You can find thermodynamics class 11 chemistry chapter 5 CBSE notes in NCERT textbooks, educational websites like Careers360, or through free PDF downloads available on online learning platforms. Many coaching institutes and YouTube channels also provide simplified notes and revision guides for quick learning.
NCERT Class 11 Chemistry Chapter 6 Notes Thermodynamics - Download PDF
Do you know how refrigerators work, why do ice cubes melt without anyone touching them and can energy be created, or is it always just transformed? The answer to all these questions lies in Thermodynamics, an important branch of chemistry that deals with the change in energy in any Physical and Chemical process. It examines how energy moves between different systems and their surroundings, with an emphasis on important ideas like work and heat, along with the basic rules that control these changes. Thermodynamics explains the reason for the occurrence of any reaction, transfer of energy, etc.
This Story also Contains
- NCERT Notes for Class 11 Chemistry Chapter 5 Download PDF
- NCERT Notes for Class 12 Chapter 5 Thermodynamics
- Thermodynamics Previous Years Questions and Answers
- How to Master Class 11 Chemistry Chapter 5 Thermodynamics
- Advantages of Using Class 11 Chemistry Chapter 6 Thermodynamics Notes
- NCERT Class 11 Notes Chapter-Wise
- NCERT Solutions for Class 11 Chemistry
- Subject Wise NCERT Exemplar Solutions
- Subject Wise NCERT Solutions
Thermodynamics is something we deal with every day. Consider how automobile engines convert fuel into action or how refrigerators keep food fresh. These NCERT Notes for Class 11 Chemistry will be helpful for a quick revision of topics. These class 11 chemistry chapter 5 thermodynamics notes are designed by our subject experts, which ensures the credibility of the content provided. It becomes difficult and time-consuming for students to read the NCERT textbooks point-to-point. So, to solve this problem, we are providing these NCERT notes that cover all the topics and concepts provided in the NCERT textbook in a very clear and comprehensive way.
NCERT Notes for Class 11 Chemistry Chapter 5 Download PDF
Students can download these well-structured thermodynamics class 11 chemistry chapter 5 CBSE notes pdf from the icon given below to study anytime, revise quickly, and strengthen your understanding.
Also, students can refer
NCERT Notes for Class 12 Chapter 5 Thermodynamics
The ncert class 11 chemistry chapter 5 thermodynamics notes contain a concise overview of the chapter, including all the important topics and formulas. These NCERT Notes for Class 11 can be used for a quick revision during exam time. It includes types of systems, work, and calculation of internal energy and enthalpy. Each topic is discussed in detail below.
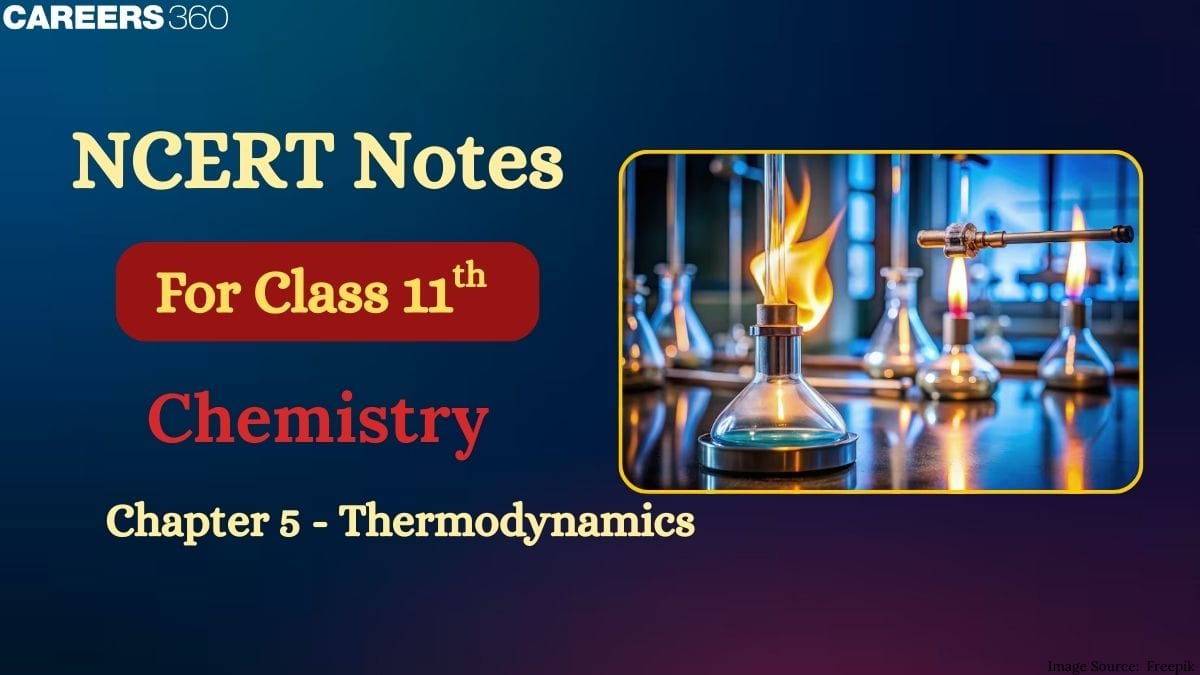
System and Surroundings
A system can be defined as where the observations were made, it is the part of the universe.
Surrounding can be defined as the part other than the system is, surrounding.
The system and surroundings can be differentiated by a wall called a boundary.
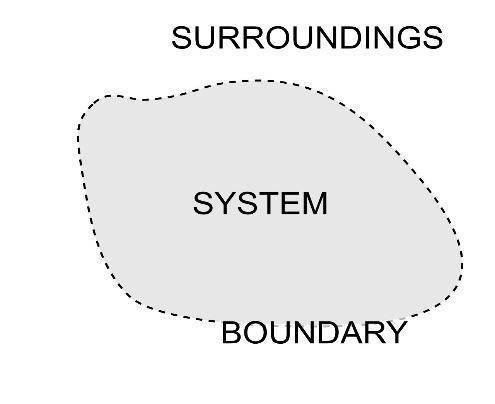
Types of the system:
The system can be further divided into three categories such as-
-
Open system- Boundary is imaginary in this system and exchange of mass or matter and energy both can be possible between system and surroundings. Example: Open beaker.
-
Closed system- No exchange of matter occurs, but the exchange of energy is possible between the system and its surroundings. Example: closed container of steel or copper.
-
Isolated system- No exchange of matter or energy can be possible. Example: Closed container like a thermos flask.
The State of the System
In general concern when we have to make any calculations we require measurable quantities or properties.
Those quantities are temperature, pressure, and the volume combined to form the composition of the system. But in order to deal with these quantities, we must have average conclusions of such measurements so we introduce a term called state functions or state variables. These quantities like pressure(p), volume(V), temperature(T), and amount(n).such variables like p, V, T, n are stated as state variables or state functions.
Internal Energy as a State Function:
The combined energy or sum of energies, such as mechanical, electrical or chemical are so-called internal energy of the system. This can be denoted in thermodynamics by the letter ‘U’. This can be affected by a change in any of the following:
-
Heat goes into and out of the system
-
Work can be done on the system or by the system.
-
Matter; Exchange of matter( entering or leaving)
General case: This can be shown using an equation.
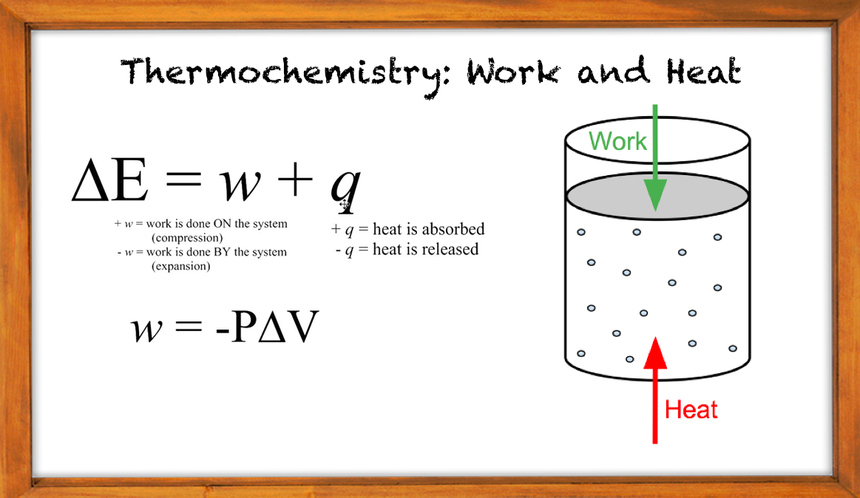
∆U=q+w
Here the change in internal energy is the sum of heat passing in and out and the work done. It is independent of the change and also depends on the initial and final state of the system. The equation that we get for change in internal energy is the mathematical form of the First law of thermodynamics. For a better understanding of these concepts, refer to the NCERT Solutions for Class 11 Chemistry Chapter 5 Thermodynamics, which explain them through a variety of solved questions.
Adiabatic system: It is the system in which the system and surroundings are separated by a wall, where no transfer or exchange of matter or energy occurs.
Applications
Work: Here we take an example of a cylinder or piston, which is fitted perfectly and is filled with a frictionless container with one mole of gas.
Let us first discuss the variables to calculate the work done.
The pressure of the gas=p
The volume of the gas=V
The external pressure of the gas=pext
Area of cross section=A
The piston is moved to a certain distance, taken as length=l
Change in volume$\Delta \mathrm{V}=\mathrm{A} \times \mathrm{I}=\left(\mathrm{V}_{\mathrm{f}}-\mathrm{V}_{\mathrm{I}}\right)$
So here, the pressure inside the piston becomes
p=FA
Case1: Expansion process
In the case of this process, work is done by the system, by expanding the volume of the system. When the work is done by the system then the negative sign convention will be used in it. so the value of work done will be
W=-p∆V
Now consider the two cases of expansion of ideal gas.
1. Work is to be done in isothermal and reversible condition, where the expansion of ideal gas occur.
Reversible work is taken from the initial volume to the final volume.
$
\mathrm{w}_{\text {rev }}=-\int_{V_i}^{V_f} p_{\text {ex }} d V=-\int_{V_i}^{V_f}\left(p_{\text {in }} \pm d p\right) d V
$
Since $d p \times dV$ is very small, we can write
$
\mathrm{w}_{r e v}=-\int_{V_i}^{V_f} p_{i n} d V
$
Now, the pressure of the gas ( $p_{\text {in }}$, which we can write as $p$ now) can be expressed in terms of its volume through the gas equation. For $n$ mol of an ideal gas i.e., $p V=n R T$
$
\Rightarrow p=\frac{n \mathrm{R} T}{V}
$
Therefore, at constant temperature (isothermal process),
$
\begin{aligned}
& \mathrm{w}_{\mathrm{rev}}=-\int_{V_i}^{V_f} n \mathrm{R} T \frac{d V}{V}=-n \mathrm{R} T \ln \frac{V_f}{V_i} \\
& =-2.303 n \mathrm{R} T \log \frac{V_f}{V_i}
\end{aligned}
$
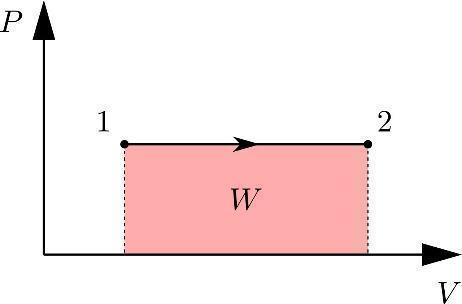
B. Isothermal condition, where the expansion of an ideal gas occurs.
-
For irreversible change:
$q=-\mathrm{w}=p_{e x}\left(V_f-V_i\right)$
-
For reversible change
$\begin{aligned} & q=-\mathrm{w}=n \mathrm{R} T \ln \frac{V_f}{V_i} \\ & =2.303 n \mathrm{R} T \log \frac{V_f}{V_i}\end{aligned}$
-
For adiabatic change, q=0,
$\Delta U=\mathrm{w}_{\mathrm{ad}}$
Enthalpy
Enthalpy can be denoted by the letter ‘H’.
Enthalpy is defined as the sum of pressure-volume and internal energy.
Change in enthalpy can be defined as the heat being evolved or absorbed at constant pressure conditions in a given system.
For finite changes at constant pressure, we can write the equation as,
$
\Delta H=\Delta U+\Delta p V
$
Since $p$ is constant, we can write
$
\Delta H=\Delta U+p \Delta V
$
For the Exothermic process, Heat is given out of the system, whereas for the Endothermic process, heat is given to the system from the surroundings.
Relationship between Change in enthalpy and change in internal energy of the system
$\mathrm{p} \Delta \mathrm{V}=\Delta \mathrm{n}_{\mathrm{g}} \mathrm{RT}$
$\Delta \mathrm{H}=\Delta \mathrm{U}+\Delta \mathrm{n}_{\mathrm{g}} \mathrm{RT}$
1. Extensive property:
Extensive property can be defined as that property in which the value depends on the quantity or the size that is present in the system.
Examples of extensive properties are mass, enthalpy, volume, etc.
2. Intensive property:
Intensive property can be defined as that property in which the value does not depend on the quantity or the size that is present in the system.
Examples of intensive properties are temperature, density, etc.
3. Heat capacity:
Heat capacity may be defined increase in temperature that is directly proportional to heat transferred.
The increase in temperature is proportional to the heat transferred
$
q=\text { coeff } \times \Delta T
$
The magnitude of the coefficient depends on the size, composition and nature of the system. We can also write it as $ q=C\Delta T$.
To find out the heat, q, required to raise the temperature of a sample, we multiply the specific heat of the substance, c, by the mass m, and temperature change, ∆T, as
$q=c \times m \times \Delta T=C \Delta T$
4. Relationship between the value of Cp and Cv.
Constant volume value of heat capacity; Cv.
Constant pressure value of heat capacity; Cp.
We can write an equation for heat, $q$
at constant volume as $q_V=C_V \Delta T=\Delta U$
at constant pressure as $q_p=C_p \Delta T=\Delta H$
The difference between $C_p$ and $C_V$ can be derived for an ideal gas as:
For a mole of an ideal gas
$
\begin{aligned}
& \Delta H=\Delta U+\Delta(p V) \\
&=\Delta U+\Delta(\mathrm{R} T) \\
&=\Delta U+\mathrm{R} \Delta T \\
& \therefore \Delta H=\Delta U+\mathrm{R} \Delta T
\end{aligned}
$
On putting the values of $\Delta H$ and $\Delta U$, we have
$
\begin{aligned}
& C_p \Delta T=C_V \Delta T+\mathrm{R} \Delta T \\
& C_p=C_V+\mathrm{R}
\end{aligned}
$
$C_p-C_v=R$
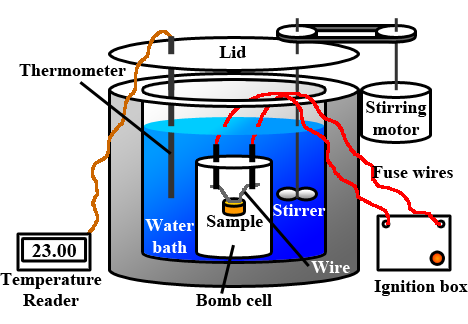
Measurement of ∆U and ∆H: Calorimetry
Measurement of the value of change in internal energy and change in enthalpy with Bomb calorimeter.
Enthalpy changes occur during the process of phase transformation.
-
Enthalpy of Fusion: Enthalpy of fusion can be defined as the conversion of one mole of a solid is accompanied to change it in liquid. The condition of fusion is governed at its melting point
-
Enthalpy of Vaporisation: Enthalpy of vaporization can be defined as the conversion of one mole of a solid accompanied to change it in liquid, the condition of fusion is governed by its boiling point.
-
Enthalpy of Sublimation: Enthalpy of sublimation is defined as the change in when one mole of a solid is converted to a gaseous state at its melting point. But the temperature is below its melting range.
-
Enthalpy of Combustion: Enthalpy of fusion is defined as the change in when one mole of substance burns in excess amount of air.
-
Standard enthalpy of formation: It is defined as inthe standard condition of temperature at 273K and pressure at 1 atm formation of one mole of substance occurs with the help of their constituent elements.
-
Thermochemical equations: It is defined as in chemical reaction when the products and reactants are present with their physical value and value of∆rH present such reaction equation is known asa Thermochemical equation.
Enthalpy change, ∆rH of a Reaction – Reaction Enthalpy
In a chemical reaction, reactants are converted into products and is represented by,
Reactants $\rightarrow$ Products
The enthalpy change accompanying a reaction is called the reaction enthalpy. The enthalpy change of a chemical reaction, is given by the symbol $\Delta_r H$
$\Delta_r H=$ (sum of enthalpies of products) - (sum of enthalpies of reactants)
$
=\sum_i \mathrm{a}_i H_{\text {products }}-\sum_i b_i H_{\text {reactants }}
$
Here symbol $\sum$ (sigma) is used for summation and $\mathrm{a}_i$ and $\mathrm{b}_i$ are the stoichiometric coefficients of the products and reactants respectively in the balanced chemical equation. For example, for the reaction
$
\begin{aligned}
& \mathrm{CH}_4(\mathrm{~g})+2 \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(l) \\
& \begin{aligned}
\Delta_r H & =\sum a_i H_{\text {Products }}-\sum b_i H_{\text {reactants }} \\
=\left[H_{\mathrm{m}}^i\left(\mathrm{CO}_2, \mathrm{~g}\right)+2 H_{\mathrm{m}}^i\left(\mathrm{H}_2 \mathrm{O}, t\right)\right] & -\left[H_{\mathrm{m}}\left(\mathrm{CH}_4, \mathrm{~g}\right)\right. \\
& \left.+2 H_{\mathrm{m}}\left(\mathrm{O}_2, \mathrm{~g}\right)\right]
\end{aligned}
\end{aligned}
$
where $H_{\mathrm{m}}$ is the molar enthalpy.
Standard Enthalpy of Reactions
The standard enthalpy of reaction is the enthalpy change for a reaction when all the participating substances are in their standard states. The standard state of a substance at a specified temperature is its pure form at 1 bar. Standard conditions are denoted by adding the superscript $\ominus$ to the symbol $\Delta H$, e.g., $\Delta H^{\ominus}$.
Enthalpy Changes During Phase Transformations
Phase transformations also involve energy changes. Ice, for example, requires heat for melting. Normally, this melting takes place at constant pressure (atmospheric pressure), and during phase change, temperature remains constant (at 273 K ).
$
\mathrm{H}_2 \mathrm{O}(\mathrm{~s}) \rightarrow \mathrm{H}_2 \mathrm{O}(t) ; \Delta_{f u s} H^{\ominus}=6.00 \mathrm{~kJ} \mathrm{~mol}^{-1}
$
Here $\Delta_{\text {fus }} H^{\ominus}$ is the enthalpy of fusion in standard state. If water freezes, then the process is reversed and an equal amount of heat is given off to the surroundings.
The enthalpies of different processes are-
- The enthalpy change that accompanies the melting of one mole of a solid substance in standard state is called the standard enthalpy of fusion or molar enthalpy of fusion, $\Delta_{f u s} \boldsymbol{H}^{\ominus}$.
- The amount of heat required to vaporise one mole of a liquid at constant temperature and under standard pressure (1bar) is called its standard enthalpy of vaporisation or molar enthalpy of vaporisation, $\Delta_{vap} H^{\ominus}$.
- Standard enthalpy of sublimation, $\Delta_{\text {sub }} H^{\ominus}$ is the change in enthalpy when one mole of a solid substance sublimes at a constant temperature and under standard pressure (1bar).
Standard Enthalpy of Formation: The standard enthalpy change for the formation of one mole of a compound from its elements in their most stable states of aggregation (also known as reference
states) is called Standard Molar Enthalpy of Formation. Its symbol is $\Delta_f\boldsymbol{H}^{\ominus}$, where the subscript ‘ f ’ indicates that one mole of the compound in question has been formed in its standard state from its elements in their most
stable states of aggregation.
Some reactions with standard molar enthalpies of formation are as follows.
$\begin{aligned} & \mathrm{H}_2(\mathrm{~g})+1 / 2 \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{H}_2 \mathrm{O}(1) ; \\ & \Delta_f H^{\ominus}=-285.8 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ & \mathrm{C}(\text { graphite, } \mathrm{s})+2 \mathrm{H}_2(\mathrm{~g}) \rightarrow \mathrm{Ch}_4(\mathrm{~g}) ; \\ & \Delta_f H^{\ominus}=-74.81 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ & 2 \mathrm{C}(\text { graphite, } \mathrm{s})+3 \mathrm{H}_2(\mathrm{~g})+1 / 2 \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}(1) ; \\ & \qquad \Delta_f H^{\ominus}=-277.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\end{aligned}$
The following general equation can be used for the enthalpy change calculation.
$\Delta_r H^{\ominus}=\sum_i \mathrm{a}_i \Delta_f H^{\ominus}$ (products) $-\sum_i \mathrm{~b}_i \Delta_f H^{\ominus}$ (reactants)
where a and b represent the coefficients of the products and reactants in the balanced equation. You can also download Class 11 Chemistry Thermodynamics notes PDF for easy offline study and quick revision.
Thermochemical Equations:
It would be necessary to remember the following conventions regarding thermochemical equations.
1. The coefficients in a balanced thermochemical equation refer to the number of moles (never molecules) of reactants and products involved in the reaction.
2. The numerical value of $\ Delta_rH^ {\ominus}$ refers to the number of moles of substances specified by an equation. Standard enthalpy change $\Delta_r H^{\ominus}$ will have units as $\mathrm{kJ} \mathrm{mol}^{-1}$.
3. When a chemical equation is reversed, the value of $\ Delta_rH^ {\ominus}$ is reversed in sign.
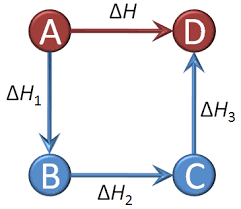
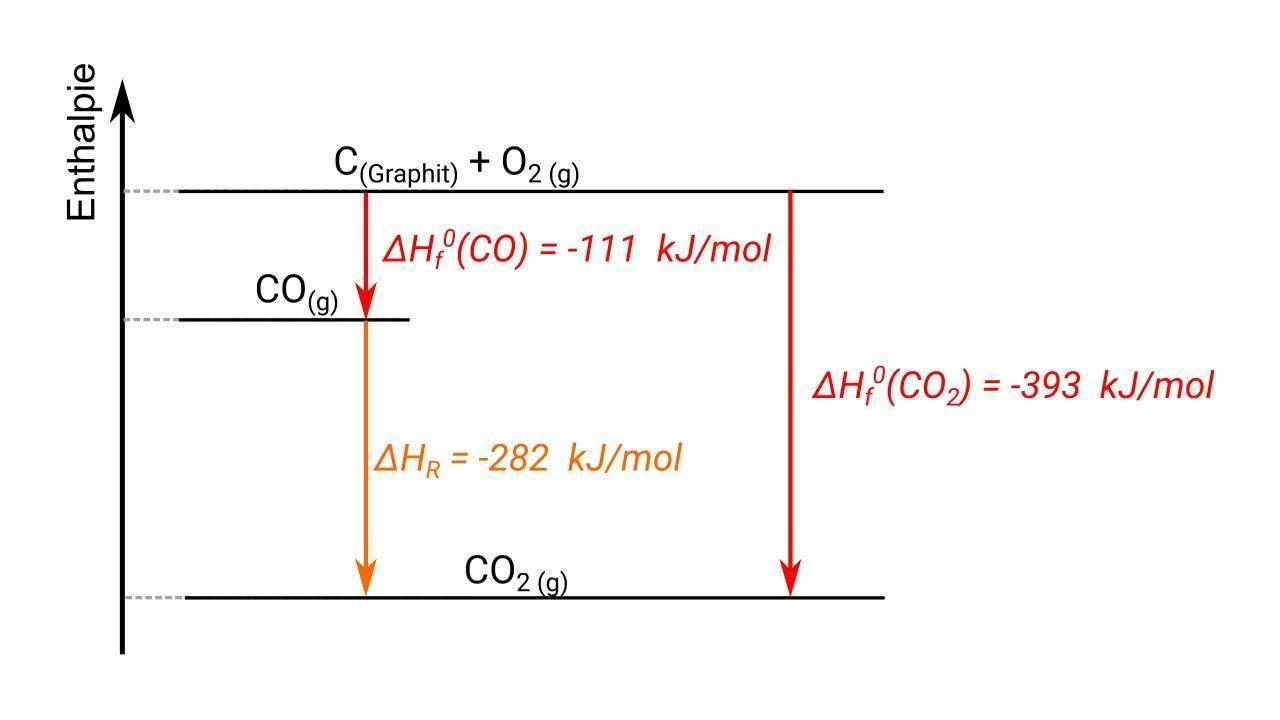
Hess’s Law
It is defined as Hess’s law for constant heat summation.
In general, to solve the questions we can write the equation as follows:
-
On combining the two reactions we get the final result as shown above.
-
The constant heat summation law suggests that whether the heat is absorbed or evolved in one or many steps, the total amount of heat is the same.
Enthalpies for Different Types of Reactions
- Standard Enthalpy of Combustion (symbol: $\Delta_c \mathbf{H}^{\ominus}$ ): Standard enthalpy of combustion is defined as the enthalpy change per mole (or per unit amount) of a substance, when it undergoes combustion and all the reactants and products being in their standard states at the specified temperature. The thermochemical reaction for the combustion of one mole of butane can be given as: $\begin{aligned} \mathrm{C}_4 \mathrm{H}_{10}(\mathrm{~g})+\frac{13}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow 4 \mathrm{CO}_2(\mathrm{~g})+5 \mathrm{H}_2 \mathrm{O}(1) \\ \Delta_C H^{\ominus}=-2658.0 \mathrm{~kJ} \mathrm{~mol}^{-1}\end{aligned}$
- Enthalpy of Atomization (symbol: $\Delta_a H^{\ominus}$ ): It is the enthalpy change on breaking one mole of bonds completely to obtain atoms in the gas phase.
- Bond Enthalpy (symbol: $\Delta_{\text {bond }} \mathbf{H}^{\ominus}$ ): Bond enthalpy refers to the energy involved in breaking or forming chemical bonds. It helps estimate the enthalpy change of a reaction based on the bonds broken and formed. It is the enthalpy change when one mole of a specific bond in a gaseous molecule is broken into atoms.
For example:
$\mathrm{H}_2(\mathrm{~g}) \rightarrow 2 \mathrm{H}(\mathrm{g}) ; \Delta \mathrm{H}^{\circ}=435 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{Cl}_2(\mathrm{~g}) \rightarrow 2 \mathrm{Cl}(\mathrm{g}) ; \Delta \mathrm{H}^{\circ}=242 \mathrm{~kJ} / \mathrm{mol}$In polyatomic molecules like $\mathrm{CH}_4$, individual bond energies vary with each successive bond breaking. So, an average (mean bond enthalpy) is used:
$
\mathrm{CH}_4(\mathrm{~g}) \rightarrow \mathrm{C}(\mathrm{~g})+4 \mathrm{H}(\mathrm{~g}) ; \quad \Delta \mathrm{aH}^{\circ}=1665 \mathrm{~kJ} / \mathrm{mol}
$Mean C-H bond enthalpy $=1 / 4 \times 1665=416 \mathrm{~kJ} / \mathrm{mol}$
Though it may slightly vary across compounds, this average is widely used in thermochemical calculations. Bond enthalpy helps estimate the standard enthalpy of a reaction in the gas phase:$
\Delta \mathbf{r} \mathbf{H}^{\circ}=\mathbf{\Sigma}(\text { bond enthalpies of bonds broken })-\mathbf{\Sigma}(\text { bond enthalpies of bonds formed })
$ -
Lattice Enthalpy: The lattice enthalpy of an ionic compound is the enthalpy change which occurs when one mole of an ionic compound dissociates into its ions in gaseous state. We use BOrn-Haber Cycle for this enthalpy.
Born-Haber Cycle
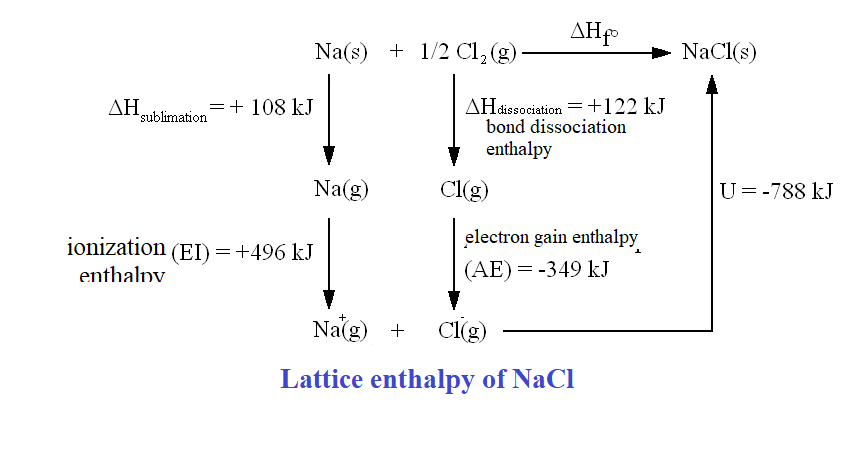
To calculate the lattice enthalpy of NaCl , we apply Hess's Law using the following steps:
Stepwise Enthalpy Changes:
1. Sublimation of $\mathrm{Na}(\mathrm{s})$
$\mathrm{Na}(\mathrm{s}) \rightarrow \mathrm{Na}(\mathrm{g})$ ; $\Delta_{\text {sub }} H^{\circ}$$=+108.4 \mathrm{~kJ} / \mathrm{mol}$
2. Ionization of $\mathrm{Na}(\mathrm{g})$
$\mathrm{Na}(\mathrm{g}) \rightarrow \mathrm{Na}^{+}(\mathrm{g})+\mathrm{e}^{-}$; $\Delta_{\mathrm{i}} H^{\circ}$$=+496 \mathrm{~kJ} / \mathrm{mol}$
3. Dissociation of $\mathrm{Cl}_2(\mathrm{~g})$
$1 / 2 \mathrm{Cl}_2(\mathrm{~g}) \rightarrow \mathrm{Cl}(\mathrm{g})$; $\Delta_{\text {bond }} H^{\circ}$$=+121 \mathrm{~kJ} / \mathrm{mol}$
4. Electron Gain by Cl(g)
$\mathrm{Cl}(\mathrm{g})+\mathrm{e}^{-} \rightarrow \mathrm{Cl}^{-}(\mathrm{g})$$\Delta_{\mathrm{eg}} H^{\circ}$$=-348.6 \mathrm{~kJ} / \mathrm{mol}$
5. Formation of $\mathrm{NaCl}(\mathrm{s})$
$\mathrm{Na}^{+}(\mathrm{g})+\mathrm{Cl}^{-}(\mathrm{g}) \rightarrow \mathrm{NaCl}(\mathrm{s})$; $\Delta_{\mathrm{f}} H^{\circ}$$-411.2 \mathrm{~kJ} / \mathrm{mol}$
Applying Hess's Law:
$
\begin{gathered}
\Delta_{\text {lattice }} H^{\circ}=\Delta_{\mathrm{f}} H^{\circ}+\Delta_{\text {sub }} H^{\circ}+\Delta_{\text {bond }} H^{\circ}+\Delta_{\mathrm{i}} H^{\circ}-\Delta_{\mathrm{eg}} H^{\circ} \\
\Delta_{\text {lattice }} H^{\circ}=-411.2+108.4+121+496-(-348.6) \\
\Delta_{\text {lattice }} H^{\circ}=+788 \mathrm{~kJ} / \mathrm{mol}
\end{gathered}
$
This value corresponds to the process:
$
\mathrm{NaCl}(\mathrm{~s}) \rightarrow \mathrm{Na}^{+}(\mathrm{g})+\mathrm{Cl}^{-}(\mathrm{g})
$
The internal energy is slightly less (+783 kJ/mol) due to Δng = 2 (2RT correction).
Using:
$
\Delta_{\text {sol }} H^{\circ}=\Delta_{\text {lattice }} H^{\circ}+\Delta_{\text {hyd }} H^{\circ}
$
Given:
Lattice enthalpy = +788 kJ/mol
Hydration enthalpy $=-\mathbf{7 8 4} \mathbf{~ k J} / \mathbf{m o l}$
$
\Delta_{\mathrm{sol}} H^{\circ}=+788-784=+4 \mathrm{~kJ} / \mathrm{mol}
$
Thus, NaCl dissolves with very little heat change.
5. Enthalpy of Solution (symbol: $\Delta_{\text {sol }} H^{\oplus}$ ): Enthalpy of solution of a substance is the enthalpy change when one mole of it dissolves in a specified amount of solvent.
The enthalpy of solution of $\mathrm{AB}(\mathrm{s}), \Delta_{\text {sol }} H^{\ominus}$, in water is, therefore, determined by the selective values of the lattice enthalpy, $\Delta_{\text {lattice }} H^{\ominus}$ and enthalpy of hydration of ions, $\Delta_{\text {hyd }} H^{\ominus}$ as
$
\Delta_{\text {sol }} H^{\ominus}=\Delta_{\text {lattice }} H^{\ominus}+\Delta_{\text {hyd }} H^{\ominus}
$
6. Enthalpy of Dilution: The enthalpy of dilution is the heat change that occurs when a solution is diluted, i.e., when more solvent (usually water) is added to a solution. It is usually expressed per mole of solute.
Spontaneity
Spontaneous process: It is the process that has been taken by itself or tends to occur on its own.
The process is not instantaneous in nature. Speed can vary from slow to fast.
Examples of such processes: Common salt dissolves in water on its own.
Entropy
Entropy is defined as the degree of disorders and randomness in a system.
The entropy of the gaseous system is found to be more than in the solid state.
Change in entropy∆S=qrevT=∆HT;
Change is entropy can be denoted as ∆S, and is zero for equilibrium condition.

Gibb’s Energy and Spontaneity
It is defined as mathematically $\Delta \mathrm{G}=\Delta \mathrm{H}-\mathrm{T} \Delta \mathrm{S}$
The above reaction is at constant temperature and pressure.
-
If the value of $\Delta \mathrm{G}$ is negative or less than zero, the process would be spontaneous.
-
If the value of $\Delta \mathrm{G}$ is positive or more than zero, the process would be non-spontaneous.
Condition of equilibrium, when all reactants and products are in standard condition, value of $\Delta \mathrm{G}$ will be:
$0=\Delta_{\mathrm{r}} \mathrm{G}-\mathrm{RT}$ InK
$\Delta \mathrm{rG}=\Delta_{\mathrm{r}} \mathrm{H}-\mathrm{T} \Delta_{\mathrm{r}} \mathrm{S}$
=-RTInK
Entropy and Second Law of Thermodynamics
We know that for an isolated system, the change in energy remains constant. Therefore, an increase in entropy in such systems is the natural direction of a spontaneous change. This, in fact is the second law of thermodynamics. Like first law of thermodynamics, second law can also be stated in several ways. The second law of thermodynamics explains why spontaneous exothermic reactions are so common. In exothermic reactions heat released by the reaction increases the disorder of the surroundings and overall entropy change is positive which makes the reaction spontaneous. Understand these concepts better with the help of class 11 chemistry chapter 5 thermodynamics notes.
Absolute Entropy and the Third Law of Thermodynamics
The entropy of any pure crystalline substance approaches zero as the temperature approaches absolute zero. This is called third law of thermodynamics. This is so because there is perfect order in a crystal at absolute zero. The statement is confined to pure crystalline solids because theoretical arguments and practical evidence have shown that the entropy of solutions and supercooled liquids is not zero at 0 K.
Gibbs Energy Change and Equilibrium
Gibbs energy for a reaction in which all reactants and products are in standard state, $\Delta_r G^{\ominus}$ is related to the equilibrium constant of the reaction as follows:
$
\begin{aligned}
0 & =\Delta_r G^{\ominus}+\mathrm{R} T \ln K \\
\text { or } \Delta_r G^{\ominus} & =-\mathrm{R} T \ln K \\
\text { or } \Delta_r G^{\ominus} & =-2.303 \mathrm{R} T \log K
\end{aligned}
$
We also know that
$
\Delta_r G^{\ominus}=\Delta_r H^{\ominus}-T \Delta_r S^{\ominus}=-\mathrm{R} T \ln K
$
Using the above equation,
- It is possible to obtain an estimate of $\Delta$$G^ {\ominus}$ from the measurement of $\Delta$$H^ {\ominus}$ and $\Delta$$S^ {\ominus}$, and then calculate $K$ at any temperature for economic yields of the products.
- If K is measured directly in the laboratory, the value of $\Delta$$G^ {\ominus}$ at any other temperature can be calculated.
Effect of Temperature on Spontaneity of Reactions is given in the table below-
| ΔH° | ΔS° | ΔG° | Description |
|---|---|---|---|
| – | + | – | Reaction spontaneous at all temperatures |
| – | – | – (at low T) | Reaction spontaneous at low temperature |
| – | – | + (at high T) | Reaction nonspontaneous at high temperature |
| + | + | + (at low T) | Reaction nonspontaneous at low temperature |
| + | + | – (at high T) | Reaction spontaneous at high temperature |
| + | – | + (at all T) | Reaction nonspontaneous at all temperatures |
Thermodynamics Previous Years Questions and Answers
Practise these previous years questions to enhance your understanding of the topic and boost your exam preparation. The concepts used to solve these questions are explained in thermodynamics class 11 chemistry chapter 5 CBSE notes.
Question 1: The hydration energies of $\mathrm{K}^{+}$and $\mathrm{Cl}^{-}$are -x and -y $\mathrm{kJ} / \mathrm{mol}$ respectively. If lattice energy of KCl is -z $\mathrm{kJ} / \mathrm{mol}$, then the heat of solution of KCl is :
(1) $+x-y-z$
(2) $x+y+z$
(3) $z-(x+y)$
(4) $-z-(x+y)$
Answer:
$\begin{aligned} & \Delta \mathrm{H}_{\text {solution }}=\left(-\Delta \mathrm{H}_{\text {L.E. }}\right)+\Delta \mathrm{H}_{\text {hydration }} \\ & =-(-z)+(-\mathrm{x}-\mathrm{y}) \\ & =\mathrm{z}-(\mathrm{x}+\mathrm{y})\end{aligned}$
Hence, the correct answer is option (3).
Question 2: Total enthalpy change for freezing of 1 mol of water at $10^{\circ} \mathrm{C}$ to ice at $-10^{\circ} \mathrm{C}$ is $\qquad$
(Given : $\Delta_{\mathrm{fus}} \mathrm{H}=\mathrm{x} \mathrm{kJ} / \mathrm{mol}$
$\begin{aligned}
& \mathrm{C}_{\mathrm{p}}\left[\mathrm{H}_2 \mathrm{O}(\mathrm{l})\right]=\mathrm{y} \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1} \\
& \mathrm{C}_{\mathrm{p}}\left[\mathrm{H}_2 \mathrm{O}(\mathrm{~s})\right]=\mathrm{z} \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}
\end{aligned}$
(1) $-x-10 y-10 z$
(2) $-10(100 x+y+z)$
(3) $10(100 x+y+z)$
(4) $x-10 y-10 z$
Answer:

$\Delta H=1 \times y(0-10)-x \times 1000+1 \times z\left(-10^{\circ}-0^{\circ}\right)$
$\Delta H=-(10 y+10 z-1000 x) \mathrm{J}$
$\Delta H=-10(100 x+y+z)$ Joule.
Hence, the correct answer is option (2).
Question 3: The enthalpies of elements in their standard states are taken as zero. The enthalpy of the formation of a compound
(1) is always negative
(2) is always positive
(3) maybe positive or negativ
(4) is never negative
Answer:
It can be positive or negative, as the reaction could be exothermic or endothermic.
Hence, the answer is option (3).
Question 4: The correct statement amongst the following is :
(1) The term 'standard state' implies that the temperature is $0^{\circ} \mathrm{C}$
(2) The standard state of pure gas is the pure gas at a pressure of 1 bar and temperature 273 K
(3) $\boldsymbol{\Delta}_{\mathrm{f}} \mathrm{H}_{298}^{\boldsymbol{\theta}}$ is zero for $\mathrm{O}(\mathrm{g})$
(4) $\boldsymbol{\Delta}_{\mathrm{f}} \mathrm{H}_{298}^{\boldsymbol{\theta}}$ is zero for $\mathrm{O}_2(\mathrm{~g})$
Answer:
Option (1)
It asserts that the term "standard state" implies that the temperature is $0^{\circ} \mathrm{C}(273 \mathrm{~K})$.
In fact, "standard state" typically specifies a standard pressure (1 bar) and a chosen temperature-commonly 298 K for tabulated thermodynamic data-not necessarily $0^{\circ} \mathrm{C}$.
Thus, Option 1 is incorrect.
Option (2)
It claims that the standard state for a pure gas is defined at 1 bar and 273 K .
In modern thermochemistry, the standard state generally means the pure substance at 1 bar, but the standard temperature most commonly used is $298 \mathrm{~K}\left(25^{\circ} \mathrm{C}\right)$, not 273 K $\left(0^{\circ} \mathrm{C}\right)$.
Thus, Option 2 is incorrect.
Option (3)
It claims that $\Delta_f H_{298}^{\circ}$ is zero for $O(g)$ (atomic oxygen).
However, the standard state of oxygen is diatomic $O_2(g)$, not atomic oxygen. Since $O(g)$ is not the most stable form of oxygen under standard conditions, its formation enthalpy is not zero.
Hence, Option 3 is incorrect.
Option (4)
$\Delta_{\mathrm{f}} \mathrm{H}_{298}^0$ for $\mathrm{O}_2$ is zero and not for O.
The standard enthalpy of formation of any element in its most stable form is zero, regardless of temperature (though typically reported at 298 K ).
Thus, option (4) is correct
Hence, the correct answer is option (4).
Question 5: One mole of an ideal gas expands isothermally and reversibly from $10 \mathrm{dm}^3$ to $20 \mathrm{dm}^3$ at $300 \mathrm{~K} . \Delta \mathrm{U}$, q and work done in the process respectively are :
Given : $\mathrm{R}=8.3 \mathrm{JK}^{-1}$ and $\mathrm{mol}^{-1}$
In $10=2.3$
$\log 2=0.30$
$\log 3=0.48$
(1) $0,21.84 \mathrm{~kJ},-1.26 \mathrm{~kJ}$
(2) $0,-17.18 \mathrm{~kJ}, 1.718 \mathrm{~J}$
(3) $0,21.84 \mathrm{~kJ}, 21,84 \mathrm{~kJ}$
(4) $0,178 \mathrm{~kJ},-1.718 \mathrm{~kJ}$
Answer:
$\begin{aligned} & (10 \mathrm{~L}, 300 \mathrm{~K}) \xrightarrow[\mathrm{n}=1]{\longrightarrow}(20 \mathrm{~L}, 300 \mathrm{~K}) \\ & -\mathrm{q}=\mathrm{w}=-\mathrm{nRT} \ln \frac{\mathrm{V}_2}{\mathrm{~V}_1} \\ & =-8.3 \times 300 \times \ln \left(\frac{20}{10}\right)\end{aligned}$
$\begin{aligned} & =-1.718 \mathrm{~kJ} \\ & \Rightarrow \mathrm{q}=1.718 \mathrm{~kJ} \\ & \mathrm{w}=-1.718 \mathrm{~kJ} \\ & \Delta \mathrm{U}=0(\because \Delta \mathrm{~T}=0)\end{aligned}$
Hence, the correct answer is option (4).
How to Master Class 11 Chemistry Chapter 5 Thermodynamics
The ncert class 11 chemistry chapter 5 thermodynamics notes explains energy changes in chemical processes. These notes help to understand the basic concepts from your NCERT textbook. Given below some points on how to master this chapter.
- Students first need to understand the basics like system, surroundings, types of systems, and state functions.
- Then learn the first law of thermodynamics and focus on internal energy, work, and heat.
- Students must clear their basics of isothermal, adiabatic, isobaric, and isochoric processes.
- Questions from Hess’s Law, enthalpy of reactions, Spontaneity, Gibbs free energy (ΔG), entropy (ΔS), and second law of thermodynamics are often asked in exams. To understand these concepts better refer to ncert class 11 chemistry chapter 5 thermodynamics notes.
- At last students can solve previous year questions from this chapter.
Advantages of Using Class 11 Chemistry Chapter 6 Thermodynamics Notes
NCERT Class 11 Chemistry Chapter 5 Notes Thermodynamics helps students understand the basic concepts from the NCERT book in a simple and organised manner. The advantages of using these notes are given below:
- These notes are designed to help students understand the basic concepts like internal energy, enthalpy, and Gibbs free energy, laws of thermodynamics, heat capacity, enthalpy changes, and spontaneity of reactions.
- Using these notes helps students to revise formulas, definitions, and laws.
- The ncert class 11 chemistry chapter 5 thermodynamics notes are prepared by subject experts in a very comprehensive and clear manner.
- They provide step by step explanations of every topic.
NCERT Class 11 Notes Chapter-Wise
Besides NCERT Class 11 Chemistry Chapter 5 Notes Thermodynamics also refer to links given below for each chapter of class 11:
NCERT Solutions for Class 11 Chemistry
Along with thermodynamics class 11 chemistry chapter 5 CBSE notes, follow the links below to get chapter-wise solutions of NCERT and make your learning better.
Subject Wise NCERT Exemplar Solutions
NCERT exemplar solutions for each chapter of class 11 are given below
Subject Wise NCERT Solutions
NCERT subject wise solutions of class 11 are given below
| NCERT Solutions for Class 11 Mathematics |
| NCERT Solutions for Class 11 Chemistry |
| NCERT Solutions for Class 11 Physics |
Frequently Asked Questions (FAQs)
Surroundings refer to everything outside the thermodynamic system. They interact with the system by exchanging energy or matter, depending on the type of system.
An open system is one that can exchange both matter and energy with its surroundings. For example, a boiling pot of water is an open system because it exchanges heat (energy) and water vapour (matter) with the surroundings.
The laws of thermodynamics describe the principles governing energy transfer. The First Law states that energy cannot be created or destroyed, only transformed. The Second Law introduces the idea of entropy, indicating that systems tend to evolve toward a state of greater disorder. The Third Law states that as temperature approaches absolute zero, the entropy of a perfect crystal approaches zero.
Exothermic reactions are chemical processes that release heat into the surroundings, leading to an increase in the temperature of the surrounding environment. In contrast, endothermic reactions absorb heat from the surroundings, resulting in a decrease in the temperature of the environment.
Heat capacity is the amount of heat required to change the temperature of a substance by one degree Celsius. In thermodynamics, it helps in calculating the heat changes associated with temperature variations in a system.
Entropy measures the degree of disorder or randomness in a system. In thermodynamics, higher entropy indicates a greater level of disorder. The Second Law of Thermodynamics states that in any energy exchange, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state.
The Class 11 thermodynamics notes are concise study materials that summarize key concepts, including the laws of thermodynamics, internal energy, enthalpy, entropy, Gibbs free energy, and spontaneity. These notes help students quickly revise definitions, formulas, and solved examples for better understanding and exam preparation.
Read the chapter step by step, note key laws and formulas, and solve NCERT questions regularly. Use flowcharts, examples, and past papers for quick revision and better clarity.
Extensive property refers to a property that depends on the amount of substance present in the system. Examples: Mass, Volume, Internal Energy, Enthalpy, Entropy.
Intensive property refers to a property that does not depend on the amount of substance present. Examples: Temperature, Pressure, Density, Molar Heat Capacity.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
