NCERT Class 11 Chemistry Chapter 3 Notes - Download PDF
How can we predict the behavior of an element just by knowing its position in the periodic table? Why do elements show repeating patterns in their properties? Why is the Mendeleev table so successful? In class 11 chemistry chapter 3 classification of elements and periodicity in properties notes, you’ll explore the logic behind the classification of elements, uncover trends in atomic structure, and understand the foundation of the modern periodic law. This chapter helps us to understand why different elements behave differently. Almost every aspect around us is linked to the periodic table, whether it is salt in our food or iron in large buildings. Periodicity in properties helps us to explain how electronic configuration and periodic trends form the basis of the arrangement of elements in the periodic table. The periodic table is divided into s-block, P- Block, D- And F- Block.
This Story also Contains
- NCERT Notes for Class 11 Chemistry Chapter 3 Download PDF
- NCERT Notes for Class 11 Chapter 3
- Classification of elements and periodicity in Properties: Previous year's Question and Answers
- How to Master Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- Advantages of Using Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties Notes
- NCERT Notes Class 11 Chapter-Wise
- NCERT Solutions for Class 11 Chemistry
- NCERT Exemplar Solutions Subject-Wise
- NCERT Solutions Subject-Wise
.jpg)
The NCERT Notes for Class 11 Chemistry will be helpful for a quick revision of topics. These notes are designed by our subject experts, which ensures the credibility of the content provided. It becomes difficult and time-consuming for students to read the NCERT textbooks point-to-point. So, to solve this problem, we are providing these NCERT notes that cover all the topics and concepts provided in the NCERT textbook in a very clear and comprehensive way. These Chapter 3 Class 11 notes are also valuable resources for various competitive exams like JEE, NEET, etc. Also, check the NCERT Solutions for all the chapters.
NCERT Notes for Class 11 Chemistry Chapter 3 Download PDF
These concise classification of elements and periodicity in properties class 11 notes cover all the key concept to help students in quick revision before exam. You can download the NCERT notes for Class 11 from the button given below:
Also Read
NCERT Notes for Class 11 Chapter 3
These classification of elements and periodicity in properties ncert notes covers key concepts, including the layout of the modern periodic table, groups and periods, and periodic trends such as Atomic Size & Atomic Radius, Ionization Enthalpy, and Electronegativity. They explain basics like chemical bonding and periodicity and is an excellent resource for quick revision, as it helps build a clear understanding of fundamental principles and their real-life applications.
Genesis of Periodic Classification
Many elements were found in the nineteenth century, and studying each one separately proved difficult. Many scientists have attempted to classify elements in a variety of ways. You can also download these notes in PDF format for easy offline study and quick revision.
Dobereiner triads
Johann Wolfgang Dobereiner, a German chemist, attempted to arrange elements with comparable properties into three groups. Triads were the name given to these groups. The atomic mass of the middle element in these triads, according to Dobereiner, should be more or less equal to the mean of the atomic masses of the other two elements in the triad. A trio including lithium, sodium, and potassium is an example of such a triad. Lithium has an atomic mass of 6.94, while potassium has a mass of 39.10. The center element in this triad, sodium, has an atomic mass of 22.99, which is about similar to the mean of lithium and potassium's atomic masses (which is 23.02).
Limitation
There was no way to classify all of the elements known at the time into triads.
Newland law of octaves
In the year 1866, English chemist John Newlands organized the 56 known elements in ascending order of atomic mass. He noticed a pattern in which every eighth element had properties that were comparable to the first.
According to Newland's Law of Octaves, when elements are ordered in ascending order of atomic mass, the periodicity in properties of two elements separated by seven elements will be comparable.
Limitations
The classification of elements using Newland's Octaves was only used up to calcium.
The discovery of noble gases added to the method's limitations because they couldn't be included in the arrangement without completely disrupting it.
Mendeleev’s periodic table
Dmitri Ivanovich Mendeleev, a Russian chemist, proposed the periodic table in 1869. He saw that the physical and chemical properties of elements were connected to their atomic masses periodically.
The chemical characteristics of elements are a periodic function of their atomic weights, according to the Periodic Law (also known as Mendeleev's Law).
Advantages of Mendeleev’s periodic table
He classified the elements according to their atomic masses.
Undiscovered elements such as Gallium and Scandium germanium were left in the gaps. They even left the entire group empty in case of undiscovered inert gases.
He was able to anticipate the proportions of certain elements based on their positions in the periodic table, such as Ga and Sc.
He was able to accurately predict inaccuracies in the atomic weights of several elements, such as gold and platinum.
Limitations
Isotope positions could not be explained
The wrong order of atomic masses could not be justified. For example, Argon has an atomic mass of 40, and K has a low atomic mass of 30, although K should be placed first due to its low atomic mass.
The position of hydrogen could to be explained.
Some aspects that are dissimilar are grouped together, while others are divided into different categories. Alkali metals such as Li, Na, K, and others (group AI) are grouped alongside coinage metals such as Cu, Ag, and Au, for example.
The main body excludes lanthanides and actinides.
Modern Periodic Law and the Present Form of the Periodic Table
Henry Moseley, an English physicist, researched the wavelength of the characteristic X-rays in 1913. Using various metals as anti-cathodes, it was demonstrated that the square root of the line's frequency is proportional to the atomic number. Moseley developed the Modern Periodic Table based on the above data, which states that “Physical and chemical properties of the elements are the periodic function of their atomic numbers”. For a better understanding of these concepts, refer to the NCERT Class 11 Chemistry Chapter 3 Notes Classification of Elements and Periodicity in properties.
As a result, there is periodicity in electronic configuration when elements are organized according to increasing atomic numbers, which leads to periodicity in chemical properties. Bohr's Periodic Table is the long form of the Periodic Table. This Periodic Table has 18 groups and seven periods. Periods refer to the horizontal rows. Groups are made up of 18 vertical columns.
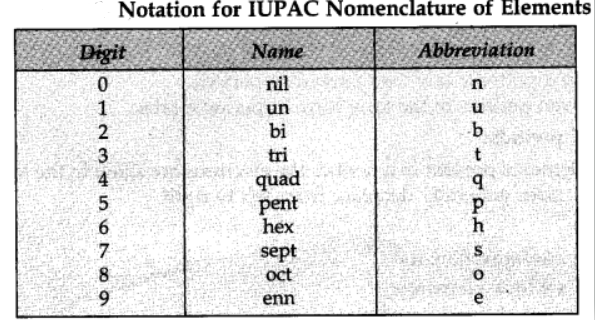
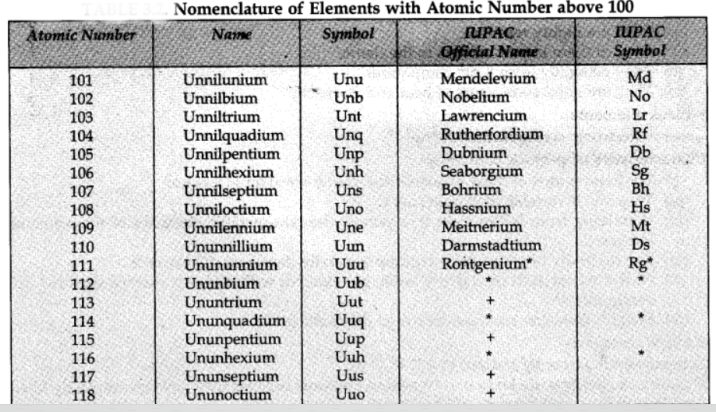
IUPAC Nomenclature of Elements with Atomic Number More Than 100
Electronic Configuration of Elements and the Periodic Table
It explains how the arrangement of electrons in shells or energy levels helps determine an element’s position in the periodic table. It also reveals trends in properties like atomic size and chemical reactivity, etc, across periods and groups.
Electronic Configurations in Periods
The period indicates the value of n for the outermost shell. In other words, successive periods in the Periodic Table are associated with the filling of the next higher principal energy level (n = 1, n = 2, etc.). As we move from left to right across a period, one electron is added to the same principal energy level, while the number of protons in the nucleus also increases. This leads to a change in properties such as atomic size, metallic character, and valency.
Groupwise Electronic Configurations
Elements in the same vertical group have similar valence shell electronic configurations, the same number of electrons in the outer orbitals, and similar chemical reactivity and physical properties. For example, the Group 1 elements (alkali metals) all have an ns1 valence shell electronic configuration.
Elements in s, p, d, f-block and Their Electronic Configurations
The s-Block Elements
- The last electron enters the s-orbitals in these elements.
- ns1-2, where n = 2-7, is a general outer shell electrical configuration of s-block elements.
- They are metals that are soft and have low melting and boiling points
- They are electropositive and have low ionization enthalpies (energies).
- They rapidly lose their valence (outermost) electrons, forming +1 (alkali metals) and +2 ions (in the case of alkaline earth metals).
- They are metals with a lot of reactivity. As we progress through the group, the metallic character and reactivity become more prominent. They are never found pure in nature due to their high reactivity.
- With the exception of beryllium, all s-block element compounds are generally ionic.
The p-Block Elements
- General electronic configuration $n s^2 n p^{1-6}$
- d-block elements’ compounds are primarily covalent in nature
- They have a wide range of oxidation states.
- The non-metallic character of the components grows as the period progresses from left to right.
- The reactivity of elements within a group tends to decrease.
- A noble gas element with a closed valence shell ns2np6configuration is present at the end of each period.
- As we move through the group, the metallic character becomes more prominent.
The d-Block Elements (Transition Elements)
- The general electronic configuration is$(\mathrm{n}-1) \mathrm{d}^{1-10} \mathrm{~ns}^{1-2}$
- They are all high-melting and boiling-point metals.
- d-block elements’ compounds are usually paramagnetic in nature.
- They mostly produce colored ions and have varied valence (oxidation states).
- Because they have incompletely filled d-orbitals in their ground state or any of the oxidation states, the d-block elements are known as transition elements
- Compounds of d-block elements are frequently used as catalysts
The f-Block Elements (Inner-Transition Elements)
- General electronic configuration is $n-2 f^{1-14}(n-2) f^{(0-14)}(n-1) d^{(0-1)} n s^2$..
- They are called inner transition elements because, in d-block transition elements, electrons are filled in (n – 1) d subshells, whereas in f-block inner transition elements, electrons are filled in (n – 2) f subshells, which is one inner subshell.
- The Lanthanoids Ce (Z = 58) – Lu (Z = 71) and Actinoids Th (Z = 90) – Lr (Z = 103), are two rows of elements near the bottom of the Periodic Table.
- They are all made of metal. The characteristics of the elements in each series are relatively similar.
- The actinoid series contains a large number of radioactive elements.
Metals, Non-metals, and Metalloids
Based on their physical and chemical properties, elements in the periodic table are broadly classified as metals, non-metals, and metalloids.
Metals, mostly found on the left and centre of the periodic table. They are typically lustrous, good conductors of heat and electricity, malleable, and ductile. They tend to lose electrons to form positive ions.
Non-metals, mostly found on the right side of the periodic table. They are generally poor conductors, brittle in solid form, and tend to gain or share electrons, forming negative ions or covalent bonds.
Metalloids, silicon, germanium, arsenic, antimony and tellurium lie along the stair-step line (between metals and non-metals) and exhibit properties intermediate between those of metals and non-metals. Their classification helps in understanding trends in physical and chemical properties.
Periodic Trends in Properties of Elements
There are many observable patterns in the physical and chemical properties of elements as we descend in a group or move across a period in the Periodic. Based on the physical and chemical properties, trends are explained as below.
Trends in Physical Properties
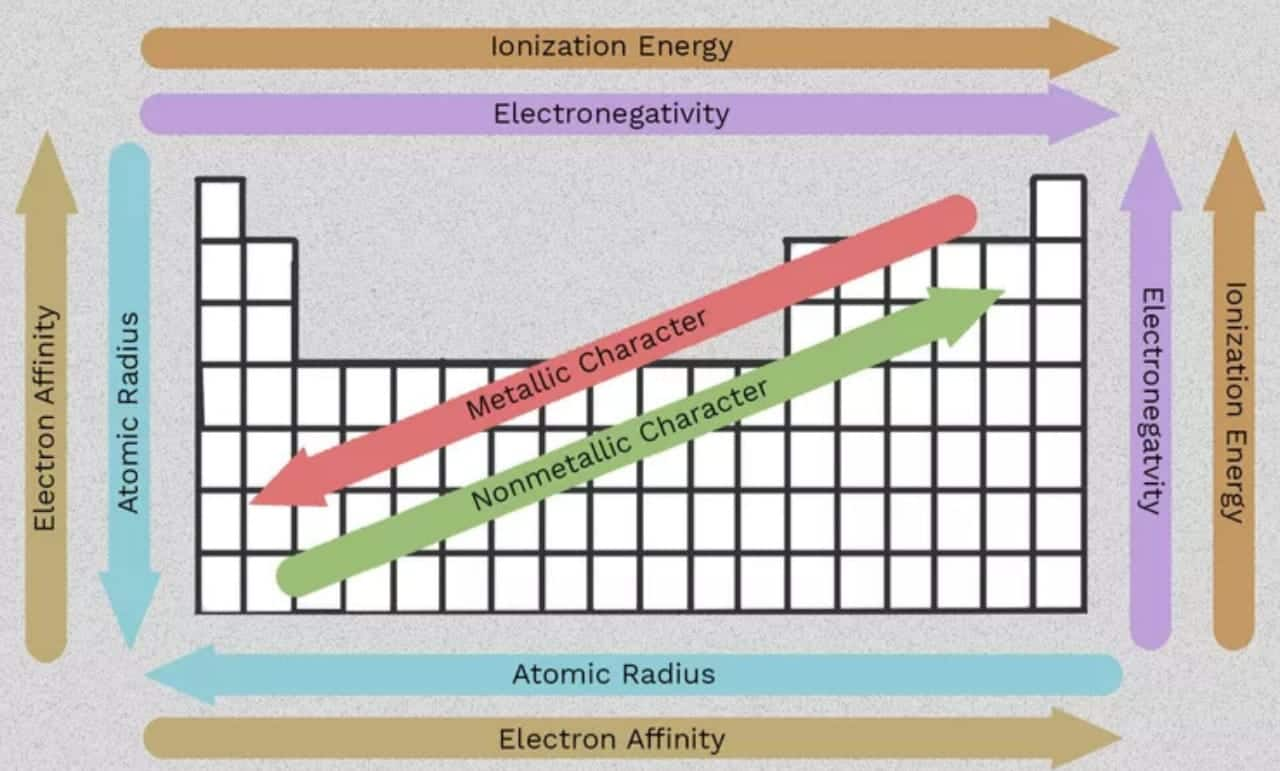
Atomic Radii
The atomic radius is the distance between the nucleus's centre and the outermost shell containing electrons. These are classified as covalent radii, van der Waals radii, and metallic radii according to the nature of bonding. Covalent radii are one-half of the distance between the centres of the nuclei of two neighbouring identical atoms linked by a single covalent bond. For example, the distance between Cl and Cl is 198 pm, and the covalent radius of Cl is 99 pm. Van der Waals radii are half of the internuclear distance between two comparable, nearby atoms belonging to two adjoining molecules of the same material in the solid state. In a metallic crystal, a metallic radius is defined as half the distance between the nuclei of two adjacent atoms. Due to an increase in effective nuclear charge, the atomic radius decreases as we move from left to right in a period. The atomic radius increases as we move down the group due to an increase in the main quantum number, which leads to an increase in the number of shells and a higher shielding effect.
Ionic Radii
When one or more electrons are lost from a neutral atom, the ions created are known as cations (positive ions). When electrons are added to the neutral atom, the ions are known as anions (negative ions). The ionic radii are the effective distances from the nucleus's centre to which the ion exerts its impact on the electron cloud. Ionic radii follow the same pattern as atomic radii. It reduces from left to right as the period progresses and increases from top to bottom as the group goes. Any natural atom's cation and anion sizes are as follows: cation< neutral atom <anion
Ionization Enthalpy
Ionisation enthalpy is the amount of energy necessary to remove an electron from an isolated gaseous atom's outermost orbit. In general, IE grows as it moves from left to right in a period, whereas it drops as it moves down the group; however, half-filled and fully filled orbitals are highly stable and hence have a high IE.
Electron Gain Enthalpy
The change in enthalpy that occurs when a gaseous atom gains an extra electron to form a monovalent anion in the gaseous state is known as the electron gain enthalpy. The enthalpy of electron gain increases across the periods but drops as the group progresses.
Electronegativity
Electronegativity is an atom's tendency to attract the shared pair of electrons in a covalent bond towards itself. The most electronegative element is fluorine, while the least electronegative one is cesium. Electronegativity increases in the periods from left to right. The electronegativity of elements in the groups reduces as they go down. Students can also refer classification of elements and periodicity in properties class 11 notes for understanding these concepts better.
All the important trends across the period and group are given below in one place:
Trends in Chemical Properties
Periodicity of Valence or Oxidation States
It is defined as the element's ability to combine. The electrical arrangement of an element's atom determines its valency, which is usually dictated by electrons in the valence shell. Valency increases from 1 to 4 and eventually declines to 0 (for noble gases) as one moves along a period from left to right, but valency remains constant as one moves along a group. Because they can use electrons from both the outer and penultimate shells, transition metals have variable valency.
Periodic Trends and Chemical Reactivity
Metal reactivity increases as the IE, electronegativity, and atomic size rise, as well as the metal's electropositive character. Increases in electronegativity, as well as electron gain enthalpy and atomic radii, increase non-metal reactivity. The atomic and ionic radii, as we know, generally decrease in a period from left to right. As a consequence, the ionization enthalpies generally increase and electron gain enthalpies become more negative across a period. In other words, the ionization enthalpy of the extreme left element in a period is the least and the electron gain enthalpy of the element on the extreme right is the highest negative (note : noble gases having completely filled shells have rather positive electron gain enthalpy values). This results into high chemical reactivity at the two extremes and the lowest in the centre. Thus, the maximum chemical reactivity at the extreme left (among alkali metals) is exhibited by the loss of an electron leading to the formation of a cation and at the extreme right (among halogens) shown by the gain of an electron forming an anion.
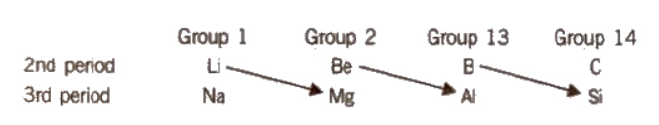
Anomalous Properties of Second-Group Elements
Certain 2nd-period elements have properties that are similar to their 3rd-period diagonal elements. As a result, Li is similar to Mg, Be is similar to Al, and B is similar to Si. This is known as a diagonal relationship, and it occurs because both elements have almost identical ionic radii and polarizing power (charge/size ratio). Mg, Al, and Si are known as bridge elements.
Classification of elements and periodicity in Properties: Previous year's Question and Answers
Practise the previous years questions of this chapter that helps to strengthen your understanding of the topic and prepare effectively for exams. The concepts used to solve these questions are explained in ncert class 11 chemistry chapter 3 classification of elements and periodicity in properties notes.
Question 1. Choose the incorrect trend in the atomic radii ( r ) of the elements :
i) $r_{B r}<r_K$
ii) $\mathrm{r}_{\mathrm{Al}}>\mathrm{r}_{\mathrm{Mg}}$
iii) $r_{\mathrm{Rb}}<\mathrm{r}_{\mathrm{Cs}}$
iv) $r_{\text {At }}<r_{C s}$
Answer:
$\mathrm{r}_{\mathrm{Mg}}>\mathrm{r}_{\mathrm{Al}}$ due to lower effective nuclear charge.
Magnesium has a larger atomic radius than aluminum, measuring about 160 pm for magnesium compared to about 143 pm for aluminum. This is primarily due to the effects of electronic configuration and effective nuclear charge.
Hence, the correct answer is option (2).
Question 2. Which of the following statements is correct?
A. The process of the addition of an electron to a neutral gaseous atom is always exothermic
B. The process of removing an electron from an isolated gaseous atom is always endothermic
C. The $1^{\text {st }}$ ionization energy of the boron is less than that of the beryllium
D. The electronegativity of C is 2.5 in $\mathrm{CH}_4$ and $\mathrm{CCl}_4$
E. Li is the most electropositive among the elements of group I
Choose the correct answer from the options given below
i) B and C only
ii)A, C, and D only
iii) B and D only
iv) B, C, and E only
Answer:
(A) The process of adding an $\mathrm{e}^{-}$to a neutral gaseous atom is not always exothermic; it may be exothermic or endothermic.
(C) Be B
$1 \mathrm{~s}^2 2 \mathrm{~s}^2 \quad 1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}^1$
The Be 2s subshell is fully filled
So, high energy is needed to remove $\mathrm{e}^{-}$as compared to B.
(D) In $\mathrm {CCl}_4 \rightarrow$ 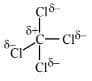 due to partially positive charge $z_{\text {eff }} \uparrow, \mathrm{EN} \uparrow$
due to partially positive charge $z_{\text {eff }} \uparrow, \mathrm{EN} \uparrow$
So, EN of $\mathrm{C} \Rightarrow \mathrm{CCl}_4>\mathrm{CH}_4$
(E) Cs is the most electropositive.
Hence, the correct answer is option (1).
Question 3. The most electronegative element in the periodic table is-
(1) Nitrogen
(2) Oxygen
(3) Chlorine
(4) Fluorine
Answer:
Fluorine has the highest electronegativity because of its small size, high nuclear charge, low shielding, and strong electron affinity.
Hence, the correct answer is option (4).
Question 4: The atomic number of the element from the following with lowest $1^{\text {st }}$ ionisation enthalpy is :
(1) 32
(2) 35
(3) 87
(4) 19
Answer:
The elements are:
- $32 \rightarrow$ Germanium (Ge)
- $35 \rightarrow$ Bromine (Br)
- $\mathbf{8 7} \rightarrow$ Francium (Fr)
- $19 \rightarrow$ Potassium $(K)$
| Atomic No. | Period No. | Group No. |
| 35 | 4 | 17 |
| 19 | 4 | 1 |
| 32 | 4 | 14 |
| 87 | 6 | 1 |
First ionization energy of an element generally decreases down the group and increases from left to right along a period. Among the given elements, Francium (Fr) and Potassium (K) are Group 1 elements, but Francium is further down the group, so it has an even lower ionization enthalpy.
Hence, the correct answer is option (3).
Question 5: Given below are two statements :
Statement (I) : The first ionisation enthalpy of group 14 elements is higher than the corresponding elements of group 13.
Statement (II) : Melting points and boiling points of group 13 elements are in general much higher than those the corresponding elements of group 14.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1) Statement I is correct but Statement II is incorrect
(2) Statement I is incorrect but Statement II is correct
(3) Both Statement I and Statement II are incorrect
(4) Both Statement I and Statement II are correct
Answer:
Statement 1 is correct since left to right, I.E. increases in general in the periodic table.
Statement 2 is incorrect since the M.P. of group 14 elements is more than that of group 13 elements.
Hence, the correct answer is option (1).
How to Master Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
This chapter helps students to understand the arrangement of elements and the trends in their chemical behaviour. These ncert class 11 chemistry chapter 3 classification of elements and periodicity in properties notes help to understand the basic concepts from your NCERT textbook. Given below some points on how to master this chapter.
- Students need to understand basic concepts like Mendeleev's and Modern Periodic Law.
- After that learn about the periodic trends such as atomic radius, ionisation enthalpy, electron gain enthalpy, electronegativity, and valency. Students can refer to classification of elements and periodicity in properties class 11 chemistry chapter 3 CBSE notes for better understanding of these concepts.
- They must practice diagrams like the modern periodic table to remember groups, periods, and classifications.
- At last students can solve previous year questions from this chapter.
Advantages of Using Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties Notes
The classification of elements and periodicity in properties class 11 notes helps students to understand the fundamental principles of the periodic table. The advantages of using these notes are given below:
- Use these notes for understanding concepts like periodic properties such as ionisation energy, atomic radius, electronegativity, and electron affinity.
- These notes are helpful for understanding concepts like Mendeleev’s periodic law, modern periodic law, and the arrangement of elements in a clear and concise manner.
- The ncert class 11 chemistry chapter 3 classification of elements and periodicity in properties notes are prepared by experts in a very clear and comprehensive manner that are useful in both boards and competitive exams.
- These notes cover every topic from the NCERT book.
NCERT Notes Class 11 Chapter-Wise
Along with the classification of elements and periodicity in properties class 11 chemistry chapter 3 CBSE notes, you can also refer to the chapter-wise class 11 notes provided below:
|
NCERT Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry Notes |
|
NCERT Class 11 Chemistry Chapter 3 classification of elements and periodicity Notes |
|
NCERT Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Notes |
|
NCERT Class 11 Chemistry Chapter 8 Organic chemistry some basic principles and techniques Notes |
NCERT Solutions for Class 11 Chemistry
Along with classification of elements and periodicity in properties ncert notes, follow the links below to get chapter-wise solutions of NCERT and make your learning better.
NCERT Exemplar Solutions Subject-Wise
NCERT Exemplar Subject-wise solutions are given below:
NCERT Solutions Subject-Wise
NCERT Subject-wise solutions are given below:
| NCERT Solutions for Class 11 Mathematics |
| NCERT Solutions for Class 11 Chemistry |
| NCERT Solutions for Class 11 Physics |
| NCERT Solutions for Class 11 Biology |
Frequently Asked Questions (FAQs)
Elements in the periodic table are primarily classified into metals, non-metals, and metalloids. Metals are characterized by their good conductivity, malleability, and ductility. Non-metals exhibit diverse properties, often being poor conductors and having more varied states at room temperature.
Ionization energy is the energy required to remove the outermost electron from a neutral atom in its gaseous state. In the periodic table, ionization energy generally increases across a period due to increased nuclear charge and decreases down a group because the outer electrons are further from the nucleus and easier to remove due to electron shielding.
The periodic law states that the properties of elements are a periodic function of their atomic numbers. This means that when elements are arranged in order of increasing atomic number, certain properties recur at regular intervals.
Transition metals are located in the center of the periodic table (groups 3 to 12) and are characterized by their ability to form various oxidation states and complex ions. They differ from other elements because they typically have partially filled d-subshells.
The gain of electrons is the most common way for an anion to develop. The size of this particle is bigger than that of a parent atom.
Periodicity of properties refers to the recurring trends observed in the chemical and physical properties of the elements as you move across or down the periodic table. For instance, atomic radius, ionization energy, electronegativity, and metallic character show periodic trends.
The classification of elements and periodicity in properties class 11 notes provide a simplified summary of how elements are arranged in the periodic table and how their physical and chemical properties show periodic trends. They highlight key concepts like periodic law, types of periodic tables, and important trends such as atomic size, ionisation enthalpy, electron affinity, and electronegativity to help students revise quickly and prepare effectively for exams.
Periodic trends in the periodic table are: Atomic radii, ionization energy, electron affinity, electronegativity, etc.
The atomic number is the fundamental basis of the periodic table, as atoms are arranged in the periodic table in order of increasing atomic number. Atomic numbers determine the element's identity, like electronic configuration, and chemical properties.
Elements are classified on the basis of atomic number, electronic configuration , physical and chemical properties.This classification helps in understanding trends and behaviour across groups and periods.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
