NCERT Exemplar class 11 chemistry chapter 10 s-block elementsprovides advanced, application-based questions that help students understand the properties, trends, and reactions of alkali and alkaline earth metals in greater depth.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 10 The s Block Elements
Did you know that sodium found in our kitchen salt can cause an explosion when dropped in water, or calcium found in our bones can light up crackers? Fascinating! This is what s-block elements are, from keeping our nerves working to constructing high-rise buildings, they play a vital role. These elements are arranged in Group 1 and Group 2 of the periodic table, also known as alkali and alkaline earth metals.
This Story also Contains
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: MCQ (Type 1)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: MCQ (Type 2)
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: Short Answer Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: Matching Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: Assertion and Reason Type
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: Long Answer Type
- Class 11 Chemistry NCERT Chapter s-Block: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Chapter 10 s-block
- Topics And Subtopics of NCERT Exemplar Class 11 Chapter 10
- General Trends in s-block Elements
- Advantages of Using NCERT Exemplar Class 11 Chemistry Solutions Chapter 10 The s-Block Elements
- NCERT Exemplar Solutions Class 11 Chemistry Chapter-Wise
- NCERT Solutions for Class 11 Chemistry
- NCERT Exemplar Class 11 Solutions Subject-wise
- NCERT Solution subject-wise
- NCERT Notes subject-wise
- NCERT Books and NCERT Syllabus
The metals of the s-block are lightweight, soft, highly reactive and rarely found in their free state in nature. In this chapter, students are going to learn about properties, trends and reactions of s-block elements. To help students, our subject experts have designed comprehensive NCERT Exemplar Class 11 Chemistry Solutions offering clear explanations and conceptual clarity. In these s-block elements class 11 chemistry chapter 10 ncert exemplar solutions higher order thinking skills (HOTs) are also added to enhance your understanding of the concepts. These NCERT exemplar solutions are beneficial for competitive exams like JEE and NEET.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: MCQ (Type 1)
At first, the MCQ questions are covered in the Class 11 Chemistry NCERT Exemplar Solutions Chapter 10 to enhance your knowledge. The concepts are explained in detail in class 11 chemistry chapter 10 notes available on our website. Students can also check NCERT Solutions to all questions chapter-wise.
Question 1 The alkali metals are low-melting. Which of the following alkali metals is expected to melt if the room temperature rises to $30^{\circ}C$?
(i) Na
(ii) K
(iii) Rb
(iv) Cs
Answer:
The answer is the option (iv)
Melting point decreases down the groups in a periodic table.
Question 2 Alkali metals react with water vigorously to form hydroxides and dihydrogen. Which of the following alkali metals reacts with water least vigorously?
(i) Li
(ii) Na
(iii) K
(iv) Cs
Answer:
The answer is the option (i)
Li has an extremely high hydrogen enthalpy. So, its reaction with water releases a high amount of energy, most of which is consumed in fusion, vaporization and ionization. Hence, its reaction with water is less vigorous.
Question 3 The reducing power of a metal depends on various factors. Suggest the factor which makes Li, the strongest reducing agent in aqueous solution.
(i) Sublimation enthalpy
(ii) Ionisation enthalpy
(iii) Hydration enthalpy
(iv) Electron-gain enthalpy
Answer:
The answer is the option (iii)
Hydration enthalpy is a measure of the tendency of an element to lose an electron in aqueous solution. More negative the hydration enthalpy, greater is the ability to lose an electron, making it a strong reducing agent.
Question 4 Metal carbonates decompose on heating to give metal oxide and carbon dioxide. Which of the metal carbonates is most stable thermally?
$(i) MgCO_{3}$
$(ii) CaCO_3$
$(iii) SrCO_3$
$(iv) BaCO_3$
Answer:
The answer is the option (iv)
Thermal stability of $MCO_{3}$ depends on the stability of MO. If MO is stable $MCO_{3}$ is unstable and vice versa
BaO is the least stable, making $BaCO_{3}$ most stable.
Question 5 Which of the carbonates given below is unstable in air and is kept in $CO_2$ atmosphere to avoid decomposition?
$(i) BeCO_{3}$
$(ii) MgCO_{3}$
$(iii) CaCO_{3}$
$(iv) BaCO_{3}$
Answer:
The answer is the option (i)
Strong polarising effect due to the small size of $Be^{2+}$ makes $BeCO_3$ unstable.
Question 6 Metals form basic hydroxides. Which of the following metal hydroxide is the least basic?
$(i) Mg(OH)_{2}$
$(ii) Ca(OH)_{2}$
$(iii) Sr(OH)_{2}$
$(iv) Ba(OH)_{2}$
Answer:
The answer is the option (i)
Basic character decreases down the group for hydroxides with an increase in the size of metal.
Question 7 Some of the Group 2 metal halides are covalent and soluble in organic solvents. Among the following metal halides, the one which is soluble in ethanol is
$(i) BeCl_2$
$(ii) MgCl_2$
$(iii) CaCl_2$
$(iv) SrCl_2$
Answer:
The answer is the option (i)
Covalent metal halides are soluble in organic solvents like ethanol.
$BeCl_{2}$ has covalent character due to small size and high effective nuclear charge.
Question 8 The order of decreasing ionisation enthalpy in alkali metals is
$(i) Na > Li > K > Rb$
$(ii) Rb < Na < K < Li$
$(iii) Li > Na > K > Rb$
$(iv) K < Li < Na < Rb$
Answer:
The answer is the option (iii)
Li > Na > K > Rb
Effective nuclear charge and ionization enthalpy decrease with increase in the size of the atom down the group.
Question 9 The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals, the lowest solubility of LiF in water is due to
(i) Ionic nature of lithium fluoride
(ii) High lattice enthalpy
(iii) High hydration enthalpy for lithium ion
(iv) Low ionisation enthalpy of lithium atom
Answer:
The answer is the option (ii)
Higher the lattice enthalpy and lower the hydration enthalpy, lower is the solubility of metal halides. In the case of LiF, high lattice enthalpy results in lower solubility.
Question 10 Amphoteric hydroxides react with both alkalies and acids. Which of the following Group 2 metal hydroxides is soluble in sodium hydroxide?
$(i) Be(OH)_{2}$
$(ii) Mg(OH)_{2}$
$(iii) Ca(OH)_{2}$
$(iv) Ba(OH)_{2}$
Answer:
The answer is option (i)
$Be(OH)_{2}$ is an amphoteric hydroxide. It reacts with base to give beryllate and water.
$\mathrm{Be}(\mathrm{OH})_2+2 \mathrm{OH}^{-} \rightarrow\left[\mathrm{Be}(\mathrm{OH})_4\right]^{2-}$
$2 \mathrm{NaOH}+\mathrm{Be}(\mathrm{OH})_2 \rightarrow \mathrm{Na}_2\left[\mathrm{Be}(\mathrm{OH})_4\right]$
When it reacts with an acid, it behaves as a base and forms a salt and water.
$\mathrm{Be}(\mathrm{OH})_2+2 \mathrm{HCl} \rightarrow \mathrm{BeCl}_2+2 \mathrm{H}_2 \mathrm{O}$
Question 11 In the synthesis of sodium carbonate, the recovery of ammonia is done by treating $NH_{4}Cl$ with $Ca(OH)_{2}$. The by-product obtained in this process is
$(i) CaCl_{2}$
$(ii) NaCl$
$(iii) NaOH$
$(iv) NaHCO_{3}$
Answer:
The answer is the option (i)
$CaCl_{2}$
On treating $NH_{4}Cl$ with $Ca(OH)_{2}$ , $CaCl_{2}$ is formed as a by product
$2NH_{4} Cl+CaOH_{2} \rightarrow 2NH_{3}+2H_{2}O+CaCl_{2}$
Question 12 When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The colour of the solution is due to
(i) ammoniated electron
(ii) sodium ion
(iii) sodium amide
(iv) ammoniated sodium ion
Answer:
The answer is the option (i)
Alkali metals, when dissolved in liquid ammonia give deep blue colour. The colour of the solution is due to ammoniated electrons which get excited to a higher energy level by absorbing red wavelength and start transmitting blue colour.
$Na+\left (x+y \right )NH_{3}\rightarrow\left [ \left (Na\left (NH_{3} \right )_{x} \right ) \right ]^{+} e \left (NH_{3} \right )_{y}^{-}$
Question 13 By adding gypsum to cement
(i) setting time of cement becomes less.
(ii) setting time of cement increases.
(iii) colour of cement becomes light.
(iv) shining surface is obtained.
Answer:
The answer is the option (ii)
Gypsum is added to cement to increase the setting time of cement to let it get hardened.
Question 14 Dead burnt plaster is
$(i)CaSO_{4}$
$(ii)CaSO_{4}. \frac{1}{2}H_{2}O$
$(iii)CaSO_{4}. H_{2}O$
$(iv)CaSO_{4}. 2H_{2}O$
Answer:
The answer is the option (i)
Plaster of Paris $CaSO_{4}.12H_{2}O$ on heating at 2000 changes to anhydrous $CaSO_{4}$ which is known as Dead burnt plaster.
Question 15 Suspension of slaked lime in water is known as
(i) lime water
(ii) quick lime
(iii) milk of lime
(iv) aqueous solution of slaked lime
Answer:
The answer is the option (iii)
Slaked lime $Ca(OH)_{2}$ gives a white suspension in water called Milk of Lime. It is a highly exothermic reaction and the milk of lime is used in whitewashing.
Question 16 Which of the following elements does not form hydride by direct heating with dihydrogen?
(i) Be
(ii) Mg
(iii) Sr
(iv) Ba
Answer:
The answer is the option $(i) BeH_{2}$ can be prepared by reacting $BeCl_{2}$ with $LiAlH_{4}$
$2BeCl_{2}+LiAlH_{4}\rightarrow 2BeH_{2}+LiCl+AlCl_{3}$ .
Question 17 The formula of soda ash is
$(i) Na_{2} CO_{3}.10H_{2}O$
$(ii) Na_{2}CO_{3}.H_{2}O$
$(iii) Na_{2}CO_{3} .2H_{2}O$
$(iv) Na_{2}CO_{3}$
Answer:
The answer is the option (iv)
Soda ash is formed when Washing soda $(Na_{2}CO_{3}.10H_{2}O )$ loses water of crystallization above 373K.
Question 18 A substance which gives brick red flame and breaks down on heating to give oxygen and a brown gas is
(i) Magnesium nitrate
(ii) Calcium nitrate
(iii) Barium nitrate
(iv) Strontium nitrate
Answer:
The answer is the option(ii)
$Ca\left (NO_{3} \right )_{2} \rightarrow CaO+NO_{2}+O_{2}$
Ca gives the brick red colour and $NO_{2}$ is a brown gas.
Question 19 Which of the following statements is true about $Ca(OH)_2$ ?
(i) It is used in the preparation of bleaching powder
(ii) It is a light blue solid
(iii) It does not possess disinfectant property.
(iv) It is used in the manufacture of cement.
Answer:
The answer is the option (i)
$2Ca(OH)_{2}+2Cl_{2} \rightarrow CaOCl_{2}+CaCl_{2}+2H_{2}O$
$CaOCl_{2}$ is the formula for bleaching powder
Question 20 A chemical A is used for the preparation of washing soda to recover ammonia. When $CO_{2}$ is bubbled through an aqueous solution of A, the solution turns milky. It is used in white washing due to disinfectant nature. What is the chemical formula of A?
$(i) Ca (HCO_{3})_{2}$
$(ii) CaO$
$(iii) Ca(OH)_{2}$
$(iv) CaCO_{3}$
Answer:
The answer is the option (iii)
On passing $CO_{2}$ through $Ca(OH)_{2}$, lime water, turns from colorless to milky due to formation of $CaCO_{3}$.
$Ca(OH)_{2}+CO_{2}\rightarrow CaCO_{3}+H_{2}O$
Also in Solvay Ammonia soda process,
$2NH_{4}Cl+Ca(OH)_{2} \rightarrow 2NH_{3}+2H_{2}O+CaCl_{2}$
Question 21 Dehydration of hydrates of halides of calcium, barium and strontium i.e., $CaCl_{2}.6H_{2}O, BaCl_{2}.2H_{2}O, SrCl_{2}.2H_{2}O$, can be achieved by heating. These become wet on keeping in air. Which of the following statements is correct about these halides?
(i) act as dehydrating agent
(ii) can absorb moisture from air
(iii) Tendency to form hydrate decreases from calcium to barium
(iv) All of the above
Answer:
The answer is the option (iv)
Alkaline earth metal chlorides are hydrated and hygroscopic in nature. Thus, all of the above options are correct.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: MCQ (Type 2)
The NCERT Exemplar class 11 chemistry chapter 10 s-block elements questions are provided here with simple explanations. These MCQ-type questions are covered to improve your conceptual thinking and problem-solving ability:
Question 22 Metallic elements are described by their standard electrode potential, fusion enthalpy, atomic size, etc. The alkali metals are characterized by which of the following properties?
(i) High boiling point
(ii) High negative standard electrode potential
(iii) High density
(iv) Large atomic size
Answer:
The answer is the option (ii) and (iv)
Alkali metals have the largest size and low boiling point and density (Since size decreases from left to right across a period). Due to less effective nuclear charge, they can easily lose the electrons from the outermost shell and have high negative standard electrode potential.
Question 23 Several sodium compounds find use in industries. Which of the following compounds are used for textile industry?
$(i) Na_{2}CO_{3}$
$(ii) NaHCO_{3}$
$(iii) NaOH$
$(iv) NaCl$
Answer:
The answer is the option (i) and (iii)
$Na_{2}CO_{3}$ is used in making soap powders, in laundry and textile industry. $NaOH$ is used in Soap industry and paper industry.
Question 24 Which of the following compounds are readily soluble in water?
$(i) BeSO_{4}$
$(ii) MgSO_{4}$
$(iii) BaSO_{4}$
$(iv) SrSO_{4}$
Answer:
The answer is the option (i) and (ii)
Hydration energy decreases down the group as size increases.
Hydration energy of $Be^{+2}$and $Mg^{+2}$ is very high. Hence their compounds are soluble.
Question 25 When Zeolite, which is hydrated sodium aluminium silicate is treated with hard water, the sodium ions are exchanged with which of the following ion(s)?
$(i) H^{+} ions$
$(ii) Mg^{2+} ions$
$(iii) Ca^{2+} ions$
$(iv) SO_{4}^{2-} ions$
Answer:
The answer is the option (ii) and (iii)
Zeolite is used to remove the hardness of the water. Zeolite is a sodium aluminosilicate.
$Ca^{2+}$ and $Mg^{2+}$ ions in hard water get exchanged with $Na^{+}$ ion of zeolite.
Question 26 Identify the correct formula of halides of alkaline earth metals from the following.
$(i) BaCl_{2}.2H_{2}O$
$(ii) BaCl_{2}.4H_{2}O$
$(iii) CaCl_{2}.6H_{2}O$
$(iv) SrCl_{2}.4H_{2}O$
Answer:
The answer is the option (i) and (iii)
The correct formulas of halides of alkaline earth metals are $\mathrm{BaCl}_2 \cdot 2 \mathrm{H}_2 \mathrm{O}$ and $\mathrm{CaCl}_2 \cdot 6 \mathrm{H}_2 \mathrm{O}$. This is because the extent of hydration of chlorides decreases down the group due to the increasing ionic size and decreasing hydration enthalpy. Calcium, being higher up in the group, forms a hexahydrate, while barium, lower in the group, forms only a dihydrate.
The extent of hydration of chlorides of alkaline earth metals decreases down the group.
Question 27 Choose the correct statements from the following.
(i) Beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal.
(ii) Beryllium sulphate is readily soluble in water as the greater hydration enthalpy of $Be^{2+}$ overcomes the lattice enthalpy factor.
(iii) Beryllium exhibits coordination number more than four.
(iv) Beryllium oxide is purely acidic in nature.
Answer:
The answer is the option (i) and (ii)
Be has diagonal relation with Al and forms a protective film of oxide and is not readily attacked by acids.
The high hydration enthalpy of $Be^{2+}$ makes $BeSO_{4}$ Soluble in water.
Question 28 Which of the following are the correct reasons for anomalous behaviour of lithium?
(i) Exceptionally small size of its atom
(ii) Its high polarising power
(iii) It has high degree of hydration
(iv) Exceptionally low ionisation enthalpy
Answer:
The answer is the option (i) and (ii)
Lithium shows anomalous behaviour mainly due to its exceptionally small atomic size and high polarising power. Its small size leads to a high charge density, allowing the Li+ ion to strongly polarise anions and form compounds with significant covalent character.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: Short Answer Type
Here some short answer type questions from NCERT Exemplar Solutions for Class 11 Chemistry Chapter 10 s-block elements are given for practice. This section contains important questions that are asked in the exams.Practice short answer types from the questions below.
Question 29 How do you account for the strong reducing power of lithium in aqueous solution?
Answer:
Li ions, due to small size, have high enthalpy of ionization and hydration. In aqueous solution, high hydration enthalpy predominates, and Li loses electrons and has strong reducing power.
$Li (s) \rightarrow Li (g)$ (Sublimation enthalpy )
$Li(g) \rightarrow Li^{+}(g)+1e^{-}$(Enthalpy of Ionization )
$Li^{+}(g)+H_{2}O \rightarrow Li^{+} (aq)$ (Enthalpy ofhydration)
Question 30 When heated in air, the alkali metals form various oxides. Mention the oxides formed by Li, Na and K.
Answer:
Reactivity of alkali metals with $O_{2}$ increases down the group. These elements give 3 types of oxides- normal oxide, peroxide and superoxide.
$4Li+O_{2} \rightarrow 2Li_{2}O$ Lithium oxide
$6Na+2O_{2} \rightarrow Na_{2}O_{2}$ Sodium peroxide$+2 Na_{2}O$ Sodium oxide
$8K+2O_{2} \rightarrow KO_{2}$ Superoxide$+K_{2}O$ Peroxide $+K_{2}O$ (Normal oxide)
Question 31 Complete the following reactions
$(i)O_{2}^{2-}+H_{2}O\rightarrow$
$(ii)O_{2}^{-}+H_{2}O\rightarrow$
Answer:
$(i)O_{2}^{2-}+H_{2}O\rightarrow$ $2OH^{-}+H_{2}O_{2}$
$(ii) 2O_{2}^{-}+ 2H_{2}O\rightarrow$ $2OH^{-}+H_{2}O_{2} +O_{2}$
Question 32 Lithium resembles magnesium in some of its properties. Mention two such properties and give reasons for this resemblance.
Answer:
Li and Mg have similar ionic radii due to which some of their properties resemble.
Both Li and Mg react slowly with water. Their oxides and hydroxides are much less soluble, and their hydroxides decompose on heating.
$2LiOH \rightarrow Li_{2}O+H_{2}O$ (On heating)
$2Mg(OH)_{2} \rightarrow Mg_{2}O+2H_{2}O$
Both form nitride, $Li_{3}N$ and $Mg_{3}N_{2}$, by direct combination with nitrogen.
$6Li+N_{2}\rightarrow 2Li_{3}N$
$3Mg+N_{2}\rightarrow 2Mg_{3}N_{2}$
Question 33 Name an element from Group 2 which forms an amphoteric oxide and a water soluble sulphate.
Answer:
Beryllium from group 2 gives an amphoteric oxide BeO and water-soluble sulphate $BeSO_4$.
Question 34 Discuss the trend of the following:
(i) Thermal stability of carbonates of Group 2 elements.
(ii) The solubility and the nature of oxides of Group 2 elements
Answer:
(i) Thermal stability increases down the group.
$BeCO_{3}<MgCO_{3}<CaCO_{3}<SrCO_{3}<BaCO_{3}$
(ii) All oxides are basic and ionic in nature except BeO, which is amphoteric and covalent. The lattice energy of oxides decreases with an increase in the size of cation. Basic nature also increases down the group. Except for BeO and MgO, all are soluble in water and produce a large amount of heat on dissolving. The insolubility of BeO and MgO can be attributed to their high lattice energy.
Question 35 Why are $BeSO_{4}$ and $MgSO_{4}$ readily soluble in water while $CaSO_{4}$, $SrSO_{4}$ and $BaSO_{4}$ are insoluble?
Answer:
The solubility of sulphates of group 2 elements depend on their hydration energy which decreases down the group. The lattice energy of group 2 sulphates is almost the same. Very high hydration enthalpy of $Be^{2+}$ and $Mg^{2+}$ ions overcome the lattice enthalpy and their sulphates are soluble. However
In other elements of group 2 the hydration enthalpy is not high enough to overcome lattice enthalpy. So, they remain insoluble in water.
Question 36 All compounds of alkali metals are easily soluble in water but lithium compounds are more soluble in organic solvents. Explain.
Answer:
Alkali metal compounds are ionic in nature and hence soluble in water. In the case of Lithium, its small size and high polarizing power give it a covalent character making it soluble in organic solvents.
Question 37 In the Solvay process, can we obtain sodium carbonate directly by treating the solution containing $(NH_{4})_{2}CO_{3}$ with sodium chloride? Explain.
Answer:
$(NH_{4})_{2}CO_{3}$ reacts with NaCl to get $Na_{2}CO_{3}$ and $NH_{4}Cl$. Both the products are highly soluble in water and equilibrium can not shift in forward direction. That is why $Na_{2}CO_{3}$ can not be prepared directly.
Answer:
Lewis structure of $O_{2}^{-}$ is $O-O^{-}$.
First oxygen atom with no charge has 6 electrons and its oxidation number O.N: 0.
But the other oxygen atom has 7 electrons and its O.N.: -1.
So, the average oxidation state is -1/2.
Question 39 Why do beryllium and magnesium not impart colour to the flame in the flame test?
Answer:
Be and Mg do not impart colour to the flame in the flame test because these 2 metals have very small atomic radii and electrons are more strongly bonded due to higher effective nuclear charge compared to other alkaline earth metals. The energy of Bunsen flame is not sufficient to excite the electrons.
Question 40 What is the structure of $BeCl_2$ molecule in gaseous and solid state?
Answer:
In the gaseous state, it exists as a monomer having a linear structure and zero dipole moment. Cl-Be-Cl
In solid state, it exists in polymeric chain structure in which each Be atom is surrounded by 4 Cl atoms, 2 through covalent bonds and other 2 through coordinate bonds to give a bridge structure.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: Matching Type
Matching-type questions are covered in s-block elements class 11 chemistry chapter 10 ncert exemplar solutions to improve conceptual clarity and topic awareness. These Class 11 chemistry chapter 10 s-block elements important questions are are generally asked in exams to test your knowledge. These exemplar solutions is quite helpful for competitive exams.
Question 41 Match the elements given in Column I with the properties mentioned in Column II.
|
Column I |
Column II |
|
(i)Li |
(a) Insoluble sulphate |
|
(ii) Na |
(b) Strongest monoacidic base |
|
(iii) Ca |
(c) Most negative $E^{\Theta }$ value among alkali metals |
|
(iv)Ba |
(d)Insoluble oxlate |
|
|
(e)$6s^{2}$ electronic onfiguration |
Answer:
(i) – (c) Due to extremely high hydration enthalpy, $E^{\Theta }$ is most negative
(ii) – (b) Na gives NaOH (strong base) which is the strongest monoacidic base.
(iii) – (d) Calcium oxalate is insoluble due to low hydration energy
(iv) – (a,e) $Ba^{2+}$ is large in size.So its hydration energy is low and barium sulphate
Question 42 Match the compounds given in Column I with their uses mentioned in Column II.
|
Column I |
Column II |
|
(i) $CaCO_{3}$ |
(a) Dentistry, Ornamental work |
|
(ii) $Ca(OH)_{2}$ |
(b) Manufacture of sodium carbonate from caustic soda |
|
(iii) CaO |
(c) Manufacture of high quality paper |
|
(iv) $CaSO_{4}$ |
(d) Used in whote washing |
Answer:
(i)- (c)
(ii)-(d)
(iii)-(b)
(iv)- (a)
- $\left(\mathrm{CaCO}_3\right)$ is used in making high-quality paper and also in the chalk industry.
- $\left[\mathrm{Ca}(\mathrm{OH})_2\right]$, known as slaked lime, is used in whitewashing due to its disinfectant nature and low solubility in water.
- (CaO) or quicklime reacts with sodium carbonate in the Solvay process.
- $\left(\mathrm{CaSO}_4\right)$ is commonly used in dentistry (as plaster of Paris) and ornamental plastering.
Question 43 Match the elements given in Column I with the colour they impart to the flame given in Column II.
|
Column I |
Column II |
|
(i) Cs |
(a) Apple green |
|
(ii) Na |
(b)Violet |
|
(iii) K |
(c) Brick red |
|
(iv) Ca |
(d) Yellow |
|
(v)Sr |
(e) Crimson red |
|
(vi) Ba |
(f) Red |
Answer:
(i) – (f); (ii)- (d); (iii)- (b); (iv)- (c); (v)-(e ); (vi) –(a)
The heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is the emission of radiation in the visible region which gives characteristic colour to Bunsen flame.
NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: Assertion and Reason Type
Assertion and Reason type questions are covered to improve your conceptual thinking and problem-solving solving ability. This is one of the most important sections covered in the NCERT exemplar solutions Class 11 chemistry chapter 10.
Question 44 In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): The carbonate of lithium decomposes easily on heating to form lithium oxide and $CO_2$.
Reason (R) : Lithium being very small in size polarises large carbonate ion leading to the formation of more stable $Li_{2}O$ and $CO_2$.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct
(iv) A is not correct but R is correct.
Answer:
The answer is the option (i)
Both Assertion and Reason are correct, and Reason is the correct explanation of Assertion.
$Li_{2}CO_{3}$ is unstable and decomposes on heating. Lithium being very small in size polarises large carbonate ion leading to $Li_{2}O$ which is more stable due to higher lattice energy.
Assertion (A): Beryllium carbonate is kept in the atmosphere of carbon dioxide.
Reason (R) : Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Answer:
The answer is the option (i)
Both Assertion and Reason are correct and Reason is the correct explanation of Assertion.
BeO is more stable due to small size and strong polarising power of $Be^{2+}$. Since BeO is stable and $BeCO_3$ is unstable, when kept in atmosphere of $CO_{2}$ a reversible process occurs and stability of $BeCO_3$ increases
NCERT Exemplar Class 11 Chemistry Solutions Chapter 10: Long Answer Type
The following are the long-answer type questions that needs more practice. These class 11 chemistry chapter 10 s-block elements NCERT Exemplar important questions are frequently asked in the exams. Long-answer type questions are covered to improve your subject knowledge and conceptual thinking:
The atoms of alkali metals have a large size due to which they readily form cations. They have +1 oxidation state, and their compounds are ionic in nature. Alkali metals give three types of oxides- Normal oxides $(M_2O)$, Peroxides $M_{2}O_{2}$ and Super oxides $MO_{2}$. Basic character of normal oxides increases down the group due to increase in their ionic character. Halides of alkali metals MX are also ionic except LiX which is covalent due to small size and high polarizing power. The Ionic character increases from Li to Cs. Oxosalts of alkali metals $M_{2}CO_{3}-MHCO_{3}$, $MNO_{3}$ are solid water soluble ionic compounds. Oxosalts of lithium show different properties due to its small size and high polarizing power.
Question 47 Present a comparative account of the alkali and alkaline earth metals with respect to the following characteristics:
(i) Tendency to form ionic / covalent compounds
(ii) Nature of oxides and their solubility in water
(iii) Formation of oxosalts
(iv) Solubility of oxosalts
(v) Thermal stability of oxosalts
Answer:
(i) Compounds of alkaline earth metals are less ionic than alkali metals because of more effective nuclear charge and small size.
(ii) Oxides of alkaline earth metals are less basic than oxides of alkali metals. These oxides are water-soluble and reactions highly exothermic. Hydroxides of alkaline earth metals are less basic than hydroxides of alkali metals.
(iii) Alkaline earth metals give Oxo salts with oxoacids, but the reactivity of alkali metals is faster.
(iv) Oxo salts of alkaline earth metals are more soluble than that of alkali metals because of the smaller size of cations and higher hydration enthalpy.
(iv) Thermal stability of Oxo salts of alkali metals is higher than that of alkaline earth metals.
$Na_{2}CO_{3}$ is stable towards heat but $MgCO_{3}$ decomposes into MgO and $CO_{2}$ on heating.
(i) Alkali metals dissolve in liquid ammonia, and ammoniated electrons get excited to a higher level which imparts a blue colour to the solution.
$M+(x+y) N H_3 \rightarrow\left[\left(M\left(N H_3\right)_x\right)\right]^{+}$ + $e\left(\mathrm{NH}_3\right)_{\bar{y}}^{-}$
(ii) In a concentrated solution, blue colour changes to bronze due to the formation of clusters of the metal ion. On keeping the solution for some time, blue solution liberates H2 gas with the formation of amide.
$M^{+}(aq)+e^{-}+NH_{3}(l)\rightarrow MNH_{2}(aq)+12H_{2}(g)$
Answer:
The stability of peroxides and superoxides increases as the size of metal ion increases. Stability increases as the size of cation increases. Peroxide ion and superoxide ion combine with large size of alkali metals.$O^{-2}<O_{2}^{-2}<O_{2}^{-}$
$4Li+O_{2}\rightarrow 2Li_{2}O$ Lithium oxide
$2Na+O_{2}\rightarrow Na_{2}O_{2}$Sodium peroxide
$K+O_{2} \rightarrow KO_{2}$ Superoxide
Solution B which turns milky on passing $CO_2$ is lime water $Ca(OH)_{2}$ (Calcium hydroxide)and Compound C which gives milky appearance is $CaCO_{3}$ (Calcium carbonate). On passing excess of $CO_2$ milkiness disappears due to the formation of $Ca(HCO_{3})_{2}$(Calcium bicarbonate). Compound A reacts with water to give B. A is CaO.
$CaO A+H_{2}O\rightarrow Ca(OH)_{2} (B)$
$Ca(OH)_{2}(B)+CO_{2} \rightarrow CaCO_{3} (C)+H_{2}O$
$Ca(OH)_{2}(B)+CO_{2}+H_{2}O\rightarrow Ca(HCO_{3})_{2} (D)$
Answer:
Beryllium hydride cannot be prepared directly by reaction with $H_{2}$. It is prepared by reacting with $LiAlH_{4}$ . The reactions that take place are given below:
$8LiH+Al_{2}Cl_{6} \rightarrow 2LiAlH_{4}+6LiCl$
$2BeCl_{2}+LiAlH_{4}\rightarrow 2BeH_{2}+LiCl+AlCl_{3}$
In group 2 only Be gives covalent oxide, which is amphoteric in nature. The rest elements in the group give ionic oxide, which is basic in nature. BeO on dissolving in water gives sparingly soluble hydroxide which reacts with acid and base to give salt.
$BeO+H_{2}O \rightarrow Be(OH)_{2}$
$Be(OH)_{2}+2OH^{-} \rightarrow \left [BeOH_{4} \right ]^{2-}$
$Be(OH)_{2}+2HCl+2H_{2}O \rightarrow \left [Be(OH)_{4} \right ] Cl_{2}$
Sodium ions impart a yellow colour to the flame in a flame test and form an oxide and a peroxide with oxygen. The element imparts colour to the flame as the electrons in it change energy levels when heated.
$4Na+O_{2}\rightarrow 2Na_{2}O$
$2Na+O_{2}\rightarrow Na_{2}O_{2}$
$2Na_{2}O+O_{2}\rightarrow 2Na_{2}O_{2}$
Class 11 Chemistry NCERT Chapter s-Block: Higher Order Thinking Skills (HOTS) Questions
Some NCERT Exemplar class 11 chemistry chapter 10 s-block elements questions and answers are given below that will help you tackle complex problems. The questions below will help you evaluate your understanding of the concepts.
Question 1: Complete the following two reactions.
(i) $4 \mathrm{LiNO}_3 \longrightarrow x+\mathrm{O}_2$
(ii) $2 \mathrm{NaNO}_3 \longrightarrow \mathrm{y}+\mathrm{O}_2$
(1). $\mathrm{x}=\mathrm{LiNO}_2 ; y=\mathrm{NaNO}_2$
(2). $\mathrm{x}=\mathrm{LiNO}_2 ; y=\mathrm{NaNO}_2$
(3). $\mathrm{x}=\mathrm{LiO}+\mathrm{NO}_2 ; y=\mathrm{Na}_2 \mathrm{O}+\mathrm{NO}_2$
(4). $\mathrm{x}=\mathrm{Li}_2 \mathrm{O}+\mathrm{NO}_2 ; y=\mathrm{NaNO}_2$
Answer:
$
\begin{aligned}
& 4 \mathrm{LiNO}_3 \longrightarrow 2 \mathrm{H}_2 \mathrm{O}+4 \mathrm{NO}_2+\mathrm{O}_2 \\
& 2 \mathrm{NaNO}_3 \longrightarrow 2 \mathrm{NaNO}_2+\mathrm{O}_2
\end{aligned}
$
Hence, the answer is option (3).
Question 2: Suppose metal reacts with the oxygen to form oxide, then aqueous solution of this oxide, when added to a solution of HI, turn yellowish brown in colour. This compound is-
(1) $\mathrm{Na}_2 \mathrm{O}$
(2) $\mathrm{Li}_2 \mathrm{O}$
(3) NaOH
(4) $\mathrm{Na}_2 \mathrm{O}_2$
Answer:
$\mathrm{Na}_2 \mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{Na}^{+}+2 \mathrm{OH}^{-}+\mathrm{H}_2 \mathrm{O}_2$ aqueous solution of $\mathrm{Na}_2 \mathrm{O}_2$ form $\mathrm{H}_2 \mathrm{O}_2$ which acts as an oxidizing agent, hence converting lodide to lodine $\left(\mathrm{I}_2\right)$.
Hence, the answer is option (4).
Question 3. Given below are two statements:
Statement I : On passing $\mathrm{HCl}_{(\mathrm{g})}$ through a saturated solution of $\mathrm{BaCl}_2$, at room temperature white turbidity appears.
Statement II : When $\mathrm{HCl}$ gas is passed through a saturated solution of $\mathrm{NaCl}$, sodium chloride is precipitated due to common ion effect.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Statement I is correct but Statement II is incorrect
(2) Both Statement I and Statement II are incorrect
(3) Statement I is incorrect but Statement II is correct
(4) Both Statement I and Statement II are correct
Answer:
$\mathrm{BaCl}_2, \mathrm{NaCl}$ are soluble but on adding $\mathrm{HCl}(\mathrm{g})$ to $\mathrm{BaCl}_2, \mathrm{NaCl}$ solutions, Sodium or Barium chlorides may precipitate out, as a consequence of the law of mass action
Hence, the answer is the option (1).
Question 4: Which one the following compounds will readily react with dilute $\mathrm{NaOH}$ ?
(1) $\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{OH}$
(2) $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$
(3) $\left(\mathrm{CH}_3\right)_3 \mathrm{COH}$
(4) $\mathrm{C}_6 \mathrm{H}_5 \mathrm{OH}$
Answer:
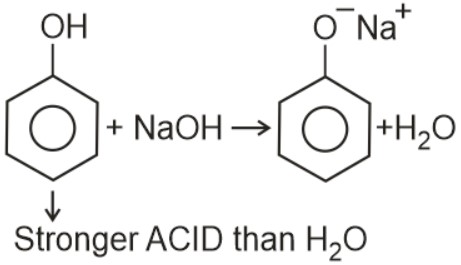
Stronger ACID than $\mathrm{H}_2 \mathrm{O}$
Hence, the answer is the option (4)
Question 5: In Solvay ammonia process, sodium bicarbonate is precipitated due to-
(1) Presence of $\mathrm{NH}_3$
(2) Reaction with $\mathrm{CO}_2$
(3) Reaction with brine solution
(4) Reaction with NaOH
Answer:
$
\mathrm{NH}_4 \mathrm{HCO}_3+\mathrm{NaCl} \longrightarrow \mathrm{NaHCO}_3 \downarrow+\mathrm{NH}_4 \mathrm{Cl}
$
Hence, the answer is the option (3).
Question 6: A: Lithium carbonate is not so stable to heat
R: Lithium being very small in size, polarizes large $\mathrm{CO}_3^{2-}$ ions, leading to the formation of more stable $\mathrm{Li}_2 \mathrm{O}$ and $\mathrm{CO}_2$
(1) A is correct, and R is the correct explanation.
(2) A is correct, and R is also correct but not the correct explanation.
(3) A is correct, and R is incorrect.
(4) A is incorrect, and R is incorrect.
Answer:
Lithium carbonate is unstable to heat; lithium being very small in size, polarises a large $\mathrm{CO}_3^{2-}$ ion leading to the formation of more stable $\mathrm{Li}_2 \mathrm{O}$ and $\mathrm{CO}_2$
Hence, the answer is the option (1).
Question 7: Li is strongest reducing agent among alkali metals due to which of the following factor?
(1) Ionisation Enthalpy
(2) Electron Affinity
(3) Hydration energy
(4) Lattice energy.
Answer:
Li due to the highest hydration energy among the alkali metals, which is the strongest reducing agent.
Hence, the answer is the option (3).
Approach to Solve Questions of Chapter 10 s-block
To solve class 11 chemistry NCERT Exemplar chapter 10 s-block elements questions, it is important to follow a systematic approach. It is recommended to strategies your study plan to solve the questions of this chapter. The following are the points that will help you build a good approach.
1. While solvinge questions from s-block elements, it's important to understand the key concepts like electronic configuration, atomic and ionic radii, ionisation enthalpy, physical and chemical properties, anomalous behaviour of lithium and beryllium, diagonal relationships, etc.
2. Many questions revolve around periodic trends. Students need to understand how and why these trends occur across and down the group. The Key Properties students should focus on are:
- Atomic and ionic radii
- Ionisation enthalpy
- Hydration enthalpy
- Reactivity with air and water
3. Some anomalies and exceptions exist from the generally observed properties. So, it's important to remember those exceptions, as questions are asked frequently about these topics.
- Anomalous behaviour of lithium and beryllium
- Diagonal relationship Li resembles Mg, and Be resembles Al.
4. Students must remember the basic chemical reactions of the NCERT Exemplar class 11 chemistry chapter 10 s-block elements like
- Reactions of s-block elements with oxygen, water, and hydrogen.
- Reactions of s-block elements with Halogens
5. Questions asked from this chapter are mostly based on trends and comparisons. A Proper understanding of basic concepts and practice helps students clear their doubts. Students can refer NCERT Exemplar textbooks for practice. For revision students can follow Class 11 chemistry chapter 10 s-block elements Notes.
Topics And Subtopics of NCERT Exemplar Class 11 Chapter 10
Following are the important topics from the chapter s-block elements. Practice more at NCERT Class 11 chemistry chapter 10 exemplar solutions.
- Group 1 Elements: Alkali Metals
- General Characteristics of the Compounds of the Alkali Metals
- Oxides And Hydroxides
- Halides
- Salts Of Oxoacids
- Anomalous Properties of Lithium
- Some Important Compounds of Sodium
- Biological Importance of Sodium and Potassium
- Group 2 Elements: Alkaline Earth Metals
- General Characteristics of Compounds of the Alkaline Earth Metals
- Anomalous Behaviour of Beryllium
- Some Important Compounds of Calcium
- Biological Importance of Magnesium and Calcium.
General Trends in s-block Elements
Atomic trends play an important role in predicting the physical and chemical properties of s-block elements. Some of the important trends are given below.
1. The Atomic Radius of s-block elements increases down the group because of the addition of electron shells.
2. Ionisation enthalpy decreases down the group because it becomes easier to remove the outermost electron due to increased atomic size and shielding effect.
3. Electronegativity decreases down the group because the tendency to attract electrons decreases down the group.
4. Metallic character increases down the group because elements become more metallic as they lose electrons more readily.
5. Melting and Boiling points decrease down the group because of weaker metallic bonding.
6. Density generally increases down the group, but group 1 shows irregular trends.
7. Reactivity increases down the group, both alkali and alkaline earth metals become more reactive, especially with water and oxygen.
8. Hydration energy decreases as we move down the group because the interaction between the ion and water molecules becomes weaker.
Advantages of Using NCERT Exemplar Class 11 Chemistry Solutions Chapter 10 The s-Block Elements
NCERT Exemplar Solutions for Class 11 Chemistry Chapter 10 s-block elements helps students to understand the concepts. Given below some points on advantages of these solutions:
- The solutions of NCERT help students to understand the topics like alkali and alkaline earth metals, their properties, trends, and reactions with the help of solved questions.
- These are higher order questions that help students to develop analytical thinking.
- Class 11 Chemistry NCERT Exemplar Solutions Chapter 10 are aligned with CBSE patterns and help students practise different types of questions expected in exams.
- These NCERT Exemplar Class 11 Solutions are prepared by subject experts in a very clear and comprehensive manner.
NCERT Exemplar Solutions Class 11 Chemistry Chapter-Wise
NCERT Exemplar Solutions play a crucial role in developing a strong conceptual foundation in Chemistry. This chapter-wise Class 11 Chemistry solutions is prepared according to the latest NCERT syllabus and examination guidelines.
NCERT Solutions for Class 11 Chemistry
NCERT Solutions for Class 11 Chemistry are designed to help students understand concepts in a clear and systematic way. Below is a list of NCERT chapter-wise solutions:
NCERT Exemplar Class 11 Solutions Subject-wise
Students can refer to the links given below for the NCERT exemplar subject-wise solutions:
NCERT Solution subject-wise
Students can refer to the links given below for the NCERT subject-wise solutions:
NCERT Notes subject-wise
Students can refer to the links given below for the NCERT subject-wise notes:
NCERT Books and NCERT Syllabus
Students can refer to the links given below for the NCERT Books and Syllabus of class 11:
Frequently Asked Questions (FAQs)
The s-block elements consist of groups 1 and 2 of the periodic table. This includes the alkali metals and the alkaline earth metals. These elements have their outermost electrons in s orbitals, which gives them distinct chemical properties.
Alkali metals are extremely reactive due to their single valence electron in the outermost shell. This makes it easy for them to lose that electron and achieve a stable noble gas configuration.
Hydration energy is crucial for s-block elements as it affects their solubility in water and their reactivity. Higher hydration energy generally means greater solubility in water and, consequently, increased reactivity.
As we move down the group, the physical properties of alkali metals exhibit notable changes.
- The melting and boiling points decrease
- Density generally increases.
- The metals become softer and more malleable
Alkaline earth metals have various uses due to their properties. For example, magnesium is used in alloys for aircraft and in fireworks, while calcium is essential in biological systems and is used in cement production. Barium is often utilized in medical imaging, and strontium has applications in pyrotechnics.
To prepare well, first master the NCERT textbook concepts, then practise exemplar questions regularly to strengthen problem-solving skills and improve your understanding of application-based topics.
NCERT Exemplar Solutions PDF provides clear, step-by-step answers to higher-level questions, helping students strengthen conceptual understanding and improve exam performance.
You can access CBSE Class 11 Chemistry solutions on trusted educational platforms like Careers360 and other educational websites which provide free, chapter-wise NCERT solution PDFs for easy study and revision.
Yes, NCERT solutions are sufficient for strong conceptual understanding and scoring well in exams, especially when combined with exemplar questions.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters

