NCERT Exemplar Class 11 Biology Solutions Chapter 9 Biomolecules
The NCERT Exemplar Class 11 Biology Solutions Chapter 9 Biomolecules explain the structure and functions of biomolecules of living organisms. Four major classes of biomolecules, such as carbohydrates, proteins, lipids, and nucleic acids, are discussed. It also describes how they interact with one another, like the way enzymes bind to substrates, to perform biological functions. The NCERT Exemplar is a perfect resource for practising questions and boosts confidence.
This Story also Contains
- NCERT Exemplar Class 11 Biology Solutions Chapter 9
- Get the NCERT Exemplar Class 11 Biology Solutions Chapter 7- MCQ
- Find Very Short Answer Questions of NCERT Exemplar Class 11 Biology Solutions Chapter 9
- Explore Short Answers of NCERT Exemplar Class 11 Biology Solutions Chapter 9
- Access the Long Answer Questions of Biomolecules Class 11 NCERT Exemplar
- Approach to Solve Questions of Biomolecules Class 11 NCERT Exemplar
- Important Topics in Class 11 Biology Chapter 9
- Important Questions from Biomolecules Class 11 NCERT Exemplar
- Advantages of NCERT Exemplar Class 11 Biology Chapter 9 Solutions
- NCERT Exemplar Class 11 Biology Solutions Chapter Wise

The Biomolecules Class 11 NCERT Exemplar is important for students who wish to perform well in both board and competitive exams. The solutions are explained briefly and connect every topic to the real world. Students can easily understand the concepts by going through the NCERT Exemplar class 11 Biology, which improves their scores. The biomolecules chapter must be studied by all who desire high marks in school exams and the NEET exam.
NCERT Exemplar Class 11 Biology Solutions Chapter 9
Practising different types of questions like MCQs, short answer questions, and long answer questions with diagrams. The NCERT Exemplar Class 11 Biology Chapter 9 will give you a clear idea of the exam pattern. Let's go through the questions to strengthen your preparation.
Get the NCERT Exemplar Class 11 Biology Solutions Chapter 7- MCQ
Question:1
Choose the correct answer from among the following: -
a. Living organisms have more gold in them than inanimate objects
b. Living organisms have more water in their body than inanimate objects
c. Living organisms have more carbon, oxygen, and hydrogen per unit mass than inanimate objects.
d. Living organisms have more calcium in them than inanimate objects.
Answer:
The correct answer is option (c): Living organisms have more carbon, oxygen, and hydrogen per unit mass than inanimate objects.
Explanation: Living organisms have more carbon, oxygen, and hydrogen per unit mass than inanimate objects because the biochemical composition of living organisms is rich in carbon, oxygen, and hydrogen.
Question:2
Many elements are found in living organisms, either free or in the form of compounds. Which of the following is not found in living organisms?
a. Silicon
b. Magnesium
c. Iron
d. Sodium
Answer:
The correct answer is option (a), Silicon
Explanation: Magnesium, iron, and sodium play important roles in biological processes, while silicon is not a major component of living beings.
Question:3
Amino acids have both an amino group and a carboxyl group in their structure. Which one of the following is an amino acid?
a. Formic acid
b. Glycerol
c. Glycolic Acid
d. Glycine
Answer:
The correct answer is option (d), Glycine
Explanation: Glycine is the simplest amino acid with both an amino and a carboxyl group.
Question:4
An amino acid, under certain conditions, has both positive and negative charges simultaneously in the same molecule. Such a form of amino acid is called
a. Acidic form
b. Basic form
c. Aromatic form
d. Zwitterionic form
Answer:
The correct answer is option (d), Zwitterionic form
Explanation: A zwitterion is a form of an amino acid that has both a positive charge on the amino acid and a negative charge on the carboxyl group at the same time.
Question:5
Which of the following sugars has the same number of carbon atoms as present in glucose?
a. Fructose
b. Erythrose
c. Ribulose
d. Ribose
Answer:
The correct answer is option (a), Fructose
Explanation: Glucose and fructose are both monosaccharides, and both have 6 carbon atoms.
Question:6
An acid-soluble compound formed by phosphorylation of a nucleoside is called:
a. Nitrogen base
b. Adenine
c. Sugar phosphate
d. Nucleotide
Answer:
The correct answer is option (d), Nucleotide
Explanation: When one or more phosphate groups are added to a nucleoside, it forms a nucleotide.
Question:7
When we homogenise any tissue in an acid, the acid-soluble pool represents
a. Cytoplasm
b. Cell membrane
c. Nucleus
d. Mitochondria
Answer:
The correct answer is option (a), Cytoplasm
Explanation: When we homogenise any tissue in acid, the acid-soluble pool it represents the Cytoplasm. The cytoplasm contains small biomolecules that dissolve in the acid and form an acid-soluble pool.
Question:8
The most abundant component of living organisms is
a. Protein
b. Water
c. Sugar
d. Nucleic acid
Answer:
The correct answer is option (b), Water
Explanation: Going by constituents present in the human body, water constitutes 70% of the entire body weight.
Question:9
A homopolymer has only one type of building block, called monomer repeated ‘n’ number of times. A heteropolymer has more than one type of monomer. Proteins are heteropolymers usually made of
a. 20 types of monomers
b. 40 types of monomers
c. 30 types of monomers
d. only one type of monomer
Answer:
The correct answer is option (a), 20 types of monomers
Explanation: Proteins are heteropolymers usually made of 20 types of monomers.
Question:10
Proteins perform many physiological functions. For example, some functions as enzymes. Which of the following represents an additional function that some proteins discharge?
a. Antibiotics
b. Pigment conferring colour to skin
c. Pigments make the colours of flowers
d. Hormones
Answer:
The correct answer is option (d), Hormones
Explanation: Some proteins function as hormones, which regulate various processes in the body, such as insulin, glucagon, etc.
Question:11
Glycogen is a homopolymer made of
a. Glucose units
b. Galactose units
c. Ribose units
d. Aminoacids
Answer:
The correct answer is option (a), Glucose units
Explanation: Glycogen is a homopolymer made of Glucose units. It serves as the primary storage form of glucose.
Question:12
The number of ‘ends’ in a glycogen molecule would be
a. Equal to the number of branches plus one
b. Equal to the number of branch points
c. One
d. Two, one on the left side and another on the right side
Answer:
The correct answer is option (a), Equal to the number of branches plus one
Explanation: The number of ends in a glycogen molecule would be equal to the number of branches plus one, as one end is a reducing end, and all other ends are non-reducing ends.
Question:13
The primary structure of a protein molecule has
a. Two ends
b. One end
c. Three ends
d. No ends
Answer:
The correct answer is option (a), Two ends
Question:14
Which of the following reactions is not enzyme-mediated in the biological system?
a. Dissolving CO2 in water
b. Untwining the two strands of DNA
c. Hydrolysis of sucrose
d. Formation of a peptide bond
Answer:
The correct answer is option (a), Dissolving CO2 in water
Find Very Short Answer Questions of NCERT Exemplar Class 11 Biology Solutions Chapter 9
Question:1
a. Penicillin ___________________________
b. Sulfonamide ___________________________
c. Vitamin C ___________________________
d. Growth Hormone ___________________________
Answer:
A. Penicillin - Natural product
B. Sulfonamide- Synthetic product
C. Vitamin C - Natural product
D. Growth Hormone - Natural product
Question:2
Select an appropriate chemical bond among ester bond, glycosidic bond, peptide bond, and hydrogen bond, and write against each of the following.
a. Polysaccharide ___________________________
b. Protein ___________________________
c. Fat ___________________________
d. Water ___________________________
Answer:
a. Polysaccharides – Glycosidic bond
b. Protein - Peptide bond
c. Fats - Ester bond
d. Water - H-bond
Question:3
Write the name of any one amino acid, sugar, nucleotide, or fatty acid.
Answer:
Nucleotide- Adenosine
Amino acid- Glycine
Fatty acid- Oleic acid
Sugar- Glucose
Question:4
Answer:
A reduced + A’ Oxidised → A oxidised + A’reduced
Question:5
How are prosthetic groups different from cofactors?
Answer:
A prosthetic group is a non-protein component that is permanently attached to an enzyme and essential for its activity in haemoglobin.
A cofactor, on the other hand, is a non-protein substance that may be loosely bound to an enzyme and helps in its function. Cofactors can be organic or inorganic ions.
Question:6
Answer:
COOH, NH2, and H are the common substituents in Glycine and Alanine.
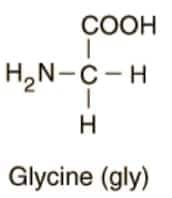
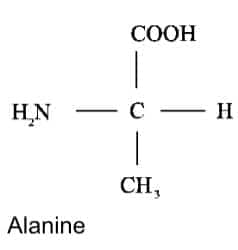
Question:7
Starch, Cellulose, Glycogen, and Chitin are polysaccharides found among the following. Choose the one appropriate and write against each.
a) Cotton fibre __________________________
b) Exoskeleton of cockroach __________________________
c) Liver __________________________
d) Peeled potato __________________________
Answer:
a) Cotton fibre - Cellulose
b) Exoskeleton of cockroach - Chitin
c) Liver - Glycogen
d) Peeled Potato - Starch
Explore Short Answers of NCERT Exemplar Class 11 Biology Solutions Chapter 9
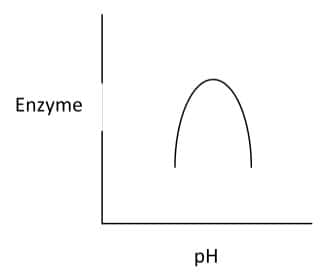
Question:1
Answer:
Explanation of this question can be summed up as follows:
1. This variation triggers changes in the charge of the amino acid.
2. Dependence of the enzyme on pH is because of the existence of a charged amino acid at its active site.
3. The enzymatic activity reduces when the pH is either lower or higher than the optimum pH.
Question:2
Is rubber a primary metabolite or a secondary metabolite? Write four sentences about rubber.
Answer:
Rubber is considered a secondary metabolite because it is not directly involved in the growth or development of the plant.
Four features of rubber are:
Rubber consists of long chains of isoprene units forming a polymer.
Rubber is stored in vessels and acts as a defence mechanism against pathogens.
It is not important for plant growth, but it is mostly used in industries due to its elasticity.
It is used for the production of various products like tyres, gloves, toys, etc.
Question:3
Answer:
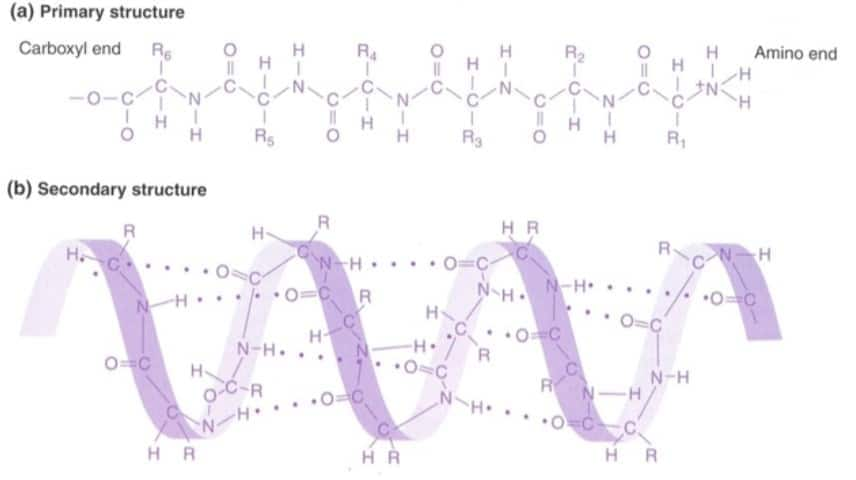
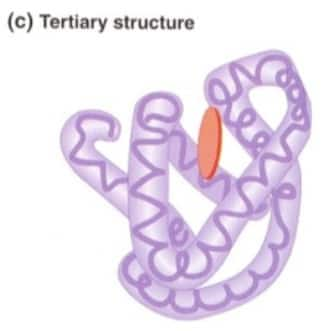
Question:4
Nucleic acids exhibit secondary structure, justify with an example.
Answer:
Nucleic acids, like DNA and RNA, show secondary structures due to the base pairing and hydrogen bonding between nucleotides.
RNA forms a single-stranded helical form while DNA forms a double helix. As a helix is regarded as a secondary structure, nucleic acids are present in the secondary structure.
Question:5
Answer:
The statement “Living state is a non-equilibrium state to be able to perform work” explains that living organisms maintain the exchange of energy and matter with their surroundings, which prevents equilibrium. If a living system reaches the state of equilibrium, all biological activity stops.
Access the Long Answer Questions of Biomolecules Class 11 NCERT Exemplar
Question:1
Answer:
After the formation of the enzyme-substrate complex, the following steps occur in an enzyme-catalysed reaction:
Formation of transition state:
The enzyme makes the substrate stable in its transition state and thus lowers its activation energy, which is required for the reaction.
Catalysis and Conversion:
The enzyme, after that, initiates the chemical reaction by breaking bonds in the substrate or helping to form new bonds.
This step transforms the substrate into the product.
Product Formation:
The substrate is fully converted into the final product.
The enzyme remains attached to the product only.
Product Release:
The enzyme releases the product because products no longer fits perfectly in the active site.
The enzyme returns to its original shape, ready to bind with another substrate.
Enzyme Recycling:
The enzyme is free to catalyze another reaction, making the process speedy.
This cycle repeats as long as substrates are available.
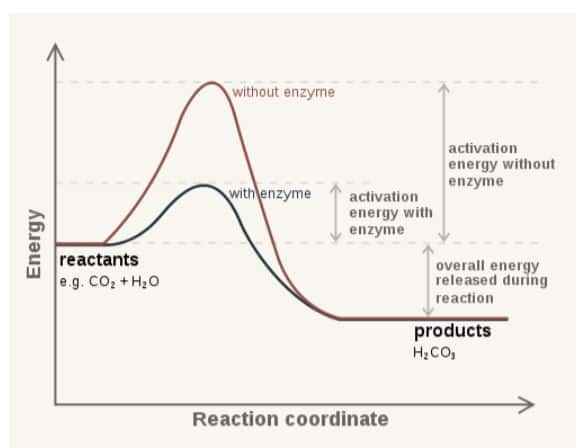
Question:2
What are the different classes of enzymes? Explain any two with the type of reaction they catalyse.
Answer:
Enzymes are the catalysts that increase the rate of biochemical reactions. They regulate the speed and accuracy of reactions without being used. Enzymes are mainly of six types, which are oxidoreductases, transferases, hydrolases, lyases, Isomerases and ligases.
a) Oxidoreductase: These enzymes oxidise the substrate by the addition of oxygen and the removal of hydrogen or electrons.
S reduced + S’reduced → S oxidised + S’ oxidised
b) Hydrolases: Hydrolases are the enzymes that catalyse the hydrolysis reaction by adding water, such as esters, peptides, etc.
Sucrose → Glucose + Fructose
Question:3
Nucleic acids exhibit secondary structure. Describe through the Watson-Crick Model.
Answer:
James Watson and Francis Crick gave the double helix model of DNA, which explains the secondary structure of nucleic acids.
Main features of the Watson and Crick Model:
Double Helix Structure:
Nucleic acids such as DNA consist of two polynucleotide chains, which are twisted around each other.
Both these chains run antiparallel, which means one strand runs in the 5' to 3' direction and the other one in the 3' to 5' direction.
Sugar-Phosphate Backbone:
The backbone is composed of sugar and phosphate groups.
The phosphate groups connect the 3' carbon of one sugar to the 5' carbon of the next sugar by bonds.
Base Pairing:
The nitrogenous bases (purines and pyrimidines) are present inside the helix
Adenine (A) pairs with Thymine (T) with two hydrogen bonds.
Guanine (G) pairs with Cytosine (C) with three hydrogen bonds.
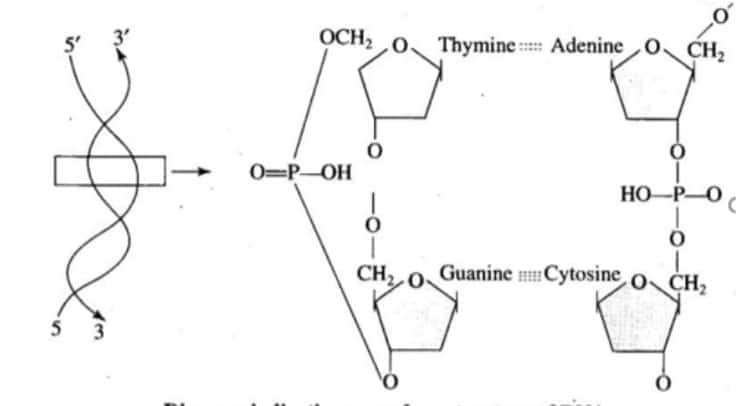
Question:4
Answer:
Nucleotide | Nucleoside |
A nucleotide consists of a nitrogenous base, sugar, and a phosphate group. | Nucleoside only contains the nitrogenous base and sugar. |
They are involved in energy transfer(ATP) and genetic information storage. | They are the precursors of nucleotides. |
Examples - AMP(Adenosine monophosphate), CMP. | Examples- Adenosine, cytidine. |
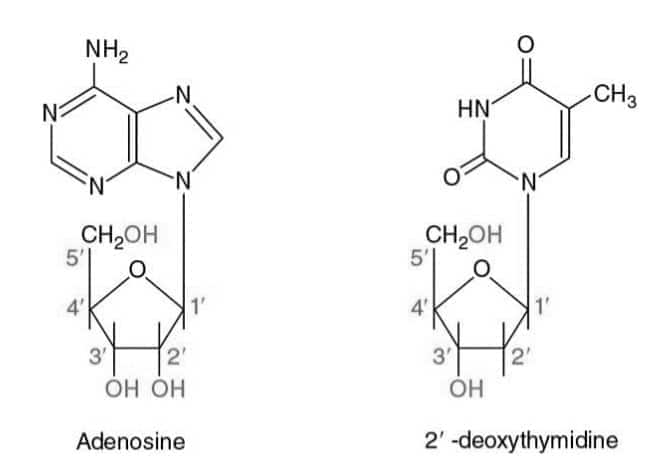
Question:5
Describe various forms of lipid with a few examples.
Answer:
Lipids are important constituents of our diet because of their high energy value. Based on their chemical composition, the classification of lipids is given below:
1- Simple lipids: These are esters of fatty acids with various alcohols. They can be further grouped into three categories:
Fats- These are esters of fatty acids with glycerol and are solid at room temperature.
Oils- They are also esters of fatty acids with glycerol, but are liquid at room temperature.
Waxes- Waxes are the esters of fatty acids other than glycerol.
2- Compound lipids: They are esters of fatty acids with alcohol, but also contain other groups in addition to the alcohol. They are subdivided into:
Phospholipids- These lipids contain fatty acids and glycerol along with phosphoric acid, nitrogen, etc.
Glycolipids- These are the compounds of fatty acids with carbohydrates and nitrogen bases, but do not contain phosphoric acid.
3- Derived lipids: These are the substances derived from simple or compound lipids by hydrolysis. They include fatty acids, alcohol, steroids, etc.
Also, read the NCERT Solution subject-wise
Approach to Solve Questions of Biomolecules Class 11 NCERT Exemplar
To answer Biomolecules questions well, students can adopt this easy-to-follow approach, which is given below:
Know what biomolecules are and why they're important for life.
Learn the major biomolecules, which are carbohydrates, proteins, lipids, and nucleic acids and their structures and functions.
Learn the enzyme structure and function, including its mode of working and what regulates its activity.
Practice to determine bonds and linkages (such as glycosidic, peptide, and phosphodiester bonds).
Read the NCERT textbook and last year's question solutions to adapt yourself to the types of questions posed.
Practice the Biomolecules Class 11 NCERT Exemplar to improve speed and accuracy.
NCERT Exemplar Class 11 Solutions
Important Topics in Class 11 Biology Chapter 9
Biomolecules explores the chemical foundation of life by detailing the structure and function of biomolecules. These molecules are important for energy storage, cellular structure, genetic information, and metabolic regulation. The NCERT Exemplar Class 11 Biology Chapter 9 also covers enzymes, their mechanisms, and the role of cofactors in facilitating biochemical reactions.
Carbohydrates
Lipids
Nucleic Acids
Enzymes
Cofactors
Structure of Biomolecules
Metabolism
Also, check the NCERT Books and the NCERT Syllabus here
Important Questions from Biomolecules Class 11 NCERT Exemplar
Biomolecules are the essential organic compounds, like carbohydrates, proteins, lipids, and nucleic acids, that make up living organisms. The NCERT Exemplar Class 11 Biology Chapter 9 explains their structures, functions, and importance in biological processes.
Question 1. This is a non-proteinaceous enzyme
Option 1. deoxyribonuclease
Option2. ligase
Option 3. Ribozyme
Option 4. lysozyme
Answer:
Ribozymes are RNA molecules that act as enzymes, unlike most enzymes, which are proteins. They catalyse specific biochemical reactions without being proteins.
Hence, the correct answer is option (3), Ribozyme.
Question 2. Glycogen is a homopolymer made of
Option 1. Glucose units
Option 2. Galactose units
Option 3. Ribose units
Option 4. Amino acids
Answer:
Glycogen is indeed a homopolymer made of glucose units. The term "glycogen" comes from the Greek words "glyco" (meaning sweet, referring to glucose) and "gen" (meaning to produce), so it essentially means "a substance that produces glucose."
Hence, the correct answer is option (1), Glucose Units
Question 3. Which of the following sugars has the same number of carbon atoms as glucose?
Option 1. Fructose
Option 2. Erythrose
Option 3. Ribulose
Option 4. Ribose
Answer:
Among the options, fructose falls in the category of monosaccharides. Fructose is often referred to as fruit sugar because it is naturally found in fruits, honey, and some vegetables. Glucose and fructose both fall in this category, and they have 6 atoms of carbon.
Hence, the correct answer is option (1), Fructose
Question 4. Which type of bond links amino acids together in a protein?
Option 1. Glycosidic bond
Option 2. Peptide bond
Option 3. Ester bond
Option 4. Hydrogen bond
Answer:
Amino acids are linked together by peptide bonds, which are formed between the carboxyl group of one amino acid and the amino group of another, releasing a molecule of water during the process.
Hence, the correct answer is option (2), Peptide bond
Question 5. Which of the following is a disaccharide?
Option 1. Glucose
Option 2. Fructose
Option 3. Sucrose
Option 4. Cellulose
Answer:
Sucrose is a disaccharide composed of one molecule of glucose and one molecule of fructose. Glucose and fructose are monosaccharides, while cellulose is a polysaccharide.
Hence, the correct answer is option (3), Sucrose
Must Read NCERT Notes subject-wise
Advantages of NCERT Exemplar Class 11 Biology Chapter 9 Solutions
Understanding biomolecules from the base requires a clear understanding of concepts and diagrams. The exemplar solutions provide several benefits to the students, some of which are listed below:
- NCERT Exemplar Class 11 Biology Chapter 9 Solutions provides the basic knowledge about carbohydrates, proteins, lipids, and nucleic acids.
- The exemplar solution allows students to apply theoretical knowledge in practical applications and to test the presence of biomolecules.
- Students can prepare effectively for competitive exams like NEET and board exams by going through the NCERT exemplar class 11 solutions.
- It also improves problem-solving skills and better retention of concepts through the different types of questions and diagrams.
- Solving different types of questions increases speed and accuracy.
NCERT Exemplar Class 11 Biology Solutions Chapter Wise
The chapter-wise links are given below:
Frequently Asked Questions (FAQs)
Biomolecules are organic molecules that are essential for life and play an important role in the structure and function of living organisms.
Biomolecules are involved in various biological processes such as energy production, growth, metabolism, reproduction, etc as explained in the NCERT Exemplar Class 11 Biology Solutions Chapter 9 Biomolecules.
Four major types of biomolecules are carbohydrates, proteins, lipids (fats, oils), and nucleic acids(DNA and RNA).
Proteins are formed from amino acids by two processes which are transcription and translation, and after that, the polypeptide chain folds into a functional protein.
Major differences between DNA and RNA are given below. For a more detailed explanation, students can use the NCERT Exemplar Class 11 Biology Solutions Chapter 9 Biomolecules.
DNA | RNA |
It is a double-stranded helix. | RNA is the single-stranded helix. |
Adenine, Guanine, Cytosine, and Thymine are present in DNA. | Adenine, Uracil, Guanine, and Cytosine are present in RNA. |
Enzymes are proteins that act as biological catalysts, increasing the speed of reaction in living organisms without being changed in the process.
Enzymes function as biological catalysts by reducing the activation energy, increasing the rate of reactions, and allowing the essential process in the body to occur.
Carbohydrates are classified as monosaccharides, disaccharides, and polysaccharides based on the number of sugars present, and they play an important role in providing energy and structural support in living beings.
Vitamins are considered essential biomolecules because they play a vital role in maintaining the different processes in the body, protect against diseases, and help in the growth of the body.
The difference between the three structures of proteins is:-
Primary Structure: Primary structure is formed by the sequence of amino acids.
Secondary Structure: Primary structures get folded into alpha-helices and beta-sheets.
Tertiary Structure: 3D folding due to side-chain interactions of the protein results in the formation of tertiary structure.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
