NCERT Solutions for Class 11 Chemistry Chapter 4 - Chemical Bonding and Molecular Structure
Ever wondered what makes atoms stick together to build molecules, how the shape of a molecule can be predicted using hybridisation and how we can determine whether the bond is polar or non-polar? The answer to all these questions lies in NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure. This chapter explains the properties of chemical bonds, their types, and how they are formed. Chemical bonding is the force that holds atoms together in molecules or crystals. We can relate this chapter to our day to day life. For example, the water we drink is a molecule formed by the bonding of hydrogen and oxygen atoms.
This Story also Contains
- NCERT Solution Of Class 11 Chemistry Chapter 4: Download PDF
- NCERT Solutions For Class 11 Chemistry Chapter 4 (Exercise Questions with Answers)
- Class 11 Chemistry NCERT Chapter 4: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Class 11 Chemistry Chapter 4
- Topics and Subtopics Covered in the NCERT Textbook
- What Extra Should Students Study Beyond the NCERT for JEE/NEET?
- What Students Learn from NCERT Solutions for Class 11 Chemistry Chapter 4
- Importance of Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT Solutions For Class 11 Chemistry
- NCERT Solutions for Class 11 Subject-Wise
- NCERT Books and NCERT Syllabus
The topics like valence bond theory, hybridisation and overlapping of orbitals are discussed in this chapter. These NCERT solutions for Class 11 Chemistry are designed by our subject experts in a systematic way to help you understand these complex topics. These solutions will help you strengthen your basics through detailed solutions and simple explanations. Various theories have also been discussed in the solutions to give you a clear idea of bonding interactions. We have also included higher-order thinking skills questions to improve your critical thinking.
NCERT Solution Of Class 11 Chemistry Chapter 4: Download PDF
You can download the chemical bonding and molecular structure ncert solutions pdf from the icon below. These NCERT solutions are designed to help you understand the fundamental concepts and solve questions with ease.
Also read
NCERT Solutions For Class 11 Chemistry Chapter 4 (Exercise Questions with Answers)
The detailed class 11 chemistry chapter 4 chemical bonding and molecular structure question answer are given below. This is a very important chapter from the point of view of boards and competitive exams.
Question 4.1: Explain the formation of a chemical bond.
Answer :
The attractive force that holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond. Different theories and concepts have been put forward from time to time to analyze the formation of the bond. These are the Kössel-Lewis approach, Valence Shell Electron Pair Repulsion (VSEPR) Theory, Valence Bond (VB) Theory, and Molecular Orbital (MO) Theory.
And every system tends to be more stable, and bonding is nature’s way of lowering the energy of the system to attain stability.
Atoms, therefore, combine with each other and complete their respective octets or duplets to attain a stable configuration of the nearest noble gases. It was seen that the noble gases are very stable and inert and do not react with others.
So, there is a sharing of electrons or transferring one or more electrons from one atom to another; as a result a chemical bond is formed known as a covalent bond or ionic bond.
Question 4.2(a) Write Lewis dot symbols for atoms of the following elements :
$Mg$
Answer :
There are two valence electrons in the Mg atom as it belongs to the second group (alkaline earth metals). So we will show two dots (electrons) on Mg.
Hence, the Lewis dot symbol for Mg is: $\ddot{Mg}$.
Question 4.2(b) Write Lewis dot symbols for atoms of the following elements :
$Na$
Answer:
The electronic configuration of Na is 2,8,1. So there is only one electron in the valence shell.
Hence, the Lewis dot structure is $\dot{Na}$.
Question 4.2(c) Write Lewis dot symbols for atoms of the following elements :
$B$
Answer :
Boron belongs to the 13th group of the periodic table (p-block). So, there are three valence electrons in $B$ atom. Hence, the Lewis dot structure is
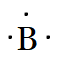
Question 4.2(d) Write Lewis dot symbols for atoms of the following elements :
$O$ ,
Answer :
The electronic configuration oxygen is 2,8,6. so, there are six valence electrons in an atom of $O$. Hence, the Lewis dot structure is
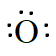
Question 4.2(e) Write Lewis dot symbols for atoms of the following elements :
$N,$
Answer :
As there are five valence electrons in an atom of $N,$. Hence, the Lewis dot structure is
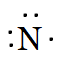
Question 4.2(f) Write Lewis dot symbols for atoms of the following elements :
Answer :
Bromine belongs to halogen family (17th group). So, there are seven valence electrons in an atom of $Br$. Hence, the Lewis dot structure is
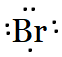
Question 4.3(a) Write Lewis symbols for the following atoms and ions:
Answer :
Sulphur belongs to the oxygen family. So the number of valence electrons in sulphur is six. Therefore, its Lewis dot symbol of sulphur(S) is
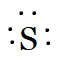
And of $S^{2-}$ is, if it has two electrons more because of its dinegative charge.
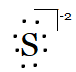
Question 4.3(b) Write Lewis symbols for the following atoms and ions:
Answer :
Al belongs to the boron family, so the number of valence electrons in aluminium is three.
Therefore, the Lewis dot symbol of aluminium(Al) is
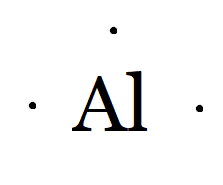
And of $Al^{3+}$, it means it has donated three electrons and acquires a tripositive charge. Hence, the Lewis symbol is

Question 4.3(c) Write Lewis symbols for the following atoms and ions:
Answer :
As the number of valence electrons in hydrogen is one.
Therefore, its Lewis dot symbol of hydrogen (H) is
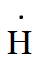
And of $H^{-}$, it has one more electron because of its negative charge. Hence, the Lewis symbol is
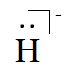
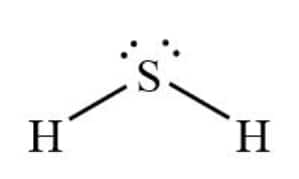
Question 4.4(a) Draw the Lewis structures for the following molecules and ions :
Answer :
Hydrogen atoms each form a single bond with sulphur. As S has six electrons, of which two are bonded to hydrogen, it is left with two lone pairs, giving it a bent shape.
Here, hydrogen completed its duplet and Sulphur its octet
The Lewis structure of $H_{2}S$ is:
Question 4.4(b) Draw the Lewis structures for the following molecules and ions :
Answer :
Silicon forms four single bonds with four chlorine atoms as Si has 4 valence electrons and chlorine has one. The Lewis structure of $SiCl_{4}$ is:
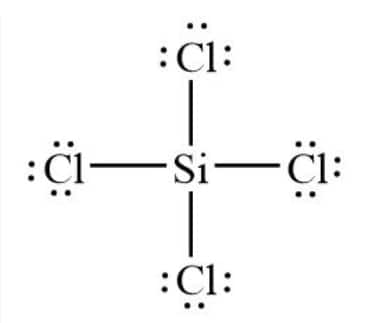
Question 4.4(c) Draw the Lewis structures for the following molecules and ions :
Answer :
Be has two valence electrons, with which it forms two single bonds with each F to complete its octet. F has seven electrons in its valence shell. So after bonding, there will be three lone pairs on each F. Beryllium forms two single bonds with two fluorine atoms and has no lone pairs, resulting in a linear shape.
The Lewis structure of $BeF_{2}$ is:
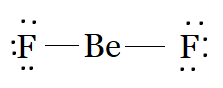
Question 4.4(d) Draw the Lewis structures for the following molecules and ions :
Answer :
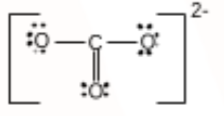
C has 4 valence electrons, and O has 6. So to complete its octet, C needs four more electrons. Carbon forms one double bond and two single bonds with oxygen atoms with a -2 charge delocalized over the three oxygens.
The Lewis structure of $CO_{3}^{2-}$ is:
Question 4.4(e) Draw the Lewis structures for the following molecules and ions :
Answer :
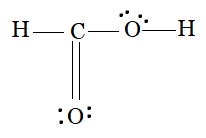
The carbon atom forms a single bond with an oxygen (bonded with a hydrogen), a double bond with one oxygen atom (=O), and a single bond with a hydrogen atom. So, both the oxygens have 2 lone pairs on them.
The Lewis structure of $HCOOH$ is:
Question 4.5 Define octet rule. Write its significance and limitations.
Answer:
Atoms can combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to have an octet in their valence shells. This is known as the octet rule.
Significance: It is quite useful for understanding the structures of most of the organic compounds and it applies mainly to the second-period elements of the periodic table
Limitations: There are three types of exceptions to the octet rule.
The incomplete octet of the central atom - the number of electrons surrounding the central atom is less than eight. Examples are $\mathrm{LiCl}, \mathrm{BeH}_2, \mathrm{BCl}_3$.
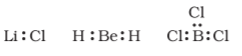
Odd-electron molecules - the octet rule is not satisfied for all the atoms in $NO\ and\ NO_{2}$.
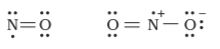
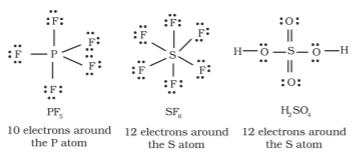
The expanded octet - there are more than eight valence electrons around the central atom. This is termed as the expanded octet. Some of the examples of such compounds are PF5, SF6, H2SO4 and a number of coordination compounds.
Question 4.6 Write the favourable factors for the formation of ionic bond.
Answer :
The formation of an ionic bond takes place by the transfer of one or more electrons from one atom to another. So, ionic bond formation mainly depends upon the ease with which neutral atoms can lose or gain electrons.
The bond formation also depends upon the lattice energy of the compound formed.
Ionic bonds will be formed more easily between elements with comparatively low ionization enthalpies and elements with a comparatively high negative value of electron gain enthalpy.
Question 4.7(a) Discuss the shape of the following molecules using the VSEPR model:
$BeCl_{2}$ ,
Answer :
Using the VSEPR model, we have $BeCl_{2}$
The central atom has no lone pair, and there are two bond pairs.
$BeCl_{2}$ is of the type $AB_{2}$
Hence, it has a linear shape.
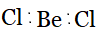
Question 4.7(b) Discuss the shape of the following molecules using the VSEPR model:
(b) $BCl_{3}$
Answer :
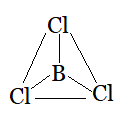
Using the VSEPR model, we have $BCl_{3}$
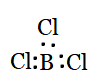
The central atom has no lone pair and there are three bond pairs.
$BCl_{3}$ is of the type $AB_{3}$
Hence, it has a trigonal planar shape.
Question 4.7(c) Discuss the shape of the following molecules using the VSEPR model:
(c) $SiCl_{4}$
Answer :
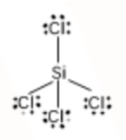
Using the VSEPR model, we have $SiCl_{4}$
The central atom has no lone pair, and there are four bond pairs.
$SiCl_{4}$ is of the type $AB_{4}$.
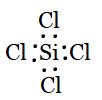
Hence, it has a tetrahedral shape.
Question 4.7(d) Discuss the shape of the following molecules using the VSEPR model:
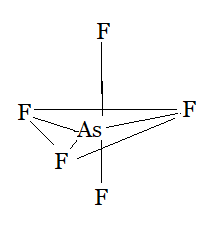
(d) $AsF_{5}$
Answer:
Using the VSEPR model, we have $AsF_{5}$
The central atom has no lone pair and there are five bond pairs.
$AsF_{5}$ is of the type $AB_{5}$
Hence, it has a trigonal bipyramidal shape.
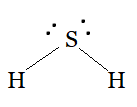
Question 4.7 (e) Discuss the shape of the following molecules using the VSEPR model:
Answer:
Using the VSEPR model we have, $H_{2}S$
The central atom has no lone pair and there are two bond pairs.
$H_{2}S$ is of the type $AB_{2}E$
Hence, it has a bent shape.
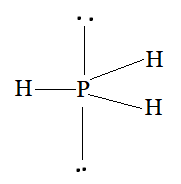
Question 4.7(f) Discuss the shape of the following molecules using the VSEPR model:
(f) $PH_{3}$
Answer :
Using the VSEPR model, we have $PH_{3}$
The central atom has no lone pair and there are three bond pairs.
$PH_{3}$ is of the type $AB_{3}E$
Hence, it has a trigonal bipyramidal shape.
Question 4.9 How do you express the bond strength in terms of bond order?
Answer :
Bond strength refers to the amount of energy required to break the bond between two atoms in a molecule.
So, with an increase in bond order, bond enthalpy increases as a result, bond strength increases.
Question 4.10 Define the bond length.
Answer :
Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
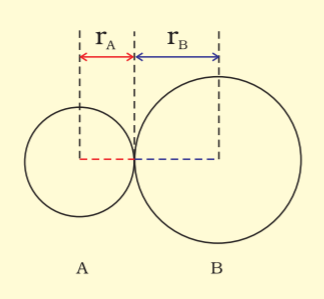
The bond length in a covalent molecule AB.
$R = r_{A}+r_{B}$ where (R is the bond length and $r_{A}$ and $r_{B}$ are covalent radii of atoms A and B respectively.
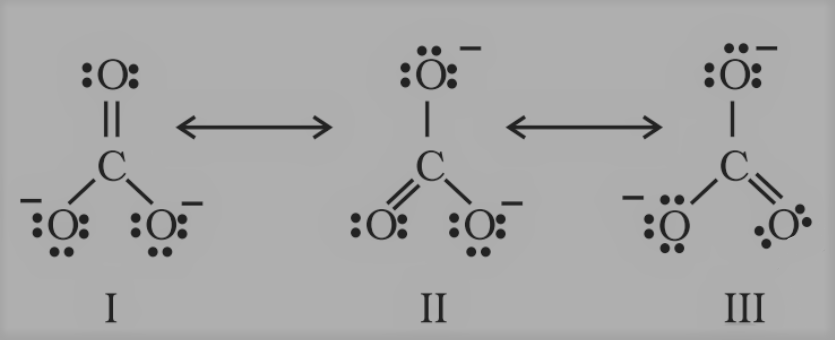
Question 4.11 Explain the important aspects of resonance with reference to the $CO_{3}^{2-}$ ion
Answer :
The single Lewis structure based on the presence of two single bonds and one double bond between carbon and oxygen atoms is inadequate to represent the molecule accurately, as it represents unequal bonds. According to the experimental findings, all carbon-to-oxygen bonds in
CO32- are equivalent. Therefore, the carbonate ion is best described as a resonance hybrid of the canonical forms I, II, and III shown below.
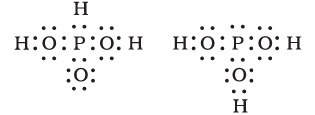
Answer :
The structures have different types of bonding. In the left one, P is doubly bonded to one oxygen, and there are 2 OH groups, while in the other, P is bonded to 3 OH groups.
Hence, the given structures cannot be taken as the canonical forms of the resonance hybrid.
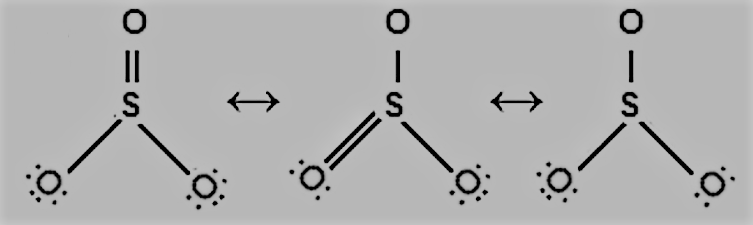
Question 4.13 Write the resonance structures for $SO_{3},$ $NO_{2},$ and $NO_{3}^{-}.$
Answer :
The resonance structures $SO_{3}$
The resonance structures $NO_{2}$
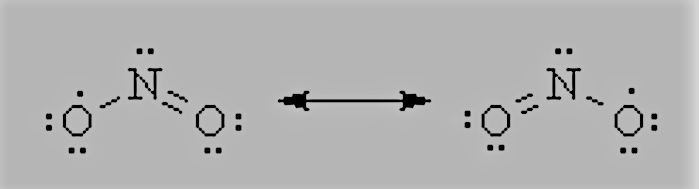
The resonance structures $NO_{3}^{-}$
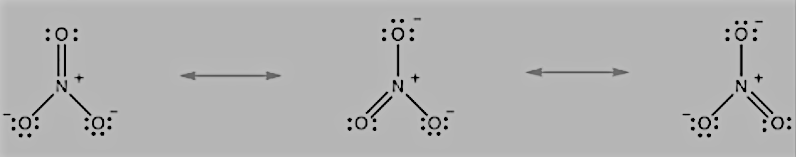
Question 4.14(a) Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
Answer :
K and S:
We have the electronic configurations of both:
$K = 2,8,8,1$ having 1 electron in the valence shell, and it can donate 1 electron to get to the nearest noble gas configuration.
$S = 2,8,6$ having 6 electrons in the valence shell, so it can complete its octet by accepting 2 more electrons.
So, there will be an electron transfer between them as follows:
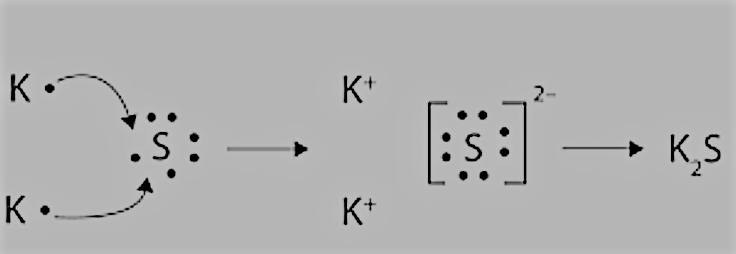
Question 4.14(b) Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
Answer :
$Ca \; and \; O$ :
We have the electronic configurations of both:
$Ca = 2,8,8,2$ has 2 electrons in the valence shell, and it can donate 2 electrons to get to the nearest noble gas configuration.
$O = 2,6$ has 6 electrons in the valence shell, so it can complete its octet by accepting 2 more electrons.
So, there will be an electron transfer between them as follows:
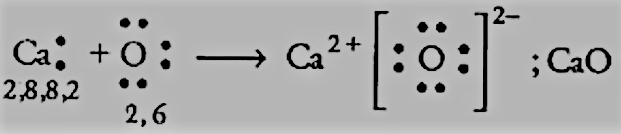
Question 4.14(c) Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
Answer :
$Al\; and\; N.$ :
We have the electronic configurations of both:
$Al = 2,8,3$ has 3 electrons in the valence shell, and it can donate 3 electrons to get to the nearest noble gas configuration.
$N = 2,5$ has 3 electrons in the valence shell, so it can complete its octet by accepting 2 more electrons.
So, there will be electron transfer between them as follows:
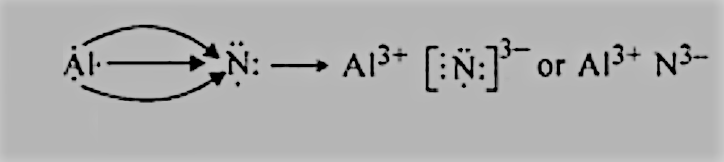
Answer :
H2O molecule, which has a bent structure, the two O–H bonds are oriented at an angle of 104.50. The net dipole moment of 6.17 × 10-30 C m is the resultant of the dipole moments of two O–H bonds.
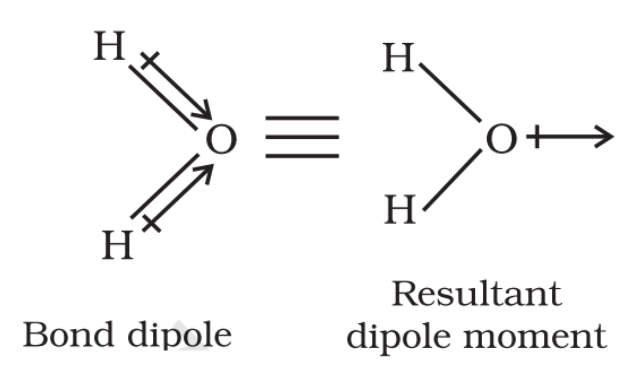
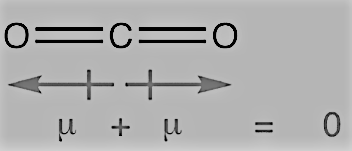
On the other hand, the dipole moment of carbon dioxide is zero. This may be because of linear shape of the molecule as it has two C-O bonds, which have opposite dipole moments cancelling each other.
Question 4.16 Write the significance/applications of dipole moment.
Answer :
Some of the important significance of the dipole moment is as follows:
1. We can determine the shape of the molecule. Symmetrical molecules like linear, etc., do have zero dipole moment, whereas if not symmetrical, then they take different shapes, such as a bent shape or some angular shapes.
2. For determining the polarity of the molecules, the greater the dipole moment value, the greater will be the polarity and vice versa.
3. We can say that if a molecule has a zero dipole moment, then it must be non-polar, and if it is non-zero, then it must have some polar character.
Question 4.17 Define electronegativity. How does it differ from electron gain enthalpy?
Answer :
Electronegativity is the ability of an atom in a compound to attract a bond pair of electrons towards itself. It cannot be measured and it is a relative number.
The electron gain enthalpy, $\triangle_{eg}H$, is the enthalpy change when a gas-phase atom in its ground state gains an electron. The electron gain process may be exothermic or endothermic.
An element has a constant value of the electron gain enthalpy that can be measured experimentally.
Question 4.18 Explain with the help of suitable example polar covalent bond.
Answer :
A polar covalent bond forms when two different atoms share electrons unequally in a covalent bond due to a difference in their electronegativities that causes the shared electron pair to shift toward the more electronegative atom.
For examples, in $H_{2}O$ ,
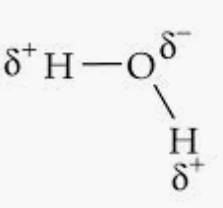
Here, slightly positive charges are developed in hydrogen atoms and slightly negative charges are developed in oxygen atoms as oxygen is more electronegative than hydrogen. Thus, opposite poles are developed in the molecule.
Hence, the bond pair lies towards the oxygen atom.
Question 4.19 Arrange the bonds in order of increasing ionic character in the molecules:
Answer :
The ionic character in a molecule depends on the electronegativity difference between the constituting atoms. The greater the difference more ionic the character of the molecule.
So, on this basis, we have the order of increasing ionic character in the given molecules.
$N_{2}<SO_{2}<ClF_{3}<K_{2}O<LiF$ .
Question 4.20 The skeletal structure of $CH_{3}COOH$ as shown below is correct, but some of the bonds are shown incorrectly
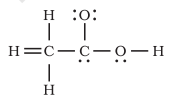
Answer :
Here hydrogen atom is bonded to carbon with a double bond, which is not possible because hydrogen has only one electron to share with carbon.
Also, the second carbon does not have its valency satisfied means it has formed five bonds instead of four.
Therefore, the correct skeletal structure of $CH_{3}COOH$ as shown below:
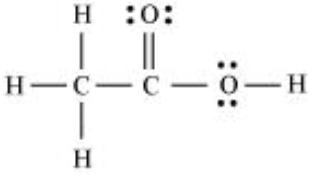
Answer :
The electronic configuration of a carbon atom is $C: 1s^22s^22p^2$.
Where it has s-orbital, p-orbital only, and there is no d-orbital present.
Hence, the carbon atom undergoes $sp^3$ hybridization in the methane molecule and takes a tetrahedral shape.
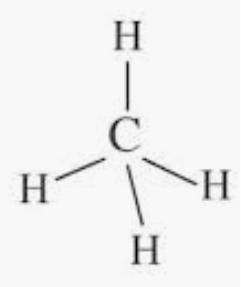
And for a molecule to have a square planar structure, it must have a d orbital present.
But here the absence of d-orbital, as a result, it does not undergo $dsp^2$ hybridization, the structure of methane cannot be square planar.
Also, the reason that the bond angle in square planar $90^{\circ}$ which makes the molecule more unstable because of repulsion between the bond pairs.
Hence, according to VSEPR theory $CH_{4}$ molecule takes a tetrahedral structure.
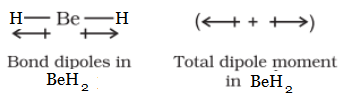
Question 4.22 Explain why $BeH_{2}$ molecule has a zero dipole moment, although the $Be-H$ bonds are polar.
Answer :
$BeH_{2}$ molecule has a zero dipole moment because the two equal bond dipoles point in opposite directions and cancel the effect of each other.
Question 4.23 Which out of $NH_{3}$ and $NF_{3}$ has a higher dipole moment and why?
Answer :
Here, both have nitrogen as a central atom, and it has a lone pair of electrons with three bond pairs.
Hence, both molecules have a pyramidal shape.
The electronegativity of fluorine is more as compared than that of hydrogen. Hence, it is expected that the net dipole moment of $NF_{3}$ is greater than $NH_{3}$.
However, $NH_{3}$ has the net dipole moment of 1.46D and $NF_{3}$ has the net dipole moment of 0.24D. which is greater than $NF_{3}$ .
This is because of the direction of the dipole moments of each bond in $NH_{3}$ and $NF_{3}$.
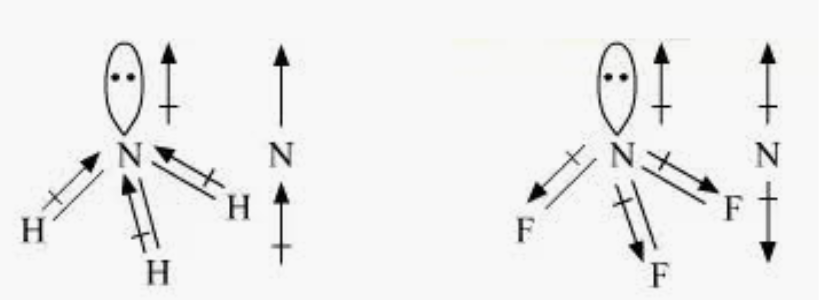
The moments of the lone pair in $NF_{3}$ partly cancel out. But in $NH_{3}$, the resultant moment adds up to the bond moment of the lone pair.
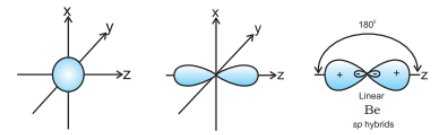
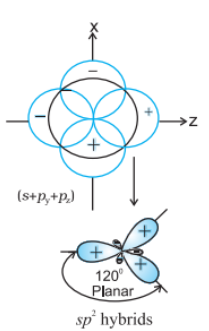
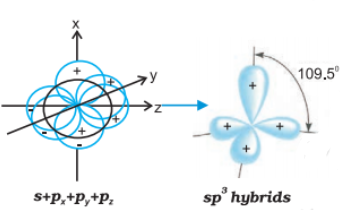
Question 4.24 What is meant by hybridisation of atomic orbitals? Describe the shapes of $sp$ , $sp^{2}$ , $sp^{3}$ hybrid orbitals.
Answer :
Hybridisation can be defined as the process of intermixing the orbitals of slightly different energies so as to redistribute their energies, resulting in the formation of a new set of orbitals of equivalent energies and shape.
The shapes of $sp$ , $sp^{2}$ , $sp^{3}$ hybrid orbitals are shown:
$sp$ hybrid orbital: It is linear in shape and formed by the intermixing of s and p orbitals.
$sp^{2}$ hybrid orbital: It is the trigonal planar shape and is formed by the intermixing of one s-orbital and two 2p-orbitals.
$sp^{3}$ hybrid orbital: It is tetrahedral in shape and is formed by the intermixing of one s-orbital and three p-orbitals.
Question 4.25 Describe the change in hybridisation (if any) of the $Al$ atom in the following reaction.
$AlCl_{3}+Cl^{-}\rightarrow AlCl_{4}^{-}$
Answer :
Initially, the aluminium is in the ground state, and the valence orbital can be shown as:
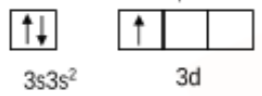
Then the electron gets excited so the valence orbital can be shown as:
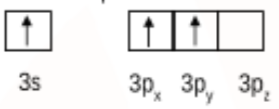
So, initially, aluminium $(AlCl_{3})$ had $sp^2$ hybridisation and hence had a trigonal planar shape.
Then it reacts with chloride ion to form $AlCl_{4}^{-}$. Where it has the empty $3p_{z}$ orbital which gets involved and the hybridisation changes from $sp^2 \rightarrow sp^3$.
Hence, there is a shape change from trigonal planar to tetrahedral.
Question 4.26 Is there any change in the hybridisation of $B$ and $N$ atoms as a result of the following reaction?
$BF_{3}+NH_{3}\rightarrow F_{3}B.NH_{3}$
Answer :
Initially boron atom $BF_{3}$ was in $sp^2$ hybridised. The valence orbital of boron in the excited state can be shown as:
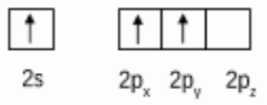
And nitrogen atom in $NH_{3}$ is $sp^3$ hybridised. The valence orbital of nitrogen in the excited state can be shown as:
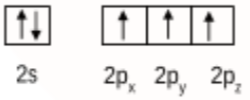
Then, after the reaction has occurred, the product $F_{3}B.NH_{3}$ is formed by the hybridisation of 'B' changes to $sp^3$. However, the hybridisation of 'N' remains unchanged.
Answer :
We have the electronic configuration of the C-atom in the excited state:
$C= 1s^22s^12p_{x}^12p_{y}^12p_{z}^1$
Formation of an ethane molecule $(C_{2}H_{4})$ by overlapping of a $sp^2$ hybridized orbital of another carbon atom, thereby forming a $C-C$ sigma bond.
The remaining two $sp^2$ orbitals of each carbon atom form a $sp^2-s$ sigma bond with two hydrogen atoms. The unhybridized orbital of one carbon atom undergoes sidewise overlap with the orbital of a similar kind present on another carbon atom to form a weak n-bond.
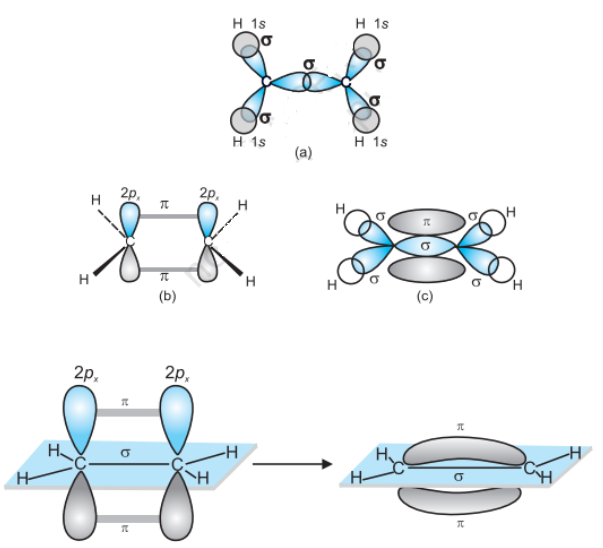
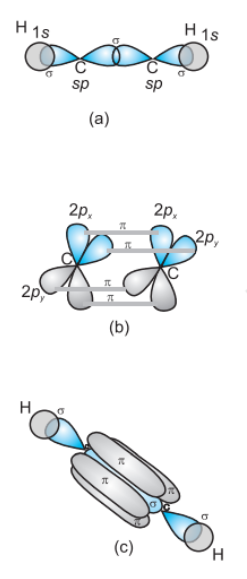
Formation of $C_{2}H_{2}$ molecule- each C-atom is sp-hybridized with two 2p-orbitals in an unhybridized state.
One sp hybrid orbital of one carbon atom overlaps axially with the sp hybrid orbital of the other carbon atom to form a C-C sigma bond, while the other hybridised orbital of each carbon atom overlaps axially with the half-filled s orbital of the hydrogen atoms forming σ bonds.
Each of the two unhybridised p orbitals of both the carbon atoms overlaps sideways to form two π bonds between the carbon atoms. So the triple bond between the two carbon atoms is made up of one sigma and two pi bonds as shown in Fig
Question 4.28(a) What is the total number of sigma and pi bonds in the following molecules?
Answer :
Given molecule $C_{2}H_{2}$ :
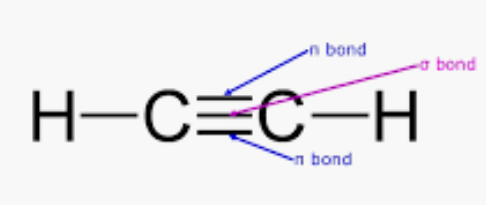
So, there are three sigma (2C-H bonds + 1 C-C bond) and two pi-bonds (2 C-C bonds) in $C_{2}H_{2}$.
Question 4.28(b) What is the total number of sigma and pi bonds in the following molecules?
Answer :
Given molecule $C_{2}H_{4}$ :
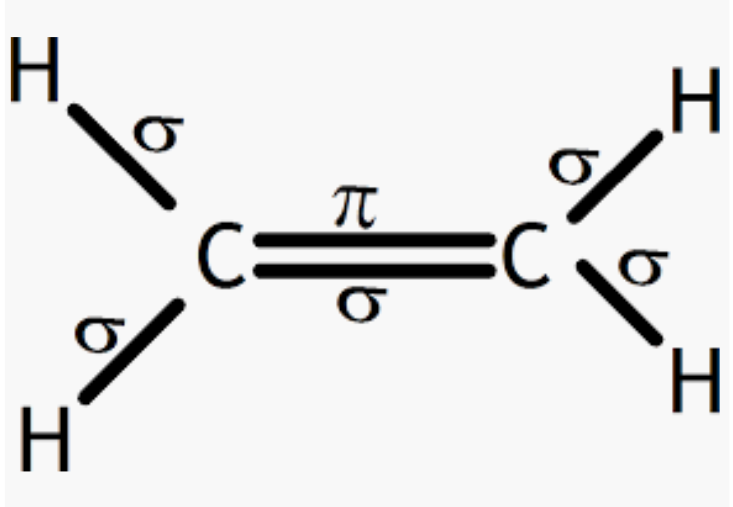
So, there are five sigma (4C-H bonds + 1 C-C bond) and one pi-bond (C-C bonds) in $C_{2}H_{4}$.
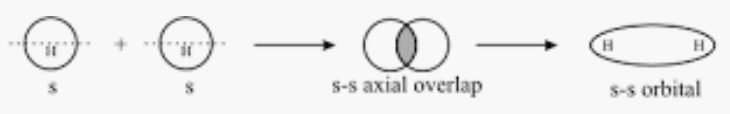
Question 4.29(a) Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
Answer :
Orbitals $1s \; and\; 1s$ will form a sigma bond as both orbitals are spherical and can combine along the x-axis as the internuclear axis.
Question 4.29(b) Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
Answer :
Orbitals $1s \; and\; 2p_{x}$ will form a sigma bond as 1s orbital and 2p x orbital are aligned such that they can combine along the x-axis as the internuclear axis.

Question 4.29(c) Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
Answer :
Orbitals $2p_{y}\; and\; 2p_{y}$ will not form a sigma bond as both 2py orbitals are aligned in y-direction but the internuclear axis is the x-axis.
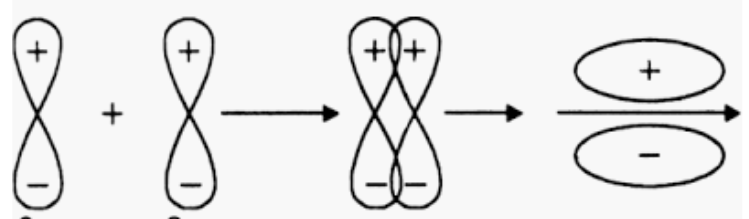
The formation of a pi bond takes place.
Question 4.29(d) Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why?
Answer :
Orbitals $1s \; and \; 2s.$ will form a sigma bond as both 1s and 2s orbitals are spherical and can combine along the x-axis as the internuclear axis.
Question 4.30(a) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
$CH_{3}-CH_{3}$
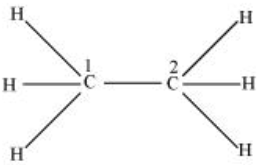
There are four sigma bonds (single bond), each with the help of one s hybrid orbital and three p hybrid orbitals. Hence, C1 and C2 are $sp^3$ hybridized.
Question 4.30(b) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
$CH_{3}-CH=CH_{2};$
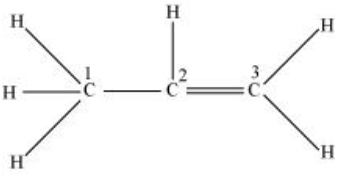
$C_{1}$ is making 4 sigma bonds (single bond) therefore it is $sp^3$ hybridised.
While $C_{2}\ and\ C_{3}$ are making a double bond. $(1 sigma\ bond + 1\ pi\ bond)$
Therefore, they both are $sp^2$ hybridized.
Question 4.30(c) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
$CH_{3}-CH_{2}-OH$
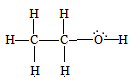
$C_{1}$ is making 4 sigma bonds (single bonds), therefore it is $sp^3$ hybridised.
and $C_{2}$ is also making a 4 sigma bond. Therefore, it is also $sp^3$ hybridised.
Therefore, they both are $sp^3$ hybridized.
Question 4.30(d) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
$CH_{3}-CHO$
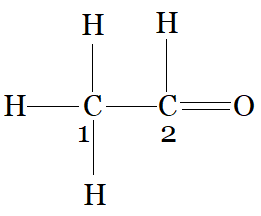
$C_{1}$ is making 4 sigma bonds (single bonds), therefore it is $sp^3$ hybridised.
and $C_{2}$ is making a 3 sigma bonds with hydrogen, carbon and oxygen. and one pi bond with oxygen,n therefore it is $sp^2$ hybridised.
Question 4.30(e) Which hybrid orbitals are used by carbon atoms in the following molecules?
Answer :
$CH_{3}COOH$
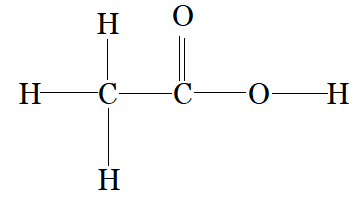
$C_{1}$ is making 4 sigma bonds (single bond) therefore it is $sp^3$ hybridised.
and $C_{2}$ is making a 2 sigma bonds with carbon and 1 sigma bond with oxygen and one pi bond with oxygen therefore, it is $sp^2$ hybridised.
Question 4.31 What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one exmaple of each type.
Answer :
The shared pairs of electrons present between the bonded atoms are called bond pairs.
And all valence electrons may not participate in bonding; those electron pairs that do not participate in bonding are called lone pairs of electrons.
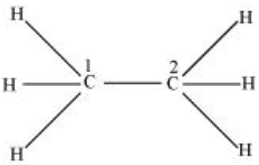
For example,
In $C_{2}H_{6}$ ethane, there are seven bond pairs but no lone pair is present.
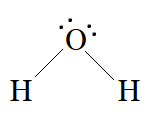
In $H_{2}O$ , there are two bond pairs and two lone pairs on the central atom (oxygen).
Question 4.32 Distinguish between a sigma and a pi bond.
Answer :
The difference between the sigma bond and the pi bond is shown in the table below:
|
Sigma $(\sigma)$ Bond
|
Pi $(\pi)$ Bond
|
|
(a) Formed by end-to-end overlapping of orbitals.
|
Formed by the lateral overlapping of orbitals
|
|
(b) Sigma bonds are stronger than the pi bond.
|
Weak bond.
|
|
(c) The orbitals involved in the overlapping are s-s, s-p, and p-p.
|
Bonds are formed only with the overlapping of p-p orbitals.
|
|
(d) The electron cloud is symmetrical about an internuclear axis.
|
The electron cloud is not symmetrical.
|
|
(e) Free rotation is possible in the case of a sigma bond.
|
Rotation is restricted in the case of pi-bonds.
|
Question 4.33 Explain the formation of $H_{2}$ molecule on the basis of valence bond theory.
Answer :
Formation of $H_{2}$ molecule:
Assume that two hydrogen atoms $(A\ and\ B)$ with nuclei $(N_{A}\ and\ N_{B})$ and electrons $(e_A\ and\ e_B)$ are taken to undergo a reaction to form a hydrogen molecule.
When the two atoms are at a large distance, there is no interaction between them. As they approach each other, the attractive and repulsive forces start operating.
An attractive force arises between:
(a) The nucleus of one atom and its electron, i.e., $N_A- e_A$ and $N_B- e_B$ .
(b) The nucleus of one atom and an electron of another atom i.e., $N_A-e_B$ and $N_B-e_A$
Repulsive force arises between:
(a) Electrons of two atoms i.e., $e_A - e_B$ .
(b) Nuclei of two atoms i.e., $N_{A} - N_{B}$ .
The force of attraction brings the two atoms together, whereas the force of repulsion tends to push them apart.
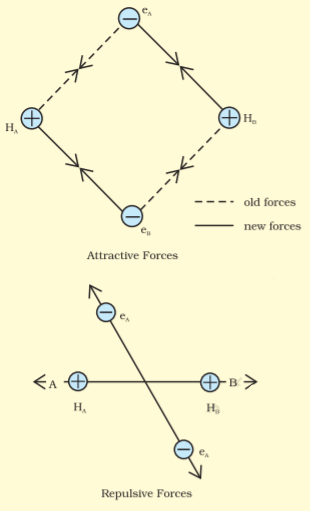
The attractive force overcomes the repulsive force. Hence, the two atoms approach each other. As a result, the potential energy decreases. Finally, a state is achieved when the attractive forces balance the repulsive forces and the system acquires minimum energy. This leads to the formation of a dihydrogen molecule.
Question 4.34 Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Answer :
The important conditions required for the linear combination of atomic orbitals to form molecular orbitals are as follows:
1. The combining atomic orbitals must have the same or nearly the same energy.
2..The combining atomic orbitals must have the same symmetry about the molecular axis.
3. The combining atomic orbitals must overlap to the maximum extent.
Question 4.35 Use molecular orbital theory to explain why the $Be_{2}$ molecule does not exist.
Answer :
The electronic configuration of Be is $1s^22s^2$ .
From the molecular orbital electronic configuration, we have for $Be_{2}$ molecule,
$\sigma_{1s}^2\sigma_{1s}^{*2}\sigma_{2s}^2\sigma_{2s}^{*2}$
We can calculate the bond order for $Be_{2}$ is $= \frac{1}{2}(N_{b}-N_{a})$ where,
$N_{b}$ is the number of electrons in bonding orbitals and $N_{a}$ is the number of electrons in anti-bonding orbitals.
So, therefore we have,
Bond order of $Be_{2} = \frac{1}{2}(4-4) = 0$
that means that the molecule is unstable.
Hence, $Be_{2}$ molecule does not exist.
Question 4.36 Compare the relative stability of the following species and indicate their magnetic properties;
$O_{2},O^{+}_{2},O^{-}_{2}-(superoxide),\ O_{2}^{2-} (peroxide)$
Answer :
The electronic configuration of $O_{2}$ molecule can be written as:
$(\sigma1s)^2(\sigma^*1s)^2(\sigma2s)^2(\sigma^*2s)^2(\sigma2p_{z})^2$
$(\pi2p_{x}^2\equiv\pi2p_{y}^2)(\pi^*2p_{x}^1 \equiv\pi^*2p_{y}^1)$
Here, the number of bonding electrons is $N_{b} = 10$ and the number of antibonding electrons is $N_{a} = 6$.
Therefore,
$Bond\ order = \frac{1}{2}(N_{b}-N_{a})$
$= \frac{1}{2}(10-6) = 2$
The electronic configuration of $O_{2}^+$ molecule can be written as:
$(\sigma1s)^2(\sigma^*1s)^2(\sigma2s)^2(\sigma^*2s)^2(\sigma2p_{z})^2$
$(\pi2p_{x}^2\equiv\pi2p_{y}^2)(\pi^*2p_{x}^1 )$
Here, the number of bonding electrons is $N_{b} = 10$ and the number of antibonding electrons is $N_{a} = 5$.
Therefore,
$Bond\ order = \frac{1}{2}(N_{b}-N_{a})$
$= \frac{1}{2}(10-5) = 2.5$
The electronic configuration of $O_{2}^-$ molecule can be written as:
$(\sigma1s)^2(\sigma^*1s)^2(\sigma2s)^2(\sigma^*2s)^2(\sigma2p_{z})^2$
$(\pi2p_{x}^2\equiv\pi2p_{y}^2)(\pi^*2p_{x}^2 \equiv\pi^*2p_{y}^1)$
Here, the number of bonding electrons is $N_{b} = 10$ and the number of antibonding electrons is $N_{a} = 7$.
Therefore,
$Bond\ order = \frac{1}{2}(N_{b}-N_{a})$
$= \frac{1}{2}(10-7) = 1.5$
The electronic configuration of $O_{2}^{2-}$ molecule can be written as:
$(\sigma1s)^2(\sigma^*1s)^2(\sigma2s)^2(\sigma^*2s)^2(\sigma2p_{z})^2$
$(\pi2p_{x}^2\equiv\pi2p_{y}^2)(\pi^*2p_{x}^2 \equiv\pi^*2p_{y}^2)$
Here, the number of bonding electrons is $N_{b} = 10$ and the number of antibonding electrons is $N_{a} = 8$.
Therefore,
$Bond\ order = \frac{1}{2}(N_{b}-N_{a})$
$= \frac{1}{2}(10-8) = 1$
Therefore, the bond dissociation energy is directly proportional to the bond order.
Thus, the higher the bond order, the greater will be the stability.
We get this order of stability:
$O_{2}^+>O_{2}>O_{2}^{-}>O_{2}^{2-}$
Question 4.37 Write the significance of a plus and a minus sign shown in representing the orbitals.
Answer :
Wave functions can be used to represent molecular orbitals. The plus and minus represent the positive wave function and negative wave function, respectively.
Question 4.38 Describe the hybridisation in case of $PCl_{5}.$ Why are the axial bonds longer as compared to equatorial bonds?
Answer :
The initial ground state and final excited state electronic configuration of phosphorus (P) are:
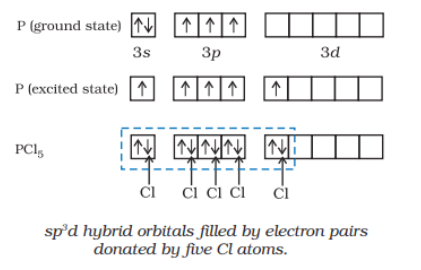
So, the phosphorus atom is $sp^3d$ hybridized in the excited state. The donated electron pairs by five Cl atoms are filled and make $PCl_{5}.$.
The resultant shape is trigonal bipyramidal and the five $sp^3d$ hybrid orbitals are directed towards the five corners.
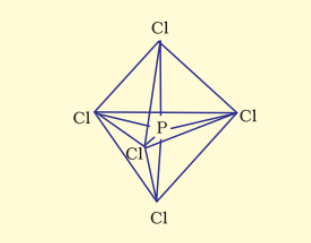
In the five P-Cl sigma bonds, three lie in one plane and make $120^{\circ}$ with each other, are equatorial bonds and the two P-Cl bonds lie above and below the equatorial plane making an angle of $90^{\circ}$ with the plane, are axial bonds.
So, because of more repulsion from the equatorial bond pairs, the axial bonds are slightly longer than the equatorial bonds.
Question 4.39 Define hydrogen bond. Is it weaker or stronger than the van der Waals forces?
Answer :
A hydrogen bond can be defined as the attractive force acting between the hydrogen atom of one molecule with the electronegative atom (F, O or N) of another molecule.
Because of the difference between electro-negativities, the bond pair between hydrogen and the electronegative atom drifts towards a more electronegative atom. As a result, the hydrogen atom becomes slightly positively charged.
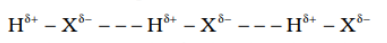
Hydrogen bonds are stronger than van der Waals forces because H-bonds are considered as an extreme form of dipole-dipole interaction.
Question 4.40 What is meant by the term bond order? Calculate the bond order of :
Answer :
Bond order (B.O.) is defined as one-half the difference between the number of electrons present in the bonding and the antibonding orbitals of a molecule.
$Bond\ Order = \frac{1}{2}(N_{b}-N_{a})$
Where $N_{b}\ and N_{a}$ are the number of electrons occupying bonding orbitals and the number occupying the antibonding orbitals, respectively.
So, the bond orders for different molecules are:
$N_{2}$ : The electronic configuration is $(\sigma1s)^2(\sigma^*1s)^2(\sigma2s)^2(\sigma^*2s)^2$
$(\pi2p_{x})^2(\pi2p_{y})^2(\sigma2p_{z})^2$
Where, the number of bonding electrons $N_{b} =10$ and number of antibonding electrons, $N_{a} =4$
So, Bond order of nitrogen molecule $= \frac{1}{2}(10-4) = 3$
$O_{2}$ : The electronic configuration is $(\sigma1s)^2(\sigma^*1s)^2(\sigma2s)^2(\sigma^*2s)^2(\sigma2p_{z})^2$
$(\pi2p_{x}^2\equiv \pi2p_{y}^2)(\pi^*2p_{x}^1\equiv \pi^*2p_{y}^1)$
Where, the number of bonding electrons $N_{b} =10$ and number of antibonding electrons, $N_{a} =6$
So, Bond order of nitrogen molecule $= \frac{1}{2}(10-6) = 2$
$O_{2}^{+}$ : The electronic configuration is $(\sigma1s)^2(\sigma^*1s)^2(\sigma2s)^2(\sigma^*2s)^2(\sigma2p_{z})^2$
$(\pi2p_{x}^2\equiv \pi2p_{y}^2)(\pi^*2p_{x}^1)$
Where, the number of bonding electrons $N_{b} =8$ and number of antibonding electrons, $N_{a} =3$
So, Bond order of $O_{2}^{+}$ molecule $= \frac{1}{2}(8-3) = 2.5$
$O_{2}^{-}$ : The electronic configuration is $(\sigma1s)^2(\sigma^*1s)^2(\sigma2s)^2(\sigma^*2s)^2(\sigma2p_{z})^2$
$(\pi2p_{x}^2\equiv \pi2p_{y}^2)(\pi^*2p_{x}^2 \equiv \pi^*2p_{y}^1 )$
Where, the number of bonding electrons $N_{b} =8$ and number of antibonding electrons, $N_{a} =5$
So, Bond order of $O_{2}^{-}$ molecule $= \frac{1}{2}(8-5) = 1.5$
Class 11 Chemistry NCERT Chapter 4: Higher Order Thinking Skills (HOTS) Questions
These Higher Order Thinking Skills questions are based on class 11 chemistry chapter 4 chemical bonding and molecular structure solutions. Practise these questions to develop conceptual understanding and problem solving ability.
Question 1. Which of the following molecules(s) show/s paramagnetic behavior ?
(A) $\mathrm{O}_2$
(B) $\mathrm{N}_2$
(C) $\mathrm{F}_2$
(D) $\mathrm{S}_2$
(E) $\mathrm{Cl}_2$
Choose the correct answer from the options given below :
1) B only
2) A & C only
3) A & E only
4) A & D only
Answer:
| No. of umpaired $e^{-}$ | ||
| (A) | $\mathrm{O}_2$ | 2 |
| (B) | $\mathrm{~N}_2$ | 0 |
| (C) | $\mathrm{~F}_2$ | 0 |
| (D) | $\mathrm{~S}_2$ | 2 |
| (E) | $\mathrm{Cl}_2$ | 0 |
If species contain unpaired electron than it is paramagnetic.
So A & D are paramagnetic.
Hence, the correct answer is option (4).
Question 2. Which of the following is not a postulate of VSEPR theory?
(1) The shape of a molecule depends upon the number of valence shell electron pairs (bonded or non-bonded) around the central atom.
(2) Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
(3) The positions of the electron pairs in space around the central atom are such that they maximise repulsion and thus minimise the distance between them.
(4) The magnitudes of the different types of electronic repulsions follow the order given below: Lone pair - Lone pair > Lone pair - Bonding pair > Bonding pair - Bonding pair
Answer:
The main postulates of VSEPR theory are:
-
The actual shape of a molecule depends upon the number of electron pairs (bonded or non–bonded) around the central atom.
-
The electron pairs tend to repel each other due to their negative charge.
-
Electron pairs arrange themselves in such a way that there exists a minimum repulsion between them.
-
The valence shell is considered as a sphere with the electron pairs placed at a distance.
-
A multiple bond is treated as if it is a single electron pair & the electron pairs that constitute the bond as a single pair.
-
The repulsive interaction of electron pairs decreases in the order as mentioned below:
Lone pair (lp) – Lone pair (lp) > Lone pair (lp) –
Bond pair (bp) > Bond pair (bp) – Bond pair (bp).
-
Double bonds cause more repulsion than single bonds, and triple bonds cause more repulsion than double bonds. This repulsion decreases sharply with increasing bond angle between the electron pairs.
The third statement is wrong as it says the elctrons arrangement is such that it maximize repulsion which is not true.
Hence, the correct answer is option (3).
Question 3. Which of the following compounds has the lowest melting point?
(1) NaCl
(2) MgCl2
(3) AlCl3
(4) BeCl2
Answer:
AlCl3 has the lowest melting point due to its covalent character, which is a result of the high charge density of Al3+. BeCl2 also has a lower melting point but higher than AlCl3.
Hence, the correct answer is option (3).
Question 4. A molecule with the formula AX4Y has all it’s elements from p-block. Element A is rarest,
monoatomic, non-radioactive from its group and has the lowest ionization enthalpy value among A, X and Y. Elements X and Y have first and second highest electronegativity values respectively among all the known elements. The shape of the molecule is :
(1) Square pyramidal
(2) Octahedral
(3) Pentagonal planar
(4) Trigonal bipyramidal
Answer:
Given A is rarest, monoatomic, non-radioactive p-block element and form $\mathrm{AX}_4 \mathrm{Y}$ type of molecule.
$\therefore$ It is concluded that it is Xe
It is given the electronegativity of A is less than X & Y
It is given the electronegativity of $\mathrm{X} \& \mathrm{Y}$ is highest and second highest respectively among all element.
$\therefore \mathrm{X} \& \mathrm{Y}$ are $\mathrm{F} \& \mathrm{O}$
$\therefore$ Compound is consider as $\mathrm{XeOF}_4$ with square pyramidal shape.
Hence, the correct answer is option (1).
Question 5. Among $\mathrm{SO}_2, \mathrm{NF}_3, \mathrm{NH}_3, \mathrm{XeF}_2, \mathrm{ClF}_3$ and $\mathrm{SF}_4$, the hybridization of the molecule with non-zero dipole moment and highest number of lone-pairs of electrons on the central atom is
(1) $\mathrm{sp}^3$
(2) $\mathrm{dsp}^2$
(3) $\mathrm{sp}^3 \mathrm{~d}^2$
(4) $\mathrm{sp}^3 \mathrm{~d}$
Answer:
| Molecule | Hybridisation | Dipole Moment | Lone pair on the central atom |
| $\mathrm{SO}_2$ | $\mathrm{sp}^2$ | Non - zero | 1 |
| $\mathrm{NF}_3$ | $\mathrm{sp}^3$ | Non - zero | 1 |
| $\mathrm{NH}_3$ | $\mathrm{sp}^3$ | Non - zero | 1 |
| $\mathrm{XeF}_2$ | $\mathrm{sp}^3 \mathrm{~d}$ | zero | 3 |
| $\mathrm{C} \ell \mathrm{~F}_3$ | $\mathrm{sp}^3 \mathrm{~d}$ | Non - zero | 2 |
| $\mathrm{SF}_4$ | $ \mathrm{sp}^3 \mathrm{~d}$ | Non - zero | 1 |
-
ClF3 has 2 lone pairs and a non-zero dipole moment, the highest among molecules with a dipole.
-
XeF2 has 3 lone pairs but zero dipole moment due to linear symmetry.
Hence, the correct answer is option (4)
Question 6:
Given below are two statements:
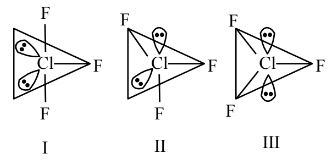
Statement (I) : for $C \ell \mathrm{F}_3$, all three possible structures may be drawn as follows.
Statement (II) : Structure III is most stable, as the orbitals having the lone pairs are axial, where the $\ell \mathrm{p}-\mathrm{bp}$ repulsion is minimum.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Statement I is incorrect but statement II is correct.
(2) Statement I is correct but statement II is incorrect.
(3) Both Statement I and statement II are correct.
(4) Both Statement I and statement II are incorrect.
Answer:
Statement 1 is correct.
Statement 2 is incorrect since in $\mathrm{sp}^3 \mathrm{~d}$ hybridization, a lone pair cannot occupy an axial position due to lone pair-bond pair repulsion. The lone pairs will be at equatorial position for maximum stability.
Hence, the correct answer is option (2).
Question 7: Consider the following species. Among them, $x$ are planar and $y$ are non-planar. Then find the value of the product of $x$ and $y$.
$
\begin{aligned}
& \mathrm{H}_2 \mathrm{O}, \mathrm{BrF}_3, \mathrm{~B}_2 \mathrm{H}_6, \mathrm{PCl}_3, \mathrm{SO}_3, \mathrm{XeF}_4, \\
& \mathrm{XeO}_3 .
\end{aligned}
$
Answer:
Planar $\rightarrow \mathrm{H}_2 \mathrm{O}, \mathrm{BrF}_3, \mathrm{XeF}_4, \mathrm{SO}_3$
Non-Planar$\rightarrow \mathrm{B}_2 \mathrm{H}_6, \mathrm{PCl}_3, \mathrm{XeO}_3$
$
x=4 \quad, y=3
$
X*Y = 12
Hence, the answer is option 12.
Question 8: In which of the following compound all the bonds are of equal length?
(1) $\mathrm{PCl}_5$
(2) $\mathrm{PF}_2 \mathrm{Cl}_3$
(3) $\mathrm{ClF}_3$
(4) $\mathrm{XeF}_4$.
Answer:
In TBP geometry, axial bonds are longer than equatorial bonds.
Hence, the correct answer is option (4).
Approach to Solve Questions of Class 11 Chemistry Chapter 4
Students can refer to the effective approaches to solve chemical bonding and molecular structure class 11 question answer. The following points will help you build a good strategy for this chapter 4 of NCERT.
1. Before solving questions it is very important to understand the structure of the chapter and break the chapter into manageable sections. Students can also refer to notes of this chapter available at our website for better understanding.
2. Try to learn and memorize key concepts like Lewis structure, octet rule, bond parameters, hybridization, etc. Most of the class 11 chemistry chapter 4 chemical bonding and molecular structure question answer are often asked directly from these topics.
3. The theories like valence bond theory, valence shell electron pair repulsion theory and molecular orbital theory are too crucial to understand. Make sure to understand their applications and limitations. Also, use NCERT solutions for class 11 chemistry chapter 4 PDF for effective learning.
4. First, read the questions thoroughly and note down the given information. Apply the concepts learned and solve in a step-wise manner.
5. Solve the NCERT in-text and exercise questions. You can also refer to the solved examples to learn how to answer the question. The NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure will make your learning feasible.
Topics and Subtopics Covered in the NCERT Textbook
All the topics and subtopics listed below are well explained in the Class 11 Chemistry Chapter 4 Notes. Students can access these notes available on our website.
4.1 Kössel-Lewis Approach to Chemical Bonding
4.1.1 Octet Rule
4.1.2 Covalent Bond
4.1.3 Lewis Representation of Simple Molecules( the Lewis Structures)
4.1.4 Formal Charge
4.1.5 Limitations of the Octet Rule
4.2 Ionic or Electrovalent Bond
4.2.1 Lattice Enthalpy
4.3 Bond Parameters
4.3.1 Bond Length
4.3.2 Bond Angle
4.3.3 Bond Enthalpy
4.3.4 Bond Order
4.3.5 Resonance Structures
4.3.6 Polarity of Bonds
4.4 The Valence Shell Electron Pair Repulsion (VSEPR) Theory
4.5 Valence Bond Theory
4.5.1 Orbital Overlap Theory
4.5.2 Directional Properties of Bonds
4.5.3 Overlapping of Atomic Orbitals
4.5.4 Types of Overlapping and Nature of Covalent Bonds
4.5.5 Strength of Sigma and Pi Bonds
4.6 Hybridisation
4.6.1 Types of Hybridisation
4.6.2 Other Examples of sp3, sp2 and sp Hybridisation
4.6.3 Hybridisation of Elements Involving d Orbitals
4.7 Molecular Orbital Theory
4.7.1 Formation of Molecular Orbitals Linear Combination of Atomic Orbitals (LCAO)
4.7.2 Conditions for the Combination of Atomic Orbitals
4.7.3 Types of Molecular Orbitals
4.7.4 Energy Level Diagram for Molecular Orbitals
4.7.5 Electronic Configuration and Molecular Behaviour
4.8 Bonding in Some Homonuclear Diatomic Molecules
4.9 Hydrogen Bonding
4.9.1 Cause of Formation of Hydrogen Bond
4.9.2 Types of H-Bonds
What Extra Should Students Study Beyond the NCERT for JEE/NEET?
What Students Learn from NCERT Solutions for Class 11 Chemistry Chapter 4
By practising the class 11 chemistry chemical bonding and molecular structure question answer , students gain a clear understanding of how atoms combine to form molecules and how the structure of these molecules determines their properties.
- These solutions help students to learn about the different types of chemical bonds ionic, covalent, and coordinate bonds and how these bonds are formed.
- The concept of the octet rule, Lewis dot structures, and formal charge calculation are explained here very well with the help of solved examples.
- Here students will learn about theories of Valence Shell Electron Pair Repulsion theory, Molecular Orbital theory
- Using these class 11 chemistry chapter 4 chemical bonding and molecular structure solutions concepts like hybridisation of atomic orbitals and its role in explaining molecular geometry are explained.
- The relationship between bond order, bond length, and bond strength and concept of polarity in bonds and molecules are explained in these solutions.
Importance of Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
The chemical bonding and molecular structure class 11 question answer is important for understanding how atoms bond to form molecules and how molecular structure affects the properties and reactivity of substances.
- This chapter helps students understand how atoms combine to form molecules through different types of chemical bonds.
- Concepts like ionic, covalent, and coordinate bonding are well explained in this chapter.
- This chapter covers various theories and concepts linking atomic orbitals to bonding.
- The class 11 chemistry chemical bonding and molecular structure question answer develops a base for understanding chemical reactions, physical properties, and reactivity of substances.
NCERT Solutions For Class 11 Chemistry
Along with class 11 chemistry chapter 4 chemical bonding and molecular structure solutions excel your exam preparations by solving chapter-wise NCERT Solutions for Class 11. Click on the link below
NCERT Solutions for Class 11 Subject-Wise
Follow the links below to get access to the NCERT solutions for other subjects as well.
NCERT Books and NCERT Syllabus
Click on the links below to get the syllabus and recommended books.
| NCERT Books Class 11 Chemistry |
| NCERT Books Class 11 |
| NCERT Syllabus Class 11 Chemistry |
| NCERT Syllabus Class 11 |
Frequently Asked Questions (FAQs)
NCERT Solutions for Chapter 4 provide detailed and accurate answers to all textbook questions based on chemical bonding and molecular structure. They help students understand the formation of chemical bonds, molecular geometry, and bonding theories.
Most of educational websites and the official NCERT site offer free access to Chapter 4 solutions in PDF format.
Start by reading the NCERT textbook thoroughly to understand the basic concepts and theories like the octet rule, VSEPR theory, and hybridisation.
A chemical bond is defined as the force of attraction that holds two or more atoms together in a molecule or compound.
Bond strength and bond order are directly proportional to each other. As bond order increases, bond strength also increases which means a higher bond order corresponds to a stronger bond between two atoms.
The octet rule states that atoms tend to bond in such a way that they have eight electrons in their valence shell, achieving a stable electron configuration similar to noble gases. This rule helps in predicting how atoms will interact and bond with each other to form molecules, thus playing a crucial role in understanding chemical reactions.
VSEPR theory suggests that electron pairs around a central atom will repel each other and arrange themselves as far apart as possible to minimize repulsion. This helps predict the geometry of molecules, such as linear, trigonal planar, tetrahedral, etc
Electronegativity is a measure of an atom's ability to attract shared electrons in a bond. It plays a crucial role in determining the bond type: if the difference in electronegativity between two atoms is large.
Chemical bonding is crucial because it helps explain how substances interact, their physical and chemical properties, and their behavior in reactions. Knowledge of bonding principles aids in predicting the structure and reactivity of molecules, which is foundational for advanced studies in chemistry and materials science.
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals that can accommodate the bonding electrons. It is important because it explains the geometry and bonding properties of molecules that cannot be described by simple atomic orbital theory.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters