Some basic concepts of Chemistry Class 11th Notes - Free NCERT Class 11 Chemistry Chapter 1 Notes - Download PDF
Some Basic Concepts of Chemistry: the real starting point of your chemistry journey! This chapter is like the ABCs of the subject, where you get to know what matter is made of, how atoms and molecules interact, and why every tiny calculation in chemistry counts. It will form the basis for other chapters that you will learn later on. Some basic concepts of Chemistry Class 11th Notes covers essential topics such as the mole concept, molar mass, chemical equations, stoichiometry, chemical calculations and the law of conservation of mass.
This Story also Contains
- NCERT Notes for Class 11 Chemistry Chapter 1 Download PDF
- NCERT Notes for Class 11 Chemistry Chapter 1
- Some Basic Concepts of Chemistry Previous Years Questions and Answers
- How to Master Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
- Advantages of Using Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Notes
- NCERT Class 11 Notes Chapter-Wise
- NCERT Solutions for Class 11 Chemistry
- NCERT Exemplar Class 11 Solutions Subject-wise
- NCERT Solution subject-wise

The NCERT notes for Class 11 Chemistry will help you revise these concepts in the easiest way possible. These some basic concepts of chemistry ncert notes will not only help you in your exams but will also improve your understanding of the topics. The important formulas are well discussed in these notes. The tables and charts are also included to help you learn better.
NCERT Notes for Class 11 Chemistry Chapter 1 Download PDF
These concise notes cover all the key concepts of Chapter 1 to help students in quick revision before exam. You can download class 11 chemistry chapter 1 some basic concepts of chemistry notes pdf from the button given below. These NCERT notes are useful either you are learning concepts for the first time or revising them before exams.
Also Read
NCERT Notes for Class 11 Chemistry Chapter 1
If you want to make your learning easier and interesting, then you are at the right place. These some basic concepts of chemistry Class 11 Chemistry Chapter 1 CBSE notes contain everything from simple to complex topics that will help you a lot in your exams. Scroll down to know more!
1.1 Importance Of Chemistry
Chemistry is a study that investigates the composition, structure, and properties of matter as well as the changes that occur during chemical reactions. It is derived from the Egyptian word kme (chem), which means earth. Because it connects physical sciences, including chemistry, with life sciences and applied sciences, chemistry is sometimes referred to as a core science, such as medicine and engineering. Students can refer to the NCERT Solutions for Class 11 Chemistry Chapter 1 to gain a clearer understanding of these concepts through detailed, solved questions.
The branches of chemistry are as follows
Physical chemistry
The branch of chemistry that studies macroscopic and physical processes in the universe. It deals with the effect of a substance's physical properties on its chemical properties as well as its structure.
Inorganic chemistry
Inorganic chemistry is the field of chemistry that investigates substances that do not contain carbon or hydrogen atoms. To put it another way, it is the reverse of organic chemistry. Metals, salts, and chemicals are examples of substances that do not have carbon-hydrogen bonds.
Organic chemistry
Organic chemistry is the branch of science that studies the structure, composition, and chemical characteristics of organic molecules. It entails the investigation of carbon and its compounds.
Biochemistry
Biochemistry is the discipline of chemistry that studies the chemical processes that occur in and around organisms. It's a science that combines biology and chemistry in a laboratory setting. Biochemists can comprehend and address biological problems by applying chemical knowledge and technology.
Analytical chemistry
It is a discipline of chemistry that employs equipment and analytical procedures to figure out a substance's structure, functionality, and qualities.
1.2 Nature of matter
Anything that has mass and occupies space is called matter.
1.2.1 States of matter
In general, matter is grouped into three categories
Solids
Solids have the least flexibility of movement and have a distinct shape and volume. E.g., sugar, stone.
Liquids
The term "liquid" refers to a substance that has the shape of a container but has a fixed volume. Liquids can also flow or be poured. Water, milk, oil, mercury, and alcohol are a few examples.
Gas
Gases are substances that have neither a defined volume nor a defined shape. Gases usually entirely fill the container in which they are stored. For example, hydrogen, oxygen, and so on.
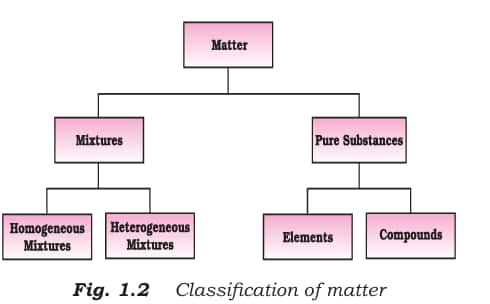
1.2.2. Classification of Matter
At the bulk or macroscopic level, matter is classified into two
-
Mixtures
-
Pure substance
Mixtures
A mixture is a material that contains two or more chemicals in any proportion. It is mostly divided into two types: heterogeneous and homogeneous mixtures
Homogeneous mixtures
Homogeneous mixtures form when two substances are mixed to form a single uniform phase. In homogeneous mixtures, the composition of substances present is uniform. Sugar solution and air are two examples of homogeneous mixtures.
Heterogeneous mixtures
Heterogeneous Mixtures are those in which two or more substances are combined to produce a combination with a non-uniform composition. Suspensions, which are made up of two solids, such as salt and sugar, are an example.
Pure substances
A pure substance is a material that contains only one type of particle. The following are the divisions of pure substances
Elements
An element is a pure material that contains only one type of atom and can't be broken down any further. Silver, hydrogen, oxygen, etc, are examples.
Compounds
A compound is a pure material made up of two or more elements combined in a certain mass proportion. Furthermore, the properties of a composite differ from those of its constituent parts. Additionally, physical methods cannot separate the parts of a complex into simpler compounds. Chemical procedures are the only way to separate them. Examples include water, ammonia, carbon dioxide, etc.
1.3 Properties of Matter and Their Measurements
Every substance has its own distinct or distinguishing qualities. The two categories of attributes that are observed are physical and chemical properties. Students can refer to the NCERT Solutions for Class 11 Chemistry Chapter 1 to gain a clearer understanding of these concepts through detailed, solved questions.
1.3.1 Physical and chemical properties
Physical properties
Physical attributes are those that may be measured or observed without changing the identity or composition of a substance. Colour, aroma, melting point, boiling temperature, density, and other physical characteristics are examples.
Chemical properties
Chemical properties are the characteristics of a substance that can be observed during a chemical reaction. Flammability, toxicity, heat of combustion, pH, radioactive decay rate, and chemical stability are some of the most important chemical properties.
1.3.2 Measurement of physical properties
Physical quantities are those that we come upon during our scientific research. A physical quantity includes a number followed by a unit. The reference standard used to measure any physical quantity is referred to as a unit.
1.3.3 The International System of Units (SI)
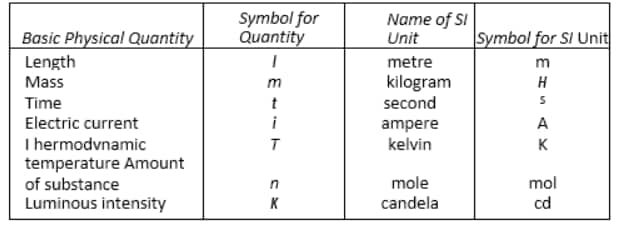
The eleventh General Conference on Weights and Measures developed the International System of Units. The CGPM is an intergovernmental body established by the Meter Convention, a diplomatic convention signed in Paris in 1875. The SI system has seven base units. The seven essential scientific quantities are represented by these units. These quantities can be used to generate other physical quantities such as speed, volume, density, and so on.
1.3.4 Mass and Weight
A substance's mass is the amount of substance it contains, whereas its weight is the force of gravity acting on the object. The mass of matter is constant, but its weight varies from place to place due to changes in gravity. The kilogram is the SI unit of mass (kg). Newton is the SI derived unit of weight (derived unit of the SI base unit).
1.3.5 Volume
The amount of three-dimensional space surrounded by specific closed boundaries, such as the space occupied or contained by a material (solid, liquid, gas, or plasma), or the space occupied or contained by a shape. The most common way to measure volume is to use SI-derived units (cubic meters).
1.3.6 Density
A material's mass density, sometimes known as density, is defined as its mass per unit volume. The density is symbolised by the symbol ρ (the lowercase Greek letter rho). The SI unit for density is kilograms per cubic metre.
1.3.7 Temperature
The term "temperature" refers to a physical attribute of matter that quantifies the concepts of heat and cold. There are three popular temperature scales: Celsius, Fahrenheit, and (Fahrenheit) (Kelvin.
${ }^{\circ} \mathrm{F}=9 / 5\left({ }^{\circ} \mathrm{C}\right)+32$
$\mathrm{K}={ }^{\circ} \mathrm{C}+273.15$
There are some prefixes that are used in the SI system. The values in the table below will help you with unit conversions.
|
Multiple |
Prefix |
Symbol |
|
10⁻²⁴ |
yocto |
y |
|
10⁻²¹ |
zepto |
z |
|
10⁻¹⁸ |
atto |
a |
|
10⁻¹⁵ |
femto |
f |
|
10⁻¹² |
pico |
p |
|
10⁻⁹ |
nano |
n |
|
10⁻⁶ |
micro |
µ |
|
10⁻³ |
milli |
m |
|
10⁻² |
centi |
c |
|
10⁻¹ |
deci |
d |
|
10¹ |
deca |
da |
|
10² |
hecto |
h |
|
10³ |
kilo |
k |
|
10⁶ |
mega |
M |
|
10⁹ |
giga |
G |
|
10¹² |
tera |
T |
|
10¹⁵ |
peta |
P |
|
10¹⁸ |
exa |
E |
|
10²¹ |
zeta |
Z |
|
10²⁴ |
yotta |
Y |
1.4 Uncertainty in Measurements
In the study of chemistry, one must frequently deal with both experimental data and theoretical computations. There are practical methods for dealing with numbers and realistically presenting facts, to the extent possible.
1.4.1 Scientific Notation
While performing mathematical operations on numbers expressed in scientific notation, the following points are to be kept in mind.
Multiplication & Division
Follow exponent rules
- Multiply the coefficients
- Add or subtract the exponents
For example,
a) Multiplication-
$
\left(5.6 \times 10^5\right) \times\left(6.9 \times 10^8\right)=38.64 \times 10^{13}$
$=3.864 \times 10^{14}
$
b) Division-
$
\frac{9.8 \times 10^{-2}}{2.5 \times 10^{-6}}=3.92 \times 10^4
$
Addition & Subtraction
Make the exponents the same, then add/subtract the coefficients
For example,
a) Addition
$
\left(6.65 \times 10^4\right)+\left(8.95 \times 10^3\right)$
$=(6.65+0.895) \times 10^4=7.545 \times 10^4
$
b) Subtraction
$
\left(2.5 \times 10^{-2}\right)-\left(4.8 \times 10^{-3}\right)$
$=(2.5-0.48) \times 10^{-2}=2.02 \times 10^{-2}
$
1.4.2 Significant Figures
All experimental measurements are subject to some degree of ambiguity. When we talk about measurement, precision and accuracy are frequently mentioned. Significant figures are digits that are certain to be relevant.
Precision
Precision refers to the consistency of different measurements for the same amount. Accuracy, on the other hand, is the agreement of a given value with the true value of the result.
1.4.3 Dimensional analysis
We frequently have to convert units from one system to another in calculations. The factor label approach, also known as the unit factor method or dimensional analysis, is used to do this.
1.5 Laws of Chemical Combination
1.5.1 law of conservation of mass
“The mass in an isolated system can neither be created nor destroyed but can be transformed from one form to another.” According to the law of conservation of mass, in a low-energy thermodynamic process, the mass of the reactants must be equal to the mass of the products. This law was proposed by Antoine Lavoisier in 1789.
1.5.2 Law of Definite Proportions
The mass proportions of the constituents in a composite sample are always the same, according to the law of definite proportions. It was given by Joseph Proust, a French chemist.
1.5.3 law of multiple proportions
When two elements are mixed to make more than one compound, the weight of one element is proportionate to the fixed weight of the other element as a whole number, according to the law of multiple proportions. Dalton proposed this law in 1803.
1.5.4 Gay Lussac’s Law of Gaseous Volumes
"The link between the volume of a gaseous reactant and a product can be represented by a simple whole number," according to Gay Lussac's law, developed in 1808
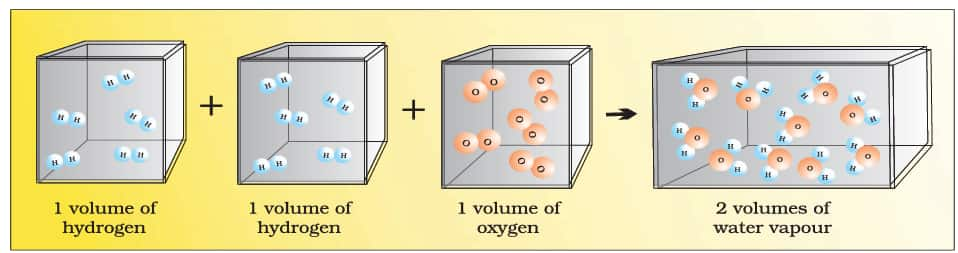
1.5.5 Avogadro Law
Avogadro postulated in 1811 that the number of molecules in equal quantities of gases at the same temperature and pressure should be the same.
Here, in the figure below, two volumes of hydrogen gas combine with one volume of oxygen gas to form two volumes of water vapour, showing that hydrogen and oxygen exist as diatomic molecules.
1.6 Dalton’s Atomic Theory
Dalton wrote 'A New System of Chemical Philosophy' in 1808, in which he proposed:
-
Atoms are the building blocks of matter.
-
A given element's atoms all share the same properties, including the same mass. The mass of atoms in different elements differs.
-
When atoms of different elements mix in a specific ratio, compounds are produced.
-
Reorganization of atoms occurs during chemical processes. In a chemical reaction, they are neither generated nor destroyed.
1.7 Atomic and Molecular Masses
1.7.1 atomic mass
Atomic mass is defined as the mass of a single atom, expressed in atomic mass units (amu) or unified mass units (u)
1 a.m.u = 1/12 th mass of 1 carbon-12 atom
$1 \mathrm{amu}=1.66056 \times 10^{-24} \mathrm{~g}$
1.7.2 Average atomic mass
The average atomic mass of an element can be estimated from distinct isotopes of an element based on their relative abundance.
For example, there are three isotopes of C – (12C, 13C, 14C) with their relative abundance (%), 98.892, 1.108, and 2 × 10-10 with their atomic mass (AMU) as 12, 13.00335, and 14.00317, respectively.
The average atomic mass of carbon will be
$=(0.98892)(12 u)+(0.01108)(13.00335 u)+\left(2 \times 10^{-12}\right)(14.00317 u)$
= 12.011 u.
1.7.3 Molecular mass
The masses of the atoms that make up a molecule are added together to determine its mass. As a result, the two methods for measuring the mass of an atom, amu and g / mol, can also be used to represent the mass of a molecule.
1.7.4 Formula mass
Formula mass is used for compounds like NaCl, in which positive and negative entities are arranged in a three-dimensional structure.
Formula mass of NaCl = Atomic mass of sodium + Atomic mass of chlorine
= 23.00 u + 35.5 u = 56.5 u.
1.8 Mole Concept
Mole is a unit of amount of substance. A mole is defined as the amount of something that includes the same number of elementary particles (atoms, molecules, or ions) as 12 g of carbon dioxide (C-12).
1 mol of any substance contains $6.023 \times 10^{23}$ particles. $6.023 \times 10^{23}$ is known as the Avogadro constant or Avogadro number.
Eg : 1 mole water = $6.023 \times 10^{23}$ water molecules
The mass of 1 mole of a substance is called the molar mass.
1.9 Percentage Composition
Mass% of an element = Mass of that element in the compound* 100/Molar mass of the compound
Eg : Molar mass of water = 18.02 g/mol
Mass % of hydrogen = 11.18
Mass % of oxygen = 88.79
1.9.1 Empirical formula for molecular formula
The compound's formula that gives the simplest whole-number ratio of the atoms of various elements present in a single molecule is called the empirical formula.
The actual ratio of the atoms of various elements present in one molecule of a compound is given by the substance's molecular formula.
1.10 Stoichiometry and Stoichiometric Calculations
Stoichiometry is the study of chemical processes and calculations. The stoichiometric coefficient is the coefficient that is utilized to balance the reaction. The stoichiometric coefficient, rather than the mass, is the ratio of moles of molecules of atoms that react. The stoichiometric ratio can only be used to forecast the number of moles of product generated when all reactants are present in a stoichiometric ratio. The real quantity of product created is always smaller than the theoretical calculation predicts.
1.10.1 Limiting reagent
If the reagents are not employed in a stoichiometric ratio, the limiting reagent, which is less than the needed amount, dictates how much product is created, and the excess reagent is called the excess reagent. When we burn carbon in the air (which has an infinite supply of oxygen), the amount of CO2 created is proportional to the amount of carbon burned. Carbon is the limiting reactant in this situation, whereas O2 is the excess reactant.
1.10.2 Reactions in Solutions
Because the two solids cannot react in the solid state, they must be dissolved in a liquid. When solutes dissolve in a solvent, they form a solution, which is a single phase in which they coexist. The strength of the solution is measured using several factors. The amount of solute in a solution is indicated by the strength of the solution.
Mass percentage
It is the mass of the solute in grams per 100 grams of solution. A 10 % solution of sodium chloride, for example, contains 10 g of NaCl per 100 g of solution.
Mass $\%$ of the solute $=\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100$
Mole fraction
The mole fraction of any component of a solution is the ratio of that component's number of moles to the total number of moles of all the solution's components.
Mole fraction of A,
$X_A=\frac{\text { No. of moles of } A}{\text { No. of moles of } A+\text { No. of moles of } B}$
$=\frac{n_{\mathrm{A}}}{n_{\mathrm{A}}+n_{\mathrm{B}}}$
and the mole fraction of B
$\mathrm{X}_{\mathrm{B}}$ = $\frac{\text { No. of moles of B }}{\text { No. of moles of A + No. of moles of B }}
$
$\begin{aligned} & =\frac{n_{\mathrm{B}}}{n_{\mathrm{A}}+n_{\mathrm{B}}} \\ \mathrm{X}_{\mathrm{A}}+\mathrm{X}_{\mathrm{B}} & =\frac{n_{\mathrm{A}}}{n_{\mathrm{A}}+n_{\mathrm{B}}}+\frac{n_{\mathrm{B}}}{n_{\mathrm{A}}+n_{\mathrm{B}}} \\ & =\frac{n_{\mathrm{A}}+n_{\mathrm{B}}}{n_{\mathrm{A}}+n_{\mathrm{B}}}=1 \\ \mathrm{X}_{\mathrm{A}}+\mathrm{X}_{\mathrm{B}} & =1 \end{aligned}$
Molarity
The number of moles of solute dissolved per dm3 (or litre, L) of a solution is referred to as its molarity. Temperature affects the molarity of any solution. As a result, any solution's molarity is specified for a specific temperature.
Molarity of solution $=\frac{\text { No. of moles of the solute }}{\text { Volume of the solution in litres (or in } \mathrm{dm}^3 \text { ) }}=\frac{n \mathrm{}}{\mathrm{V}}$
Molality
The number of moles of solute per kg of solvent is the molality of a solution. If n moles of a solute are dissolved in W kg of solvent to make a solution,
Molality, $(m)=\frac{\text { No. of moles of solute }}{\text { Mass of the solvent in kg. }}$
Some Basic Concepts of Chemistry Previous Years Questions and Answers
Given below important questions that will help you understand the chapter better and strengthen your exam preparation. The concepts used to solve these questions are explained in some basic concepts of chemistry Class 11 Chemistry Chapter 1 CBSE notes.
Question 1: On combustion 0.210 g of an organic compound containing $\mathrm{C}, \mathrm{H}$ and O gave $0.127 \mathrm{~g} \mathrm{H}_2 \mathrm{O}$ and $0.307 \mathrm{~g} \mathrm{CO}_2$. The percentages of hydrogen and oxygen in the given organic compound are:
(1) $53.41,39.6$
(2) $6.72,53.41$
(3) $7.55,43.85$
(4) $6.72,39.87$
Answer:
Mass of organic compound $=0.210 \mathrm{~g}$
Mass of water formed $=0.127 \mathrm{~g}$
Mass of $\mathrm{CO}_2$ formed $=0.307 \mathrm{~g}$
Mass of hydrogen $=\frac{0.127 \times 2}{18}=0.014 \mathrm{~g}$
Percentage of hydrogen $=\frac{0.014 \times 100}{0.210}=6.72 \%$
Mass of carbon $=\frac{0.307 \times 12}{44}=0.084 \mathrm{~g}$
Percentage of carbon $=\frac{0.084 \times 100}{0.210}=39.87 \%$
Percentage of oxygen $= 100- (39.87 + 6.72) = 53.41 \%$
Hence, the correct answer is option (2).
Question 2: Among $10^{-9} \mathrm{~g}$ (each) of the following elements, which one will have the highest number of atoms?
Element : $\mathrm{Pb}, \mathrm{Po}, \mathrm{Pr}$ and Pt
(1) Po
(2) Pr
(3) Pb
(4) Pt
Answer: $\text { No. of atoms }=\frac{\text { Mass}}{\text { MolarMas }(\mathrm{g} / \mathrm{mol})} \times \mathrm{N}_{\mathrm{A}}$
From this formula, it's clear that for a given mass, the element with the smallest molar mass will have the greatest number of atoms, as it appears in the denominator.
$\mathrm{M}_{\mathrm{P}_{\mathrm{o}}}=209$
$\mathrm{M}_{\mathrm{pr}}=141$
$\mathrm{M}_{\mathrm{Pb}}=207$
$\mathrm{M}_{\mathrm{Pt}}=195$
Pr has the lowest molar mass ( $M_{\mathrm{Pr}}=141 \mathrm{~g} / \mathrm{mol}$ ), $10^{-9}$ grams of Pr will contain the highest number of atoms compared to the other elements listed.
Hence, the correct answer is option (2).
Question 3: 16 g of oxygen has the same number of molecules as
(i) 16 g of CO
(ii) 28 g of $N_2$
(iii) 14 g of $N_2$
(iv)) 1.0 g of $\mathrm{H}_2$
Choose the answer from the options below
1) (i) and (ii)
2) (ii) and (iv)
3) (iii) and (iv)
4) None of the above
Answer:
The number of molecules of oxygen in 16g of oxygen is
$
\begin{aligned}
& \frac{16}{32} \times\left(6.023 \times 10^{23}\right) \\
& =0.5 \times\left(6.023 \times 10^{23}\right)_{\text { }}
\end{aligned}
$
Number of molecules of $\mathrm{N}_2$
$
\begin{aligned}
& =\frac{14}{28} \times\left(6.023 \times 10^{23}\right) \\
& =0.5 \times\left(6.023 \times 10^{23}\right)_{\text {}}
\end{aligned}
$
Number of molecules of $\mathrm{H}_2$
$
\begin{aligned}
& =\frac{1}{2} \times 6.023 \times 10^{23} \\
& =0.5 \times\left(6.023 \times 10^{23}\right)_{\text { }}
\end{aligned}
$
Hence, the correct answer is option (3).
Question 4: x mg of $\mathrm{Mg}(\mathrm{OH})_2($ molar mass $=58)$ is required to be dissolved in 1.0 L of water to produce a pH of 10.0 at 298 K . The value of x is ______ mg. (Nearest integer)
(Given : $\mathrm{Mg}(\mathrm{OH})_2$ is assumed to dissociate completely in $\mathrm{H}_2 \mathrm{O}$ )
Answer:
$\begin{aligned}
& \mathrm{pH}=10 \\
& \mathrm{pOH}=4 \\
& {\left[\mathrm{OH}^{-}\right]=10^{-4}}
\end{aligned}$
no. of moles of $\mathrm{OH}^{-}=10^{-4}$
$\begin{aligned}
& \text { no. of moles of } \mathrm{Mg}(\mathrm{OH})_2=\frac{10^{-4}}{2}=5 \times 10^{-5} \\
& \begin{aligned}
\text { mass of } \mathrm{Mg}(\mathrm{OH})_2 & (x)= moles \times molar mass \\
&5 \times 10^{-5} \times 58 \times 10^3 \mathrm{mg} \\
& =2.9 ~mg
\end{aligned}
\end{aligned}$
Hence, the answer is 2.9
Question 5: The amount of calcium oxide produced on heating 150 kg limestone ( $75 \%$ pure) is ______ kg. (Nearest integer)
Given : Molar mass (in $\mathrm{g} \mathrm{mol}^{-1}$ ) of $\mathrm{Ca}-40, \mathrm{O}-16, \mathrm{C}-12$
Answer:
Given that
Mass of limestone $=150 \mathrm{~kg}$
Purity $=75 \%\left(\right.$ pure calcium carbonate, $\left.\mathrm{CaCO}_3\right)$
Molar masses:
- $\mathrm{Ca}=40 \mathrm{~g} / \mathrm{mol}$
- $C=12 \mathrm{~g} / \mathrm{mol}$
- $O=16 \mathrm{~g} / \mathrm{mol}$
$ \mathrm{CaCO}_3 \rightarrow \mathrm{CaO}+\mathrm{CO}_2 $
${ mass ~of } ~\mathrm{CaCO}_3=\frac{150 \times 75}{100}=112.5 \mathrm{~kg}$
molar mass of $\mathrm{CaCO}_3$
$M_{\mathrm{CaCO}_3}=40+12+(3 \times 16)=40+12+48=100 \mathrm{~g} / \mathrm{mol}$
Moles of $\mathrm{CaCO}_3$
Mass in grams $=112.5 \times 1000=112500 \mathrm{~g}$
$
n=\frac{112500}{100}=1125 \mathrm{~mol}
$
Molar mass of CaO :
$$
M_{\mathrm{CaO}}=40+16=56 \mathrm{~g} / \mathrm{mol}
$$
Mass of CaO produced:
$
m=n \times M_{\mathrm{CaO}}=1125 \times 56=63000 \mathrm{~g}=63 \mathrm{~kg}
$
Hence, the answer is 63 kg.
How to Master Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry
Mastering this chapter requires a strong foundation in the fundamental concepts of chemistry. These NCERT notes Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry help to understand the basic concepts from your NCERT.
- First, start by understanding the basics like the definition of chemistry, matter, elements, compounds, and mixtures.
- Then learn the concept of mole, like the importance of mole, Avogadro’s number, molar mass, and how to calculate the number of particles, atoms, or molecules in a substance.
- After that, practice questions related to mole concept, stoichiometry, empirical and molecular formulas. Students can refer to some basic concepts of chemistry class 11 ncert notes for understanding these concepts better.
- Students must learn how to calculate molecular masses for various compounds.
- After that students can solve previous year questions from this chapter.
Advantages of Using Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry Notes
The ncert class 11 chemistry chapter 1 some basic concepts of chemistry notes provide a clear and concise explanation of basic concepts. The advantages of using these notes are given below:
- Students can use these notes to understand the concepts like mole concept, atomic and molecular masses, and chemical equations.
- These notes cover formulas, definitions, and units.
- NCERT notes Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry notes are prepared by subject experts in a very clear and comprehensive manner.
- They are useful for developing a strong foundation for Class 12 and competitive exams.
NCERT Class 11 Notes Chapter-Wise
Along with class 11 chemistry chapter 1 some basic concepts of chemistry notes follow the links below and get access to the NCERT notes for class 11 of other chapters. Make your learning better!
NCERT Solutions for Class 11 Chemistry
Along with ncert class 11 chemistry chapter 1 some basic concepts of chemistry notes, students can also refer to solutions of NCERT for Class 11 Chemistry chapters.
NCERT Exemplar Class 11 Solutions Subject-wise
Students can refer to the links given below for the NCERT exemplar subject-wise solutions.
NCERT Solution subject-wise
The links provided in the table below will help give you the NCERT solutions for other subjects as well.
Frequently Asked Questions (FAQs)
Concentration is the measure of how much solute is dissolved in a specific volume of solvent or solution. It can be expressed in various ways, such as molarity (moles of solute per liter of solution), mass percent, or volume percent.
The limiting reactant is the substance that is completely consumed first in a chemical reaction, limiting the amount of product that can be formed.
Balancing chemical equations is important because it reflects the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
A chemical reaction is a process where reactants convert into products through the rearrangement of atoms. It is represented by a chemical equation, where the reactants are shown on the left side and the products on the right, often separated by an arrow.
The empirical formula represents the simplest whole-number ratio of the elements in a compound, while the molecular formula shows the actual number of atoms of each element in a molecule of the compound.
PDFs make it easy for students to access, download, and study chapter-wise notes anytime, anywhere. They provide a convenient, organised, and portable format for quick learning and exam revision.
Class 11 Chemistry NCERT notes are concise study materials based on the NCERT textbook. They summarise important concepts, definitions, formulas, and reactions chapter-wise, helping students understand the subject quickly, revise efficiently, and prepare effectively for exams.
You can download some basic concepts of chemistry Class 11 Chemistry Chapter 1 CBSE notes in PDF form from Careers360, which provides concise NCERT-based summaries for easy learning and quick revision.
Stoichiometry deals with the calculation of the quantities of products and reactants involved in chemical reactions. It involves determining the proportions in which substances react or are produced using the relationships between the amounts of substances in a balanced chemical equation.
The atomic mass of an element is the mass of one atom of that element compared to 1/12th the mass of a carbon-12 atom. It is expressed in atomic mass units (amu) or unified atomic mass units (u).
The molar mass of a substance is the mass of one mole of that substance. It is expressed in grams per mole (g/mol) or grams (g).
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
