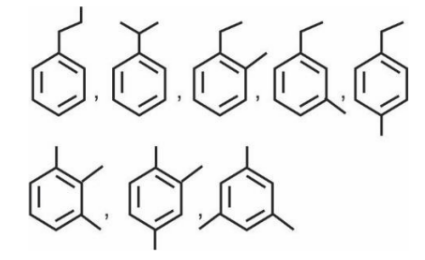
These organic chemistry some basic principles and techniques ncert solutions provide step by step answers to all exercise questions from the chapter. These solutions of NCERT help students understand key concepts clearly, practise numerical problems, and prepare effectively for exams.
Question8.1(i) What are the hybridization states of each carbon atom in the following compounds? $C H_2=C=O$
Answer:
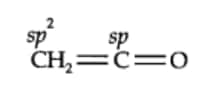
Here $C_1$ carbon is $sp^2$ hybridised and the $C_2$ carbon is $sp$ hybridised.
Question 8.1(ii) What are the hybridisation states of each carbon atom in the following compounds?
$(ii)\; CH_{3}CH=CH_{2}$
Answer:
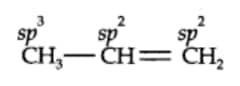
Here $C_1$ caron is $sp^3$ hybridised, $C_2$ carbon is $sp^2$ and the third carbon $C_3$ atom is $sp^2$ hybridise
Question 8.1(iii) What are the hybridisation states of each carbon atom in the following compounds?
Answer:
Here is the structure
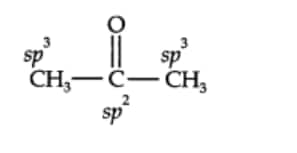
Here $C_1$ and $C_3$ carbon atom is in $sp^3$ hybridistaion and the $C_2$ carbon is $sp^2$
Question 8.1(iv) What are the hybridisation states of each carbon atom in the following compounds ?
$(iv)CH_{2}=CHCN$
Answer:
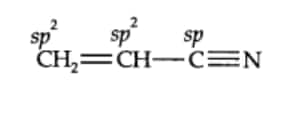
Here, in the above compound the $C_1$ carbon atom is $sp^2$ hybridised, $C_2$ carbon atom is $sp^2$ hybridised and the $C_3$ carbon is $sp$ hybridiesd
Question 8.1(v) What are the hybridisation states of each carbon atom in the following compounds ?
Answer:
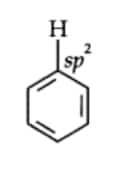
In benzene, all six carbon atoms are $sp^2$ hybridised.
Question 8.2(i) Indicate the $\sigma$ and $\pi$ bonds in the following molecule :
Answer:

There are six C-C sigma bonds, six C-H sigma bonds and three $\pi$ -bonds between the carbon atoms in the benzene.
Question 8.2(ii) Indicate the $\sigma$ and $\pi$ bonds in the following molecule:
Answer

In $C_{6}H_{12}$, there are six sigma bonds between C-Cand and twelve sigma bonds between C-H bonds in the given compound.
Question 8.2(iii) Indicate the $\sigma$ and $\pi$ bonds in the following molecule :
$(iii)\; CH_{2}Cl_{2}$
Answer:
In $CH_{2}Cl_{2}$ ,
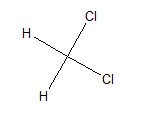
There are two sigma C-H bonds and two C-Cl sigma bonds.
Question 8.2 (iv) Indicate the $\sigma$ and $\pi$ bonds in the following molecule :
Answer:
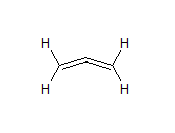
In this compound, there are two C-C sigma bonds, four C-H sigma bonds and two $\pi$ bonds between carbon atoms.
Question 8.2(v) Indicate the $\sigma$ and $\pi$ bonds in the following molecule :
Answer:

There are three C-H sigma bonds, one C-N sigma bond, one N-O sigma bond and one N-O $\pi$ -bond.
Question 8.2(vi) Indicate the $\sigma$ and $\pi$ bonds in the following molecule :
Answer:

Two C-N sigma bonds, four C-H sigma bonds, one N-H sigma bond and one $C=O$ $\pi$ -bond in the above compound.
Question 8.3 Write bond line formulas for : Isopropyl alcohol, 2,3-Dimethylbutanal, Heptan-4- one.
Answer:
Bond line formula of the following compounds-
|
Isopropyl alcohol
|
 |
|
2, 3-Dimethyl butanal
|
 |
|
Heptan-4-one
|
 |
Question 8.4(a) Give the IUPAC names of the following compounds:

Answer:

The IUPAC name of the above compound is 1-phenylpropane
Question 8.4(b) Give the IUPAC names of the following compounds:
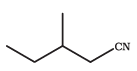
Answer:

The IUPAC name of the above compound is 3-Methylpentanenitrite.
Question 8.4(c) Give the IUPAC names of the below compound :
Answer:
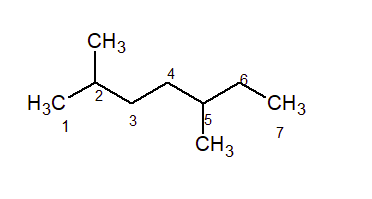
The IUPAC name of the above structure is 2, 5-dimethyl heptane.
Question 8.4(d) Give the IUPAC names of the following compounds:
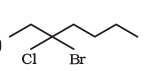
Answer:
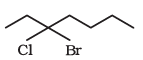
The IUPAC name of the above compound is 3-Bromo- 3-chloroheptane
Question 8.4(e) Give the IUPAC names of the following compounds:
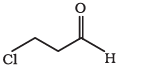
Answer:

The IUPAC name of the compound is 3-chloropropanal.
Question 8.4(f) Give the IUPAC names of the following compounds :
Answer:
$(e) \; Cl_{2}CHC\! H_{2}OH$
The IUPAC name of the compound is 1, 1-dichloro-2-ethanol
Question 8.5 Which of the following represents the correct IUPAC name for the compounds concerned?
(a) 2,2-Dimethylpentane or 2-Dimethylpentane
(b) 2,4,7- Trimethyloctane or 2,5,7-Trimethyloctane
(c) 2-Chloro-4-methylpentane or 4-Chloro-2-methylpentane
(d) But-3-yn-1-ol or But-4-ol-1-yne .
Answer:
(a) The prefix $di$ in the IUPAC nomenclature indicates two identical substituent groups in the parent chain. Since two methyl groups are present at $C_2$ of the parent chain. So, the correct IUPAC name is 2, 2-dimethylpentane.
(b) In IUPAC nomenclature, the sum of locant numbers should be a minimum. Here, the sum of 2,4,7 (=13)is less than the sum of 2, 5, 7 (=14). Thus, the correct IUPAC name is 2,4,7- Trimethyloctane
(c) In IUPAC nomenclature, if the substituent group acquires the equivalent position of the parent chain, then the lower number is given to the one that comes first in alphabetical order. Hence, 2-chloro-4-methyl pentane is the correct IUPAC name of the compound.
(d) If the two functional groups are present in the parent chain, then the suffix of the IUPAC name depends on the principal functional group. Here, alcohol is the principal functional group, so the suffix should be $-ol$, and the alkyne group is considered as a substituent group. Therefore, the correct IUPAC name of the compound is But-3-yn-1-ol.
Question 8.6(a) Draw formulas for the first five members of each homologous series beginning with the following compound.
Answer:
The first five members of each homologous series beginning with the following compound are shown as
$(a)\; H-COOH$ ---methanoic acid
- ethanoic acid
$CH_{3}-COOH$ - propanoic acid
$CH_{3}-CH_{2}-COOH$ - butanoic acid
$CH_{3}-CH_{2}-CH_{2}-COOH$ - pentanoic acid
$CH_{3}-CH_{2}-CH_{2}-CH_{2}-COOH$
Question 8.6 (b) Draw formulas for the first five members of each homologous series beginning with the following compound.
$(b) CH_{3}COCH_{3}$
Answer:
The first five members of each homologous series beginning with the following compound are shown as-
$(b) CH_{3}COCH_{3}$
(propanone)
- Butanone
$CH_{3}-CO-CH_{2}CH_{3}$ - Pentan-2-one
$CH_{3}-CO-CH_{2}CH_{2}CH_{3}$ - hexan-2-one
$CH_{3}-CO-CH_{2}CH_{2}CH_{2}CH_{3}$ - Hept-2-one
$CH_{3}-CO-CH_{2}CH_{2}CH_{2}CH_{2}CH_{3}$
Question 8.6 (c) Draw formulas for the first five members of each homologous series beginning with the following compound.
Answer:
The first five members of each homologous series beginning with the following compound are shown as-
$(c)\; H-CH=CH_{2}$ ..........(Ethene)
- Propene
$CH_{3}-CH=CH_{2}$ - 1-Butene
$CH_{3}-CH_{2}-CH=CH_{2}$ - 1-Pentene
$CH_{3}-CH_{2}-CH_{2}-CH=CH_{2}$ - 1-Hexene
$CH_{3}-CH_{2}-CH_{2}-CH_{2}-CH=CH_{2}$
Question 8.7(a) Give condensed and bond line structural formulas and identify the functional group(s) present, if any, for :
2,2,4-Trimethylpentane
Answer:
2,2,4-Trimethylpentane
Condensed formula- $(CH_{3})_2CHCH_{2}C(CH_{3})_{3}$
Bond line formula-
.png)
Answer:
The condensed formula of 2-Hydroxy-1,2,3-propanetricarboxylic acid
$(COOH)CH_{2}C(OH)(COOH)CH_{2}(COOH)$
The bond line structure -

Question 8.7(c) Give condensed and bond line structural formulas and identify the functional group(s) present, if any, for :
Hexanediol
Answer:
The condensed formula of hexanediol
$(CHO)(CH_{2})_4(CHO)$
The bond line structure is-
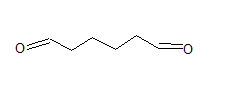
Question 8.8 Identify the functional groups in the following compounds
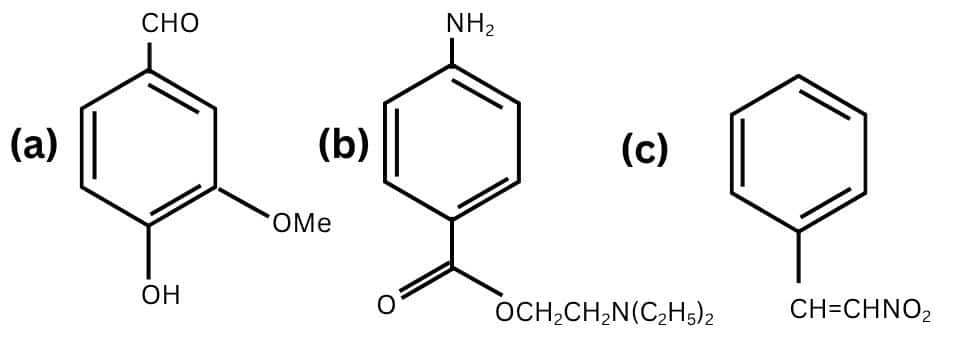
Answer:
The functional groups in the above structure are-
- Aldehyde ( $-CHO$ )
- HYdroxyl ( $-OH$ )
- Methoxy ( $-OMe$ )
- $C=C$ double bond
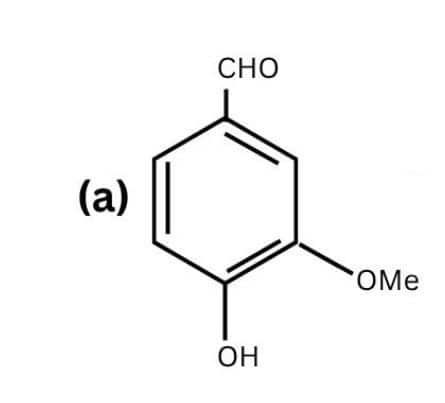
(b)
 The following functional groups are present-
The following functional groups are present-
- Amino, primary amine ( $-NH_{2}$ )
- Ester ( $-O-CO-$ )
- Tertiary amine ( $-NR_2$ ) R = ethyl group

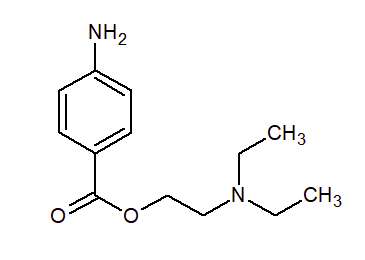
Here,
- Nitro group ( $-NO_{2}$ )
- $C=C$ double bond
Question 8.9 Which of the two: $O_{2}NCH_{2}CH_{2}O^{-}$ or $CH_{2}CH_{2}O^{-}$ is expected to be more stable and why ?
Answer:
Since the Nitro group is an electron-withdrawing group. So, it shows $-I$ effect. By reducing the electron negative charge of the compound, it stabilises the compound. On the other hand, the methyl group is an electron donor group so it shows $+I$ effect. This increases the negative charge on the compound and destabilises the compound.
Hence $O_{2}NCH_{2}CH_{2}O^{-}$ is more stable than the $CH_{2}CH_{2}O^{-}$ .
Question 8.10 Explain why alkyl groups act as electron donors when attached to a $\pi$ system.
Answer:
When an alkyl group is attached to the $\pi$ system, it acts as an electron donor group by the property of hyperconjugation.
For example, in propene,

In the figure, you can see that the sigma electrons of C-H bonds of the alkyl group are delocalised because of the partial overlap of the $sp^3-s$ sigma bond orbital with the empty p orbital of the $\pi$ bond of the adjacent carbon atom. It is also known as no-bond resonance.

Question 8.11(a) Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
Answer:
The resonating structure of the phenol--
The lone pair of electrons starts shifting to an oxygen-carbon bond and forms a double bond character and $\pi$ electron of the C-C bond shifts to the next C-C single bond.

Question 8.11 (b) Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
Answer:
The resonating structure of the nitrobenzene-
Here, the electrons of N-O bonds shift to the Oxygen atom (more electronegative), and then the $\pi$ electron of carbon-carbon double bonds starts delocalising towards the N atom.

Question 8.11(c) Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
Answer:
The resonating structure of the But-2-ene-1-al-
Here, the $\pi$ electrons of C-O bonds shift to the Oxygen atom (more electronegative), and then the $\pi$ electron of carbon-carbon double bonds starts shifting towards the next C-C bond, introducing a partial double bond character.
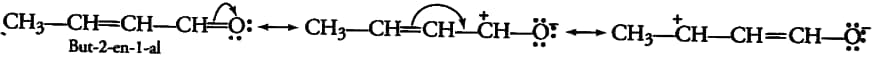
Question 8.11(d) Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
Answer:
The resonating structure of the benzaldehyde--
Here the $\pi$ electron of the carbon-oxygen double bond starts shifting towards the electronegative oxygen atom and the $\pi$ -electrons of the C-C double bonds shift towards the carbonyl group (as shown in figures).
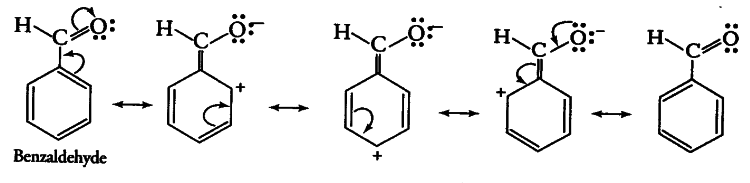
Question 8.11(e) Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
Answer:
The resonating structure of Benzyl carbocation-
The $\pi$ -electrons of the C-C double bond shift towards the $C-C^+$ bond to minimise the deficiency of electron density and the rest of the $\pi$ -electrons follow the same process.
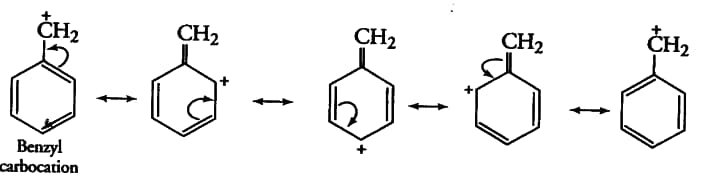
Question 8.11(f) Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
Answer:
The resonating structure of But-2-ene-1-yl carbocation-
The $\pi$ -electrons of the C-C double bond shift towards the $C-C^+$ bond to minimise the deficiency of electron density at $CH_2^+$. So, this way only one resonant is possible.
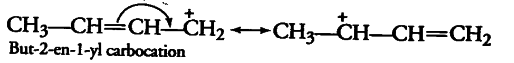
Question 8.12 What are electrophiles and nucleophiles? Explain with examples.
Answer:
Electrophile- It is an electron-deficient species, which seeks an electron pair. This reagent takes away an electron pair. It is denoted as $E^{+}$. For example, carbocations $CH_{3}CH_{2}^+$ neutral molecules having functional groups such as the carbonyl group are an example of the electrophile.
A nucleophile is a reagent that brings an electron pair. In other words, it is the nucleus, seeking a reagent called a nucleophile.
For examples- $-OH, -NC$ and carbanions ( $R_{3}C^-$ ) etc. Ammonia and water also act as a nucleophiles due to the presence of a lone pair of electrons.
Question 8.13 Identify the reagents shown in bold in the following equations as nucleophiles or electrophiles:
$(a)CH_{3}COOH+HO^{-}\rightarrow CH_{3}COO^{-}+H_{2}O$
Answer:
Electrophiles are electron-deficient species and can seek an electron pair. On the other hand, the nucleophile is electron electron-rich reagent and can electron donor(nucleophile seeking).
Therefore,
(a) $OH^-$ has one lone pair of an electrons, an electron-rich species. So, it is a nucleophile
(b) $C^-N$ acts as a nucleus-seeking reagent and acts as a nucleophile
(c) $CH_{3}C^+O$, it is an electron-deficient species, So, it is an electrophile.
Question 8.14. (a) Classify the following reactions in one of the reaction types studied in this unit.
$(a)\; CH_{3}CH_{2}Br+HS^{-}\rightarrow CH_{3}CH_{2}SH+Br^{-}$
Answer:
$(a)\; CH_{3}CH_{2}Br+HS^{-}\rightarrow CH_{3}CH_{2}SH+Br^{-}$
It is a substitution reaction, as in the above equation, the bromine group is replaced by the $-SH$ group.
Question 8.14.(b) Classify the following reactions in one of the reaction types studied in this unit.
$(b)\; (CH_{3})_{2} C= CH_{2}+HCl\rightarrow (CH_{3})_{2}ClC-CH_{3}$
Answer:
$(b)\; (CH_{3})_{2} C= CH_{2}+HCl\rightarrow (CH_{3})_{2}ClC-CH_{3}$
The given reaction is an example of an addition reaction because in this reaction, the two reactant molecules combine to form a single product. Also, we can say that the Hydrogen and chlorine of $HCl$ is added in the two different carbon atoms of the same compound.
Question 8.14(c) Classify the following reactions in one of the reaction types studied in this unit.
$(c)CH_{3}CH_{2}Br+HO^{-}\rightarrow CH_{2}=CH_{2}+H_{2}O+Br^{-}$
Answer:
$(c)CH_{3}CH_{2}Br+HO^{-}\rightarrow CH_{2}=CH_{2}+H_{2}O+Br^{-}$
In this reaction, the hydrogen and bromine are removed from the original compound and formed ethene. So, this is an elimination reaction.
Question 8.14(d) Classify the following reactions in one of the reaction type studied in this unit.
$(d)(CH_{3})_{3}C-CH_{2}OH+HBr\rightarrow (CH_{3})_{2}CBrCH_{2}CH_{2}CH_{3}+H_{2}O$
Answer:
$(d)(CH_{3})_{3}C-CH_{2}OH+HBr\rightarrow (CH_{3})_{2}CBrCH_{2}CH_{2}CH_{3}+H_{2}O$
In the above reaction, the substitution takes place between the two reactants, followed by the rearrangement of atoms and the groups of atoms.
Question 8.15(a) What is the relationship between the members of the following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
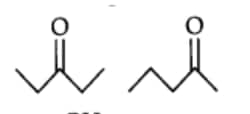
Answer:

These pairs are structural isomers.
Compounds having the same molecular formula but different structures are called structural isomers. The above compounds have the same molecular formula but the structures are different due to the difference in the position of the carbonyl group.
In structure one -CO group is present at $C_3$ position and in second the -CO group is present at $C_2$ position.
Question 8.15(b) What is the relationship between the members of the following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
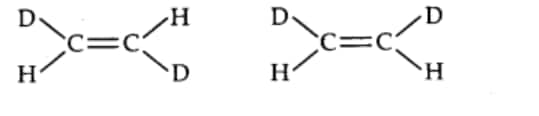
Answer:

The given compounds are a pair of geometrical isomers since they have the same molecular formula, sequence of covalent bonds and same constitution but differ in the relative positioning of the atoms in space. These compounds differ in the positioning of the Deuterium and Hydrogen.
Question 8.15(c) What is the relationship between the members of the following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
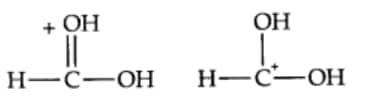
Answer:
(c) The given compounds are a pair of contributing structures or canonical structures. They do not represent any real molecule and are purely hypothetical. They are also called resonance isomers.
(a) 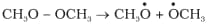
Answer:
Bond cleavage by using curved arrows to show the electron flow of the given reaction can be represented as
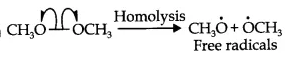
It is an example of hemolysis because the two electrons are equally divided into the products. (see in fig)
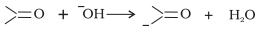
Answer:
Bond cleavage by using curved arrows to show the electron flow of the given reaction can be represented as;
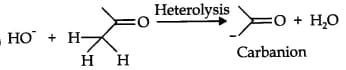
It is an example of heterolytic cleavage as the bond breaks in such a manner that the electron pair will remain with the carbon of propanone. The reaction intermediate is a carbanion.
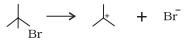
Answer:
Bond cleavage by using curved arrows to show the electron flow of the given reaction can be represented as;
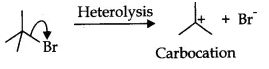
It is heterolysis as the shared pair of an electrons is distributed to only the bromine ion. Here, the reaction intermediate is a carbocation.
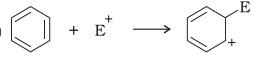
Answer:
Bond cleavage by using curved arrows to show the electron flow of the given reaction can be represented as
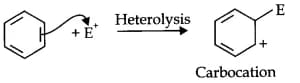
The reaction intermediate is a carbocation. It is a heterolytic cleavage as the bonds break in such a manner that the shared electron pair will remain with the one species.
$(a) Cl_{3}CCOOH> Cl_{2}C\! H\! \, COOH> ClC\! H_{2}COOH$
$(b) CH_{3}CH_{2}COOH> (CH_{3})_{2}CHCOOH> (CH_{3})_{3}C.COOH$
Answer:
Inductive effect- The permanent displacement of sigma ( $\sigma$ ) electrons along the saturated chain, whenever an electron-donating or withdrawing group is present, is known as the inductive effect. It could be $+I/-I$ effect.
Electromeric effect- The complete transfer of the shared pair of $\pi$ -electrons to one of the atoms linked by multiple bonds on the demand of the attacking reagent. It is a temporary effect. It may be +E(electrons transferred towards the attacking reagent) or -E effect (electrons transferring away from the attacking reagent).
(a) The order of acidity can be explained by the negative inductive effect ( $-I$ ). As the no. of chlorine atoms increases, the $-I$ effect also increases and so, the acidic strength also increases.
$Cl_{3}CCOOH> Cl_{2}C\! H\! \, COOH> ClC\! H_{2}COOH$
(b) This can be explained by the $+I$ effect of the alkyl group. As the number of electron donor groups increases, the $+I$ effect will also increase. With the increases in the $+I$ effect, the acidic character decreases accordingly.
$CH_{3}CH_{2}COOH> (CH_{3})_{2}CHCOOH> (CH_{3})_{3}C.COOH$
Question 8.18 (a) Give a brief description of the principles of the following techniques taking an example in each case.
Crystallisation
Answer:
Crystallisation- It is one of the most commonly used techniques for the purification of solid organic compounds. Its principle is based on the difference in the solubilities of the compounds and the solvent's impurities. The impure compounds are dissolved in a solvent, but they are sparingly soluble at room temperature, but soluble at higher temperatures. On cooling the compound, the pure compounds crystallise and are removed by filtration.
For example, pure aspirin is obtained by recrystallising crude aspirin. Around 2 - 4 g of crude aspirin is dissolved in 20 mL of ethyl alcohol, and the solution is heated for complete dissolution. Then, after crystal formation, they can be filtered out and dried.
Question 8.18 (b) Give a brief description of the principles of the following techniques taking an example in each case.
Distillation
Answer:
Distillation- This method is used for the purification of liquids from non-volatile impurities. It is based on the fact that fluids with different boiling points vaporise at different temperatures. The impure liquid is boiled in a flask, and initially, the vapours of lower lower-boiling-point component are formed. The vapours are condensed by using a condenser, and the liquid is collected in a receiver.
For example, Organic liquids such as benzene, toluene, xylene, etc, can be purified by this method.
Question 8.18 (c) Give a brief description of the principles of the following techniques taking an example in each case.
Chromatography
Answer:
Chromatography- It is one of the extensive methods for the separation and purification of organic compounds. It is based on the difference in the movements of components of mixtures through the stationary phase under the influence of the mobile phase. The stationary phase can be solid or liquid. While the mobile phase is only liquid or gas.
For example, this technique can separate a mixture of blue and red ink.
Question 8.19 Describe the method, which can be used to separate two compounds with different solubilities in a solvent S.
Answer:
Fractional distillation can be used to separate the two compounds with different solubilities in a solvent S. The following steps are carried out in this process-
- The powdered mixture is taken in a flask, and the solvent is added to it and stirred simultaneously. We have to prepare a saturated solution and then heat it.
- After heating, this hot saturated solution can be filtered with filter paper in a china dish.
- Now the solution is allowed to cool. The less soluble compounds crystallise first, andthe more soluble compounds remain in the solution. After removing these crystals, the latter is concentrated once again. The hot solution is allowed to cool, and then the crystals of the more soluble compound are obtained.
- The last step is the isolation and drying of crystals from the mother liquor.
Question 8.20 What is the difference between distillation, distillation under reduced pressure and steam distillation ?
Answer:
The differences between distillation, distillation under reduced pressure and steam distillation are-
|
Distillation
|
Distillation under reduced pressure
|
Steam Distillation
|
|
1. Used for the purification of compounds that are non-volatile impurities or liquids, which don't decompose on boiling.
|
1. Used to purify liquids, which tend to decompose on boiling. Under the condition of reduced pressure.
|
1. Used to purify the organic compounds, which are steam-volatile and immiscible with water.
|
|
Simply, to separate the volatile liquids from non-volatile impurities. Or a mixture of liquids having a sufficient Boiling point difference.
|
The liquid will boil at a lower temperature than its boiling point; therefore, it does not decompose.
|
The mixture of water and aniline can be separated by this method.
|
|
The mixture of petrol and kerosene is separated by this method.
|
Glycerol is purified by this method.
|
|
Question 8.21. Discuss the chemistry of Lassaigne’s test.
Answer: Lassaigne’s test.-
This test is used to detect the presence of nitrogen, halogens and sulphur in organic compounds. These elements are present in the covalent form in an organic compound. So, they are converted into the ionic form by fusing the compound with the sodium metal.
Cyanide, sulphide or halide of sodium are extracted from the fused mass by boiling it with distilled water. This Is known as the sodium extract method or Lassaigne's extract.
Question 8.22(i) Differentiate between the principle of estimation of nitrogen in an organic compound by
Dumas method
Answer:
In the Dumas method, a Nitrogen-containing organic compound is heated with copper oxide in a carbon dioxide atmosphere, yielding free nitrogen in addition to $CO_{2}$ and water.
Traces of nitrogen oxides can also be formed in the reaction, which can be reduced to nitrogen by passing the gaseous mixture over the heated copper gauge. The produced mixture of gases is collected over an aqueous solution of $KOH$ , which absorbs $CO_{2}$. Nitrogen is collected in the upper part of the graduated tube.

Question 8.22(ii) Differentiate between the principle of estimation of nitrogen in an organic compound by
Kjeldahl’s method.
Answer:
In Kjeldahl’s method, the nitrogen-containing organic compound is heated with concentrated sulfuric acid. Nitrogen is converted into ammonium sulphate. It is then passed into the known volume of sulphuric acid. The amount of ammonia produced can be estimated by the amount of $H_{2}SO_{4}$ consumed in the reaction.
It is done by estimating the amount of unreacted $H_{2}SO_{4}$ left after the absorption of ammonia by titrating it with a standard alkali solution. This method is not applicable for compounds containing nitrogen in -nitro form or nitrogen present in the ring structure.

Question 8.23 Discuss the principle of estimation of halogens, sulphur and phosphorus present in an organic compound.
Answer:
Estimation of halogen is done by the Carius method. In this method, a known quantity of organic compound is heated with fuming $HNO_{3}$ (nitric acid) with the presence of silver nitrate, contained in a hard glass tube, known as a carius tube. C and H present in the compound are oxidised to carbon dioxide and water. Halogens are into to form $AgX$, and then it is filtered, dried, and weighed.
Let the mass of the organic compound be $m$ grams.
Mass of $AgX$ formed = $m_1$ gram
1 mol of $AgX$ contains 1 mol of X.
Therefore, Mass of halogen inm1 g of AgX = (Atomic mass of $X*m_1$ ) $/$ (Molecular mass of $AgX$ )
Thus, % of halogen will be = (Atomic mass of $X(m_1\times 100)$$/$ mo l. wt of $AgX*(m)$
Estimation of sulphur- In this method, an Organic compound is heated with either fuming nitric acid or sodium peroxide in a hard glass tube called a carius tube. Sulphur present in the compound is oxidised to form sulphuric acid. It is precipitated as barium sulphate by adding barium chloride solution to water. Then the ppt is filtered, washed and weighed.
Let the mass of the organic compound taken = $m$ g
and the mass of barium sulphate formed = $m_1$ g
1 mol of $BaSO_{4}$ = 233 g $BaSO_{4}$ = 32 g sulphur
$m_1$ g $BaSO_{4}$ contains = $\frac{32\times m_1}{233}$ g sulphur
Percentage (%)of sulphur =
$\frac{32\times m_1\times 100}{233\times m}$
Estimation of phosphorus- In this process, a known mass of an organic compound is heated with fuming nitric acid and the phosphorus gets oxidised to phosphoric acid. By adding ammonia and ammonium molybdate, phosphorus can be precipitated as ammonium phosphomolybdate, $(NH_4)_3PO_4.12MoO_{3}$. It can also be estimated by precipitating it as $MgNH_{4}PO_{4}$ by adding a magnesia mixture, which on ignition yields $Mg_2P_2O_{7}$.
Let the mass of organic compound taken = $m$ g and the mass of ammonium phosphomolybdate = $m_1$ g
Molar mass of $(NH_4)_3PO_4.12MoO_{3}=1877$ g
Percentage(%) of phosphorus = $\frac{31\times m_1\times 100}{1877\times m}$
If phosphorus is estimated as $Mg_2P_2O_{7}$ ,
Percentage(%) of phosphorus = $\frac{62\times m_1\times 100}{222\times m}$ %
Question 8.24 Explain the principle of paper chromatography.
Answer: In paper chromatography, chromatography paper is used. It contains water trapped in it, which acts as the stationary phase. The solution of the mixture is spotted based on chromatography paper. The strip of paper is suspended in a suitable solvent, which is the mobile phase. Due to capillary action, the solvent rises up in the paper, and it flows over the spot. The spots of the different components travel with the mobile phase to different levels of heights. The obtained paper is called a chromatogram.

Question 8.25 Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens?
Answer:
Nitric acid was added to the sodium extract before adding silver nitrate for testing halogens to decompose the $NaCN$ to $HCN$ and $Na_2S$ to $H_2S$ and to expel these gases. If any amount of nitrogen and sulphur are present in the form of $NaCN$ and $Na_2S$, then they are removed.
$\\NaCN+HNO_{3}\rightarrow NaNO_{3}+HCN\\$
$Na_{2}S+HNO_{3}\rightarrow 2NaNO_{3}+H_{2}S$
Question 8.26: Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur and halogens.
Answer:
In Organic compounds, nitrogen, sulphur, and halogens are covalently bonded. To detect them, they have to convert into ionic form. This is done by the fusion of an organic compound by sodium metal. This is known as Lassaigne's test.
The following chemical reactions are-
$\\Na+C+N\rightarrow NaCN$
$Na+S+C+N\rightarrow NaSCN$
$2Na+S\rightarrow Na_2S$
$Na+X\rightarrow NaX$
Here X = halogen atoms
Question 8.27. Name a suitable technique for the separation of the components from a mixture of calcium sulphate and camphor.
Answer:
To separate a mixture of calcium sulphate and camphor, we use the sublimation method. In this method, the sublimable compound is converted to a vapour state from the solid state without achieving its liquid state. Here, camphor is a sublimable compound, and calcium sulphate is not.
Question 8.28. Explain, why an organic liquid vaporises at a temperature below its boiling point in its steam distillation ?
Answer:
In steam distillation, an Organic liquid starts boiling when the total sum of the vapour pressure of the organic liquid (P') and the of water (P'') becomes equal to the atmospheric pressure (P),
it means P = P' + P''. since P'< P'', the organic liquid will vaporise at a lower temperature than its boiling point.
Question 8.29. Will $CCl_{4}$ givea white precipitate of $AgCl$ on heating it with silver nitrate? Give reason for your answer.
Answer:
No, $CCl_{4}$ does not give white ppt of $AgCl$ on heating with the silver nitrate. This is because the chlorine atoms are covalently bonded to the carbon atom. To get ppt, it should be present in ionic form.
Answer:
Carbon dioxide is acidic in nature and $KOH$ is a strong base. Thus, carbon dioxide reacts with the potassium hydroxide by acid-base reaction and form a salt known as potassium carbonate and water as a by-product.
$KOH+CO_{2}\rightarrow K_{2}CO_{3}+H_{2}O$
Thus, due to the increase in KOH, the mass of the U-tube also increases. This increased mass of the tube gives the mass of carbon dioxide produced. From its mass, the % of carbon in the Organic compound can be estimated.
Answer:
It is necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test, because in case of sulphuric acid, the complete ppt formation of lead sulphate does not take place. While the addition of acetic acid will ensure a complete ppt. formation of sulphur in the form of lead sulphate due to the common ion effect.
Answer:
Given,
Percentage of carbon = 69%
% of Hydrogen = 4.8%, and
% of Oxygen = 26.2 %
So, 0.2 g of Organic Compound have $(0.2\times 0.69)$ g of Carbon = 0.138g
we know that,
molecular weight of $CO_{2}$ = 44 g
12 g of C is present in 44 g of $CO_{2}$
Therefore, 0.138 g of C present in $0.138\times \frac{44}{12}$ = 0.506 g of $CO_{2}$
Hence, from 0.2 g of Organic Compound, 0.506 g of $CO_{2}$ will be produced.
Similarly,
100 g of Organic Compound contains 4.8 g of H
So, 0.2 g of Organic Compound contains $(0.2\times 0.048)$ = 0.0096 g of H
We know the mol. wt. of water = 18 g
Therefore 0.0096 g of H will present in $\frac{18\times 0.0096}{2}$ = 0.0864 g of water
Hence, 0.2 grams of Organic Compound will produce 0.0864 g of water on complete combustion of
Answer:
Given that,
The total mass of the organic compound = 0.50 g
60 mL of 0.5 M solution of $NaOH$ is required for neutralisation by residual acid.
60 mL of 0.5 M $NaOH$ solution = $\frac{60}{2}$ mL of 0.5M $H_{2}SO_{4}$
= 30 mL of 0.5 M $H_{2}SO_{4}$
Therefore,
Acid consumed in the absorption of evolved ammonia is (50-30) mL = 20 mL
Again, 20 mL of 0.5 M $H_{2}SO_{4}$ = 40 mL of 0.5 M $NH_3$
Also, since 1000 mL of 1 M $NH_3$ contains 14 g of nitrogen,
So, 40 mL of 0.5 M $NH_3$ will contain
= $\frac{14\times 40\times 0.5}{1000}$
$= 0.28$ g of Nitrogen
Therefore, percentage(%) of nitrogen in 0.50 g of organic compound = $(0.28/0.5)\times 100$ = 56 %
Answer:
Given that,
Mass of organic compound = 0.3780 g.
Mass of AgCl formed = 0.5740 g
It is known that,
1 mol of $AgCl$ contains 1 mol of Cl.
Thus, the mass of chlorine in 0.5740 g of $AgCl$
$\\=\frac{35\times 0.5740}{143.32}$
= 0.1421 g
∴ Percentage(%) of chlorine = $=\frac{0.1421}{0.3780}\times 100$
= 37.59%
Hence, the percentage of chlorine present in the given organic compound is 37.59%.
Answer:
Given data,
The total mass of the Organic Compound = 0.468 g
Mass of barium sulphate formed = 0.668 g
We know that,
1 mol of BaSO4 = 233 g of $BaSO_{4}$
= 32 g of sulphur
Thus, 0.668 g of $BaSO_{4}$ contains = $\frac{32\times 0.668}{233}$ g of sulphur
= 0.0917 g of sulphur
Therefore, the percentage(%) of sulphur
$= \frac{0.0197}{0.468}\times 100$
= 19.59 %
Hence, the percentage of sulphur in the given compound is 19.59 %.
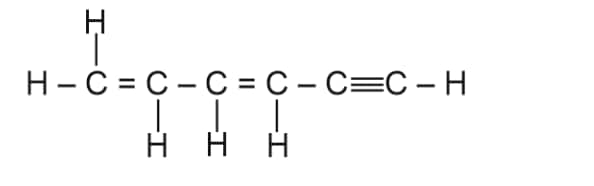
$(a) sp-sp^{2}$ $(b) sp-sp^{3}$ $(c) sp^{2}-sp^{3}$ $(d) sp^{3}-sp^{3}$
Answer:
In the organic compounds-
$C\! H_{2}=C\! H-C\! H_{2}-C\! H_{2}- C\equiv \! CH$
In $C_2$ and $C_3$ carbon atom the hybridisation is $sp^2-sp^3$
Hence, the correct answer is option (3)
Question 8.37. In the Lassaigne’s test for nitrogen in an organic compound, the Prussian blue colour is obtained due to the formation of:
Answer:
The Prussian blue colour is due to the formation of $Fe_4[Fe(CN)_6]_3.xH_{2}O$ in Lassaigne's test.
$6CN^-+Fe^{2+}\rightarrow [Fe(CN)_6]^{4-}$
$3[Fe(CN)_6]^{4-}+4Fe^{3+}\rightarrow Fe_4[Fe(CN)_6]_3.xH_{2}O$
Hence, the correct answer is option (b).
Question 8.38. Which of the following carbocation is most stable?
$(a)\; (CH_{3})_{3}C.C\, H_{2}$ $(b)\; (C\! H_{3})_{3}C$ $(c)\; C\! H_{3}C\! H_{2}C\! H_{2}$ $(d)\; CH_{3}\, C\! H C\! H_{2}C\! H_{3}$
Answer:
We know the stability of carbocation order is-
$3^o>2^o>1^o>CH_3^+$ (It is due to the tendency of the methyl group to electron release and stabilise the carbocation)
Hence, the correct answer is option (b).
Answer:
Chromatography is the latest technique of separation and purification of organic compounds.
Hence, the correct answer is option (d).
Question 8.40. The reaction:
$C\! H_{3}C\! H_{2}I+K\! O\! H(aq)\rightarrow C\! H_{3}C\! H_{2}O\! H+K\! I$ is classified as :
(a) electrophilic substitution (b) nucleophilic substitution (c) elimination (d) addition
Answer:
$C\! H_{3}C\! H_{2}I+K\! O\! H(aq)\rightarrow C\! H_{3}C\! H_{2}O\! H+K\! I$
Here, $OH^-$ attacks ethyl iodide and substitutes the iodide ion. $OH^-$ has a lone pair of electrons, so it acts as a nucleophile. Hence, it is a nucleophilic substitution reaction.
Hence, the correct answer is option (b).