Students can find Class 11 Chemistry Hydrocarbons notes on educational websites, learning apps, and CBSE portals that provide chapter wise study material. Many platforms offer free PDF downloads, allowing easy access for revision and quick reference anytime, anywhere.
NCERT Class 11 Chemistry Chapter 13 Notes Hydrocarbons - Download PDF
Ever wondered what powers your car, lights your gas stove, or even forms the backbone of many medicines and plastics? The answer lies in hydrocarbons. It is the simplest yet most powerful organic compound made of just carbon and hydrogen! Hydrocarbons are compounds that consist of carbon and hydrogen only. Have you ever wondered what the world would look like if fuel were not available? Fuel is required to provide a source of energy to vehicles, to cook food, or to generate electricity. If fuel is not available, then it becomes difficult for us to carry on our day-to-day activities. Hydrocarbons play a very important role in our day-to-day lives as they serve as the primary source of energy. Petrol and Diesel are hydrocarbons that are used as fuel for vehicles, and LPG is also a hydrocarbon that is used for cooking. Some of the important topics covered in this chapter are types of hydrocarbons, their properties, formation and reactions. Hydrocarbons are the simplest organic compounds which form the basis of many complex compounds.
This Story also Contains
- NCERT Notes for Class 11 Chapter 9 Hydrocarbons: Download PDF
- NCERT Notes for Class 11 Chapter 9 Hydrocarbons
- Hydrocarbons Previous year Question and Answer
- How to Master Class 11 Chemistry Chapter 9 Hydrocarbons
- Advantages of Using Class 11 Chemistry Chapter 9 Hydrocarbons Notes
- CBSE Class 11 Chemistry Chapter-wise Notes
- NCERT Solutions for Class 11 Chemistry
- Subject-Wise NCERT Exemplar Solutions
- Subject-Wise NCERT Solutions
NCERT Class 11 chemistry notes are designed by our subject experts to make learning simple, clear, and easy. Hydrocarbon notes are provided in a very comprehensive way, which will help students clarify the concept. These notes will help students prepare for their final exams and competitive exams, such as JEE Mains, NEET, BITSAT, etc. NCERT notes also cover the basic equations as well as the substitution reactions, which include some named reactions, aromaticity, Huckel’s rule, and preparation of benzene, which is required for the deep knowledge to score well in the examination.
NCERT Notes for Class 11 Chapter 9 Hydrocarbons: Download PDF
Download the hydrocarbons class 11 chemistry chapter 9 CBSE notes pdf to access a clear explanation, important reactions of this chapter. These NCERT notes for class 11 cover all the key concepts of Hydrocarbons. You can download these notes from the download pdf icon given below.
Also Read
NCERT Notes for Class 11 Chapter 9 Hydrocarbons
In this article, we talk about alkanes, alkenes, alkynes, and aromatic compounds, from their structures, nomenclature and reactions to their industrial uses and environmental impact. These hydrocarbons class 11 chemistry notes cover the different types of hydrocarbons, their properties, and important reactions in a clear and easy way. You can also download the notes in PDF format for quick revision and offline study. Students can also refer to the NCERT Solutions for additional practice and understanding.
Discussing the Main Terms Related to Hydrocarbon
This section explains the key terms and concepts that will help you understand hydrocarbons chapter 9 class 11 chemistry more easily.
Hydrocarbon
Compounds formed with the help of carbon and hydrogen are known as Hydrocarbons.
a)Saturated hydrocarbon:
Saturated hydrocarbons are those that contain a single bond in between two carbon atoms. Example: methane
b)Unsaturated hydrocarbon:
The hydrocarbon with double or triple bonds other than single bond is so called unsaturated hydrocarbon. Example: ethyne, ethene.
c)Aromatic Hydrocarbon:
The aromatic hydrocarbon is defined as compounds of benzene and their derivatives.
Example: Benzene and Aniline.
d)Alicyclic compounds:
The cyclic ring only consisting of carbon atoms is called alicyclic compounds.
Example:
e)Heterocyclic compounds:
The cyclic ring of such a compound is composed of carbon as well as atoms other than carbon.
Example:
Classification
Hydrocarbons are classified into three different categories, depending on the type of carbon-carbon bond present in them, viz., saturated, unsaturated and aromatic hydrocarbons. Alkanes are saturated hydrocarbons, alkenes and alkynes are unsaturated hydrocarbons. Aromatic hydrocarbons are a special type of cyclic compound. You can download the hydrocarbons class 11 chemistry chapter 9 CBSE note pdf of these notes for easy revision and offline access.
Alkanes
The simplest form of organic compound that are made up of carbon and hydrogen. Carbon atoms are made up of single bond; covalent. As the chain of carbon is composed of single bonds only so they are fully saturated hydrocarbons. This class of alkanes are chemically inert in nature, so they are called paraffins.
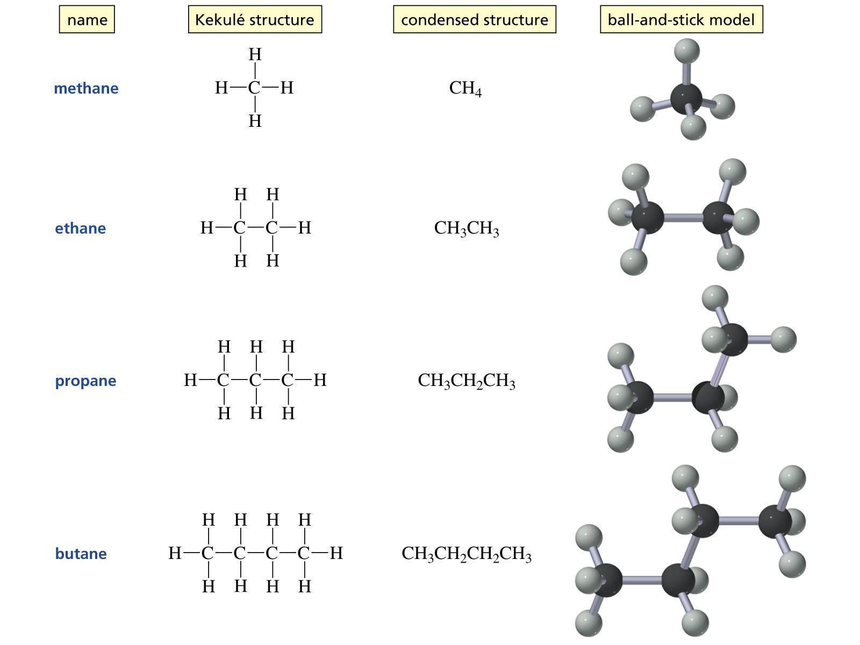
Nomenclature
We proceed further with steps to understand the IUPAC nomenclature or how to write the structural formula of different hydrocarbons.
Step1. First to identify the longest chain among all the carbon.
Step2. In the second step, give the number to the selected chain. Try to give the number from left to right. As this should also be kept in mind that the lowest numbers attached to the alkyl group come first.
Step3. Identifying the alkyl group attached to the main chain.
Step4. Finally writing the IUPAC names.
Based upon the number of carbon atoms attached to a carbon atom, the carbon atom is termed as primary (1°), secondary (2°), tertiary (3°) or quaternary (4°).
Sawhorse Projections
In this projection, the molecule is viewed along the C–C bond axis. The front carbon is shown at the lower end and the rear carbon at the upper end of a slightly tilted line. Each carbon has three bonds drawn at 120° angles to represent attached hydrogen atoms.
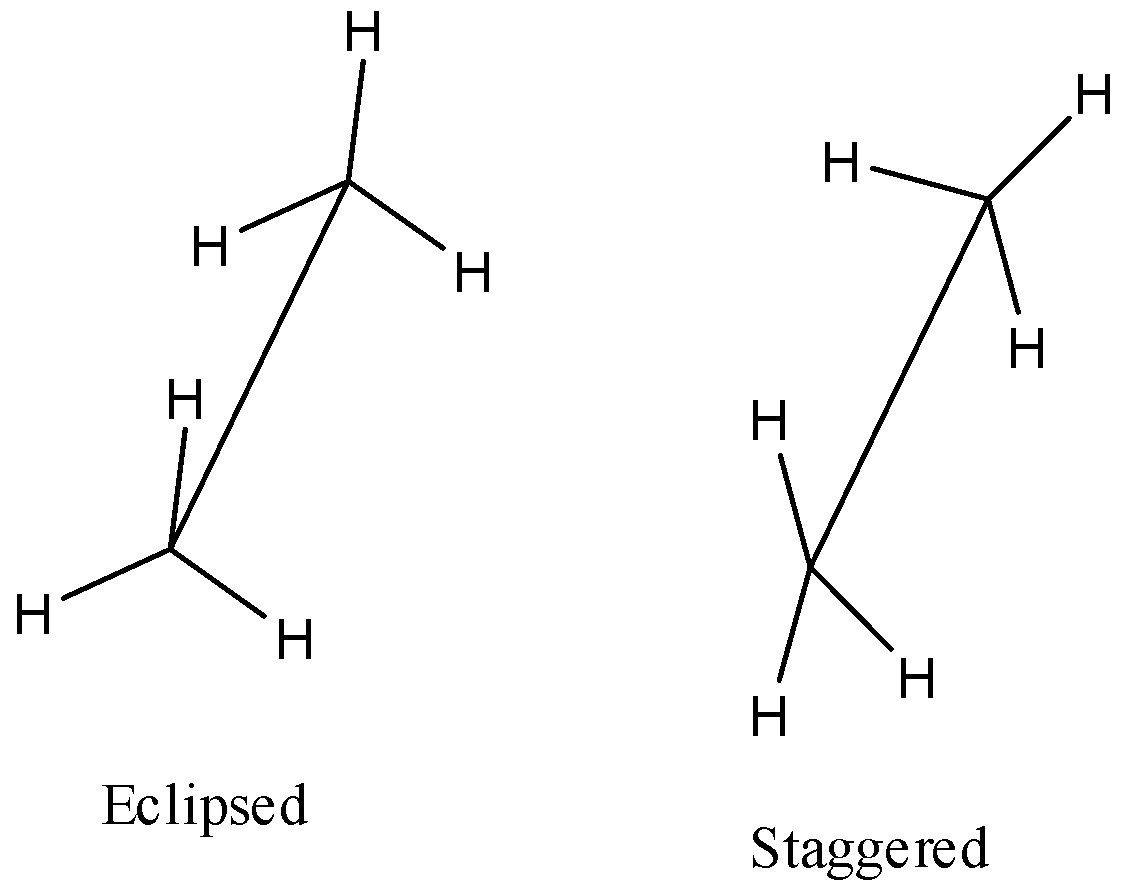
Newman Projections
In this projection, the molecule is viewed head-on along the C–C bond. The front carbon is shown as a point with three bonds at 120°, and the rear carbon as a circle with three attached hydrogen atoms also spaced at 120°.
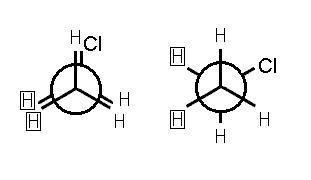
Relative stability of conformations
The two forms, decide the stability and energy of the molecule. When the alkane(ethane) is present in a staggered form, maximum repulsion occurs, which creates minimum energy and maximum stability. Whereas in the eclipsed form of ethane, the stability reduces as the electrons of the carbon cloud and hydrogen clouds come closer, and it possesses more energy than the staggered form, thus the stability in this form reduces.
Preparations
1. From unsaturated hydrocarbons:
Dihydrogen gas adds to alkenes and alkynes in the presence of finely divided catalysts like
platinum, palladium or nickel to form alkanes. This process is called hydrogenation.
.png)
2. From alkyl halides:
(i) Alkyl halides (except fluorides) on reduction with zinc and dilute hydrochloric acid give alkanes.
$\mathrm{CH}-\mathrm{Cl}+\mathrm{H}_2 \xrightarrow{\mathrm{Zn}, \mathrm{H}^{+}} \mathrm{CH}_4+\mathrm{HCl}$
(ii) Alkyl halides, on treatment with sodium metal in dry ethereal (free from moisture) solution, give higher alkanes. This reaction is known as the Wurtz reaction.
$\begin{array}{ll}\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+2 \mathrm{Na}+\mathrm{BrC}_2 \mathrm{H}_5 \xrightarrow{\text { dry ether }} & \mathrm{C}_2 \mathrm{H}_5-\mathrm{C}_2 \mathrm{H}_5 \\ \text { Bromoethane } & \text { n-Butane }\end{array}$
3. From carboxylic acids
(i) When a carboxylic acid is heated with soda lime (a mixture of sodium hydroxide and calcium oxide), it undergoes decarboxylation, that is, carbon dioxide is eliminated. As a result, an alkane is formed that contains one carbon atom less than the original carboxylic acid.
$
\mathrm{CH}_3 \mathrm{COO}^{-} \mathrm{Na}^{+}+\mathrm{NaOH} \xrightarrow[\Delta]{\mathrm{CaO}} \mathrm{CH}_4+\mathrm{Na}_2 \mathrm{CO}_3
$
Sodium ethanoate
(ii) Kolbe's electrolytic method: In Kolbe’s electrolytic method, aqueous sodium or potassium salt of a carboxylic acid is electrolysed. At the anode, decarboxylation occurs, and the resulting radicals dimerise to form an alkane with an even number of carbon atoms.
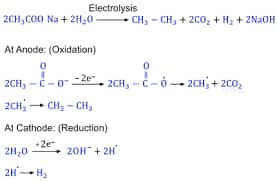
Properties
Physical Properties
Alkanes are non-polar due to covalent C-C and C-H bonds and a small electronegativity difference between carbon and hydrogen. They exhibit weak van der Waals forces, making:
- $\mathrm{C}_1-\mathrm{C}_4$ gases,
- $\mathrm{C}_5-\mathrm{C}_{17}$ liquids,
- $\mathrm{C}_{18}$ and above solids at 298 K .
They are insoluble in water but dissolve in non-polar solvents like petrol. Grease, also non-polar, is cleaned using petrol-illustrating the rule "like dissolves like."
Boiling points increase with molecular size due to stronger van der Waals forces. Branching lowers boiling points as it reduces surface area and intermolecular attraction.
E.g., pentane > 2-methylbutane > 2,2-dimethylpropane.
Chemical Properties
Although alkanes are inert towards acids, bases, oxidising and reducing agents but give following reactions:
1. Halogenation
$\mathrm{CH}_2+\mathrm{Cl} \xrightarrow{h \nu} \underset{\text { Chloromethane }}{\mathrm{CH}_3 \mathrm{Cl}}+\mathrm{HCl}$
2. Combustion
$\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}+2}+\left(\frac{3 \mathrm{n}+1}{2}\right) \mathrm{O}_2 \rightarrow \mathrm{nCO}_2+(\mathrm{n}+1) \mathrm{H}_2 \mathrm{O}$
$\begin{aligned} \mathrm{CH}_4(\mathrm{~g})+2 \mathrm{O}_2(\mathrm{~g}) \rightarrow & \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(1) ; \\ & {\mathrm{}} \mathrm{H}^{\mathrm{e}}-890 \mathrm{~kJ} \mathrm{~mol}^{-1}\end{aligned}$
Sometimes, during incomplete combustion, carbon black is formed.
$\mathrm{CH}_4(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow[\text { combustion }]{\text { incomplete }} \mathrm{C}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(1)$
3. Controlled Oxidation
$\begin{array}{r}\mathrm{CH}_4+\mathrm{O}_2 \xrightarrow[\Delta]{\mathrm{Mo}_2 \mathrm{O}_3} \mathrm{HCHO}+\mathrm{H}_2 \mathrm{O} \\ \Delta\end{array}$
4. Isomerisation
.png)
5. Aromatisation
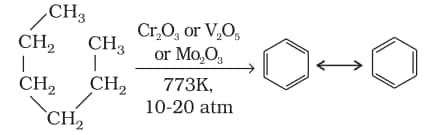
6. Reaction with steam
$\mathrm{CH}_4+\mathrm{H}_2 \mathrm{O} \xrightarrow[\Delta]{\mathrm{Ni}} \mathrm{CO}+3 \mathrm{H}_2$
7. Pyrolysis
$\mathrm{C}_{12} \mathrm{H}_{26} \xrightarrow[973 \mathrm{~K}]{\mathrm{Pt} / \mathrm{Pd} / \mathrm{Ni}} \mathrm{C}_7 \mathrm{H}_{16}+\mathrm{C}_5 \mathrm{H}_{10}+$ Other Products
Alkenes
The carbon that contains a double bond is an alkene with the general formula $\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}}$.
Points to remember while writing the formula for alkenes:
-
Two bonds are present here in alkenes in one is the sigma bond and the other is a pi bond.
-
The availability of a number of electrons in alkenes is higher, so they are more reactive than alkanes.
Orbital diagrams of the ethene molecule are shown in the figure below.
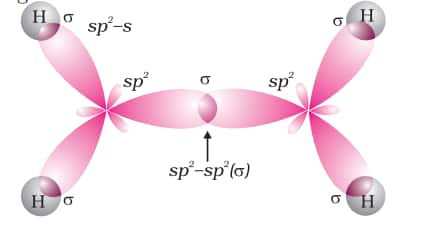
Nomenclature for alkenes
For writing the names of alkenes the same steps should be followed up which we studied in the earlier sections of nomenclature, where the parent or main chain is the longest one and indication of number started from one end.
If in case in one chain two or more double bonds are present than diene suffix should be used for it.
Isomerism
Basically, we go through different types of isomerism, some of which are listed below.
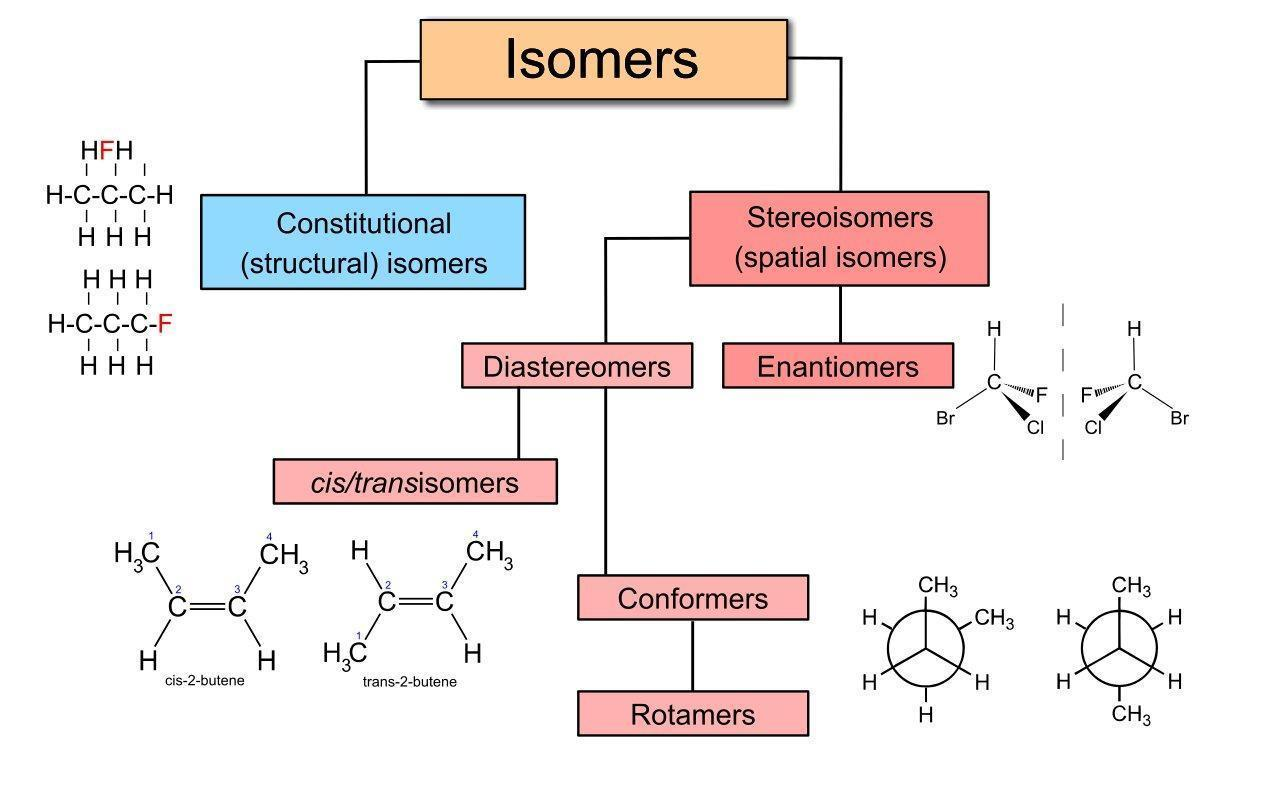
1. Structural Isomerism:

2. Geometrical Isomerism:

Preparations
1. From Alkynes
$\begin{array}{ll}\mathrm{CH} \equiv \mathrm{CH}+\mathrm{H}_2 \xrightarrow{\mathrm{Pd} / \mathrm{C}} \mathrm{CH}_2=\mathrm{CH}_2 \\ \text { Ethyne } & \text { Ethene }\end{array}$
$\mathrm{CH}_3-\mathrm{C} \equiv \mathrm{CH}+\mathrm{H}_2 \xrightarrow{\mathrm{Pd} / \mathrm{C}} \mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}_2$
2. From alkyl halides
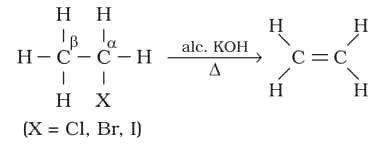
3. From vicinal dihalides
$\mathrm{CH}_2 \mathrm{Br}-\mathrm{CH}_2 \mathrm{Br}+\mathrm{Zn} \longrightarrow \mathrm{CH}_2=\mathrm{CH}_2+\mathrm{ZnBr}_2$
4. From alcohols by acidic dehydration:
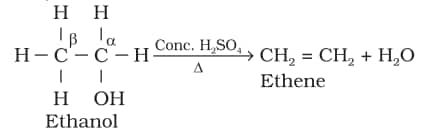
Properties
Physical Properties
Alkenes are similar to alkanes in physical properties but differ in isomerism and polarity. The first three are gases, the next fourteen are liquids, and higher ones are solids. They are colourless, generally odourless, insoluble in water, but soluble in non-polar solvents. Boiling points increase regularly with molecular size, and straight-chain alkenes have higher boiling points than their branched isomers.
Chemical Properties
Alkenes are a rich source of loosely held pi (π) electrons, due to which they show addition reactions in which the electrophiles add on to the carbon-carbon double bond to form the addition products.
1. Addition of dihydrogen: Alkenes add up one molecule of dihydrogen gas in the presence of finely divided nickel, palladium or platinum to form alkanes.
2. Addition of halogens:
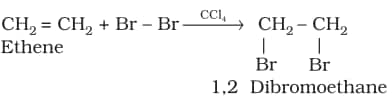
3. Addition of hydrogen halides: The addition of halides to unsymmetrical alkenes follows Markovnikov's rule. The rule states that the negative part of the addendum (adding molecule) gets attached to that carbon atom which possesses the lesser number of hydrogen atoms. Thus, according to this rule, product I, i.e., 2-bromopropane, is expected.
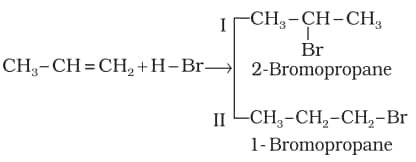
In the presence of peroxide, the addition of HBr to unsymmetrical alkenes like propene takes place contrary to the Markovnikov rule. This happens only with HBr but not with HCl and HI. This reaction is known as the peroxide or Kharash effect, or an addition reaction contrary to the Markovnikov rule.
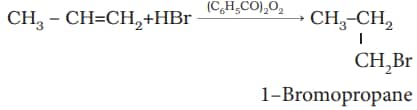
4. Addition of sulphuric acid:
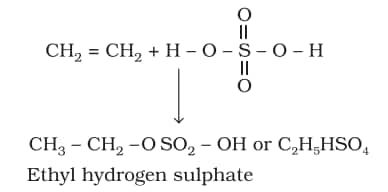
5. Addition of water:
.png)
6. Oxidation:
.png)
Decolourisation of $\mathrm{KMnO}_4$ solution is used as a test for unsaturation.
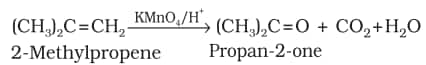
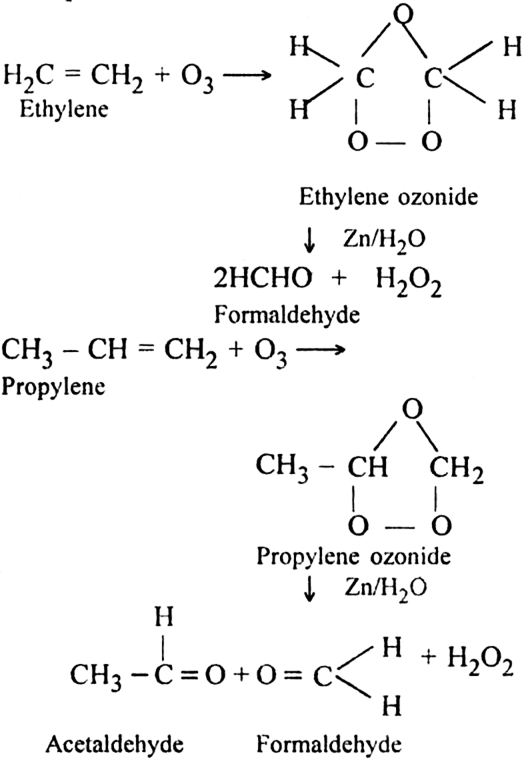
7. Ozonolysis:
8. Polymerisation:

Alkynes
Alkynes are the chain of carbon having triple bonds with the general formula $\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}-2}$.
An important member of the alkyne group of hydrocarbons is acetylene.
Points to remember while writing the formula for alkenes:
-
In this triple bond two of them are pi bonds and one is sigma bond.
-
Similar to alkenes, the pi electrons are found more, so they will go in addition reaction.
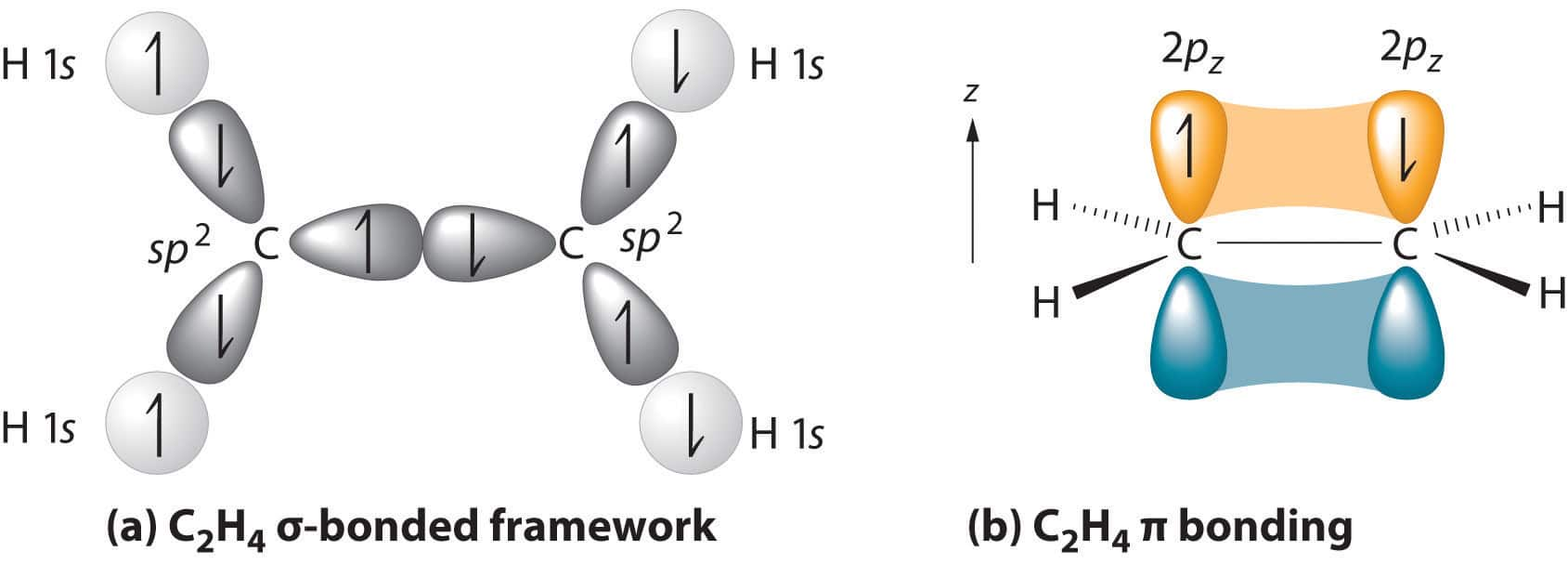
Each carbon has two unhybridised p orbitals which are perpendicular to each other as well as to the
plane of the C-C sigma bond. The 2p orbitals of one carbon atom are parallel to the 2p orbitals of the other carbon atom, which undergo lateral or sideways overlapping to form two pi (π) bonds between two carbon atoms.
Nomenclature for Alkynes
For writing the names of alkenes the same steps should be followed up which we studied in the earlier sections of nomenclature, where the parent or main chain is the longest one and indication of number started from one end.
While writing the names of alkyne hydrocarbon we use suffix as yne and first numbering the carbon which is attached to triple bond.
Preparation
1. From calcium carbide: By treating calcium carbide with water formation of ethyne can be possible.
$\mathrm{CaCO}_3 \xrightarrow{\Delta} \mathrm{CaO} \quad+\quad \mathrm{O}_2$
$\mathrm{CaC}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{C}_2 \mathrm{H}_2$
2. From vicinal dihalides: Dehydrohalogenation occur alcoholic potassium
Properties
Physical Properties
Physical properties of alkynes follow the same trend of alkenes and alkanes. First three members are gases, the next eight are liquids and the higher ones are solids. All alkynes are colourless. Ethyene has characteristic odour. Other members are odourless. Alkynes are weakly polar in nature. They are lighter than water and immiscible with water but soluble in organic solvents like ethers, carbon tetrachloride and benzene. Their melting point, boiling point and density increase with increase in molar mass.
Chemical Properties
A. Acidic character of alkyne:
$\begin{aligned} \mathrm{HC} \equiv \mathrm{CH}+\mathrm{Na} \rightarrow & \mathrm{HC} \equiv \mathrm{C}^{-} \mathrm{Na}^{+}+1 / 2 \mathrm{H}_2 \\ & \text { Monosodium } \\ & \text { ethynide }\end{aligned}$
B. Addition reactions:
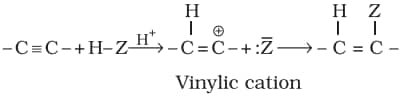
The addition product formed depends upon stability of vinylic cation. Addition in unsymmetrical alkynes takes place according to Markovnikov rule.
(i) Addition of dihydrogen:
$\mathrm{HC} \equiv \mathrm{CH}+\mathrm{H}_2 \xrightarrow{\mathrm{Pt} / \mathrm{Pd} / \mathrm{Ni}}\left[\mathrm{H}_2 \mathrm{C}=\mathrm{CH}_2\right] \xrightarrow{\mathrm{H}_2} \mathrm{CH}_3-\mathrm{CH}_3$
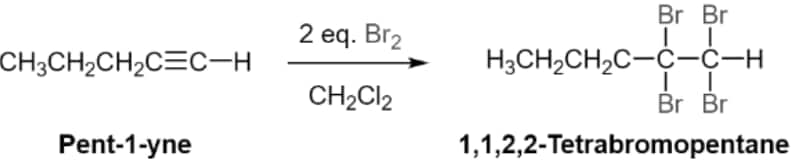
(ii) Addition of halogens:
(iii) Addition of hydrogen halides:
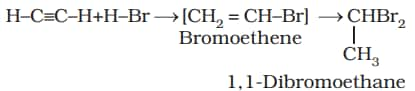
(iv) Addition of water:
.jpg)
Aromatic Hydrocarbons
The important feature of aromatic hydrocarbon is that most of the compounds containing the benzene ring and are also called arenes. The compounds having benzene rings are called benzenoids and those who have to benzene ring in their structure are termed as non-benzenoids.
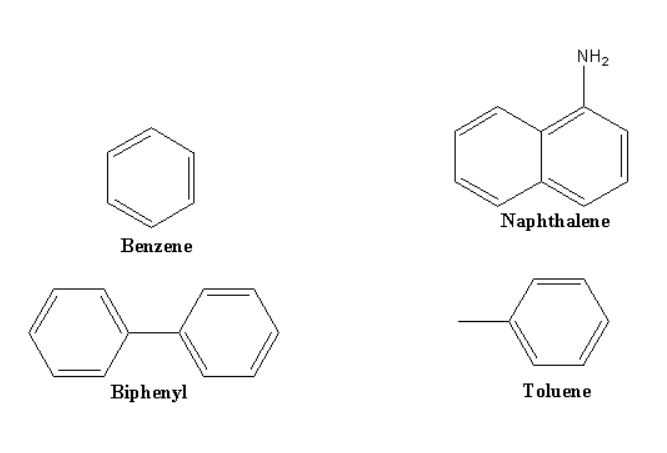
Above mentioned are the examples of Hydrocarbon.
Nomenclature of Aromatic compounds
The common names according to IUPAC are accepted for naming the benzene and its homologous.
Structure of benzene:
The molecular formula of benzene is C6H6, and are highly unsaturated compound.
According to Kekule’s , he had given benzene structure in the year 1865, in that he showed single and double bonds are present on alternate positions in the ring.
He also gave that the benzene ring would present in two forms.
Failures of Kekule’s benzene ring:
He wont able to explain how the stability of benzene ring can be achieved and also why the substitution reactions are preferred than addition reactions.
Resonance structure:
Resonance is defined as the where we can make two or more structures for the same compound and the atoms in that are also identical such structure is termed as Resonance structure.
Below showing you the resonance structure of benzene.
Orbital structure:
S-orbitals of hydrogen atom in Benzene ring form sigma bonds with carbon in condition of overlapping of hybrid orbitals.
Important points to remember:
-
Benzene is found as planar in structure and this can be detected by the technique called X-ray diffractions.
-
The bond length of carbon-carbon (139) which is intermediated between C-C of bond length(133pm) and C-C of bond length (154) is of same bond order. So, under normal benzene will go for addition reactions, which is the unusual behaviour of benzene.
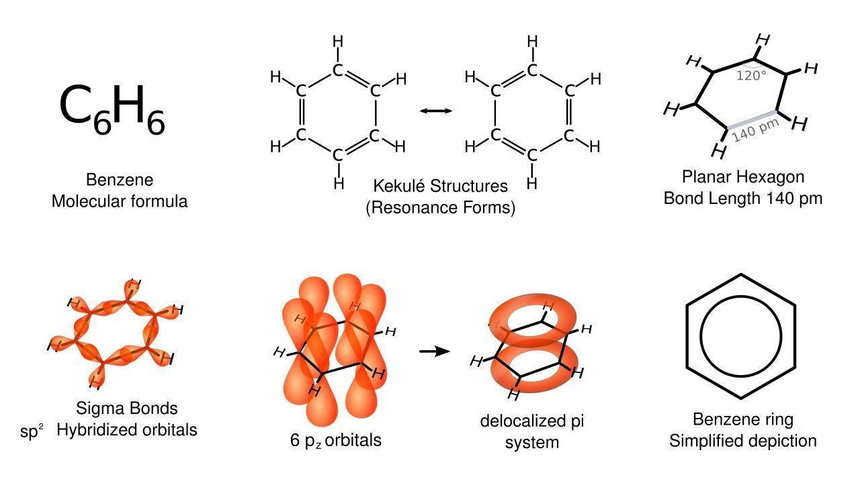
Aromaticity
This is the property of $s p^2$ hybridized planar rings, where p orbitals allow cyclic delocalization of pi electrons.
Huckle’s Rule:
When cyclic pi electron cloud overlap, with p orbitals which contains(4n+2) pi electrons, such rule is termed as Huckle rule. Here n denotes the positive integer and 0.
Preparation of Benzene using different methods and compounds:
-
It can be prepared commercially by isolating coal tar.
-
There are some of the synthetic methods through which we can prepare the benzene.
-
The synthetic methods are listed below:
Physical Properties
-
Colourless liquid.
-
Insoluble in water.
-
Soluble in alcohol, ether, etc.
-
Good solvent for organic and inorganic substances.
-
Sooty flame with blue in colour.
Chemical Properties
-
To show the chemical properties of benzene it must go through some reactions.
-
Addition reaction
-
Electrophilic substitution reaction
-
Synthetic Methods:
Preparation
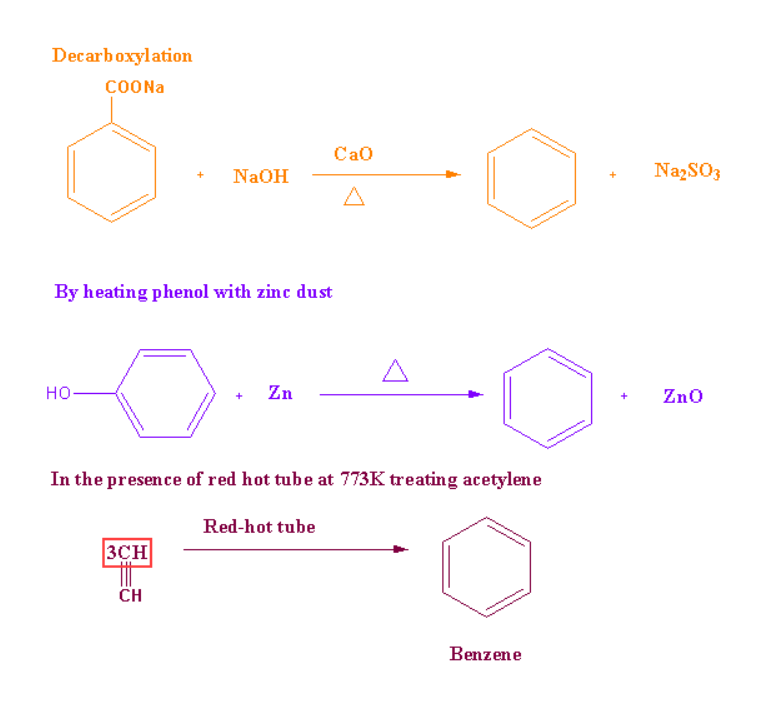
Properties
Physical Properties
Aromatic hydrocarbons are non- polar molecules and are usually colourless liquids or solids with a characteristic aroma. You are also familiar with naphthalene balls which are used in toilets and for preservation of clothes because of unique smell of the compound and the moth repellent property. Aromatic hydrocarbons are immiscible with water but are readily miscible with organic solvents. They burn with sooty flame.
Chemcial Properties
Arenes are characterised by electrophilic substitution reactions. However, under special conditions they can also undergo addition and oxidation reactions.
Electrophilic Substitution Reactions is as Follows
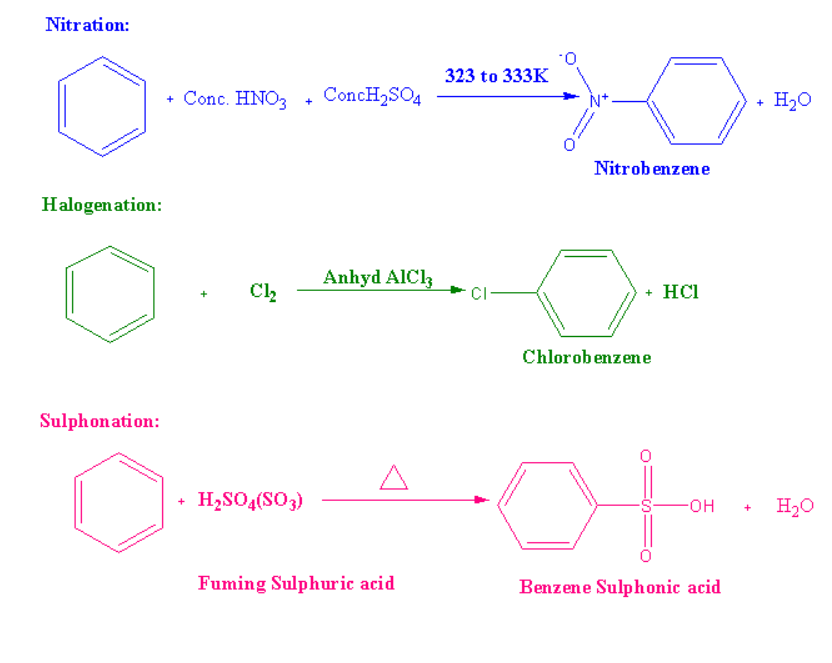
Friedel-Crafts Alkylation Reaction
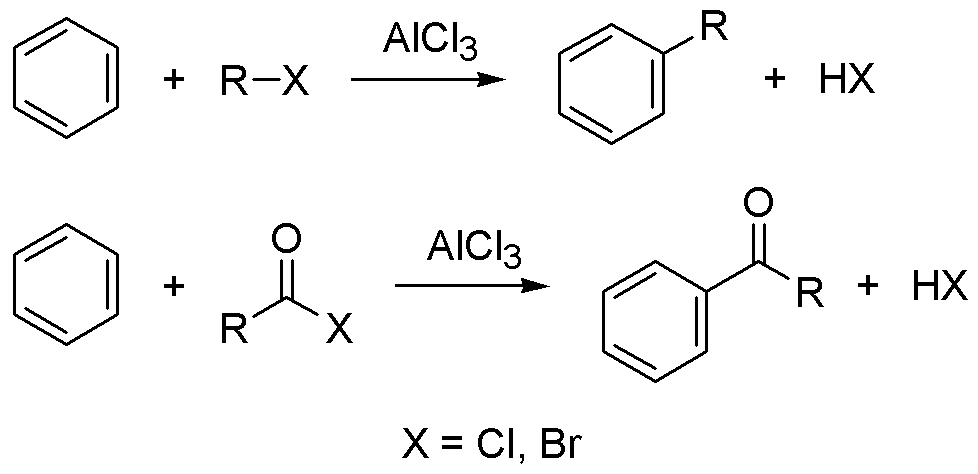
Mechanism Related to Electrophilic Substitution Reaction:
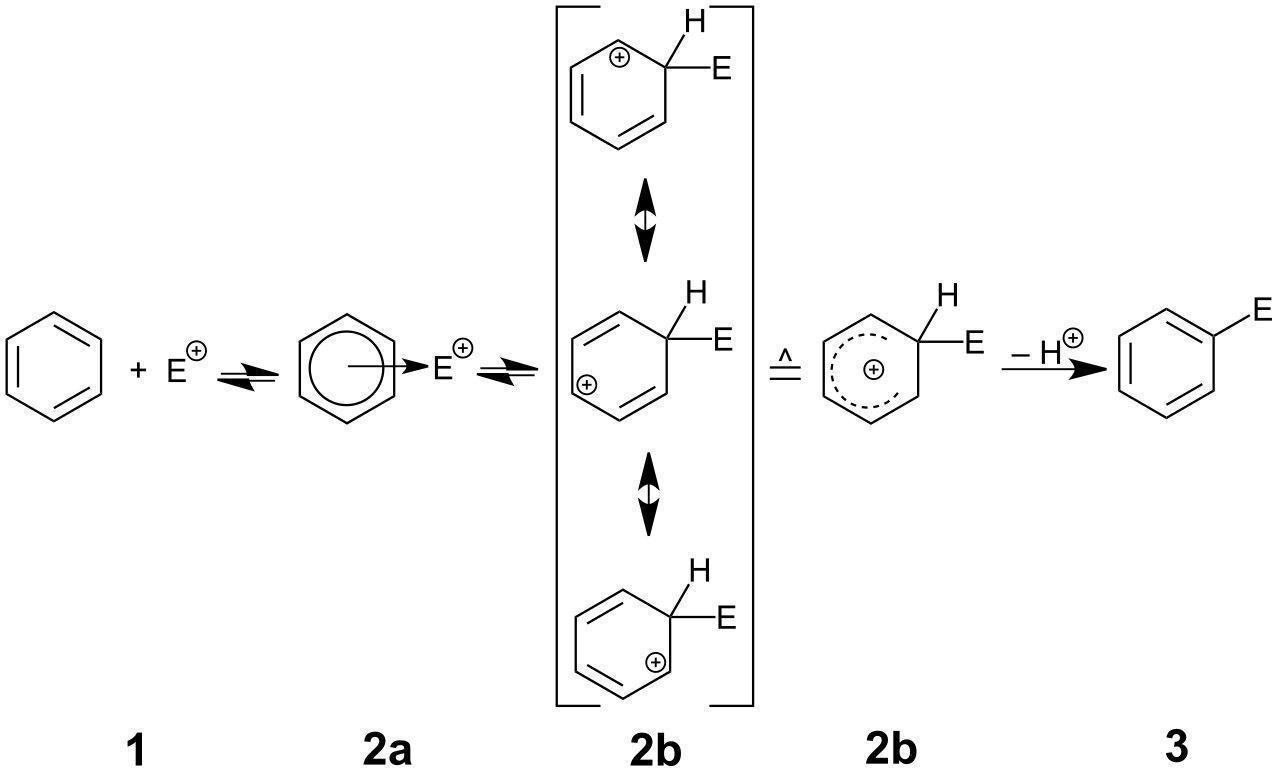
Steps included in the mechanism of electrophilic substitution reactions:
Step 1: In the first step formation of electrophiles occurs.
Step 2: To form the carbonium ion electrophile needs to attack the aromatic ring.
Step 3: Loss in a number of protons gives us the substitution product.
Addition reactions
.png)
.png)
Combustion Reaction
$\mathrm{C}_x \mathrm{H}_y+\left(x+\frac{y}{4}\right) \mathrm{O}_2 \rightarrow x \mathrm{CO}_2+\frac{y}{2} \mathrm{H}_2 \mathrm{O}$
$\mathrm{C}_6 \mathrm{H}_6+\frac{15}{2} \mathrm{O}_2 \rightarrow 6 \mathrm{CO}_2+3 \mathrm{H}_2 \mathrm{O}$
Carcinogenicity and Toxicity
Some of the polynuclear hydrocarbons contain benzene rings more than two in their chains, and when they fuse together they produce property that creates cancer.
They are formed by the incomplete combustion of tobacco, coal, etc.
Examples are as follows:
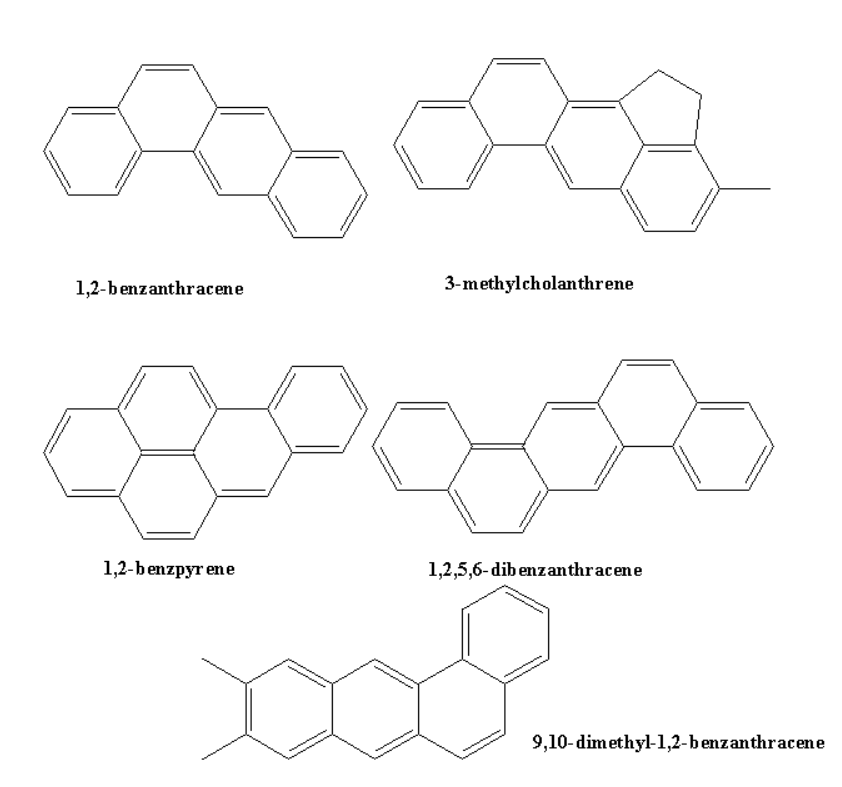
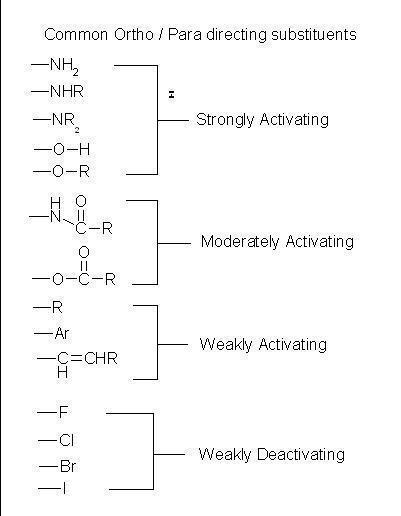
Activating Groups and Deactivating Groups
The activating groups are those who activate the benzene ring to attack with the help of an electrophile.
Deactivating groups are those who due to strong -I effect are formed which decreases the overall density and makes substitution difficult.
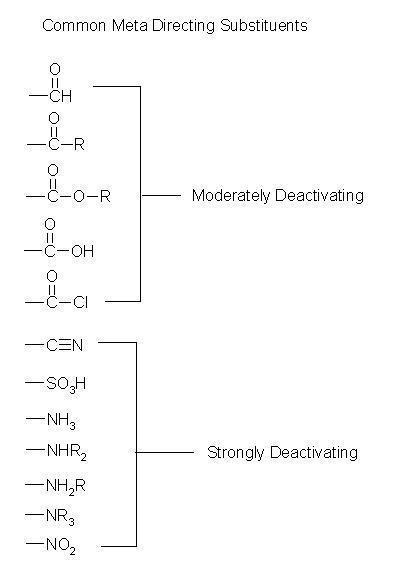
Meta-Directing Groups
These are the groups who helps to direct the incoming group to meta position are termed as meta-directing groups.
Hydrocarbons Previous year Question and Answer
Previous year questions and answers on hydrocarbons help students identify important topics and commonly asked concepts in exams. These solved questions are highly useful for Chemistry exam preparation and students can also refer to ncert class 11 chemistry chapter 9 hydrocarbons notes for better understanding.
Question 1: Benzene is treated with oleum to produce compound (X) which when further heated with molten sodium hydroxide followed by acidification produces compound (Y).The compound Y is treated with zinc metal to produce compound (Z).
Identify the structure of compound (Z) from the following option.
Answer:

Hence, the correct answer is option (2).
Question 2: Which compound would give 3-methyl-6-oxoheptanal upon ozonolysis ?
Answer:

3-methyl-6-oxoheptanal
Ozonolysis breaks the carbon–carbon double bonds in alkenes. Here, p-cymene undergoes ozonolysis, and the double bonds in the aromatic ring are cleaved. Each broken double bond is replaced by a carbonyl group (either an aldehyde or a ketone), resulting in a linear compound with aldehyde and ketone groups.
Hence, the correct answer is option (2).
Question 3: Arrange the following in decreasing order of their boiling points.
(A) n–butane
(B) 2–methylbutane
(C) n-pentane
(D) 2,2–dimethylpropane
Select the correct option:
- A > B > C > D
- B > C > D > A
- D > C > B > A
- C > B > D > A
Answer: The Boiling point is α molar mass & Boiling point is α surface area. It means that the boiling point will decrease on branching (surface area decreases on branching). Therefore, the highest boiling point is that of n-pentane and the lowest is of n-butane. The other two options have branches; therefore, 2-methyl butane has a higher boiling point than 2,2-dimethyl propane.
Hence, the answer is the option (4).
Question 4:
Choose the correct set of reagents for the following conversion.
(1)
$\mathrm{Br}_2 / \mathrm{Fe} ; \mathrm{Cl}_2, \Delta$; alc. KOH
(2)
$\mathrm{Cl}_2 / \mathrm{Fe}$; $\mathrm{Br}_2 /$ anhy. $\mathrm{AlCl}_3$; aq. KOH
(3)
$\mathrm{Br}_2 /$ anhy. $\mathrm{AlCl}_3 ; \mathrm{Cl}_2, \Delta$; aq. KOH
(4)
$\mathrm{Cl}_2 /$ anhy. $\mathrm{AlCl}_3 ; \mathrm{Br}_2 / \mathrm{Fe}$; alc. KOH
Answer:
Step 1 is Electrophilic aromatic substitution, in which bromination of ethylbenzene is done in the presence of iron. Step 2 is the free radical halogenation of the above obtained product, followed by an elimination reaction in the presence of alcoholic KOH, and the obtained product is 4-bromostyrene.

Hence, the correct answer is option (1).
Question 5:
Consider the following molecule ( X ).
The structure of X is
$
\text { The major product formed when the given molecule }(X) \text { is treated with } \mathrm{HBr}(1 \mathrm{eq}) \text { is : }
$
(1)
(2)
(3)
(4)
Answer:

Hence, the correct answer is option (2).
How to Master Class 11 Chemistry Chapter 9 Hydrocarbons
This chapter is a fundamental part of Class 11 Chemistry that explains the structure, properties, and reactions of carbon-based compounds. Students can refer to class 11 chemistry chapter 9 hydrocarbons notes to understand the basic concepts from your NCERT textbook. Given below some points on how to master this chapter.
- First understand the classification of hydrocarbons as alkanes, alkenes, alkynes, and aromatic compounds.
- Then learn about the nomenclature rules for naming different hydrocarbons using IUPAC conventions.
- Students must study structure, bonding, and isomerism of each type of hydrocarbon. They can understand these concepts with the help of Hydrocarbon notes.
- Questions related to methods of preparation and physical properties of hydrocarbons are often asked in exams.
- Learn about substitution, addition, and elimination reactions.
- At last students can solve previous year questions from this chapter.
Advantages of Using Class 11 Chemistry Chapter 9 Hydrocarbons Notes
NCERT Class 11 Chemistry Chapter 9 Hydrocarbons Notes covers all important concepts from the NCERT book in a simple and organised manner. Advantages of using these notes are given below:
- Students can use these notes to understand the concepts like alkanes, alkenes, alkynes, aromatic hydrocarbons, methods of preparation, physical and chemical properties.
- These ncert class 11 chemistry chapter 9 hydrocarbons notes are written in a systematic manner that provide clear concepts to help students understand how hydrocarbons behave and react.
- Science students can use these notes for clear and comprehensive explanations of the concepts covered in NCERT Book.
- Hydrocarbon notes are prepared by subject experts in an organised manner to help students understand the concepts.
CBSE Class 11 Chemistry Chapter-wise Notes
In addition to hydrocarbons class 11 chemistry notes, students can refer to the NCERT notes of other Class 11 chapters provided below.
NCERT Solutions for Class 11 Chemistry
Along with hydrocarbons class 11 chemistry chapter 9 CBSE notes, follow the links below to get chapter-wise solutions of NCERT and make your learning better.
Subject-Wise NCERT Exemplar Solutions
Below is the chapter-wise list of NCERT exemplar solutions:
Subject-Wise NCERT Solutions
Below is the subject-wise list of NCERT solutions:
| NCERT Solutions for Class 11 Mathematics |
| NCERT Solutions for Class 11 Chemistry |
| NCERT Solutions for Class 11 Physics |
| NCERT Solutions for Class 11 Biology |
Frequently Asked Questions (FAQs)
Isomerism is a phenomenon where compounds have the same molecular formula but different arrangements of atoms. In hydrocarbons, isomerism can be structural, like chain, position, or functional group isomers or geometric, cis-trans isomerism.
Effectively study hydrocarbons, focusing on understanding the fundamental concepts outlined in the chapter, including their structures and types. Utilise diagrams to visualise molecular structures, practice problems and exercises in the textbook, and make use of summary notes and flashcards to reinforce key terms and reactions.
Common reactions of hydrocarbons include combustion, where hydrocarbons react with oxygen to produce carbon dioxide and water; substitution reactions mainly for alkanes and aromatics; and addition reactions, which are typical for alkenes and alkynes.
Aromatic hydrocarbons, such as benzene, contain one or more rings that exhibit resonance stability due to alternating double bonds. This is different from aliphatic hydrocarbons, which do not possess this special structure and stability.
Hydrocarbons are primarily divided into two categories: saturated hydrocarbons alkanes and unsaturated hydrocarbons alkenes and alkynes. Saturated hydrocarbons contain only single bonds between carbon atoms, while unsaturated hydrocarbons have one or more double or triple bonds.
Class 11 Chemistry Hydrocarbons notes help students quickly revise key concepts, reactions, and properties of alkanes, alkenes, alkynes, and aromatic compounds. They simplify complex topics, highlight important formulas and mechanisms, and provide a structured approach for effective exam preparation.
The chapter 9 hydrocarbons notes deals with the study of compounds made up of carbon and hydrogen. It covers the classification, structure, properties, and reactions of alkanes, alkenes, alkynes, and aromatic hydrocarbons. The chapter also explains isomerism, methods of preparation, and important reactions like substitution, addition, and elimination, forming a foundation for organic chemistry in higher classes.
In alkanes Carbon is bonded by a single bond. Alkanes are saturated hydrocarbons, and they are also known as paraffins.
Their general formula is CnH2n+2
Where n is representing the number of carbon atoms.
Physical properties of alkanes are:
- Lower alkanes are in gaseous state, middle alkanes are in liquid state while higher alkanes are solid.
- Alkanes are insoluble in water but soluble in ether and benzene.
- Density of alkanes is less than water.
- Alkanes are odourless and colourless
- Alkanes are poor conductor of electricity
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters














