NCERT Class 11 Chemistry Chapter 4 notes are available on Careers 360 and other educational websites, providing free, concise PDFs that cover key topics such as bonding, VSEPR, hybridization, and molecular orbitals, ideal for quick revision and exam preparation.
NCERT Class 11 Chemistry Chapter 4 Notes Chemical Bonding and Molecular Structure - Download PDF
Have you ever wondered why the shape of a molecule influences its behaviour in chemical reactions? Why do atoms combine to form molecules? Why do certain elements react violently while others remain stable? What makes water such a stable compound while sodium and chlorine alone are highly reactive? The answer lies in chemical bonding and molecular structure ncert notes. This chapter explains how atoms combine to form compounds and how the arrangement of atoms affects a substance's properties. It also explains that the way atoms bond together, whether through ionic, covalent, or metallic bonds, determines the strength, flexibility, and reactivity of the resulting compound. Apart from this Chemical Bonding explains about properties of chemical bonds, types of bonds, their nature, formation, and the molecular structure of different compounds.
This Story also Contains
- NCERT Notes for Class 11 Chemistry Chapter 4 Download PDF
- NCERT Notes for Class 11 Chapter 4
- Chemical Bonding and Molecular Structure: Previous Years' Questions and Answers
- How to Master Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- Advantages of Using Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Notes
- NCERT Notes Class 11 Chapter-Wise
- NCERT Solutions for Class 11 Chemistry
- NCERT Exemplar Solutions Subject-Wise
- NCERT Solutions Subject-Wise
The NCERT Notes Class 11 Chemistry will be helpful for a quick revision of topics. These notes are designed by our experienced subject matter experts, which ensures the credibility of the content provided. It becomes difficult and time-consuming for students to read the NCERT textbooks point-to-point. So, to solve this problem, we are providing these NCERT notes that cover all the topics and concepts provided in the NCERT textbook in a very clear and comprehensive way. These chemical bonding and molecular structure class 11 notes are also valuable resources for various competitive exams like JEE, NEET, etc. Also, check the NCERT Solutions for all the chapters.
NCERT Notes for Class 11 Chemistry Chapter 4 Download PDF
These concise notes cover all the key concept of chapter 4 to help students in quick revision before exam. You can download ncert class 11 chemistry chapter 4 chemical bonding and molecular structure notes pdf from the button given below:
Also Read
NCERT Notes for Class 11 Chapter 4
This chapter explains several basic concepts of Chemistry that help students develop a strong base in the subject, which will help them not just in Board exams but also in competitive exams like JEE and NEET. Some of the main topics covered in chemical bonding and molecular structure class 11 chemistry chapter 4 CBSE notes are KÖssel-Lewis’s approach to chemical bonding, the octet rule, and Limitations Of The Octet Rule, drawing Lewis Electron Dot Structures, VSEPR Theory, Valence Bond Theory, Molecular Orbital Theory of homonuclear diatomic molecules, and concepts of Hydrogen Bonding.
Chemical Bonding and Molecular Structure: Introduction
Chemical Bond: A Chemical bond is formed when various constituents are held together in chemical species by the force of attraction.
Type of intermolecular forces
1. Ion - dipole
2. Ion-induced dipole or debye forces
3. Dispersion forces or London forces
4. Dipole-dipole force
5. Hydrogen bonding
KÖssel-Lew Approach to Chemical Bonding
-
In the periodic table, noble gases separate the highly electronegative halogens from the highly electropositive alkali metals.
-
The formation of a negative ion from a halogen atom is with the gain of an electron and a positive ion from an alkali metal atom is with the loss of an electron.
-
The negative and positive ions formed attain stable noble gas electronic configurations. The noble gases (except helium,$n s^2$) have a stable outer shell configuration of eight electrons,$n s^2 n p^6$.
-
Electrostatic attraction stabilizes the negative and positive ions.
Electrovalent bond: The bond is formed, as a result of the electrostatic attraction between the positive and negative ions.
Ionic bond: The bond is formed when an atom loses an electron and another atom gains an electron.
Electronic theory of chemical bonding: It states that atoms can combine either by sharing valence electrons or by transfer of valence electrons from one atom to another, so as to have an octet in their valence shells. This is known as the octet rule.
Covalent bond: It is a bond that is formed when two atoms share a pair of electrons between them. Molecules can have a single covalent bond, a double bond, or even a triple bond.
Lewis Representation of Simple Molecules (the Lewis Structures)
In terms of shared pairs of electrons and the octet rule, the Lewis dot structures depict bonding in molecules and ions. Electrons are represented by the dots. Such structures are called Lewis dot structures.
The number of electrons present in the outermost shell are known as valence electrons. For example, the electronic configuration of sodium (Na) is 2, 8, 1, thus, sodium has one valence electron. According to the long form of the periodic table, in the case of representative elements, the group number is equal to the number of valence electrons. The valence electrons in atoms are shown in terms of Lewis symbols. To write Lewis symbol for an element, we write down its symbol surrounded by a number of dots or crosses equal to the number of valence electrons. Paired and unpaired valence electrons are also indicated. Understand these concepts better with the help of chemical bonding and molecular structure class 11 notes which provide detailed explanations.
For example, lewis dot structure of the water molecule:

Formal Charge
Lewis dot structures do not represent the real shapes of molecules. In polyatomic ions, the net charge is held by the ion as a whole, rather than by a single atom. However, possible to give each atom a formal charge. The formal charge of an atom is the difference between the number of valence electrons of an atom in an isolated or free state and the number of electrons assigned to that atom in the Lewis structure.
Thus, we calculate the formal charge as follows:
formal charge = valence shell electrons − lone pair electrons − 1/2 bonding electrons
Limitations of the Octet Rule
1. Sometimes the number of electrons around the central atom is less than eight, hence resulting in an incomplete octet of the central atom.
2. In certain molecules, such as NO there is an odd number of electrons which results in an incomplete octet of the atoms.
3. Sometimes the number of electrons around the central atom is more than eight, hence resulting in an expanded octet of the central atom.
Lattice Enthalpy: The Lattice Enthalpy is an ionic solid is defined as the formation of one mole or change of ionic compound in its gaseous ions which keeps all the other things standard. We can say that to check the strength of an ionic compound, lattice enthalpy is used.
Bond Parameters
Bond Length: It is the distance between the nuclei of two bonded atoms of a molecule. Or we can say that it is the sum of two bonded atoms covalent radii.
Bond Angle: It is the angle made in between two covalent bonds which are having the same origin from a similar atom.
Bond Enthalpy: It is the amount of energy needed to separate all the covalent bonds of a specific type. This exists in between two atoms in a gaseous state.
Bond Order: It is the number of bonds a molecule has between two atoms.
Resonance Structures
Resonance is a concept wherein, whenever a single Lewis structure cannot describe the structure of a molecule, then various possible canonical structures are used to accurately describe the molecule, which are called Resonance Structures.
Polarity of Bonds
In heteronuclear bonds, the shared electron pair shifts towards the more electronegative atom, resulting in polarity between the atoms, and the molecule is said to be a polar molecule.
Due to polarity, the molecule develops a Dipole Moment, which is defined as the product of charge and distance of separation between the two bonded atoms.
Dipole moment(µ):
IT is vector sum of all the individual bond moments
Mathematically $\mu=q \times d$
$q=$ magnitude of charge
$d=$ distance between the centres of opposite charge
It is denoted by a small arrow with a tail on the positive centre and head pointing towards negative center
Fazan’s Rule and Covalent Character in Ionic Bond :
The covalent character in ionic bonds is determined by Fajan’s Rule. It simply says that no ionic bond is completely ionic, there is always some covalent character in ionic bond. When a cation approaches an anion, then the electron cloud of the anion is distorted and shifted towards the cation, this distortion is known as the polarisation of the anion.
The ability of the cation to distort the anion is known as polarising power and the ability of the anion to get distorted is known as polarisability.
The covalent character in ionic bond depends on the following factors:
- Size of the cation: Smaller the size of the cation, larger will be its polarisability.
- Size of the anion: Larger the size of the anion, larger will be its polarisability.
- Charge on cation and anion: More the charge on cation more will be its polarising power. Further, more the charge on anion, more will be its polarisability.
Thus covalent character for chlorides follows this order:
$\mathrm{NaCl}<\mathrm{MgCl}_2<\mathrm{AlCl}_3$
In this case, the charge on the cation increases, thus its polarising power also increases.
Further, for cation size, the covalent character follows the below order:
$\mathrm{LiCl}>\mathrm{NaCl}>\mathrm{KCl}>\mathrm{CsCl}$
In this case, as the size of the cation increases, its polarising power decreases.
Valence Shell Electron Pair Repulsion Theory (VSEPR Theory)
The VSEPR Theory predicts the shape of the covalent molecules.
Below are the postulates of this theory:
-
The number of valence shell electron pairs decides the shape of a molecule around the central atom.
-
Pairs of electrons in the valence shell repel one another because of the negatively charged electron clouds.
-
The pairs of electrons occupy such positions in space where there is minimum repulsion so it maximises the distance between them.
-
The valence shell is assumed as a sphere with the electron pairs present on the spherical surface at a maximum distance from one another.
-
Multiple bonds are considered as a single electron pair and the two/three electron pairs of multiple bonds are taken as a single super pair.
-
When two or more resonance structures represent a molecule, the VSEPR theory is applied to any such structure.
The repulsive interaction of electron pairs is given as:
Lone pair – Lone pair > Lone pair – Bond pair > Bond pair – Bond pair.
As given in the table below, two regions of electron density around a central atom in a molecule form a linear geometry, three regions form a trigonal planar geometry, four regions form a tetrahedral geometry, five regions form a trigonal bipyramidal geometry, and six regions form an octahedral geometry.

The ideal shapes of molecules, which are predicted on the basis of electron pairs and lone pairs of electrons are mentioned in the table below:
Valence Bond Theory
When two atoms approach each other, at a large distance there is no force; however, as the molecule starts approaching closer, they experience a force of attraction, and after a certain distance, when the two atoms come close enough, they experience a repulsive force.
So, when two atoms approach each other and form a bond, energy is released, and this energy is called bond enthalpy.
Orbital Overlap Concept
Two hydrogen atoms come close enough that their atomic orbitals partially merge during the formation of a hydrogen molecule. This process is known as orbital overlapping. This allows the electrons from each atom to pair up with opposite spins, resulting in the formation of a covalent bond. The strength of this bond depends on the extent of overlap — the greater the overlap, the stronger the bond.
Directional Properties of Bonds
Covalent bonds are formed through the overlap of atomic orbitals, as we have seen in the hydrogen molecule, where two hydrogen atoms bond by overlapping their 1s orbitals. In polyatomic molecules like $\mathrm{CH}_4, \mathrm{NH}_3$, and $\mathrm{H}_2 \mathrm{O}$, understanding the molecular shape becomes equally important. For example, why does methane ($\mathrm{CH}_4$) adopt a tetrahedral structure, and why does ammonia (NH3) have a pyramidal shape? These questions are explained by Valence Bond Theory. VBT describes how atomic orbitals not only overlap but also undergo hybridisation.
Overlapping of Atomic Orbitals
When two atomic orbitals come close to form a bond, the nature of their overlap depends on the phase (sign) and spatial orientation of their wave functions, whether it is positive, negative, or zero. To form an effective bond, the orbitals must have the same phase and be properly aligned in space. You can also download NCERT Class 11 Chemistry Chapter 4 Notes Chemical Bonding and Molecular Structure PDF for easy offline study and quick revision.
Types of Overlapping and Nature of Covalent Bonds
Sigma(σ) bond: Also called head-on overlap or axial overlap, this type of covalent bond is formed when the bonding orbitals overlap end to end along the internuclear axis.
• s-s overlapping: The type of overlapping can be seen in half-filled s-orbitals of one atom and half-filled s-orbitals of another atom.
• s-p overlapping: The type of overlapping can be seen in half-filled s-orbitals of one atom and half-filled p-orbitals of another atom.
• p–p overlapping: The type of overlapping can be seen in p-orbitals of the two approaching atoms.
Pi Bonds: The type of overlapping can be seen in a covalent bond that is formed when the bonding orbitals overlap sideways, such that their axes are parallel to each other but perpendicular to the internuclear axis.
Strength of Sigma and Pi Bonds
The strength of a bond depends on the extent of overlapping. The sigma bond is stronger as compared to the pi bond because the overlapping of orbitals takes place to a larger extent in the sigma bond. It is important to note that in the formation of multiple bonds between two atoms of a molecule, pi bonds (s) are formed in addition to a sigma bond.
Hybridization
The features of hybridisation are:
1. The number of hybrid orbitals is equal to the number of atomic orbitals that hybridize.
2. The hybridized orbitals are always the same in energy and shape.
3. The hybrid orbitals form more stable bonds than pure atomic orbitals.
4. The hybrid orbitals are directed in a minimum repulsion space, giving a stable arrangement.
Types of Hybridisation
sp hybridization: This hybridization involves the mixing of one s and one p orbital to form equivalent sp hybrid orbitals.
$s p^2$ hybridization: This hybridization involves the mixing of one and two p-orbitals to form three equivalent $s p^2$ hybridized orbitals.
$s p^3$ hybridization: This hybridization involves the mixing of one s-orbital and three p-orbitals of the valence shell to form four $s p^3$ hybrid orbitals.
$d s p^2$ hybridization: This hybridization involves the mixing of one d-orbital, one s-orbital, and two p-orbitals of the valence shell to form four $d s p^2$hybrid orbitals.
$d^2 s p^3$ hybridization: This hybridization involves the mixing of two d-orbitals, one s-orbital, and three p-orbitals of the valence shell to form six $d^2 s p^3$ hybrid orbitals.
$s p^3 d$ hybridisation: This hybridization involves the mixing of one s-orbital, three p-orbitals, and one d-orbital to form five $s p^3 d$hybrid orbitals.
$s p^3 d^2$ hybridisation: This hybridization involves the mixing of one s-orbital, three p-orbitals, and two d-orbitals to form six $s p^3 d^2$hybrid orbitals.
Other Examples of $s p^3, s p^2$ and sp Hybridisation
$s p^3$ hybridisation in $\mathrm{C}_2 \mathrm{H}_6$ molecule: To form a $\mathrm{sp}^3$-$\mathrm{sp}^3$ sigma bond, both the carbon atoms assume a $\mathrm{sp}^3$ hybrid state. One of the four $\mathrm{sp}^3$ hybrid orbitals of a carbon atom overlaps axially with similar orbitals of other atoms in the ethane molecule, while the other three hybrid orbitals of each carbon atom are used in forming $\mathrm{sp}^3$ sigma bonds with hydrogen atoms.
$\mathrm{sp}^2$ Hybridisation in $\mathrm{C}_2 \mathrm{H}_4$: To form the C-C sigma bond, one of the $\mathrm{sp}^2$ hybrid orbitals of the carbon atom overlaps axially with the $\mathrm{sp}^2$ hybridised orbital of another carbon atom in the formation of the ethene molecule. While the other two $\mathrm{sp}^2$ hybrid orbitals of each carbon atom are used for making $\mathrm{sp}^2$ sigma bond with two hydrogen atoms.
sp Hybridisation in $\mathrm{C}_2 \mathrm{H}_2$ : In the formation of the ethyne molecule, both the carbon atoms undergo sp-hybridisation, having two unhybridised orbitals, i.e., 2py and 2px.
Hybridisation of Elements Involving d Orbitals
The elements present in the third period contain d orbitals. The energy of the 3d orbitals is comparable to the energy of the 3s and 3p orbitals. The energy of 3d orbitals is also comparable to that of 4s and 4p orbitals. As a consequence, the hybridisation involving either 3s, 3p and 3d or 3d, 4s and 4p is possible. However, since the difference in energies of 3p and 4s orbitals is significant, no hybridisation involving 3p, 3d and 4s orbitals is possible.
Molecular Orbital Theory
-
As the electrons of atoms are present in the various atomic orbitals, in the same way, the electrons in a molecule are present in the various molecular orbitals.
-
The molecular orbitals are formed by the combination of atomic orbitals that have comparable energies and proper symmetry.
-
A nucleus influences an electron in an atomic orbital, however, in a molecular orbital, it is influenced by two or more nuclei. Therefore, an atomic orbital is monocentric and a molecular orbital is polycentric.
-
The number of molecular orbitals is equal to the number of combining atomic orbitals. Two atomic orbitals combine to form two molecular orbitals, where one is a bonding molecular orbital and the other is an antibonding molecular orbital.
-
The bonding molecular orbital has lower energy, and the antibonding molecular orbital has more energy; hence, it is less stable.
-
In an atom, an atomic orbital gives the electron probability distribution around a nucleus, and in a molecule molecular orbital gives the electron probability distribution around a group of nuclei.
-
The molecular orbitals are also filled in accordance with the following rules and principles: the Aufbau principle, Pauli’s exclusion principle, and Hund’s rule.
Molecular orbitals are formed by a Linear Combination of Atomic Orbitals (LCAO) where atomic orbitals have the same energy or nearly the same, the same symmetry and the overlap is maximum.
Formation of Molecular Orbitals: Linear Combination of Atomic Orbitals (LCAO)
The atomic orbitals can be expressed by wave functions (ψ’s) according to wave mechanics, which represent the amplitude of the electron waves. These are obtained from the solution of the Schrödinger wave equation. Since it cannot be solved for any system containing more than one electron, molecular orbitals, which are one-electron wave functions for molecules, are difficult to obtain directly from the solution of the Schrödinger wave equation. To overcome this problem, an approximate method known as linear combination of atomic orbitals (LCAO) has been adopted.
Wave functions of individual atomic orbitals as shown below:
$\Psi _{MO}=\Psi _{A}\pm \Psi _{B}$
The two molecular orbitals σ and σ* are formed as :
$\sigma =\Psi _{A}+\Psi _{B}$
$\sigma^{*} =\Psi _{A}-\Psi _{B}$
The molecular orbital σ formed by the addition of atomic orbitals is called the bonding molecular orbital, while the molecular orbital σ* formed by the subtraction of atomic orbitals is called the antibonding molecular orbital.
Conditions for the Combination of Atomic Orbitals
1. The combining atomic orbitals must have the same or nearly the same energy.
2. The combining atomic orbitals must have the same symmetry about the molecular axis.
3. The combining atomic orbitals must overlap to the maximum extent.
Types of Molecular Orbitals
Molecular orbitals of diatomic molecules are designated as σ (sigma), π (pi), δ (delta), etc. In this nomenclature, the sigma (σ ) molecular orbitals are symmetrical around the bond axis, while the pi ( π) molecular orbitals are not symmetrical.
Energy Level Diagram for Molecular Orbitals
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram. As given in the figure below, for a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the centre. Dashed lines show which of the atomic orbitals combine to form the molecular orbitals. For each pair of atomic orbitals that combine, one lower-energy (bonding) molecular orbital and one higher-energy (antibonding) orbital result. Thus, we can see that combining the six 2p atomic orbitals results in three bonding orbitals (one σ and two π) and three antibonding orbitals (one σ* and two π*).
22
molecular orbital diagram
The molecular orbitals are filled in the same manner as atomic orbitals, using the Aufbau principle and Hund’s rule.
Electronic Configuration and Molecular Behaviour
The distribution of electrons among various molecular orbitals is called the electronic configuration of the molecule.
Stability of Molecules: If Nb is the number of electrons occupying bonding orbitals and Na the number occupying the antibonding orbitals then,
- The molecule is stable if Nb is greater than Na
- The molecule is unstable if Nb is less than Na.
Nature of the bond:
- Integral bond order values of 1, 2 or 3 correspond to single.
- Double or triple bonds respectively as studied in the classical concept.
Bond-length: It may be taken as an approximate measure of the bond length.
- The bond length decreases as bond order increases.
Magnetic nature:
- If all the molecular orbitals in a molecule are doubly occupied, the substance is diamagnetic.
- If one or more molecular orbitals are singly occupied it is paramagnetic e.g., O2 molecule.
Bonding in Some Homonuclear Diatomic Molecules
1. Hydrogen molecule ($\mathrm{H}_2$): It is formed by the combination of two hydrogen atoms. Each hydrogen atom has one electron in the 1s orbital. The electronic configuration of the hydrogen molecule is
$H_2:(\sigma 1 s)^2$
2. Helium molecule ($\mathrm{He}_2$ ): The electronic configuration of helium atom is 1s2. Each helium atom contains 2 electrons. These electrons will be accommodated in σ1s and σ*1s molecular orbitals; therefore electronic configuration of helium molecules is
$\mathrm{He}_2:(\sigma 1 \mathrm{~s})^2\left(\sigma^* 1 \mathrm{~s}\right)^2$
3. Lithium molecule $\left(\mathrm{Li}_2\right.$ ): The electronic configuration of lithium is 1s2, 2s1. There are six electrons in Li2. Therefore, electronic configuration of Li2 molecule is
$\mathrm{Li}_2:(\sigma 1 \mathrm{~s})^2\left(\sigma^* 1 \mathrm{~s}\right)^2(\sigma 2 \mathrm{~s})^2$
Hydrogen Bonding
Hydrogen bonds are strong forces which occur when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom, such as O, N, F, etc. A greater electronegativity of the atom will result in an increase in hydrogen-bond strength. The hydrogen bond is a stronger intermolecular force, but it is weaker than a covalent or an ionic bond. Hydrogen Bonding is responsible for holding together DNA, proteins, and other macromolecules.
Formation of Hydrogen Bond
A hydrogen bond is an electromagnetic attraction that occurs between a partially positively charged hydrogen atom attached to a highly electronegative atom and another nearby electronegative atom. A hydrogen bond is a type of dipole-dipole interaction; it is not a true chemical bond. This hydrogen bond attraction can occur between different molecules (intermolecularly) or within different parts of a single molecule (intramolecularly).
Types of Hydrogen Bonding
There are two types of hydrogen bonding, i.e:
-
Intermolecular Hydrogen Bonding: Intermolecular hydrogen bonding occurs when the H-atom of one molecule and an electronegative atom of another molecule are close to each other. For example, hydrogen bonds exist between the molecules of hydrogen fluoride. Intermolecular hydrogen bonding results in an association of molecules. Thus, it increases the melting point, boiling point, solubility, etc.
-
Intramolecular Hydrogen Bonding: Intramolecular hydrogen bonding occurs between the hydrogen atom and an electronegative atom of the same molecule. Intramolecular hydrogen bonding results in the cyclisation of the molecules and prevents their association. Thus, the properties of these compounds, like melting point, boiling point, etc,. are usually low. For example, intramolecular hydrogen bonding is present in molecules such as o-nitrophenol, o-nitrobenzoic acid, etc.
Chemical Bonding and Molecular Structure: Previous Years' Questions and Answers
Practice these previous years questions of chapter 4 to strengthen your understanding of the topic and prepare effectively for exams. The concepts used to solve these questions are explained in ncert class 11 chemistry chapter 4 chemical bonding and molecular structure notes.
Question 1. In $\mathrm{SO}_2, \mathrm{NO}_2^{-}$and $\mathrm{N}_3^{-}$the hybridizations at the central atom are respectively :
Options
i) $\mathrm{sp}^2, \mathrm{sp}^2$ and sp
ii) $\mathrm{sp}^2, \mathrm{sp}$ and sp
iii) $\mathrm{sp}^2, \mathrm{sp}^2$ and $\mathrm{sp}^2$
iv) $\mathrm{sp}, \mathrm{sp}^2$ and sp
Answer:
$\mathrm{SO}_2 \Rightarrow 2 \sigma$ bond +1 l.p. $\Rightarrow s p^2$ hybridisation
$\mathrm{NO}_2^{-} \Rightarrow 2 \sigma$ bond +1 l.p. $\Rightarrow s p^2$ hybridisation
$\mathrm{N}_3^{-} \Rightarrow 2 \sigma$ bond $\Rightarrow s p$ hybridisation
Hence, the correct answer is option (1).
Question 2. Among $\mathrm{SO}_2, \mathrm{NF}_3, \mathrm{NH}_3, \mathrm{XeF}_2, \mathrm{ClF}_3$ and $\mathrm{SF}_4$, the hybridisation of the molecule with non-zero dipole moment and the highest number of lone-pairs of electrons on the central atom is
Options
i) $\mathrm{sp}^3$
ii) $\mathrm{dsp}^2$
iii) $\mathrm{sp}^3 \mathrm{~d}^2$
iv) $\mathrm{sp}^3 \mathrm{~d}$
Answer:
|
Molecule |
Hybridisation |
Dipole Moment |
Lone pair on the central atom |
|
$\mathrm{SO}_2$ |
$\mathrm{sp}^2$ |
Non - zero |
1 |
|
$\mathrm{NF}_3$ |
$\mathrm{sp}^3$ |
Non - zero |
1 |
|
$\mathrm{NH}_3$ |
$\mathrm{sp}^3$ |
Non - zero |
1 |
|
$\mathrm{XeF}_2$ |
$\mathrm{sp}^3 \mathrm{~d}$ |
zero |
3 |
|
$\mathrm{C} \ell \mathrm{~F}_3$ |
$\mathrm{sp}^3 \mathrm{~d}$ |
Non - zero |
2 |
|
$\mathrm{SF}_4$ |
$ \mathrm{sp}^3 \mathrm{~d}$ |
Non - zero |
1 |
- $\mathrm{ClF}_3$ has 2 lone pair and a non-zero dipole moment, the highest among molecules with a dipole
- $\mathrm{XeF}_2$ has 3 lone pair but zero dipole moment due to linear symmetry
Hence, the correct answer is option (iv)
Question 3. Which hybridisation leads to a trigonal planar shape?
(1) sp
(2) sp2
(3) sp3
(4) dsp2
Answer:
sp2 hybridisation involves 3 hybrid orbitals arranged in a plane at 120° angles, which leads to a trigonal planar geometry (for example, BF3).
Hence, the correct answer is option (2).
Question 4: Given below are two statements:
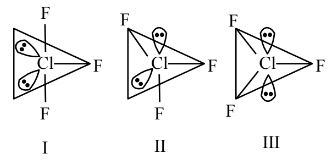
Statement (I) : for $C \ell \mathrm{F}_3$, all three possible structures may be drawn as follows.
Statement (II) : Structure III is most stable, as the orbitals having the lone pairs are axial, where the $\ell \mathrm{p}-\mathrm{bp}$ repulsion is minimum.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1) Statement I is incorrect but statement II is correct.
(2) Statement I is correct but statement II is incorrect.
(3) Both Statement I and statement II are correct.
(4) Both Statement I and statement II are incorrect.
Answer:
Statement 1 is correct.
Statement 2 is incorrect since in $\mathrm{sp}^3 \mathrm{~d}$ hybridization, a lone pair cannot occupy an axial position due to lone pair-bond pair repulsion. The lone pairs will be at equatorial position for maximum stability.
Hence, the correct answer is option (2).
Question 5: Match the LIST - I with LIST - II.
| LIST - I Molecule/ion |
LIST - II | ||
| A. | $\mathrm{ICl}_2^{-}$ | I. | 4 : 2 |
| B. | $\mathrm{H}_2 \mathrm{O}$ | II. | 4 : 1 |
| C. | $\mathrm{SO}_2$ | III. | 2 : 3 |
| D. | $\mathrm{XeF}_4$ | IV. | 2 : 2 |
Choose the correct answer from the options given below :
(1) A-IV, B-III, C-II, D-I
(2) A-III, B-IV, C-II, D-I
(3) A-III, B-IV, C-I, D-II
(4) A-II, B-I, C-IV, D-III
Answer:
(A) 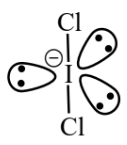 B.P : L.P = 2 : 3
B.P : L.P = 2 : 3
(B) 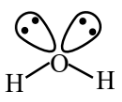 B.P : L.P = 2 : 2
B.P : L.P = 2 : 2
(C) 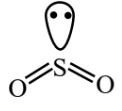 B.P : L.P = 4 : 1
B.P : L.P = 4 : 1
(D)  B.P : L.P = 4 : 2
B.P : L.P = 4 : 2
Hence, the correct answer is option (2).
How to Master Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
This class 11 chemistry chapter 4 chemical bonding and molecular structure notes helps students to understand the chemical bonding that forms the foundation of chemistry. These NCERT notes for Class 11 help to understand the basic concepts from your NCERT textbook. Given below some points on how to master this chapter.
- Students must understand the basic concepts first like octet rule, Lewis symbols, and ionic bond formation.
- Learn how lattice energy and Born Haber cycle explains stability of ions.
- Then understand the covalent bonding, theories, Lewis structures, resonance, and formal charge.
- Questions related to Valence Bond Theory and Hybridisation are often asked in exams to understand these concepts better refer to class 11 chemistry chapter 4 chemical bonding and molecular structure notes.
- Also read molecular shape and geometry in detail for predicting molecular geometry.
- Learn about molecular orbital theory, bond order, stability, magnetism of molecules, polarity and intermolecular forces
- At last students can solve previous year questions from this chapter.
Advantages of Using Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Notes
The chemical bonding and molecular structure class 11 notes cover all important concepts from the NCERT book in a simple and organised manner. The advantages of using these notes are given below:
- Students can use these notes to understand the concepts like valence bond theory, hybridisation, molecular orbital theory, and VSEPR theory.
- These notes cover all the topics from NCERT and they are written in a very clear and comprehensive manner.
- The chemical bonding and molecular structure class 11 chemistry chapter 4 CBSE notes provide step by step explanations of every topic.
- These notes are prepared by subject experts in a concise format that helps students in both boards and competitive exams.
NCERT Notes Class 11 Chapter-Wise
Along with chemical bonding and molecular structure class 11 chemistry chapter 4 CBSE notes also refer to links given below for Chemistry chapter-wise notes:
|
NCERT Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry notes Notes |
|
NCERT Class 11 Chemistry Chapter 3 classification of elements and periodicity Notes |
|
NCERT Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Notes |
|
NCERT Class 11 Chemistry Chapter 8 Organic chemistry some basic principles and techniques Notes |
NCERT Solutions for Class 11 Chemistry
Along with chemical bonding and molecular structure class 11 notes, follow the links below to get chapter-wise solutions of NCERT and make your learning better.
NCERT Exemplar Solutions Subject-Wise
NCERT Exemplar Subject-wise solutions are given below:
NCERT Solutions Subject-Wise
NCERT Subject-wise solutions are given below:
Frequently Asked Questions (FAQs)
Ionic bonds are formed through the transfer of electrons from one atom to another, resulting in the formation of oppositely charged ions. For example, in sodium chloride (NaCl), sodium donates an electron to chlorine, leading to the formation of Na⁺ and Cl⁻ ions.
Electronegativity is a measure of an atom's ability to attract and hold onto electrons within a bond. The difference in electronegativity between two bonding atoms can determine the type of bond that forms. A large difference usually indicates an ionic bond, while a small difference suggests a covalent bond.
Polar covalent bonds occur when there is a significant difference in electronegativity between the two atoms, leading to an unequal sharing of electrons. This creating partial charges within the molecule. Non-polar covalent bonds, on the other hand, form when the electronegativities of the bonding atoms are equal or very similar, resulting in an even distribution of electron density.
Lone pairs, or non-bonding electron pairs, influence molecular geometry by repelling bonding electron pairs. They take up space and can change the angle between bonds, typically leading to alterations in the predicted shape based on VSEPR theory.
The chemical bonding and molecular structure ncert notes are concise study materials that summarise the core concepts of the chapter. These notes cover important topics such as the octet rule, ionic and covalent bonds, resonance, VSEPR theory, hybridisation, molecular orbital theory (MOT), bond parameters, polarity, and intermolecular forces.
A chemical bond is a force that holds atoms together in a molecule or compound. It results from the attraction between the positively charged nucleus of one atom and the negatively charged electrons of another.
To download NCERT Class 11 Chemistry Chapter 4 notes, visit trusted websites or NCERT official site, navigate to Class 11 Chemistry resources, locate Chapter 4 “Chemical Bonding and Molecular Structure,” and click on the PDF download link. The notes are free and can be saved on your device for offline study and quick revision.
Resonance in chemistry refers to the delocalization of electrons in a molecule, where the actual structure is a blend of two or more possible structures (called resonance forms) rather than a single fixed structure.
Bond parameters affect molecular structure by determining the Bond Length, Bond Angle, and Bond Energy.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters