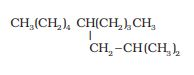Get comprehensive class 11 chemistry chapter 9 hydrocarbons question answer in an easy to download PDF format. These solutions of NCERT will help you understand key concepts and practise questions effectively. Click on the icon below to get detailed solutions to all the questions that are given in the textbook.
NCERT Solutions for Class 11 Chemistry Chapter 9 - Hydrocarbons
Ever wondered what powers your car, cooks your food, and heats your home? The answer to all these questions lies in hydrocarbons ncert solutions. They are the compounds that consist of carbon and hydrogen only. They play a vital role in our day to day lives as they are the primary source of energy. Petrol and diesel are hydrocarbons that are used as fuel for vehicles, LPG is also a hydrocarbon that is used for cooking. This is one of the important chapters in organic chemistry.
This Story also Contains
- NCERT Solutions for Class 11 Chemistry Chapter 9: Download PDF
- NCERT Solutions for Class 11 Chemistry Hydrocarbons- Exercise Questions
- Class 11 Chemistry NCERT Chapter 9: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Class 11 Chemistry Chapter 9
- Topics and Subtopics of NCERT Class 11 Chemistry Chapter 9
- What Extra Should Students Study Beyond NCERT for JEE/NEET?
- What Students Learn from NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrocarbons
- Importance of Class 11 Chemistry Chapter 9 Hydrocarbons
- NCERT Solutions for Class 11 Chemistry
- NCERT Class 11 Solutions
- NCERT Books and NCERT Syllabus

The important topics like the preparation and properties of these compounds, are well discussed in this chapter. The NCERT solutions for class 11 chemistry are prepared by our subject experts to help you master the topics discussed in Hydrocarbons. These solutions offer detailed solutions with simple explanations that will help you develop a clear understanding of the topics. The higher-order thinking skills (HOTS) questions are included to improve your critical thinking. We have also highlighted some points that will help you build a good approach to solve the questions. Students can also go through the hydrocarbons class 11 notes available on our website.
NCERT Solutions for Class 11 Chemistry Chapter 9: Download PDF
Also Read
NCERT Solutions for Class 11 Chemistry Hydrocarbons- Exercise Questions
Access well structured class 11 chemistry chapter 9 hydrocarbons solutions, designed to clarify important concepts and enhance problem-solving skills. These step by step NCERT solutions will help you practise exercise questions and strengthen your understanding for exams.
Question 9.1 How do you account for the formation of ethane during the chlorination of methane?
Answer:
Chlorination of methane occurs by a free radical mechanism and it takes place in three steps-
(i) Initiation-
First, homolytic cleavage of the $Cl-Cl$ bond as

(ii) Propagation-
Chlorine-free radicals attack methane molecules and generate methyl-free radicals as

This methyl radical reacts with other molecules of chlorine ( $Cl-Cl$ ) to form methyl chloride and liberate chlorine-free radicals.

Then the chlorine free radical reacts with methyl chloride and this way propagation occurs.

(iii) Termination-
Ethane will be produced as a final product in this step. When two methyl free radicals react with each other, ethane will be formed.

Question 9.2(a) Write IUPAC names of the following compounds
Answer:
The parent chain has 4 carbons and the double bond is at C-2, so it but-2-ene and also methyl group is also present C-2.
$CH_{3}CH=C(CH_{3})_{2}$
The IUPAC name of the given compound is 2-methylbut-2-ene
Question 9.2(b) Write IUPAC names of the following compounds
Answer:
Both alkene and alkyne are present but the priority will go to the alkene group.
$CH_{2} =CH-C\equiv C-CH_{3}$
The IUPAC name of the compound is Pent-1-ene-3-yne
Question 9.2(c) Write IUPAC names of the following compounds
Answer:
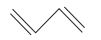
The IUPAC name of the compound is 1, 3-Butadiene or Buta-1, 3-diene. It is a symmetrical compound so the numbering cam be done from either end.
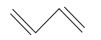
Question 9.2(d) Write IUPAC names of the following compounds :
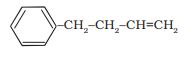
Answer:
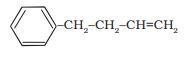
The parent chain will be the aliphatic carbon and benzene will be the side group. The IUPAC name of the compound is 4-phenylbut-1-ene.
Question 9.2(e) Write IUPAC names of the following compounds :

Answer:
Here there are two functional groups hydroxy and methyl. The priority will go to the hydroxy group.

The IUPAC name of the compound is 2-methylphenol
Question 9.2 (f) Write IUPAC names of the following compounds :
Answer:
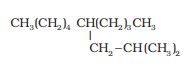
The parent chain has ten carbons so decane (longest chain). The IUPAC name of the compound is 5-(2-methylpropyl)-decane.
Question 9.2(g) Write IUPAC names of the following compounds :

Answer:

The parent chain has ten carbons and the numbering starts where the alkene give the least number. The IUPAC name of the compound is 4-ethyl-1,4,8-deca triene.
$C_{4}H_{8}$ ( one double bond)
Answer:
$C_{4}H_{8}$ the following isomers are possible with one double bond ;
 | But-1-ene |
 | But-2-ene |
 | 2-Methylprop-1-ene |
Question 9.4(i) Write IUPAC names of the products obtained by the ozonolysis of the following compounds :
Pent-2-ene
Answer:
Ozonolysis of Pent-2-ene gives two product,s both are aldehyde compounds. In this process, the ozone molecules attach to the double bond of the molecule and break it into two products.

The IUPAC name of the compounds-(i) ethanal (ii) propanal
Question 9.4 (ii) Write IUPAC names of the products obtained by the ozonolysis of the following compounds :
3,4-Dimethylhept-3-ene
Answer:
Ozonolysis of 3,4-dimethylhept-3-ene gives two products of keto-compounds. The ozone molecules attach to the double bond.

The IUPAC name of the compound is -(i) Butan-2-one (ii) Pentan-2-one
Question 9.4(iii) Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
2-Ethylbut-1-ene
Answer:
Ozonolysis of 2-ethylbut-1-ene gives two products, one is a keto compound and another is an aldehyde.
.jpg)
.
The IUPAC name of one compound is Pentan-3-one and the other compound is methanal.
Question 9.4 (iv) Write IUPAC names of the products obtained by the ozonolysis of the following compounds :
1-Phenylbut-1-ene
Answer:
Ozonolysis of 1-Phenylbut-1-ene gives two aldehydes. One is aromatic in nature and the other is an aliphatic aldehyde.

The IUPAC name of the compound is-(i) Benzaldehyde (ii) propanal
Question 9.5 An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3- one. Write structure and IUPAC name of ‘A’.
Answer:
In the process of ozonolysis, an ozonide, a cyclic ring structure intermediate, is formed, which undergoes cleavage to give the product. The compound A produces pentan-3-one and ethanal. So, the possible structure of A should be

Thus, by removing the ozone from ozonide, we can get the parent alkene structure.

Answer:
As per the given data, compound A on ozonolysis gives two moles of aldehyde, having a molar mass of 44u. It indicates that the compound is symmetrical. So, the possible general structure of
A = $YC=CY$
There are eight $C-H$ sigma bonds, which means eight hydrogen atoms in the structure A. Also, three $C-C$ bonds that indicates the presence of four carbon atoms in A.
Now, by combining all the observations, the structure of the A would be

The ozonolysis reaction is shown below-

The atomic mass of ethanal is 44 u.
Question 9.7 Propanal and pentane-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
Answer:
In the process of ozonolysis, an ozonide (a cyclic ring structure intermediate) is formed, which undergoes cleavage to give the product. The parent compound produces pentan-3-one and propanal. So, the possible structure should be

Here in the above structure, the right side will give Pent-3-one and the left-hand side will give propanal.
Thus, by removing the ozone from ozonide, we can get the parent alkene structure.
Therefore, the structure of the parent alkene is

(3-ethyl-3-hexene)
Question 9.8(i) Write chemical equations for the combustion reaction of the following hydrocarbons:
Butane
Answer:
Combustion is a chemical reaction in which a substance reacts with oxygen releasing energy in the form of heat and light. It is commonly seen in burning fuels like wood, petrol or gas.
Combustion means the reaction of a compound with dioxygen( $O_2$)
$CH_{3}-(CH_{2})_2-CH_{3}(g)+13O_2(g)\rightarrow 8CO_{2}(g)+10H_{2}O(g)+Heat$
Question 9.8(ii) Write chemical equations for combustion reaction of the following hydrocarbons:
Pentene
Answer:
Combustion means the reaction of a compound with dioxygen( $O_2$ ). Combustion reaction of the Pentene-
$CH_{3}-(CH_{2})_3-CH_{3}(g)+15O_2(g)\rightarrow 10CO_{2}(g)+10H_{2}O(g)+Heat$
Question 9.8 (iii) Write chemical equations for combustion reaction of the following hydrocarbons:
Hexyne
Answer:
Combustion means the reaction of the given compound with dioxygen($O_2$) that gives carbon dioxide, water molecules and produces some amount of heat.
Structure of hexyne
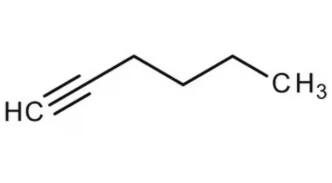
The reaction
$2C_{6}H_{10}+17O_{2}\rightarrow 12CO_{2}+10H_{2}O+heat$
Question 9.8(iv) Write chemical equations for combustion reaction of the following hydrocarbons:
Toluene
Answer:
Combustion is the reaction of the given compound with dioxygen( $O_2$ ) to give carbon dioxide, water molecules and produces some amount of heat.
Structure of toluene
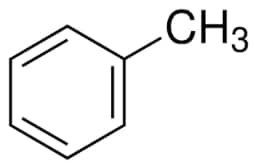
$C_{6}H_{5}(CH_{3})+9O_{2}\rightarrow 7CO_{2}+4H_{2}O+heat$
Question 9.9 Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Answer:
The structure of the hex-2-ene is shown here-

Now, the Geometrical, cis and trans isomers -

Cis-isomer has a higher boiling point than trans-isomers due to more dipole-dipole interactions between the molecules. The cis-form is more polar than the trans-form because it has a higher dipole moment. The trans-form is almost non-polar.
Question 9.10 Why is benzene extraordinarily stable though it contains three double bonds?
Answer:
Benzene is a hybrid of various resonating structures.

Each carbon of benzene is in $sp^2$ hybridization. Two $sp^2$ hybrid orbitals of each carbon overlap with the adjacent carbon atoms' orbital, resulting in six C-C sigma bonds that all are in a hexagonal plane and the remaining $sp^2$ orbital overlaps with the s-orbitals of hydrogen and forms C-H sigma bonds.
In benzene, six C-H sigma bonds present. And the remaining unhybridised $p$-orbital (which are perpendicular to the plane) formed $\pi$-bond by lateral overlapping. The possibility of forming $\pi$ -bond is six ($C_1-C_2,C_3-C_4,C_6-C_1, / C_2-C_3,C_4-C_6, C_6-C_1$).
There are 6 $\pi$ electrons, whichare delocalised and move freely about the six carbon nuclei, and the presence of these delocalised $\pi$ electrons in benzene makes it more stable.
Question 9.11 What are the necessary conditions for any system to be aromatic?
Answer:
The necessary conditions for any system to be aromatic are
- The cyclic compound should be a planner
- The complete(continuous) delocalisation of $\pi$ -electrons in the ring
- Follow the Huckel rule- it states that the ring has (4n+2) $\pi$ -electrons, where n =integer(n = 0, 1, 2, 3....)
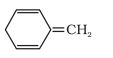
Question 9.12 (i) Explain why the following systems are not aromatic?
Answer:
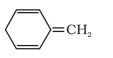
Not an aromatic compound because the $\ pi$-electrons in the ring are not in complete conjugation. And it is a non-planner structure.
Question 9.12(ii) Explain why the following systems are not aromatic?

Answer:
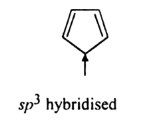
There is no complete conjugation of a $\pi$ electron in the ring as there is sp3 hybridized carbon between them. And also it does not obey huckle rule [(4n+2) $\pi$ ]electron.
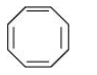
Question 9.12(iii) Explain why the following systems are not aromatic?
Answer:
It disobeys the Hückel rule of (4n+2) $\pi$ electrons. According to this rule, it should have 2, 6, 10 .... number of $\pi$ electrons but it has 8 $\pi$ electrons. Also, this structure becomes non-planar.
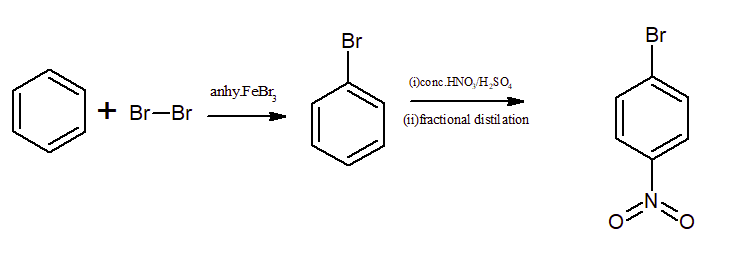
Question 9.13(i) How will you convert benzene into
p-nitrobromobenzene
Answer:
Bromination of a benzene ring in the presence of anhydrous $FeCl_{3}$ and $Br_{2}$ gives bromobenzene. Then, treating bromobenzene with conc. nitric acid in the presence of sulphuric acid gives p-nitrobenzene. Halogens are ortho and para-directing groups.
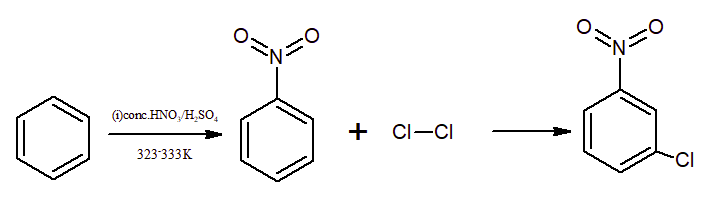
Question 9.13 (ii) How will you convert benzene into
m- nitrochlorobenzene
Answer:
Benzene on treatment with conc. nitric acid and sulphuric acid give nitrobenzene, which on further treatment with chlorine in the presence of anhydrous aluminium chloride ($AlCl_{3}$) gives m-nitrochlorobenzene. Here, Cl will attach at meta position as NO2 is a meta directing group.
Question 9.13(iii) How will you convert benzene into
p - nitrotoluene
Answer:
Alkylation of benzene in the presence of anhydrous aluminium chloride gives methylbenzene and $HCl$. When methylbenzene reacts with conc. nitric acid and sulphuric acid it gives a mixture of para and ortho products of nitrotoluene, which on distillation gives $p$-nitrotoluene.

Question 9.13 (iv) How will you convert benzene into
acetophenone
Answer:
Benzene on reacting with an acyl chloride in the presence of anhydrous aluminium chloride, gives acetophenone with hydrochloric acid as a by-product.

Answer:

$1^0$ carbon are those that are directly bonded with only one carbon atom; the given structure has five $1^0$ carbon atoms and 15 hydrogens attached to it.
$2^0$ carbon are those that are connected with only two carbon atoms. In the above structure, there are two $2^0$ carbons present, and four hydrogens attached to them.
$3^0$ are those that connect with three carbon atoms. In the above structure, 1 carbon atom is $3^0$ carbon atom and only one hydrogen is attached to it.
Question 9.15 What effect does branching of an alkane chain has on its boiling point?
Answer:
With an increase in the branching of the alkane, the boiling point of the alkane decreases. Alkanes experience intermolecular van der Waals forces. The strong is the force, strong will be the boiling point. When we increase the branching, the surface area of the molecule decreases; as a result, the van der Waals force also decreases.
Answer:
Addition of $HBr$ to propene-
In this addition, an electrophile $H^+$ attacks the double bond of the alkene to form $1^0$ and $2^0$ carbocations-

A secondary carbocation is more stable than a primary carbocation. Thus bromide ion attacks the carbocation to form 2-bromopropane as a major product. (This mechanism is followed by Markovnikov's rule)

Answer:
Ortho-xylene has two resonant structures, so,

Since all three products, methylglyoxal, 1,2-methylglyoxal and glyoxal cannot be obtained from either of the two structures (i and ii). Hence, we can say that o-xylene is a resonant hybrid of two Kekule structures (I and II)
Question 9.18 Arrange benzene, $n$-hexane and ethyne in decreasing order of acidic behaviour. Also, give a reason for this behaviour.
Answer:
The acidic character of a species is defined on the basis of the ease with which it can lose its H+ ions.
The acidic character decreases in the order: Ethyne > Benzene > Hexane.
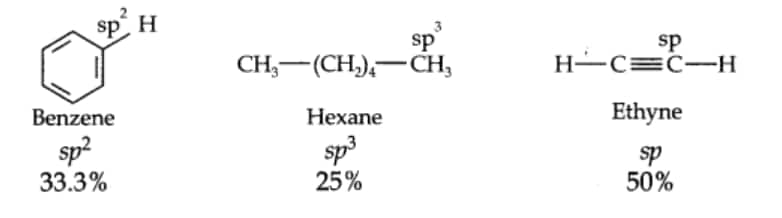
As the s-character decreases, carbon electronegativity decreases. Higher the s-character, more will be the negativity so the negative charge developing on carbon will be more stabilized. So the H that is attached to the alkyne will leave more readily.
Question 9.19 Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Answer:
In benzene, the $\ pi$-electrons are delocalised above and below the ring. Thus, it is an electron-rich species. In nucleophilic substitution, the attacking species is electron-rich in nature, so it becomes challenging to attack benzene because there will be repulsion. On the other hand, when the attacking agent is an electrophile, which is electron-deficient, they are easily attracted by the benzene.
Question 9.20 (i) How would you convert the following compounds into benzene?
Ethyne
Answer:
Benzene can be formed by the cyclic polymerisation of ethyne. Ethyne on passing through a red-hot tube(made of iron) at 873K forms benzene. Three molecules of ethyne polymerise to form benzene.

Question 9.20(ii) How would you convert the following compounds into benzene?
Ethene
Answer:
By converting ethene to ethyne by reacting with bromine in the presence of carbon tetrachloride. And then heating in the presence of alc. KOH followed by $NaNH_{2}/liq.\ NH_{3}$. Cyclic polymerisation of ethyne gives us benzene.

Question 9.20(iii) How would you convert the following compounds into benzene?
Hexane
Answer:
The cyclisation of hexane in the presence of $Cr_{2}O_{3}$ produces cyclohexane, which on aromatisation gives benzene.

Question 9.21 Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Answer:
Structures of all the alkenes that on hydrogenation, give 2-methylbutane.
The general structure of the 2-methylbutane is shown here;

As per the above structure, the following alkene compounds produce 2-methylbutane by hydrogenation are



Question 9.22 (a) Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E +
Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
Answer:
Electrophiles are electron-deficient species, so they want a nucleophile that donates electrons to them. The higher the electron density on a benzene ring, the higher the reactivity towards electrophiles.
$NO_{2}$ is an electron-withdrawing group; it deactivates the benzene ring towards electrophiles by decreasing the electron density of the ring.
Decreasing order of their reactivity with an electrophile($E^+$)
Chlorobenzene > p-nitrochlorobenzene > 2,4-dinitrochlorobenzene
Question 9.22 (b) Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E +
Toluene, $p-\mathrm{H}_3 \mathrm{C}-\mathrm{C}_6 \mathrm{H}_4-\mathrm{NO}_2, p-\mathrm{O}_2 \mathrm{~N}-\mathrm{C}_6 \mathrm{H}_4-\mathrm{NO}_2$.
Answer:
Electrophiles are electron-deficient species, so they want a nucleophile that donates electrons to them. The higher the electron density on a benzene ring, the higher the reactivity towards electrophiles.
Since the methyl group is an electron-donating group, it increases the electron density on the benzene ring. The more the number of electron withdrawing groups, the less reactive towards electrophiles.
Therefore, the decreasing order of reactivity towards electrophile -
toluene $>p-\mathrm{CH}_3 \mathrm{C}_6 \mathrm{H}_4-\mathrm{NHO}_2>p-\mathrm{O}_2 \mathrm{NC}_6 \mathrm{H}_4-\mathrm{NO}_2$
Question 9.23 Out of benzene, m–dinitrobenzene and toluene which will undergo nitration most easily and why?
Answer:
Nitration occurs by an electrophilic substitution reaction, in which an electron-rich species is attacked by an electron-deficient molecule known as an electrophile. In nitration, $NO_{2}^+$ is used as an electrophile. Here methyl group is electron electron-donating group, and the nitro group is electron electron-withdrawing group. So, the benzene ring attached with the $-CH_{3}$ group has high electron density, and the ring that is attached with the nitro group has the least electron density. Hence, toluene undergoes nitration most easily.
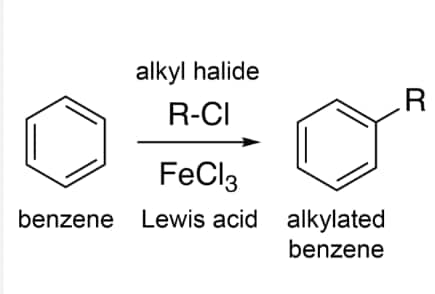
Question 9.24 Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Answer:
Lewis acid like anhydrous ferric chloride $(FeCl_{3})$, stannic chloride $(SnCl_{4})$ , $BF_{3}$ etc. can be used instead of aluminium chloride.
Answer:
Wurtz reaction-

Wurtz reaction is not preferred for the preparation of alkanes containing an odd number of carbon atoms because if we take two dissimilar alkyl halides as reactants, the product will be a mixture of alkanes and alkene may also form due to free radical mechanism. example- bromomethane and iodoethane.

All the products in the mixture have nearly the same boiling point. So, the separation will be difficult.
Class 11 Chemistry NCERT Chapter 9: Higher Order Thinking Skills (HOTS) Questions
These Higher Order Thinking Skills (HOTS) questions from this chapter will help students apply their concepts in real life situations. These class 11 chemistry chapter 9 hydrocarbons solutions are designed to improve critical thinking, problem-solving skills, and deepen understanding of the topic.
Question 1.Given below are two statements :
Statement (I) : On nitration of $m$-xylene with $\mathrm{HNO}_3, \mathrm{H}_2 \mathrm{SO}_4$ followed by oxidation, 4-nitrobenzene-1, 3-dicarboxylic acid is obtained as the major product.
Statement (II) : $\mathrm{CH}_3$ group is $\mathrm{o} / \mathrm{p}$-directing while$\mathrm{NO}_2$ group is m-directing group.
In light of the above statements, choose the correct answer from the options given below:
1) Both Statement I and Statement II are false
2) Statement I is false but Statement II is true
3) Both Statement I and Statement II are true
4) Statement I is true but Statement II is false
Answer:
Statement-I

Statement-II $-\mathrm{CH}_3$ group is $\mathrm{o} / \mathrm{p}$ directing while $-\mathrm{NO}_2$ group is meta directing.
Hence, the correct answer is option (3).
Question 2. Given below are two statements :
Statement I : Ozonolysis followed by treatment with $\mathrm{Zn}, \mathrm{H}_2 \mathrm{O}$ of cis-2-butene gives ethanal.
Statement II : The production obtained by ozonolysis followed by treatment with $\mathrm{Zn}, \mathrm{H}_2 \mathrm{O}$ of 3, 6-dimethyloct-4-ene has no chiral carbon atom.
In the light of the above statements, choose the correct answer from the options given below :
(1) Both Statement I and Statement II are true
(2) Statement I is false but Statement II are true
(3) Statement I is true but Statement II is false
(4) Both Statement I and Statement II are false
Answer:
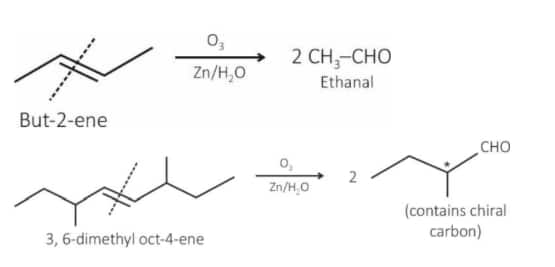
Statement I: Correct statement
Statement II: Incorrect statement because the product has a chiral center.
Hence, the correct answer is option (3).
Question 3. Choose the correct set of reagents for the following conversion.
1) $\mathrm{Br}_2 / \mathrm{Fe} ; \mathrm{Cl}_2, \Delta$; alc. KOH
2) $\mathrm{Cl}_2 / \mathrm{Fe}$; $\mathrm{Br}_2 /$ anhy. $\mathrm{AlCl}_3$; aq. KOH
3) $\mathrm{Br}_2 /$ anhy. $\mathrm{AlCl}_3 ; \mathrm{Cl}_2, \Delta$; aq. KOH
4) $\mathrm{Cl}_2 /$ anhy. $\mathrm{AlCl}_3 ; \mathrm{Br}_2 / \mathrm{Fe}$; alc. KOH
Answer:
The first step is electrophilic aromatic substitution, in which bromination of ethylbenzene is done in the presence of iron. The second step is the free radical halogenation of the above obtained product, followed by an elimination reaction in the presence of alcoholic KOH, and the obtained product is 4-bromostyrene.

Hence, the correct answer is option (1).
Question 4. Which compound would give 3-methyl-6-oxoheptanal upon ozonolysis?
(1)
(2)
(3)
(4)
Answer:


3-methyl-6-oxoheptanal
Ozonolysis breaks the carbon–carbon double bonds in alkenes. Here, p-cymene undergoes ozonolysis, and the double bonds in the aromatic ring are cleaved. Each broken double bond is replaced by a carbonyl group (either an aldehyde or a ketone), resulting in a linear compound with aldehyde and ketone groups.
Hence, the correct answer is option (2).
Question 5: Benzene is treated with oleum to produce compound (X) which when further heated with molten sodium hydroxide followed by acidification produces compound (Y).The compound Y is treated with zinc metal to produce compound (Z).
Identify the structure of compound (Z) from the following option.
(1)

(2)

(3)

(4)

Answer:

Hence, the correct answer is option (2).
Question 6: Consider the following molecule ( X ).
The structure of X is
$
\text { The major product formed when the given molecule }(X) \text { is treated with } \mathrm{HBr}(1 \mathrm{eq}) \text { is : }
$
(1)
(2)
(3)
(4)
Answer:

Hence, the correct answer is option (2).
Question 7: For the following conversion, which set of reagents are required:
$
\text { But-2-ene } \longrightarrow \text { 2-hydroxy-2-methyl butanoic acid}
$
Select the correct set of reagent:
(1) $\mathrm{HBr} ;{ }^{\ominus} \mathrm{CN}, \mathrm{HCN}, \mathrm{H}_3 \mathrm{O}^{+}$
(2) $\mathrm{H}_3 \mathrm{O}^{+} ; \mathrm{CrO}_3, \mathrm{H}^{+} ;{ }^{\ominus} \mathrm{CN}, \mathrm{HCN} ; \mathrm{LiAlH}_4$
(3) $\mathrm{H}_3 \mathrm{O}^{\oplus} ; \mathrm{CrO}_3, \mathrm{H}^{\oplus} ;{ }^{\mathrm{-}} \mathrm{CN}, \mathrm{HCN} ; \mathrm{H}_3 \mathrm{O}^{\oplus}$
(4) $\mathrm{HBr} ; \mathrm{PCC} ;{ }^{\ominus} \mathrm{CN}, \mathrm{HCN} ; \mathrm{H}_2 / \mathrm{Pd}$
Answer:

Hence, the correct answer is option (3).
Question 8: In the reaction

The final product obtained shows that:
the nucleophilic attack occurs at the less substituted carbon, and inversion of configuration is observed at the carbon where ring opening takes place.
Which of the following correctly identifies the reagents X and Y?
(1) $\mathrm{X}=\mathrm{NaOCH}_3, \quad \mathrm{Y}=$ dilute acid
(2) X = CHMgBr, Y = dry ether
(3) X = conc. HSO, Y = HO
(4) X = AgO, Y = CHOH
Answer:
Alkene $\xrightarrow{\text { (i) } \mathrm{PhCO}_3 \mathrm{H}}$ Epoxide $\xrightarrow{\text { (ii) } \mathrm{X}}$ Intermediate $\xrightarrow{\text { (iii) } \mathrm{Y}}$ Final product

Hence, the correct answer is option (1).
Approach to Solve Questions of Class 11 Chemistry Chapter 9
A structured approach for hydrocarbons class 11 question answer that works well for both theory based and numerical problems is given below. It is advisable to follow the NCERT solutions for class 11 for deep learning:
1. Students can start by identifying the key topics covered like types of hydrocarbons- alkanes, alkenes, alkynes, aromatic hydrocarbons, nomenclature rules, Isomerism, preparation and reactions of hydrocarbons, and their physical and chemical properties.
2. Learn to categorise questions into conceptual/theoretical questions, structural drawing, reaction-based questions, IUPAC nomenclature or numerical questions. This will help you prepare for them accordingly.
3. While solving class 11 chemistry chapter 9 hydrocarbons question answer it is very important to read the question carefully and identify exactly what is being asked. Relate it to the concept learned.
4. While solving questions of organic chemistry, it is very important to note down the information given and you can also use bullet points for clarity when writing answers.
5. Students can refer to the solved examples in the textbook and then solve the in-text questions. Students can also access the hydrocarbons ncert solutions for quick revision of the concepts.
Topics and Subtopics of NCERT Class 11 Chemistry Chapter 9
Given below are topics that are covered in the NCERT textbook. Learn these concepts through class 11 hydrocarbons notes available on our website.
9.1 Classification
9.2 Alkanes
9.2.1 Nomenclature and Isomerism
9.2.2 Preparation
9.2.3 Properties
9.2.4 Conformations
9.3 Alkenes
9.3.1 Structure of Double Bond
9.3.2 Nomenclature
9.3.3 Isomerism
9.3.4 Preparation
9.3.5 Properties
9.4 Alkynes
9.4.1 Nomenclature and Isomerism
9.4.2 Structure of Triple Bond
9.4.3 Preparation
9.4.4 Properties
9.5 Aromatic Hydrocarbon
9.5.1 Nomenclature and Isomerism
9.5.2 Structure of Benzene
9.5.3 Aromaticity
9.5.4 Preparation of Benzene
9.5.5 Properties
9.5.6 Directive influence of a functional group in monosubstituted benzene
9.6 Carcinogenicity and Toxicity
What Extra Should Students Study Beyond NCERT for JEE/NEET?
Given below is a comparison table highlighting what to study beyond the NCERT for JEE. For detailed learning refer the NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrocarbons.
What Students Learn from NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrocarbons
These class 11 chemistry chapter 9 hydrocarbons question answer help students to develop a strong foundation in this important topic of organic chemistry. These solutions also help students practice questions and prepare well for exams.
- Using these solutions students can understand the classification and types of hydrocarbons
- Here they will learn about the alkanes, alkenes, alkynes, and aromatic hydrocarbons.
- The structures, physical properties, and chemical properties of these compounds are explained very well in class 11 chemistry chapter 9 hydrocarbon solutions class 11 chemistry hydrocarbons question answer through a series of solved questions.
- Various methods of preparation and important reaction mechanisms are explained in these solutions.
Importance of Class 11 Chemistry Chapter 9 Hydrocarbons
The class 11 chemistry chapter 9 hydrocarbons question answer develops the foundation of organic chemistry that are important for understanding the structure and reactions of organic compounds.
- Students will learn about the fundamental of Organic Chemistry.
- They will learn about types of hydrocarbons and their structural differences.
- Reaction Mechanisms like substitution, addition, elimination, and combustion are explained well in class 11 chemistry hydrocarbons question answer.
- Environmental relevance and industrial applications also plays important role here.
NCERT Solutions for Class 11 Chemistry
Along with the class 11 chemistry chapter 9 hydrocarbons solutions , here are chapter-wise solutions links designed to strengthen your understanding and help you score well in exams.
NCERT Class 11 Solutions
The hyperlinks of the NCERT solution of class 11 are given below.
NCERT Books and NCERT Syllabus
Students can refer to the links given below for the NCERT books and Syllabus.
Frequently Asked Questions (FAQs)
NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrocarbons are detailed answers to all the textbook questions. They help students understand different types of hydrocarbons, their properties, reactions, and preparation methods, making it easier to learn and revise the chapter.
Chapter 9 of Class 11 Chemistry is Hydrocarbons. It deals with different types of hydrocarbons—alkanes, alkenes, alkynes, and aromatic hydrocarbons their structures, properties, methods of preparation, and important reactions.
Chapter 9 Hydrocarbons is not very hard if studied with proper understanding. It mainly involves learning reaction mechanisms, properties, and preparation methods. With regular practice and clear concepts, most students find it manageable and interesting.
Isomerism in hydrocarbons refers to compounds that have the same molecular formula but different structural arrangements. It is significant because it leads to variations in physical and chemical properties, influencing reactivity and usability in different applications. You can learn more topic like this from our NCERT solutions for class 11 chemistry chapter 9 PDF.
Hydrocarbons can undergo a variety of reactions, including combustion, substitution, and addition reactions.
Alkanes are defined as saturated hydrocarbons containing only single carbon-carbon bonds. Alkanes are characterized by their relatively low reactivity and are commonly found in natural gas and petroleum.
Isomers are compounds that have the same molecular formula but differ in structural arrangement or bonding. In hydrocarbons, isomerism can occur in both alkanes and alkenes, resulting in different properties and reactivities.
Aromatic hydrocarbons are unique due to their stable ring structure that adheres to specific rules regarding the number of π electrons. This unique structure grants them distinctive chemical properties, such as unusual reactivity patterns and resonance stability.
Alkanes are saturated hydrocarbons with single bonds (C-C) between carbon atoms, while alkenes are unsaturated hydrocarbons that contain at least one double bond (C=C), and alkynes contain at least one triple bond (C≡C). This difference in bonding affects their chemical reactivity, stability, and properties.
Hybridisation is a concept that describes the mixing of atomic orbitals to form new hybrid orbitals, which can affect the shape and bonding of molecules. In hydrocarbons, different types of hybridisation correspond to the bonding configurations of alkanes, alkenes, and alkynes, respectively, helping to explain their molecular geometry and reactivity.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters