These redox reactions class 11 question answer help you think deeper and apply what you’ve learned in new ways. They are meant to make you solve problems creatively and understand the chapter better, not just memorize facts.
NCERT Solutions for Class 11 Chemistry Chapter 7 - Redox Reactions
Have you ever thought about how the process of rusting of iron takes place? How does metabolism in our body take place? The answer to all these questions lies in NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reactions. These reactions play a crucial role in understanding important phenomena, industrial processes, workings of batteries, combustion of fuels, etc. The topics discussed in Redox Reactions provide detailed descriptions of various topics, such as the process of oxidation and reduction, the change in oxidation state of elements during chemical reactions, and different types of oxidation reactions.
This Story also Contains
- NCERT Solutions for Class 11 Chemistry Chapter 7: Download PDF
- NCERT Solutions for Class 11 Chemistry Chapter 7 (Exercise Questions)
- Class 11 Chemistry NCERT Chapter 7: Higher Order Thinking Skills (HOTS) Questions
- Approach to Solve Questions of Class 11 Chemistry Chapter 7
- Topics and Subtopics Covered in the NCERT Textbook Class 11 Chemistry Chapter 7
- What Extra Should Students Study Beyond the NCERT for JEE/NEET?
- What Students Learn from NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reactions
- Importance of Class 11 Chemistry Chapter 7 Redox Reactions Solutions
- NCERT Solutions for Class 11 Chemistry Chapter-Wise
- NCERT Solutions for Class 11 Subject-Wise
- NCERT Books and NCERT Syllabus:

The NCERT Solutions are prepared by our subject experts, who provide all the answers to the exercise questions given in the NCERT textbook. By referring to this article, students can understand all the important concepts through a series of solved questions. These NCERT Solutions for Class 11 Chemistry will help students to clear their doubts, improve analytical thinking, enhance application skills, and build confidence in chemistry. Some selected HOTS questions and approaches to solve questions are also included in this article.
NCERT Solutions for Class 11 Chemistry Chapter 7: Download PDF
Students can download the class 11 chemistry chapter 7 redox reactions solutions pdf for free. These solutions of NCERT are designed to help you understand the fundamental concepts and solve textbook questions with ease.
Also Read,
NCERT Solutions for Class 11 Chemistry Chapter 7 (Exercise Questions)
Given below the detailed and accurate class 11 chemistry chapter 7 redox reactions question answer. These NCERT Solutions for Class 11 will help in better concept clarity and exam preparation.
Question 7.1 Assign oxidation number to the underlined elements in each of the following species
$(a) NaH_{2}\bar{P}O_{4}$
$(b)NaH\bar{S}O_{4}$
$(c) H_{4}\bar{P_{2}}O_{7}$
$(d) K_{2}\bar{Mn}O_{4}$
$(e) Ca\bar{O_{2}}$
$(f) Na\bar{B}H_{4}$
$(g) H_{2}\bar{S_{2}}O_{7}$
$(h) KAl(\bar{S}O_{4})_{2}.12H_{2}O$
Answer :
O.N is the oxidation number
O.N of Oxygen( $O$ ) = -2 ( In case of peroxide and superoxide it wil be different ON)
O.N of hydrogen( $H$ )= +1 (In case of metalic hydride, -1)
O.N of sodium ( $Na$ ) = +1
O.N of aluminium ( $Al$ ) = +3
O.N of potassium ( $K$ )= +1
O.N of calcium ( $Ca$ ) = +2
In neutral compounds, the sum of O.N of all the atoms is zero.
(a) Let the O.N of P be x
$\therefore\:\:\:1\ast 1+2\ast 1+x+4\ast (-2) = 0 \Rightarrow x = +5$
(b) Let the O.N. of S be x
$\therefore \:\:1\ast 1 + 1\ast 1+x +4\ast (-2) = 0\Rightarrow x = +6$
(c) Let the O.N. of P be x
$\therefore \:\:4*1 +2*x +7*(-2) = 0 \Rightarrow x = +5$
(d) Let the O.N. of Mn be x
$\therefore \:\:2*1 + x + 4*(-2) = 0\Rightarrow x = +6$
(e) Let the O.N. of O be x
Ca is an alkaline earth metal, so its O.N. is +2
$\therefore \:\:1\ast 2 + 2\ast x = 0\Rightarrow x = -1$
(f) Let the O.N. of B be x
Note that in this, H exists as a hydride ion, H− so its O.N. is -1
$\therefore \:\:1*1+x+4*(-1) = 0 \Rightarrow x = +3$
(g) Let the O.N. of S be x
$\therefore \:\: 2*1 +2*x+7*(-2) =0 \Rightarrow x = +6$
(h) Let the O.N. of S be x
$\therefore \:\:1*1+1*3+[x+(-2)*4]*2 +12*[1*2+(-2)] = 0\Rightarrow x = +6$
$(a) K\underline{I}_{3}$
$(b) H_{2}\underline{S_{4}}O_{6}$
$(c) \underline{Fe}_{3}O_{4}$
$(d) \underline{C}H_{3}\underline{C}H_{2}OH$
$(e) \underline{C}H_{3}\underline{C}OOH$
Answer :
Solution-
O.N of potassium ( $K$ )= +1
O.N of hydrogen( $H$ )= +1 (In case of metalic hydride, -1)
O.N of Oxygen( $O$ ) = -2 ( In case of peroxide and superoxide, it will be different ON)
$(a) K\underline{I}_{3}$
1*1 + 3*x = 0
x = (-1/3)
average O. N. of $I$ is $-\frac{1}{3}$
But it is wrong because O.N. cannot be fractional. So let's try with the structure of $KI_{3}$
O.N of I = -1 (because a coordinate bond is formed between $I_{2}$ and I− ion. Hence, O.N of three I atoms are 0,0 and -1, O.N 0 in $I_{2}$ molecule and -1 in I− ion.)
$K^+(I-I\leftarrow I)^{-1}$
O.N of $I$ = -1 (because a coordinate bond is formed between $I_{2}$ and $I^{-}$ ion. Hence O. N of three $I$ atoms are 0,0 and -1, O.N 0 in $I_{2}$ molecule and -1 in $I^{-}$ ion.)
$(b) H_{2}\underline{S_{4}}O_{6}$
Assume O.N of S is x
$2*1 + 4*x + 6*(-2) = 0 \Rightarrow x=2.5$
Fractional O.N. is not possible, so try with structure -
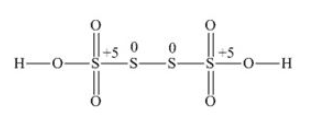
The two S atoms present in the middle has zero (0) O.N and other two have (+5) O.N [ -2 from the two O atoms and -1 from OH]
$(c) \underline{Fe}_{3}O_{4}$
If you calculate the oxidation number of Fe in $Fe_{3}O_{4}$ it would be 8/3 and however, O.N cannot be in fractional.
$Fe_{3}O_{4} \:\:\: is \:an\:equimolar \:mixture\: of \:(FeO)\: and \:(Fe_{2}O_{3})$ Here one iron atom has +2 O.N and the other two are of +3 O.N.
$(d) \bar{C}H_{3}\bar{C}H_{2}OH$
let assume carbon has x oxidation Number
So,[ x + 1(3) +x +1(2) +(-2)+1 = 0]
2x = - 4
x = - 2
In this molecule, two carbon atoms are present in different environments. Hence, they cannot have the same O.N. Thus, C exhibits the O.S. of -3 and -1.
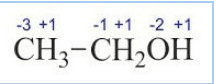
$(e) \bar{C}H_{3}\bar{C}OOH$
Suppose the oxidation number of Carbon is x.
If we calculate the O.N of x, we get x=0
However, 0 is the average O.N. of C atoms. In this molecule, two carbon atoms are present in different environments. Hence, they cannot have the same O.N. Thus, C exhibits the O.S of +3 and -3 and +2 in CH3COOH. This can be better understood by the structure-
.png)
Here we can see that at right C, -3 O.N (-1 from OH and -2 from O atom) and in left C, +3 O.N(contribution from H atom only)
Question 7.3(a) Justify that the following reactions are redox reaction
$\mathrm{CuO}(s)+\mathrm{H}_2(g) \rightarrow \mathrm{Cu}(s)+\mathrm{H}_2 \mathrm{O}(g)$
Answer: Let us write the O.N. of each element
$\overset{\:\:2+\:\:-2}{CuO}\:+\:\overset{0}{H_{2}}\rightarrow \overset{0}{Cu}\:+ \:\overset{+1\:-2}{H_{2}O}$
Here, the O.N of Cu decreases from +2 to 0, i.e., CuO is reduced to Cu. Also, the O.N. of H increases from 0 to +1, i.e., H2 is oxidised to H2O. Hence, it is a redox reaction.
Question 7.3(b) Justify that the following reactions are redox reactions
$\mathrm{Fe}_2 \mathrm{O}_3(\mathrm{~s})+3 \mathrm{CO}(\mathrm{g}) \rightarrow 2 \mathrm{Fe}(\mathrm{s})+3 \mathrm{CO}_2(\mathrm{~g})$
Answer: Let us write the O.N. of each element
$\overset{\:\:+3\:\:-2}{Fe_{2}O_{3}}\:+ 3\overset{+2\:-2}{CO}\rightarrow 2\overset{0}{Fe}\:\:+$ $3\overset{+4\:\:-2}{CO_{2}}$
Here, the O.N. of Fe decreases from +3 to 0. Also, the O.N. of C increases from +2 to +4. Hence, it is a redox reaction.
Question 7.3(c) Justify that the following reactions are redox reaction
$4 \mathrm{BCl}_3(g)+3 \mathrm{LiAlH}_4(s) \rightarrow 2 \mathrm{~B}_2 \mathrm{H}_6(g)+3 \mathrm{LiCl}(s)+3 \mathrm{AlCl}_3(s)$
Answer: Let us write the O.N. of each element
$4\overset{+3\:\:-1}{BCl_{3}}\:\:+\:3\overset{+1\:\:+3\:\:-1}{LiAlH_{4}}\:\rightarrow\:2\overset{-3\:\:+1}{B_{2}H_{6}}\:\:+\:\:3\overset{+1\:-1}{LiCl}\:\:+\:3\overset{+3\:-1}{AlCl_{3}}$
Here, the O.N. of Fe decreases from +3 in BCl3 to –3 in B2H6. And, the O.N. of H increases from -1 in LiAlH4 to +1 in B2H6. Hence, it is a redox reaction.
Question 7.3(d) Justify that the following reactions are redox reaction
$2 \mathrm{~K}(\mathrm{~s})+\mathrm{F}_2(\mathrm{~g}) \rightarrow 2 \mathrm{KF}(\mathrm{s})$
Answer: We know that oxidation = loss of electrons by an atom
and reduction = gaining of e− by another atom
Here, K lose its electron and F accept it. Hence, it is a redox reaction
Question 7.3(e) Justify that the following reactions are redox reactions
$4 \mathrm{NH}_3(\mathrm{~g})+5 \mathrm{O}_2(\mathrm{~g}) \rightarrow 4 \mathrm{NO}(\mathrm{g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{g})$
Answer: Here, N (−3) → N (+2) oxidation reaction
and O (0) → O (−2) reduction reaction (oxidation state of oxygen is zero at molecular state )
Hence, it's a redox reaction
Question 7.4 Fluorine reacts with ice and results in a change
$\mathrm{H}_2 \mathrm{O}(\mathrm{s})+\mathrm{F}_2(\mathrm{~g}) \rightarrow \mathrm{HF}(\mathrm{g})+\mathrm{HOF}(\mathrm{g})$
Justify that this reaction is a redox reaction
Answer :
$\begin{array}{rcc}F_2 & \mathrm{HF} & \mathrm{HOF} \\ \text { Oxidation state of } F \rightarrow 0 & -1 & +1\end{array}$
Here, F is oxidised and reduced as well. So, it is a redox reaction.
Answer :
(i) $H_{2}SO_{5}$ let the oxidation number of sulphur be x
So,
$2*1+x+5(-2)= 0$
$2 + x - 10 = 0$
$x= +8$
There is a fallacy, Sulphur cannot have +8 oxidation state because it has a maximum +6 oxidation number, not more than that. The structure of $H_{2}SO_{5}$ is shown as follows:
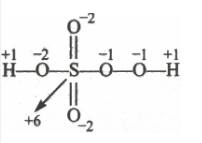
$2(H)+1(S)+3(O)+2(O \:\:in \:peroxy\: linkage)$
$\Rightarrow 2(+1) + 1(x) + 3(-2) + 2(-1) = 0$
$\Rightarrow x = +6( Answer))$
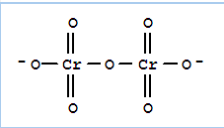
(ii) $Cr_{2}O_{7}^{2-}$
Let the oxidation number of chromium be x
now
$\\2x+7*(-2)=-2\\ $
$= (-2+14)=2x$
$x = 12/2$
$x= +6$
There is no fallacy here
(iii) $NO_{3}^{-}$
Let's assume the oxidation number of N is x
Now, $\\x+(-2)*3=-1$
$x-6 = -1$
$x= +5$
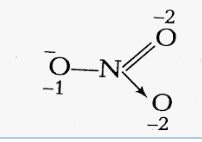
There is no fallacy about the O.N. of N in $NO_{3}^{-}$
Question 7.6(a) Write formulas for the following compounds:
Mercury(II) chloride
Answer :
$HgCl_{2}$
In this formula, we can see that mercury has $+2$ oxidation state
Question 7.6(b) Write formulas for the following compounds:
Nickel(II) sulphate
Answer:
NiSO4
Sulphate has a −2 oxidation state
Question 7.6(c) Write formulas for the following compounds:
Tin(IV) oxide
Answer:
SnO2
Oxygen has a −2 oxidation state
Question 7.6(d) Write formulas for the following compounds:
Thallium(I) sulphate
Answer:
$Tl_{2}SO_{4}$
Question 7.6(e) Write formulas for the following compounds:
Iron(III) sulphate
Answer:
Formula of the compounds: Iron(III) sulphate is
$Fe_{2}(SO_{4})_{3}$
Question 7.6(f) Write formulas for the following compounds:
Chromium(III) oxide
Answer :
The Formula of the Chromium(III) oxide compounds is
$Cr_{2}O_{3}$
Question 7.7 Suggest a list of the substances where carbon can exhibit oxidation states from –4 to +4 and nitrogen from –3 to +5
Answer :
The substance of carbon -
|
$CH_{4}$ |
-4 |
|
$C_{2}H_{6}$ |
-3 |
|
$C_{2}H_{4}\: or\: CH_{3}Cl$ |
-2 |
|
$C_{2}H_{2}$ |
-1 |
|
$CH_{2}Cl_{2}$ |
0 |
|
$C_{6}Cl_{6}$ |
+1 |
|
$CHCl_{3}$ |
+2 |
|
$(COOH)_{2}$ |
+3 |
|
$CO_{2} \: or\: CCl_{4}$ |
+4 |
|
Substance |
O.N. Of C |
|---|
Substance for Nitrogen-
|
$NH_{3}$ |
-3 |
|
$N_{2}H_{4}$ |
-2 |
|
$N_{2}H_{2}$ |
-1 |
|
$N_{2}$ |
0 |
|
$N_{2}O$ |
+1 |
|
$NO$ |
+2 |
|
$N_{2}O_{3}$ |
+3 |
|
$N_{2}O_{4}$ |
+4 |
|
$N_{2}O_{5}$ |
+5 |
|
Substance |
O.N. Of N |
|---|
Answer :
- Sulphur dioxide (SO2) here oxidation state of sulphur is +4, and the range of oxidation number of S is from -2 to +6. It means it can accept an electron and lose as well; therefore, it can behave as an oxidant and a reductant.
- In case of hydrogen peroxide $(H_{2}O_{2})$ oxidation state is -1, and the oxidation state of O can vary from 0 to -2. So it shows both oxidising and reducing properties.
- For $HNO_{3}$, Nitrogen has a +5 oxidation state and it varies from +5 to -3. So it only accepts electrons. The oxidation state of N only decreases. Hence, it acts as the only oxidant.
- And in case of $O_{3}$, the oxidation state of O is zero(0), and the range of oxidation number of O is 0 to -2. It only decreases in this case also so it acts as only an oxidant.
Question 7.9 Consider the reactions:
(a) $6 \mathrm{CO}_2(g)+6 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(a q)+6 \mathrm{O}_2(g)$
(b) $\mathrm{O}_3(g)+\mathrm{H}_2 \mathrm{O}_2(l) \rightarrow \mathrm{H}_2 \mathrm{O}(l)+2 \mathrm{O}_2(g)$
Why is it more appropriate to write these reactions as :
(a) $6 \mathrm{CO}_2(g)+12 \mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(a q)+6 \mathrm{H}_2 \mathrm{O}(l)+6 \mathrm{O}_2(g)$
(b) $\mathrm{O}_3(g)+\mathrm{H}_2 \mathrm{O}_2(l) \rightarrow \mathrm{H}_2 \mathrm{O}(l)+\mathrm{O}_2(g)+\mathrm{O}_2(g)$
Also, suggest a technique to investigate the path of the above (a) and (b) redox reactions.
Answer :
(a) In the photosynthesis process-
Step 1- the liberation of $O_{2}$ and $H_{2}$ --> $2H_{2}O\rightarrow O_{2}+2H_{2}$
Step-2 The $H_{2}$ produced in above reduces the $CO_{2}$ into glucose $(C_{6}H_{12}O_{6})$ and water $(H_{2}O)$
$6CO_{2}+12H_{2}\rightarrow C_{6}H_{12}O_{6}+6H_{2}O$
So, the final net reaction is
$2H_{2}O\rightarrow CO_{2}+2H_{2}]*6\\+6CO_{2}+12H_{2}\rightarrow C_{6}H_{12}O_{6}+6H_{2}O\\$
------------------$\\6CO_{2}+12H_{2}O\rightarrow C_{6}H_{12}O_{6}+6H_{2}O$
It is more appropriate to write the reaction as above because the water $(H_{2}O)$ molecule also produced in the photosynthesis reaction.
The path of reaction can be investigated by using the radioactive $H_{2}O^{18}$ instead of $(H_{2}O)$
(b)
$\\O_{3}(g)\rightarrow O_{2}(g)+O(g)\\ H_{2}O_{2}(l)+O(g)\rightarrow H_{2}O(l)+O_{2}(g)\\$ ---------------$\\ H_{2}O_{2}(l)+O_{3}(g)\rightarrow H_{2}O(l)+O_{2}(g)+O_{2}(g)$ (the final net reaction)
Dioxygen is produced from both steps, one from the decomposition of ozone ( $O_{3}$ ) and the other is from the reaction of hydrogen peroxide with(O)
- The path of the reaction can be investigated by using $O^{18}_{3}/ H_{2}O^{18}$.
Answer :
These can be understood by the following examples-
- $P_{4}$ is reducing agent and $Cl_{2}$ is an oxidizing agent
$P_{4}+10Cl_{2}(Excess)\rightarrow PCl_{5}$ [O.N of phosphorus +5] $\Rightarrow$ Higher O.S of P
$P_{4}(Excess)+6Cl_{2}\rightarrow 4PCl_{3}$ [O.N of phosphorus +3] $\Rightarrow$ Lower O.S of P
- K is a reducing agent and $C$ oxidising agent
$C+O_{2}\rightarrow CO_{2}$ (O is in excess) [O.N of C is +4]
$C+O_{2}\rightarrow CO$ (C is in excess) [O.N of C +2]
- $K$ is a reducing agent and $O_{2}$ is an oxidizing agent
$K+O_{2}\rightarrow K_{2}O$ (K is in excess) [O.N of O is -2] (lower O.S.)
$K+O_{2}\rightarrow K_{2}O_{2}$ (O is in excess) [O.N of O is -1] (lower O.S.)
Question 7.12(a) How do you count for the following observations?
Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene, we use alcoholic potassium permanganate as an oxidant. Why? Write a balanced redox equation for the reaction.
Answer :
Alcohol and KMnO4 both are polar in nature and alcohol is homogenous with toluene because both are organic compounds. So the reaction is faster in the homogeneous medium than in the heterogeneous medium. And hence all the compounds react at a faster rate.
Chemical equation-
$C_{6}H_{6}CH_{3}+MnO_{4}^{-}(alc.)\rightarrow C_{6}H_{6}COO^{-}+MnO_{2}+H_{2}O(l)+OH^{-}(aq)$
Question 7.12(b) How do you count for the following observations?
When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get colourless pungent pungent-smelling gas HCl, but if the mixture contains bromide, then we get red vapour of bromine. Why?
Answer :
- Concentrated sulphuric acid is added to an inorganic mixture containing chloride-
$2NaCl\:+\:2H_{2}SO_{4}\rightarrow \: 2NaHSO_{4}\:+\: 2HCl$
HCl is a weak reducing agent, and it cannot reduce $H_{2}SO_{4}$ to $SO_{2}$ that's why we get colourless pungent pungent-smelling gas HCl.
- Concentrated sulphuric acid is added to an inorganic mixture containing bromide-
$2NaBr\:+\:2H_{2}SO_{4}\rightarrow \: 2NaHSO_{4}\:+\: 2HBr$
$2HBr + H_{2}SO_{4}\:\rightarrow \:Br_{2} ( Red \:Vapour)\:+\:SO_{2}\:+\:2H_{2}O$
When conc. Sulphuric acid is added to an inorganic mixture containing bromide initially, it produces $HBr$ and it is a strong reducing agent so it reduces $H_{2}SO_{4}$ to $SO_{2}$ with the evolution of a red vapour of bromine.
Question 7.13(a) Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions
$(a)2AgBr(s)+C_{6}H_{6}O_{2}(aq)\rightarrow 2Ag(s)+2HBr(aq)+C_{6}H_{4}O_{2}(aq)$
Answer :
Substance reduced/oxidising agent- $AgBr$
Substance oxidised/reducing agent- $C_{6}H_{6}O_{2}]$
Question 7.13(b) Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions
$(b) HCHO(l)+2[Ag(NH_{3})]^+(aq) +3OH^-(aq)\rightarrow 2Ag(s)+HCOO^- (aq)+4NH_{3}(aq) +2H_{2}O(l)$
Answer :
Substance reduced/oxidising agent- $[Ag(NH_{3})_{2}]^+$
Substance oxidised/reducing agent- HCHO
Question 7.13(d) Identify the substance oxidised reduced, oxidising agent and reducing agent for each of the following reactions
$(d) N_{2}H_{4}(l)+2H_{2}O_{2}(l)\rightarrow N_{2}(g)+4H_{2}O(l)$
Answer :
Substance oxidised/reducing agent-
$N_{2}H_{4}$
Substance reduced/oxidizing agent- $H_{2}O_{2}$
Question 7.13(e) Identify the substance oxidised reduced, oxidising agent and reducing agent for each of the following reactions
$(e)Pb(s)+PbO_{2}(s)+2H_{2}SO_{4}(aq)\rightarrow 2PbSO_{4}(s)+2H_{2}O(l)$
Answer :
Substance oxidised/reducing agent- Pb
Substance reduced/oxidising agent- PbO2
Question 7.14 Consider the reactions :
$2S_{2}O_{3}^{2-}(aq)+I_{2}(s)\rightarrow S_{4}O_{6}^{2-}(aq)+2I^{-}(aq)$
$2S_{2}O_{3}^{2-}(aq)+2Br_{2}(l)+5H_{2}O(l)\rightarrow SO_{4}^{2-}(aq)+4Br^{-}(aq)+10H^{+}(aq)$
Why does the same reductant, thiosulfate, react differently with iodine and bromine?
Answer :
$F_{2}>Cl_{2}>Br_{2}>I_{2}$ oxidizing power order
Bromine is a stronger oxidising agent than iodine. So in the case of bromine (avg. oxidation number of sulphur is changed from +2 to +6)
and in case of iodine it (+2 to +2.5). So that's why thiosulfate reacts differently with bromine and iodine.
Answer :
part(i)
Fluorine can oxidise other halogen ions. On the other hand $Br_{2},I_{2},Cl_{2}$ cannot oxidize
$2F^{-} \rightarrow F_{2}$
$F_{2}+2I^{-}\rightarrow 2F^{-}+I_{2}$
$F_{2}+2Br^{-}\rightarrow 2F^{-}+Br_{2}$
$F_{2}+2Cl^{-}\rightarrow 2F^{-}+Cl_{2}$
And hence we say that fluorine is the better oxidant among halogens.
part(ii)
$HI$ & $HBr$ are able to reduce $H_{2}SO_{4}\rightarrow SO_{2}$ but $HCl,HF$ are unable to reduce sulphuric acid.
So here we can say that $HI$ & $HBr$ are better reducing agents than $HCl,HF$ .
Again $I^{-}$ can only able to reduce $Cu^{2+}\rightarrow Cu^{+}$ but $Br^{-}$ cannot.
$4I^{-}+2Cu^{2+}\rightarrow Cu_{2}I_{2}+I_{2}$
Hence, among hydrohalic compounds, hydroiodic acid is the best reducing agent.
Question 7.16 Why does the following reaction occur?
$XeO_{6}^{4-}(aq)+2F^{-}(aq)+6H^{+}(aq)\rightarrow XeO_{3}(g)+F_{2}(g)+3H_{2}O(l)$
What conclusion about the compound $Na_{4}XeO_{6}$ (of which $XeO_{6}^{4-}$ is a part) can be drawn from the reaction?
Answer :
We conclude that the oxidation state of Xenon changes from +8 to +6
$XeO_{6}^{4-} (+8)\rightarrow XeO_{3}(+6)$
and the oxidation state of F changes from -1 to 0
$F^{-}(-1)\rightarrow F_{2}(0)$
$Na_{4}XeO_{6}$ is reduced by accepting an electron.
It is a stronger oxidising agent than F
Question 7.17(a) Consider the reactions:
(a) $H_{3}PO_{2}(aq)+4AgNO_{3}(aq)+2H_{2}O(l)\rightarrow H_{3}PO_{4}(aq)+4Ag(s)+4HNO_{3}(aq)$
$(b)H_{3}PO_{2}(aq)+2CuSO_{4}(aq)+2H_{2}O(l)\rightarrow H_{3}PO_{4}(aq)+2Cu(s)+4H_{2}SO_{4}(aq)$
What inference do you draw about the behaviour of $Ag^{+}$ and $Cu^{+}$ from these reactions?
Answer :
In the first reaction, we can see that $Ag^{+}$ oxidizes the phosphorus from (+1 $\rightarrow$ +5) also in second, we clearly see that $Cu^{+}$ oxidize the phosphorus from (+1 $\rightarrow$ +5).
Both are oxidising agents.
Question 7.17(b) Consider the reactions:
$(d)C_{6}H_{5}CHO(l)+2Cu^{2+}(aq)+5OH^{-}(aq)\rightarrow$ No change observed
Answer :
Here, by looking at the reaction, we conclude that $Ag^{+}$ oxidises $C_{6}H_{5}CHO$ and in the second reaction $Cu^{+}$ is not able to oxidise. So we can say that $Ag^{+}$ is stronger oxidizing agent than $Cu^{+}$
Question 7.18(a) Balance the following redox reactions by ion – electron method
$MnO_{4}^{-}(aq)+ I^{-}(aq)\rightarrow MnO_{2}(s)+ I_{2}(s)$ (In basic medium)
Answer :
reduction half reaction
$MnO_{4}^{-}\rightarrow MnO_{2}$ (+7 to +4)
Add 3 electrons on the LHS side, and after that, to balance charge, add OH ions. And to balance O atom add a water molecule on whichever side it needs
Balance it
$MnO_{4}^{-}+2H_{2}O+3e^{-}\rightarrow MnO_{2} +4OH^{-}$
oxidation half
$I\rightarrow I_{2}$
Balance it
$2I^{-}\rightarrow I_{2}+2e^{-}$
equalising the no. of electrons by multiplying the oxidation half by 3 and the reduction half by 2, and then adding them.
$
6 \mathrm{I}^{-} \rightarrow 3 \mathrm{I}_2+6 e^{-}
$
$
2 \mathrm{MnO}_4^{-}+4 \mathrm{H}_2 \mathrm{O}+6 e^{-} \rightarrow 2 \mathrm{MnO}_2+8 \mathrm{OH}^{-}
$
Overall Reaction:
$
2 \mathrm{MnO}_4^{-}+4 \mathrm{H}_2 \mathrm{O}+6 \mathrm{I}^{-} \rightarrow 2 \mathrm{MnO}_2+3 \mathrm{I}_2+8 \mathrm{OH}^{-}
$
Question 7.18(b) Balance the following redox reactions by ion – electron method
$MnO_{4}^{-}(aq)+SO_{2}(g) \rightarrow Mn^{2+}(aq)+HSO_{4}^{-}$
(In Acidic medium)
Answer -
Oxidation half-reaction
$SO_{2}+2H_{2}O\rightarrow HSO_{4}^{-}+3H^{+}+2e^{-}$
Reduction half reaction
$MnO_{4}^{-}+8H^{+}+5e^{-}\rightarrow Mn^{2+}+4H_{2}O$
Balancing the reaction
Multiply the oxidation half by 5 and the reduction half by 2 and then add these two reactions
$2MnO_{4}^{-}+5SO_{2}+2H_{2}O+H^{+}\rightarrow 2Mn^{2+}+5HSO_{4}^{-}$
Question 7.18(c) Balance the following redox reactions by ion – electron method
(c) $H_{2}O_{2}(aq)+Fe^{3+}(aq)\rightarrow Fe^{3+}(aq)+H_{2}O(l)$
In acidic medium
Answer :
In acidic medium
oxidation half reaction-
$Fe^{2+}\rightarrow Fe^{3+}+e^{-}$
reduction half reaction-
$H_{2}O_{2}+2H^{+}+2e^{-}\rightarrow 2H_{2}O$
Balancing the reaction
Multiply by 2 on the oxidation half-reaction, then add it with reduction half-reaction
$H_{2}O_{2}+2Fe^{2+}+2H^{+}\rightarrow 2Fe^{3+}+2H_{2}O$
Question 7.18(d) Balance the following redox reactions by ion – electron method
$Cr_{2}O_{7}^{2-}+SO_{2}(g) \rightarrow Cr^{3+}(aq)+SO_{4}^{2-}(aq)$
In acidic medium
Answer :
Half-reaction
Oxidation half $SO_{2}+2H_{2}O\rightarrow SO_{4}^{2-}+4H^{+}+2e^{-}$
Reduction half $Cr_{2}O_{7}^{2-}+14H^{+}+6e^{-}\rightarrow 2Cr^{3+}+3SO^{2-}+H_{2}O$
Balancing them by multiplying the oxidation half by 3 and adding the reaction
$Cr_{2}O_{7}^{2-}+3SO_{2}+2H^{+}\rightarrow 2Cr^{3+}+3SO_{4}^{2-}+H_{2}O$
Question 7.20 What sorts of information can you draw from the following reaction?
$(CN)_{2}(g)+2OH^{-}(aq)\rightarrow CN^{-}(aq) +CNO^{-}(aq)+H_{2}O(l)$
Answer :
Carbon shows different oxidation states according to the compound formula.
Here we can clearly say that Carbon is in its +3 oxidation state.
$(CN)_{2}(+3)\rightarrow CN^{-}(+2)$
$(CN)_{2}(+3)\rightarrow CNO^{-}(+4)$
The oxidation state of carbon is increased(oxidised) and decreased(reduced) as well on the product side. So it is a redox reaction and more specifically, we can say it disproportion redox reaction.
Question 7.21
Answer :
The base equation
$Mn^{3+}\rightarrow Mn^{2+}+MnO_{2}+H^{+}$
write the oxidation half with their oxidation state
$Mn^{3+}(+3)\rightarrow MnO_{2}(+4)$
Balance the charge on $Mn$ by adding 1 $e^{-}$ on the RHS side. To balance charge, add $H^{+}$ ions on the RHS side and then for oxygen balance, add $H_{2}O$ molecule on the LHS side.
$Mn^{3+}+2H_{2}O\rightarrow MnO_{2}+1e^{-}+4H^{+}$
reduction half
$Mn^{3+}(+3)\rightarrow Mn^{2+}(+2)$
balancing the reduction half by adding 1 $e^{-}$ on LHS side
$Mn^{3+}+1e^{-}\rightarrow Mn^{2+}$
Add both balanced reduction half and oxidation half
$2Mn^{3+}+2H_{2}O\rightarrow Mn^{2+}+MnO_{2}+4H^{+}$
Question 7.22 Consider the elements: Cs, Ne, I and F
(a) Identify the element that exhibits only a negative oxidation state.
(b) Identify the element that exhibits only a positive oxidation state
(c) Identify the element that exhibits both positive and negative oxidation states
(d) Identify the element which exhibits neither the negative nor the positive oxidation state.
Answer :
(a) F (because of its highly electronegative in nature)
(b) Cs (highly electropositive)
(c) I (has empty d orbitals)
(d) Ne (inert gas)
Answer :
Base equation- $SO_{2}+Cl_{2}\rightarrow Cl^{-}+SO_{4}^{2-}$ --------------(have to remember)
Now we have to balance the oxidation half and the reduction half.
oxidation half = $SO_{2}\rightarrow SO_{4}^{2-}$
balancing - Oxygen is balanced by adding water molecule, Hydrogen is balanced by $H^{+}$ ion and for charge add $e^{-}$ (electron) $SO_{2} +2H_{2}O\rightarrow SO_{4}^{2-}+4H^{+}+2e^{-}$
Reduction half = $Cl_{2}\rightarrow Cl^{-}$
Balancing - to balance charge add an electron $Cl_{2}+2e^{-}\rightarrow 2Cl^{-}$
Now add both balanced oxidation half and reduction half, we get
$Cl_{2}+SO_{2}+2H_{2}O\rightarrow 2Cl^{-}+SO_{4}^{2-}+4H^{+}$
Question 7.24 Refer to the periodic table given in your book and now answer the following questions:
(a) Select the possible non-metals that can show a disproportionation reaction.
(b) Select three metals that can show a disproportionation reaction
Answer :
(a) Phosphorus, sulphur and chlorine can show disproportionation reaction.
(b) Manganese, copper, indium and gallium can show disproportionation reaction.
Answer :
we have,
No. of moles of ammonia $(NH_{3})$ = 10/17 = 0.588
No. of moles of oxygen $(O_{2})$ = 20/32= 0.625
Balanced Reaction $4NH_{3}+5O_{2}\rightarrow 4NO + 6H_{2}O$
Here we see that 4 moles of ammonia require 5 moles of oxygen. So
0.588 moles of ammonia = $(5/4)*0.588 = 0.735$ moles of $(O_{2})$ . But we have only 0.625 moles of $(O_{2})$ .
It means oxygen is a limiting reagent and the maximum weight of nitric oxide $(NO)$ can be produced by 0.635 moles of $(O_{2})$
So, 5 moles of $(O_{2})$ produced 4 moles of C.
therefore 0.625 moles of $(O_{2})$ = $(4/5)*0.625 = 0.5$ moles of $(NO)$ .
from Eq. 1
mass of $(NO)$ = number of moles $(NO)$ * molecular weight $(NO)$
= $0.5* 30$
= 15 g
Alternate Method
directly consider the molecular weight
(17*4) g of NH3 required (5*32) g of O to produce (30*4) g of NO
So, 10g of NH3 required (5*32/17*4)*10 = 23.5g of O. But we have only 20g (means O is limiting reagent) whatever the max. NO produce is from 20g of O.
and we know that 5*32g of O produce 30*4 g of NO
So, 20g of O produce = (30*4/5*32)*20 g of NO
= 15g of NO
Question 7.26 Using the standard electrode potentials given in the Table 8.1, predict if the reaction between the following is feasible
(a) $Fe^{3+}(aq)$ and $I^{-}(aq)$
(b) $Ag^{+}(aq)$ and Cu(s)
(c) $Fe^{3+}$ (aq) and Cu(s)
(d) Ag(s) and $Fe^{3+}$ (aq)
(e) $Br_{2}(aq)$ and $Fe^{2+}(aq)$
Answer :
If $E^{0}$ for the overall reaction is positive $\rightarrow$ feasible
negative $\rightarrow$ not feasible
(a) $[Fe^{3+}+ e^{-}\rightarrow Fe^{2+} ]*2: E^{0} = 0.77V$
$2I^{-}\rightarrow I_{2}+2e^{-}: E^{0} = -0.54V$
---------------------------------------------------------------------------------------
$2Fe^{3+}+2I^{-}\rightarrow Fe^{2+}+I_{2}: E^{0} = +0.23V$
(b) $Ag^{+}+e^{-}\rightarrow Ag(s)]*2: E^{0} = +0.80V$
$Cu\rightarrow Cu^{2+}+2e^{-}:E^{0} =-0.34V$
---------------------------------------------------------------------------------------
$2Ag^{+}+Cu\rightarrow 2Ag+Cu^{2+}: E^{0}=+0.46V$
(c) $Fe^{3+}+e^{-}\rightarrow Fe^{2+}]*2 :E^{0}=+0.77V$
$2Br^{-}\rightarrow Br+2e^{- }:E^{0}=-1.09V$
-------------------------------------------------------------------------
$2Fe^{3+}+2Br^{-}\rightarrow 2Fe^{3+}+Br_{2}:E^{0}=-0.32$
(d) $Ag\rightarrow Ag^{+}+e^{-}:E^{0}=-0.80V$
$Fe^{3+}+e^{-}\rightarrow Fe^{2+}:E^{0}==0.77V$
------------------------------------------------------------------------------
$Ag+Fe^{3+}\rightarrow Ag^{+}+Fe^{2+}:E^{0}=-0.03V$
(e) $Br_{2}+2e^{-}\rightarrow 2Br^{-}:E^{0}=+1.09V$
$Fe^{2+}\rightarrow Fe^{3+}+e^{-}]*2:E^{0}=-0.77V$
-------------------------------------------------------------------------------
$Br_{2}+2Fe^{2+}\rightarrow 2Br^{-}+2Fe^{3+}:E^{0}=+0.32V$
Question 7.27(i) Predict the products of electrolysis in each of the following:
(i) An aqueous solution of $AgNO_{3}$ with silver electrodes
Answer :
(i) $AgNO_{3}$ dissociate into $Ag^{+}$ and $NO_{3}^{-}$
Cathode - $Ag^{+}(aq)+e^{-}\rightarrow Ag(s)$ (reduction potential of silver is higher than $H_{2}O$ ) $E^{0} = 0.80V$
Anode - $Ag(s)\rightarrow Ag^{+}(aq)+e^{-}$ ( oxidation potential of silver is higher than water molecule. So silver electrode oxidized ) $E^{0} = -0.83V$
Question 7.27(ii) Predict the products of electrolysis in each of the following:
An aqueous solution $AgNO_{3}$ with platinum electrodes
Answer :
(ii) since platinum $(Pt)$ electrode cannot easily oxidize. So at the anode $H_{2}O$ will oxidize and liberate oxygen and at cathode $Ag$ will be deposited.
At cathode- $Ag^{+}+e^{+}\rightarrow Ag$
At anode- $H_{2}O\rightarrow O_{2}+4H^{+} +4e^{-}$
Question 7.27(iii) Predict the products of electrolysis in each of the following:
(iii) A dilute solution of $H_{2}SO_{4}$ with platinum electrodes
Answer :
Given that sulphuric acid is dilute.
ionize into $H_{2}SO_{4}\rightarrow 2H^{+}+ SO_{4}^{2-}$
At cathode $H^{+}+e^{-}\rightarrow 1/2H_{2}(g)$
At anode, There will be -(liberation of oxygen gas)
$H_{2}O\rightarrow 1/2O_{2}+4H^{+}+4e^{-}$
Question 7.27(iv) Predict the products of electrolysis in each of the following:
An aqueous solution of CuCl2 with platinum electrodes
Answer :
(iv) In aqueous solution $CuCl_{2}$ ionise into $Cu^{2+}$ and $2Cl^{-}$
At the cathode, the copper ion will be deposited because it has a higher reduction potential than the water molecule
At the anode, the lower electrode potential value will be preferred but due to the overpotential of oxygen, the chloride ion gets oxidised at the anode.
Anode- $2Cl^{-}\rightarrow Cl_{2}+2e^{-}$
cathode- $Cu^{2+}+2e^{-}\rightarrow Cu$
Answer :
In order to displace a metal from its metal salt is done only when the other metal has a higher electrode potential.
Mg(−2.36), Al(−1.66), Zn(−0.76), Fe(−0.44), Cu(+0.34)
Question 7.29 Given the standard electrode potentials
$K^{+}/K = -2.93 V$ $Ag^{+}/Ag = 0.80V$
$Hg^{2+}/Hg= 0.79V$
$Mg^{2+}/Mg = -2.37V$ $Cr^{3+}/Cr = -0.74V$
Arrange these metals in their increasing order of reducing power.
Answer :
A negative electrode potential $(E^{0})$ means the redox couple is a stronger reducing agent. So, as per data, the increasing order of the following is-
$Ag<Hg<Cr<Mg<K$
Question 7.30 Depict the galvanic cell in which the reaction
$Zn(s)+2Ag^{+}\rightarrow Zn^{2+}(aq)+2Ag(s)$
takes place. Further show:
(i) Which of the electrodes is negatively charged?
(ii) the carriers of the current in the cell, and
(iii) individual reaction at each electrode.
Answer :
(i) Zn electrode is negatively charged because it loses electrons (acts as an anode)
(ii) electron flow from a negatively charged electrode to a positively charged electrode (anode to cathode) and the flow of current is just reversed. So current flows through the silver cathode to the zinc anode.
(iii) At Anode- $Zn\rightarrow Zn^{2+}+2e^{-}$
At Cathode $Ag^{+}+e^{-}\rightarrow Ag$
Class 11 Chemistry NCERT Chapter 7: Higher Order Thinking Skills (HOTS) Questions
Question 1. Compounds that should not be used as primary standards in titrimetric analysis are :
A. $\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7$
B. Oxalic acid
C. NaOH
D. $\mathrm{FeSO}_4 \cdot 6 \mathrm{H}_2 \mathrm{O}$
E. Sodium tetraborate
Choose the most appropriate answer from the options given below:
1) B and D Only
2) D and E Only
3) C, D and E Only
4) A, C and D Only
Answer:
The primary standard is a highly pure stable compound with a known exact composition that can be accurately weighed and dissolved to creat a solution of known concentration.
Compounds like $\mathrm{NaOH}, \mathrm{FeSO}_4 \cdot 6 \mathrm{H}_2 \mathrm{O}, \mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7$ are not suitable as primary standards in titrimetric analysis because,
NaOH is hygroscopic and can't be used.
$\mathrm{FeSO}_4 \cdot 6 \mathrm{H}_2 \mathrm{O}$ is unstable and can be easily oxidised.
$\mathrm{Na}_2 \mathrm{Cr}_2 \mathrm{O}_7$ is hygroscopic and can't be used.
Hence, the correct answer is option (4).
Question 2. Based on the data given below:
$\begin{array}{ll}
\mathrm{E}_{\mathrm{Cr}_2 \mathrm{O}_7^{2-} / \mathrm{Cr}^{3+}}^0=1.33 \mathrm{~V} & \mathrm{E}_{\mathrm{Cl}_2 / \mathrm{Cl}^{(-)}}^0=1.36 \mathrm{~V} \\
\mathrm{E}_{\mathrm{MnO}_4^{-} / \mathrm{Mn}^{2+}}^0=1.51 \mathrm{~V} & \mathrm{E}_{\mathrm{Cr}^{3+} / \mathrm{Cr}}^0=-0.74 \mathrm{~V}
\end{array}$
the strongest reducing agent is :
1) $\mathrm{Mn}^{2+}$
2) Cr
3) $\mathrm{MnO}_4^{-}$
4) $\mathrm{Cl}^{-}$
Answer:
For the strongest reducing agent
Reduction potential should be the lowest. Hence, Cr is the strongest reducing agent.
Hence, the correct answer is option (2)
Question 3. Oxidation state of Cr in chromyl chloride is:
1) +5
2) +3
3) +7
4) +6
Answer:
The formula of Chromyl chloride is CrO2Cl2.
Let the oxidation state of Cr be X, then
X+ 2×(–2) + 2×(–1) = 0
X–4–2 = 0
X= +6
Hence, the correct answer is option (4)
Question 4. In which of the following reactions does both oxidation and reduction occur simultaneously?
(1) $\mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{ZnSO}_4+\mathrm{H}_2$
(2) $2 \mathrm{KClO}_3 \rightarrow 2 \mathrm{KCl}+3 \mathrm{O}_2$
(3) $\mathrm{CaCO}_3 \rightarrow \mathrm{CaO}+\mathrm{CO}_2$
(4) $2 \mathrm{HgO} \rightarrow 2 \mathrm{Hg}+\mathrm{O}_2$
Answer:
$\ln 2 \mathrm{KClO}_3 \rightarrow 2 \mathrm{KCl}+3 \mathrm{O}_2:$
Oxidation state of Cl changes from +5 in $\mathrm{KClO}_3$ to -1 in KCl and 0 in $\mathrm{O}_2$. Hence, its a disproportionation redox reaction.
Question 5. In the reaction $2 \mathrm{Na}+\mathrm{H}_2 \rightarrow 2 \mathrm{NaH}$, which statement is correct?
(1) Na is oxidised, $\mathrm{H}_2$ is reduced
(2) Na is reduced, $\mathrm{H}_2$ is oxidised
(3) Both are oxidised
(4) Both are reduced
Answer:
$\mathrm{Na}(0 \rightarrow+1)$ is oxidised
$\mathrm{H}(0 \rightarrow-1)$ is reduced
So, it is a redox reaction with Na as reducing agent and $\mathrm{H}_2$ as oxidising agent.
Question 6: Which of the following oxidation reactions are carried out by both $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ and $\mathrm{KMnO}_4$ in acidic medium?
A. $\mathrm{I}^- \rightarrow \mathrm{I}_2$
B. $\mathrm{S}^{2-} \rightarrow \mathrm{~S}$
C. $\mathrm{Fe}^{2+} \rightarrow \mathrm{Fe}^{3+}$
D. ${I }^{-} \rightarrow \mathrm{IO}_3^{-}$
E. $\mathrm{S}_2 \mathrm{O}_3{ }^{2-} \rightarrow \mathrm{SO}_4{ }^{2-}$
Choose the correct answer from the options given below :
(1) B, C and D only
(2) A, D and E only
(3) A, B and C only
(4) C, D and E only
Answer:
$\begin{array}{ll}\mathrm{I}^{-} \xrightarrow{\mathrm{H}^{+}} \mathrm{I}_2 & \mathrm{I}^{-} \xrightarrow{\mathrm{OH}^{-}} \mathrm{IO}_3^{-} \\ \mathrm{S}^{-2} \xrightarrow{\mathrm{H}^{+}} \mathrm{S} & \mathrm{S}_2 \mathrm{O}_3^{2-} \xrightarrow{\mathrm{OH^-}} \mathrm{SO}_4^{2-} \\ \mathrm{Fe}^{+2} \longrightarrow \mathrm{Fe}^{+3} \\ \mathrm{~S}_2 \mathrm{O}_3^{2-} \xrightarrow{\mathrm{H}^{+}} \mathrm{S} \downarrow+\mathrm{SO}_4^{2-}\end{array}$
Oxidation reactions are carried out by both $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ and $\mathrm{KMnO}_4$ in acidic medium by: A, B and C
Hence, the correct answer is option (3).
Question 7: In the reaction
$\mathrm{Zn}+\mathrm{Cu}^{2+} \rightarrow \mathrm{Zn}^{2+}+\mathrm{Cu}$
Which of the following statements is correct?
(1) Zn is reduced
(2) $\mathrm{Cu}^{2+}$ is oxidized
(3) Zn acts as a reducing agent
(4) Cu acts as a reducing agent
Answer:
Oxidation states
Zn: $0 \rightarrow+2 \rightarrow$ oxidation
$\mathrm{Cu}^{2+}:+2 \rightarrow 0 \rightarrow$ reduction
Zn acts as a reducing agent
Hence, the correct answer is option (3).
Question 8: The oxidation number of S in $\mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_8$ is:
(1) +6
(2) +7
(3) +5
(4) +4
Answer:
Let oxidation number of each $\mathrm{S}=x$
Known values:
$\mathrm{H}=+1(2$ atoms $\rightarrow+2)$
$O=-2(8$ atoms $\rightarrow-16)$
Sum of oxidation numbers = 0
$2(+1)+2 x+8(-2)=0$
$2 x=14 \Rightarrow x=+7$
Hence, the correct answer is option (2).
Approach to Solve Questions of Class 11 Chemistry Chapter 7
Sometimes class 11 chemistry chapter 7 redox reactions question answer seem difficult, but once we understand the basic rules and strategy, it becomes very easy to solve all the questions related to this chapter. We can follow the steps given below to solve questions.
1) First of all, we need to understand the type of question asked as the questions of Redox Reactions can be divided into the following categories-
- Calculation of oxidation number
- Balancing of Redox Reaction
- Identifying the oxidising and reducing agents
- Equivalent weight n-factor
- Calculating the amount of the substance using Redox titration
Practice is key, so improve your accuracy by solving more redox reactions class 11 question answer.
2. Practice assigning oxidation numbers to all elements in a compound or ion. It’s key for identifying what is oxidized and reduced. Also, learn to identify redox changes.
3. Focus on two major methods that are,
- Oxidation number method
- Half-reaction (ion-electron) method
Practice balancing both acidic and basic medium redox reactions.
4. Attempt all questions by writing steps clearly like identifying oxidation numbers, changes, electrons lost/gained and then balancing the reaction. Some questions may involve mole concepts or equivalent weight in redox reactions, so practice a few numericals to get accuracy.Students can also refer class 11 chemistry chapter 7 redox reactions solutions.
Topics and Subtopics Covered in the NCERT Textbook Class 11 Chemistry Chapter 7
All the topics and subtopics covered in the NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reactions are listed below:
7.1 Classical Idea of Redox Reactions-Oxidation and Reduction Reactions
7.2 Redox Reactions in Terms of Electron Transfer Reactions
7.2.1 Competitive Electron Transfer Reactions
7.3 Oxidation Number
7.3.1 Types of Redox Reactions
7.3.2 Balancing of Redox Reactions
7.3.3 Redox Reactions as the Basis for Titrations
7.3.4 Limitations of the Concept of Oxidation Number
7.4 Redox Reactions and Electrode Processes
What Extra Should Students Study Beyond the NCERT for JEE/NEET?
Beyond the class 11 chemistry chapter 7 redox reactions solutions, students need to focus on important concepts and theories according to the table given below. The table shows a comparison between topics that are included in the JEE syllabus and topics that are given in the NCERT textbook:
What Students Learn from NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reactions
These class 11 chemistry redox reactions question answer help students to understand the concepts of oxidation and reduction clearly. These solutions also help students practice questions and prepare well for exams
- These solutions help students to understand the basic concepts of oxidation and reduction and identify oxidizing and reducing agents.
- Balancing of redox reactions using oxidation number and half-reaction methods are explained in these class 11 chemistry chapter 7 redox reactions solutions.
- Using these solutions students get to know how these concepts of redox reactions are applicable in real life chemical processes and in the laboratory.
Importance of Class 11 Chemistry Chapter 7 Redox Reactions Solutions
The redox reactions class 11 question answer explains how substances undergo oxidation and reduction through electron transfer. Given below are some points on the importance of these solutions:
- This chapter is important as it helps to understand how chemical reactions occur.
- The concepts studied in this chapter form the basis for Electrochemistry, Metallurgy, Coordination Compounds, and Organic Chemistry
- Phenomena like rusting of iron, photosynthesis, respiration, bleaching, and corrosion control are well explained in class 11 chemistry redox reactions question answer.
- Energy generation in batteries, fuel cells, and electrolysis are also discussed
NCERT Solutions for Class 11 Chemistry Chapter-Wise
Besides NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reactions, chapter-wise solutions are given below:
NCERT Solutions for Class 11 Subject-Wise
Follow the links below to get the NCERT solutions for other subjects as well
NCERT Books and NCERT Syllabus:
Get your hands on the NCERT syllabus and books to ace your preparation.
Frequently Asked Questions (FAQs)
In NCERT Class 11 Chemistry, redox reactions are chemical reactions in which oxidation and reduction occur simultaneously. In these reactions, one substance loses electrons (oxidation) while another gains electrons (reduction).
Topics covered in this chapter are Oxidation and Reduction Reactions, Redox Reactions in Terms of Electron Transfer Reactions, Oxidation Number, Types of Redox Reactions, Balancing of Redox Reactions and Redox Reactions and Electrode Processes
Balancing redox reactions is essential because it ensures that mass and charge are conserved during the reaction. This balance helps in accurately representing the chemical process and understanding the stoichiometry involved.
Half-reactions are a way to separate the oxidation and reduction reactions involved in a redox process. By breaking down the overall reaction into two half-reactions, it's easier to balance the electrons transferred and better understand the thermodynamics and kinetic aspects of the reaction.
Electrode potential is the measure of the tendency of a metallic electrode to lose or gain electrons when in contact with a solution of its ions. It is a measure of the reduction potential.
In Chapter 7 Redox Reactions, the oxidation number (or oxidation state) of an element is the charge that an atom would have if the compound were purely ionic. It helps in identifying which atoms are oxidized and which are reduced in a redox reaction and is essential for balancing redox equations.
In Class 11 Chemistry Chapter 7, redox titration is a quantitative analysis method in which the amount of a substance is determined by a redox reaction with a titrant of known concentration. It involves electron transfer between the analyte and the titrant, and indicators may be used to detect the end point of the reaction.
To identify the oxidising and reducing agents, you need to examine the oxidation states of the elements involved in the reaction. The substance that undergoes reduction is the oxidising agent, and the substance that undergoes oxidation is the reducing agent.
Oxidation states are hypothetical charges assigned to atoms in a molecule based on a set of rules. They help in determining how many electrons an atom can lose, gain, or share during a chemical reaction.
The activity series is a list of metals arranged according to their ability to displace other metals in solution during a redox reaction. More active metals can reduce ions of less active metals, which helps predict the feasibility of certain redox reactions.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
