2$ and $\mathrm{sp}^2$
(4) $\mathrm{sp}, \mathrm{sp}^2$ and sp
Answer:
$\mathrm{SO}_2 \Rightarrow 2 \sigma$ bond +1 l.p. $\Rightarrow s p^2$ hybridisation

$\mathrm{NO}_2^{-} \Rightarrow 2 \sigma$ bond +1 l.p. $\Rightarrow s p^2$ hybridisation

$\mathrm{N}_3^{-} \Rightarrow 2 \sigma$ bond $\Rightarrow s p$ hybridisation

Hence, the correct answer is option (1).
Approach to Solve Questions of Chapter 4 Chemical Bonding and Molecular Structure
Chemical Bonding and Molecular Structure is one of the most conceptually rich chapters as it lays the foundation for understanding how atoms combine to form compounds. Here is a descriptive and detailed approach to help you effectively study and solve questions from Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure :
1. Before solving questions it is very important to understand the structure of the chapter and break the chapter into manageable sections. Students can also refer to Class 11 Chemistry Chapter 4 Notes for better understanding.
2. Try to learn and memorize key concepts like Lewis structure, octet rule, bond parameters, hybridization, etc. Most of the Chemical Bonding and Molecular Structure questions and answers are often asked directly from these topics. You can revise these concepts from notes available on our website.
3. The theories like valence bond theory, valence shell electron pair repulsion theory and molecular orbital theory are too crucial to understand. Make sure to understand their applications and limitations.
4. First, read the questions thoroughly and note down the given information. Apply the concepts learned and solve in a step-wise manner.
5. Solve NCERT examples, back exercises, and exemplar problem. You can also refer to the solved examples to learn how to answer the question. The NCERT Exemplar Solutions Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure will make your learning feasible.
Advantages of Using Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Solutions
The class 11 chemistry chapter 4 chemical bonding and molecular structure question answer cover all important concepts from the NCERT book in a simple and organised manner. The advantages of using these NCERT Exemplar solutions for Class 11 are given below:
- Students can use these solutions to understand the concepts like valence bond theory, hybridisation, molecular orbital theory, and VSEPR theory with the help of solved examples.
- These solutions cover all the topics from NCERT Exemplar and they are written in a very clear and comprehensive manner.
- The Chapter 4 Chemical Bonding and Molecular Structure NCERT Exemplar Solutions provide step by step explanations of every question.
- These solutions of NCERT are prepared by subject experts in a concise format that helps students in both boards and competitive exams.
Topics Of NCERT Exemplar for Class 11 Chemistry Chapter 4
Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure covers key concepts. Understanding these concepts are essential for building a strong foundation in Chemical Bonding and Molecular Structure.
-
VSEPR theory
-
Lewis structures
-
Valence bond theory
-
The polar character of covalent bonds
-
The concept of hybridization
-
The molecular orbital theory of homonuclear diatomic molecules
NCERT Exemplar Class 11 Chemistry Solutions Chapter-Wise
Students can follow the links given below to access all NCERT Exemplar Solutions for Class 11.
NCERT Solutions for Class 11 Chemistry
Students can follow the links given below to access all NCERT Solutions for Class 11 Chemistry
NCERT Exemplar Class 11 Solutions
Students can follow the links given below to access NCERT Exemplar Solutions for other subjects for Class 11.
NCERT Solutions subject-wise
Given below are links for Class 11 NCERT Solutions
NCERT Notes subject-wise
Given below are links for Class 11 NCERT Notes
NCERT Books and NCERT Syllabus
Given below are links for Class 11 NCERT Books and Syllabus
Frequently Asked Questions (FAQs)
A chemical bond is important because it determines the structure and properties of compounds, influencing everything from the stability of molecules to their reactivity and physical characteristics.
The type of bond that forms can generally be determined by the difference in electronegativity between the two elements. A large difference typically greater than 1.7 suggests ionic bonding, a small difference indicates covalent bonding, and if the difference is negligible, it points towards non-polar covalent bonding.
In NCERT Exemplar Class 11 Chemistry, isostructural species are those having the same shape and number of bonding pairs of electrons.
Chapter 4 Chemical Bonding and Molecular Structure NCERT Exemplar Solutions provide detailed answers and explanations to advanced questions from the NCERT Exemplar book. These solutions help students understand key concepts like bond formation, molecular geometry, and hybridisation, and are useful for exam preparation and conceptual clarity.
The different types of chemical bonds are covalent bonds, ionic bonds, and metallic bonds.
Ionic bond: This is a type of chemical bond that results from the electrostatic attraction between oppositely charged ions. It is formed when one atom donates one or more electrons to another atom, resulting in a cation and an anion.
Covalent bond: This is a type of chemical bond that results from the sharing of electrons between two atoms. It is formed when two atoms come close enough to share their valence electrons in order to achieve a stable electronic configuration.
Resonance is generally used in molecular structure to describe the delocalization of electrons in a molecule. It occurs when there is more than one valid Lewis structure for a molecule, and the actual electron distribution is a hybrid of these structures.
The molecular structure of molecules can be determined using various experimental techniques, such as electron diffraction, X-ray crystallography, and infrared spectroscopy. It can also be predicted using theoretical methods, such as quantum chemistry and molecular mechanics.
It is a type of chemical bond that exists in metals. In this type of bond, valence electrons are not associated with specific atoms but are free to move throughout the entire crystal structure of the metal.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters


























