NCERT Notes for Class 11 Biology Chapter 9 Biomolecules- Download PDF Notes
Did you know our body is mainly made up of carbon, hydrogen, oxygen, and nitrogen? The NCERT Class 11 Biology Chapter 9 Notes Biomolecules are written in simple and detailed language. These notes provide a clear understanding of the biomolecules that are formed from these elements. All the major classes of biomolecules and the functions they play in the body are covered easily. Students can use these NCERT Notes to score better in school exams and other competitive exams like NEET.
This Story also Contains
- NCERT Class 11 Biology Chapter 9 Biomolecules Notes: Download PDF
- Class 11 Biology Chapter 9 Biomolecules Notes
- Biomolecules Previous Year Questions and Answers
- How to Use Biomolecules Class 11 Notes Effectively?
- Advantages of Class 11 Biology Chapter 9 Biomolecules Notes
- Chapter-Wise NCERT Class 11 Notes Biology
Biomolecules are involved in important processes such as energy generation, growth, repair, and metabolism. Biomolecules Class 11 Notes include carbohydrates, proteins, lipids, and nucleic acids, which help in regulating various metabolic activities. Students can download the PDF of NCERT Class 11 Biology Chapter 9 Notes for quick revision. Understanding the biomolecules allows them to understand how cells grow and respond to their environment. The NCERT Notes for Class 11 help students in improving their overall understanding of the subject in an easy and effective manner.
NCERT Class 11 Biology Chapter 9 Biomolecules Notes: Download PDF
Students can get well-organized notes for the Biomolecules chapter in PDF format. These notes explain important terms, concepts, and reactions clearly. Students can access the Biomolecules Class 11 Notes anytime, even without the internet. By using the NCERT Notes for Class 11 Biology, they can perform well in the exam without any further help.
Also, Read:
Class 11 Biology Chapter 9 Biomolecules Notes
Biomolecules are the essential compounds that make up every living organism and play an important role. These notes help students understand topics like carbohydrates, proteins, lipids, nucleic acids, and enzymes in a simple and clear manner. Each topic is explained step by step with easy definitions and examples, following the latest NCERT curriculum. The Biomolecules Class 11 Notes also highlight key terms for quick revision and better exam preparation.
What Are Biomolecules?
Biomolecules are organic molecules essential for the structure and function of living organisms. These molecules are primarily composed of carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur. Biomolecules are involved in various biological processes, such as energy production, growth, metabolism, and genetic information transfer.
Definition and Importance of Biomolecules
Biomolecules are chemical compounds present in living organisms that contribute to cellular functions and life-sustaining processes. Their importance includes:
Providing Energy: Carbohydrates and lipids serve as energy sources.
Structural Components: Proteins and lipids contribute to cell membranes and tissues.
Genetic Information: Nucleic acids (DNA & RNA) store and transmit genetic instructions.
Catalysis of Reactions: Enzymes (proteins) speed up biochemical reactions.
Cell Communication: Hormones and neurotransmitters regulate body functions.
Classification of Biomolecules
Biomolecules are categorised into two major types:
Organic Biomolecules: Contain carbon and hydrogen and form the backbone of life. Examples include carbohydrates, proteins, lipids, and nucleic acids.
Inorganic Biomolecules: Do not contain carbon-hydrogen bonds and are essential for biological functions. Examples include water, minerals, and gases like oxygen and carbon dioxide.
Types of Biomolecules
The different types of biomolecules are described as follows:
1. Carbohydrates
Carbohydrates are organic compounds composed of carbon (C), hydrogen (H), and oxygen (O) in a ratio of 1:2:1. Their general formula is (CH2O)n.
Classification of Carbohydrates
Carbohydrates are categorised based on their complexity:
Monosaccharides (Simple Sugars): The smallest carbohydrate units, e.g., glucose, fructose, galactose.
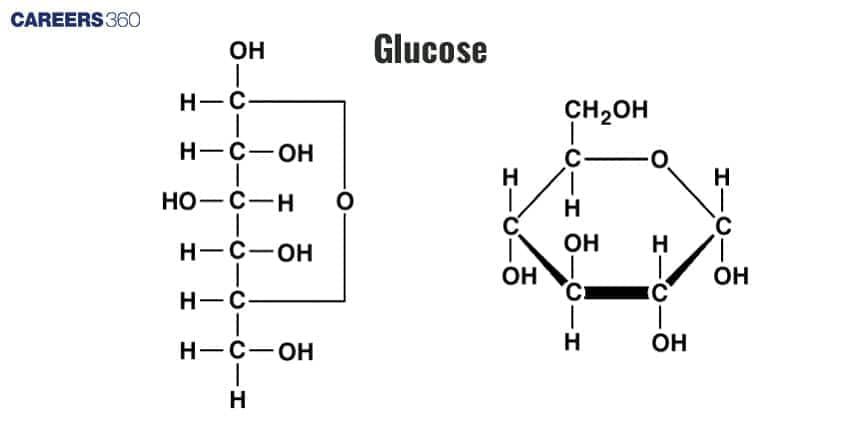
Disaccharides: Formed by the condensation of two monosaccharides, e.g., sucrose (glucose + fructose), lactose (glucose + galactose).
Polysaccharides: Long chains of monosaccharides, e.g., starch (plant storage), glycogen (animal storage), and cellulose (plant cell wall).
Functions of Carbohydrates
Provide immediate energy through glucose metabolism.
Act as structural components (e.g., cellulose in plants, chitin in fungi).
Play a role in cell recognition and signalling (glycoproteins).
2. Proteins
Proteins are macromolecules composed of amino acids linked by peptide bonds. Each amino acid contains an amino group (-NH₂), a carboxyl group (-COOH), and a variable side chain (R-group).
Classification of Proteins
Proteins are classified based on their structure and function:
Structural Proteins: Provide support, e.g., collagen in connective tissues.
Functional Proteins: Enzymes, hormones, and antibodies perform specific biological functions.
Fibrous Proteins: Insoluble, providing structural support, e.g., keratin in hair.
Globular Proteins: Soluble, involved in metabolism, e.g., haemoglobin.
Functions of Proteins
Catalyzes biochemical reactions (enzymes).
Provide structural support (collagen, keratin).
Transport molecules (haemoglobin carries oxygen).
Regulate body functions (hormones like insulin).
Defend against infections (antibodies in immunity).
3. Lipids
Lipids are hydrophobic molecules composed mainly of carbon, hydrogen, and oxygen. They are insoluble in water but soluble in organic solvents like ethanol.
Classification of Lipids
Simple Lipids: Fats and oils (triglycerides) are composed of glycerol and fatty acids.
Compound Lipids: Contain additional groups, e.g., phospholipids in cell membranes.
Derived Lipids: Steroids (cholesterol), terpenes, and fat-soluble vitamins.
Functions of Lipids
Store energy for long-term use.
Act as structural components of cell membranes (phospholipids).
Serve as insulation and protection (fat in the body).
Function as signaling molecules (steroids like hormones).
4. Nucleic Acids
Nucleic acids are polymers of nucleotides, where each nucleotide consists of:
A pentose sugar (ribose in RNA, deoxyribose in DNA).
A phosphate group.
A nitrogenous base (adenine, guanine, cytosine, thymine in DNA, uracil in RNA).
Types of Nucleic Acids
Nucleic acids are classified as:
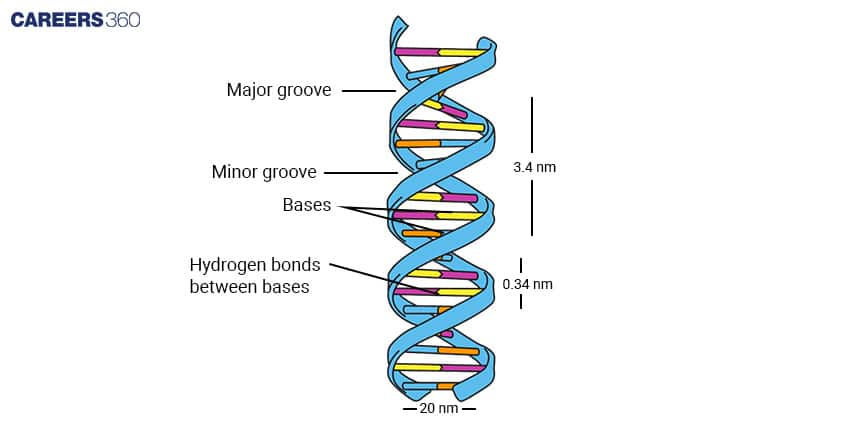
- DNA (Deoxyribonucleic Acid):
Double-stranded helix.
Stores genetic information.
Composed of adenine (A), thymine (T), cytosine (C), and guanine (G).
2. RNA (Ribonucleic Acid):
Single-stranded.
Involved in protein synthesis.
Contains uracil (U) instead of thymine.
Functions of Nucleic Acids
DNA stores and transmits genetic information.
RNA helps in protein synthesis by translating the genetic code into proteins.
Regulates gene expression and cellular activities.
Also, Read
Biomolecules Previous Year Questions and Answers
Here are some important previous year questions from this chapter to help students practice and test their knowledge and understanding. Students can easily solve these questions by using the NCERT Class 11 Biology Chapter 9 Notes Biomolecules.
Question 1. The primary structure of a protein molecule has
Option 1. Two ends
Option 2. One end
Option 3. Three ends
Option 4. No ends
Answer:
The primary structure of a protein molecule consists of a specific sequence of amino acids linked by peptide bonds. This sequence is determined by the gene encoding the protein and dictates its overall structure and function. Any change or mutation in the sequence can alter the protein's properties, potentially affecting its biological activity. The primary structure serves as the foundation for the protein's higher-order structures, including secondary, tertiary, and quaternary forms.
Hence, the correct answer is option (1), Two ends
Question 2. Glycogen is a homopolymer made of
Option 1. Glucose units
Option 2. Galactose units
Option 3. Ribose units
Option 4. Amino acids
Answer:
Glycogen is indeed a homopolymer made of glucose units. The term "glycogen" comes from the Greek words "glyco" (meaning sweet, referring to glucose) and "gen" (meaning to produce), so it essentially means "a substance that produces glucose."
Hence, the correct answer is option (1), Glucose Units
Question 3. Which of the following sugars has the same number of carbon atoms as glucose?
Option 1. Fructose
Option 2. Erythrose
Option 3. Ribulose
Option 4. Ribose
Answer:
Among the options, fructose falls in the category of monosaccharides. Fructose is often referred to as fruit sugar because it is naturally found in fruits, honey, and some vegetables. Glucose and fructose both fall in this category, and they have 6 atoms of carbon.
Hence, the correct answer is option (1), Fructose
Question 4. The bond formed between two amino acids during protein synthesis is called:
Option 1. Glycosidic bond
Option 2. Peptide bond
Option 3. Phosphodiester bond
Option 4. Hydrogen bond
Answer:
During protein synthesis, two amino acids are linked when the carboxyl group of one amino acid reacts with the amino group of another, releasing a molecule of water. This covalent linkage is known as a peptide bond. It forms the backbone of all protein structures and is essential for forming polypeptide chains.
Hence, the correct answer is option (2), Peptide bond
Question 5. Which of the following is an example of a saturated fatty acid?
Option 1. Oleic acid
Option 2. Linoleic acid
Option 3. Palmitic acid
Option 4. Arachidonic acid
Answer:
Saturated fatty acids have no double bonds in their hydrocarbon chain. Palmitic acid is a common saturated fatty acid found in animals and plants. In contrast, oleic, linoleic, and arachidonic acids contain one or more double bonds and are classified as unsaturated fatty acids.
Hence, the correct answer is option (3), Palmitic acid
Also Read:
How to Use Biomolecules Class 11 Notes Effectively?
Understanding biomolecules is important for understanding how living organisms function at the molecular level. Students can follow the steps given below to use the notes effectively.
Go through the chapter into smaller sections like carbohydrates, proteins, lipids, nucleic acids, and enzymes for better understanding.
Regularly revise Class 11 Biology Chapter 9 Biomolecules Notes to remember the structure, functions, and types of biomolecules.
Make flow charts for properties of different biomolecules, as visual learning helps in faster recall during exams.
Use the Class 11 Biology Chapter 9 Biomolecules Notes PDF to solve problems that appeared frequently in the exams.
Highlight definitions such as monosaccharides, amino acids, peptide bonds, and enzyme activity for quick revision before exams.
Advantages of Class 11 Biology Chapter 9 Biomolecules Notes
Understanding biomolecules is important to build a strong foundation in biology. Well-structured and organised notes allow students to understand the difficult topics easily.
- Class 11 Biology Chapter 9 Biomolecules Notes PDF summarises topics like carbohydrates, proteins, and lipids, for quick understanding.
- All the concepts are covered in a proper sequence, which allows students to study easily without getting confused.
- Diagrams, flow charts, and tables are included in the notes, which improves visual memory and better retention of concepts.
- Students can use these quick last-minute revision notes to save time during board exams and competitive exams like NEET.
- Regular revision of these notes improves the conceptual clarity of the biomolecules and boosts overall performance in biology.
Chapter-Wise NCERT Class 11 Notes Biology
Students can find detailed notes for every chapter of Class 11 Biology in the section below. These notes make complex topics easy to understand.
Frequently Asked Questions (FAQs)
Carbohydrates consist of carbon, hydrogen, and oxygen in a 1:2:1 ratio. They function as energy sources (glucose), storage molecules (starch, glycogen), and structural components (cellulose in plants, chitin in fungi).
Lipids are hydrophobic molecules that include fats, oils, and steroids. Unlike carbohydrates, they do not follow a fixed ratio of elements and serve as long-term energy stores, cell membrane components, and hormonal precursors.
The four major biomolecules are carbohydrates (energy sources), proteins (structural and functional roles), lipids (energy storage and membrane components), and nucleic acids (DNA and RNA, responsible for genetic information storage and transfer). All are well-explained in the NCERT Notes for Class 11 Biology Chapter 9 Biomolecules.
Proteins are polymers of amino acids linked by peptide bonds. They function in structural support (collagen), enzymatic activity (enzymes), transport (hemoglobin), immunity (antibodies), and hormonal regulation (insulin). The functions are well-explained in the NCERT Notes for Class 11 Biology Chapter 9 Biomolecules
Biomolecules are organic molecules essential for life, including carbohydrates, proteins, lipids, and nucleic acids. They are involved in cellular structure, metabolism, and genetic information transfer, playing key roles in energy production, growth, and biochemical reactions.
Primary metabolites are essential for growth and development (e.g., amino acids, nucleotides). Secondary metabolites are non-essential but aid in defense and signaling (e.g., alkaloids, flavonoids). They play significant roles in medicine and plant protection.
Enzymes are biological catalysts that speed up biochemical reactions by lowering activation energy. They work through the lock and key or induced fit model, binding specific substrates at their active site to form products.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
