NCERT Class 11 Physics Chapter 11 Notes Thermal Properties of Matter - Download PDF
Have you ever asked yourself why a thermometer is so accurate in measuring the temperature of your body, why ice melts or why metals are colder than wood at the same temperature? These phenomena in everyday life are described in a simple and interesting manner in Chapter 10, Class 11 Physics: Thermal Properties of Matter. These NCERT notes are designed by highly qualified faculty members based on the updated 2025-26 CBSE syllabus and are perfect in terms of board exams, JEE, NEET, and other competitive exams.
This Story also Contains
- NCERT Notes for Class 11 Physics Chapter 10: Download PDF
- NCERT Notes for Class 11 Physics Chapter 10
- Class 11 Physics Chapter 10 Thermal Properties of Matter: Previous Year Questions and Answers
- How to Master Class 11 Physics Chapter 10 Thermal properties of Matter?
- Importance of Thermal Properties of Matter Class 11 Notes
- How to Use NCERT Class 11 Physics Chapter 10: Thermal Properties of Matter Notes Effectively?
- NCERT Class 11 Notes Chapter-Wise
- Subject-Wise NCERT Exemplar Solutions
- Subject-Wise NCERT Solutions
- NCERT Books and Syllabus

The topics that are found to be important in NCERT notes are heat transfer (conduction, convection, and radiation), specific and latent heat, the law of cooling by Newton, and thermal expansion. These NCERT Notes for Class 11 Physics Chapter 10 Thermal Properties of Matter make the learning process simple and quick with well-organised formulas, clear explanations, and examples of real life. The NCERT Notes for Class 11 Physics Chapter 10 Thermal Properties of Matter can be used by students to revise quickly, understand concepts, and practice exam-oriented studies so that they can be able to master both theoretical and numerical problems.
Also Read
NCERT Notes for Class 11 Physics Chapter 10: Download PDF
These Class 11 Physics Chapter 10 Thermal Properties of Matter Notes are easy and quick to revise, and provide step-by-step explanations, key formulas, and solved problems. The PDF is free to download, and the students can study it anywhere and at any time to sharpen their knowledge and gain enough confidence to have a successful experience at the board exams, JEE and NEET. The Thermal Properties of Matter Class 11 Physics notes are useful to facilitate the conceptualisation, training in the practical mathematical tasks, as well as reviewing effectively.
NCERT Notes for Class 11 Physics Chapter 10
These Class 11 Physics Chapter 10 Thermal Properties of Matter Notes are a concise and clear summary of all the significant concepts, formulas and solved examples. The Thermal Properties of Matter Class 11 Physics Chapter 10 CBSE notes make students learn the thermal properties of matter with ease and have a revision with ease. These NCERT notes Class 11 Physics Chapter 10 Thermal Properties of Matter are perfect for the preparation of CBSE board exams, JEE, and NEET, and help to learn at a faster and more efficient rate.
Temperature and Heat
Temperature is the measurement of a body's degree of hotness or coolness.
Example:- A cup of hot soup or an ice cream scoop
Heat is a kind of energy that is exchanged via the temperature difference between two (or more) systems or between a system and its surroundings.
For example, a cup of hot coffee might be regarded as an object or system, whereas everything else is the environment.
As a result of the temperature difference, heat will move from one thing to another.
The SI unit of Heat is the joule.
Measurement of Temperature
A thermometer is used to determine the temperature.
In liquid-in-glass thermometers, mercury and alcohol are regularly used liquids.
The following graph depicts the relationship between temperature in Celsius and Fahrenheit graphically:-
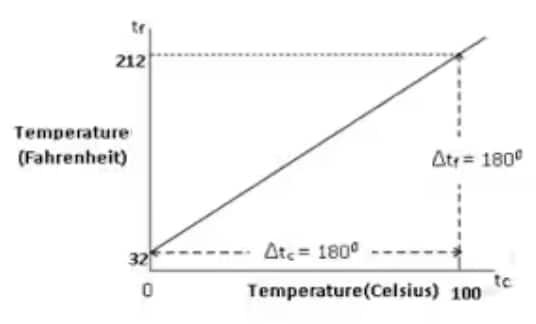
$\left(t_{\mathrm{f}}-32\right) / 180=t_{\mathrm{c}} / 100$
Where
tf means Fahrenheit temperature
tc means Celsius temperature
Absolute Temperature and the Ideal Gas Equation
A thermometer that employs any gas, on the other hand, will produce the same readings regardless of the gas used since all gases expand at the same rate at low temperatures.
The following variables are used to characterise the behaviour of gas:
-
Quantity(mass)
-
Pressure
-
Volume
-
Temperature
Gases with a low density obey the following rules: -
1. Boyle’s Law– In this law, the product of PV = constant(when temperature T is constant)
2. Charles’ Law- In this law, the ratio of V/T = constant (when pressure P is constant)
When both of the preceding principles are combined, the equation becomes PV = RT, where R stands for the universal gas constant.
Absolute Zero
The minimal absolute temperature of an ideal gas is defined as Absolute Zero.
We get a straight line if we plot pressure versus temperature and then extend the line backwards to the x-axis, as seen in the graph below.
The lowest temperature measured (experimentally) was -273.15 °C, which is known as absolute zero.
The relationship between temperature in Kelvin and temperature in Celsius is provided by
$\mathbf{T}=\mathbf{t}_c+273.15$
Thermal Expansion
Thermal expansion is the phenomenon of a body's dimensions expanding as its temperature rises.
Thermal Expansion Types
Expansion in length is known as linear expansion.
Expansion in the area is known as area expansion.
Volume Expansion: This is a term that refers to the increase in volume of anything.
Linear Expansion
The term "linear expansion" refers to the length of anything expanding as the temperature rises.
The term "linear expansion" refers to a fractional change in length or how the length changes in relation to the original length.
$\Delta L / L=\alpha_L \Delta T$
ΔL is the length change, L is the initial length, ΔT is the temperature change, and αL is the linear coefficient of thermal expansion.
Area Expansion
The term "area expansion" refers to the expansion of an area as a result of an increase in temperature.
There is an increase in both length and width when an area is expanded.
$\Delta \mathbf{A} / \mathbf{A}=\alpha_{\mathrm{a}} \Delta \mathbf{T}$
Where αa = coefficient of area expansion.
Volume Expansion
It is defined as the expansion of volume as a result of an increase in temperature.
This indicates that a substance's length, width, and height have increased.
$\Delta V / V=\alpha_v \Delta T$
Where αv = coefficient of volume expansion.
Anomalous Behaviour of Water
At 4 °C, water has the highest density.
When the temperature drops below 4 °C, the water expands, and the density falls.
Because of this feature, water in lakes and ponds only freezes at the top layer and not at the bottom, but if the water freezes at the bottom as well, animal and plant life would be unable to survive.
Specific heat capacity:
Heat Capacity
A quantity termed the heat capacity of a substance characterises the change in temperature of a substance when a particular amount of heat is absorbed or rejected by that substance.
$S=\Delta Q / \Delta T$
Where ΔQ is the amount of heat supplied to the substance and ΔT change in its temperature.
Specific heat is the amount of heat received or rejected per unit mass by a substance to change its temperature by one degree.
$\mathbf{s}=\mathbf{S} / \mathbf{m}$
Molar specific heat capacity: -
The heat capacity per mole is the amount of heat (in moles) absorbed or rejected by a substance to change its temperature by one unit.
$C=S / \mu=\Delta Q / \mu \Delta T$
The molar specific heat capacity (Cp) is defined as follows:
The equivalent molar specific heat capacity at constant pressure is called molar specific heat capacity at constant pressure if the gas is retained at constant pressure during the heat transfer (Cp).
Molar specific heat capacity (Cv):-
The equivalent molar specific heat capacity at constant volume is called molar specific heat capacity at constant volume if the volume of the gas is maintained during the heat transfer (Cv).
Calorimetry
The word calorimetry comes from two parts:
-
Calorie, which means "heat"
-
Metry, which means "measuring"
So, calorimetry is the process of measuring heat.
It involves the transfer of heat from a hotter body to a cooler body, while making sure that no heat is lost to the surroundings.
The basic idea behind calorimetry is that the heat lost by one body is equal to the heat gained by another. The device used to measure this heat transfer is called a calorimeter.
Change of State
A change of state is when a substance transitions from one form (solid, liquid, or gas) to another. For example:
-
Melting is when a solid (like ice) turns into a liquid (like water).
-
Fusion is the process by which a liquid (like water) turns into a solid (like ice).
Thermal Equilibrium
In thermal equilibrium, there’s no heat gain or loss. The melting point of a substance is the temperature at which its solid and liquid states are in balance (equilibrium).
-
Normal Melting Point: This is the temperature at which a substance melts under standard atmospheric pressure.
Regelation
Regelation is the process where pressure lowers the freezing point of water, allowing ice to melt under pressure and refreeze when the pressure is reduced.
Vaporization
Vaporisation is the process by which a liquid changes into a gas (for example, water turning into steam).
Sublimation
Sublimation is when a solid directly turns into a gas without becoming a liquid first. During sublimation, both the solid and gas forms exist in thermal equilibrium.
Latent Heat
Latent heat is the amount of heat energy required to change the state of a substance without changing its temperature. For example, during melting or boiling, a substance absorbs or releases heat, but its temperature stays constant. There are two main types: latent heat of fusion (solid to liquid) and latent heat of vaporisation (liquid to gas). This concept helps explain why ice melts or water boils at specific temperatures.
Heat Transfer
Heat transfer refers to the process by which thermal energy moves from a body at a higher temperature to one at a lower temperature. This transfer can occur in three primary ways:
- Conduction – Transfer of heat through direct contact, mainly in solids. For example, a metal rod gets hot when one end is placed in a flame.
- Convection – Transfer of heat through the movement of fluids (liquids or gases). Warm fluids rise, and cooler fluids sink, creating a convection current. For instance, boiling water in a pot.
- Radiation – Transfer of heat without a medium, in the form of electromagnetic waves. This is how the Sun heats the Earth.
Newton's Law of Cooling
Newton's Law of Cooling states that the rate at which a hot object cools down is directly proportional to the difference in temperature between the object and its surroundings, provided this difference is small. In simple words, the hotter the object compared to its surroundings, the faster it loses heat.
$
\frac{d T}{d t}=-k\left(T-T_{\text {surrounding }}\right)
$
Where:
- $\frac{d T}{d t}$ is the rate of change of temperature,
- $T$ is the temperature of the object,
- $T_{\text {surrounding }}$ is the surrounding temperature,
- $k$ is a constant depending on the nature of the object and its surroundings.
Class 11 Physics Chapter 10 Thermal Properties of Matter: Previous Year Questions and Answers
The chapter Thermal Properties of Matter explains how substances respond to heat—expanding, changing temperature, and undergoing phase transitions. It introduces key ideas such as thermal expansion, heat capacity, calorimetry, and heat transfer. These concepts are widely used in daily life, from thermometers to refrigerators, making the chapter both practical and engaging. A strong conceptual base and numerical practice help you solve exam questions with ease.
Q1: Water falls from a height of 200 m into a pool. Calculate the rise in temperature of the water, assuming no heat dissipation from the water in the pool.
(Take $\mathrm{g}=10 \mathrm{~m} / \mathrm{s}^2$, specific heat of water $=4200 \mathrm{~J} /(\mathrm{kg} \mathrm{K})$ )
Answer:
Setting the potential energy loss equal to the heat gained:
$\begin{aligned} & m g h=m s \Delta T \\ & \Delta T=\frac{g h}{S}=\frac{10 \times 200}{4200}=0.48 \mathrm{~K}\end{aligned}$
Q2:
Two cylindrical rods, A and B, made of different materials, are joined in a straight line. The ratio of lengths, radii and thermal conductivities of these rods are: $\frac{L_A}{L_B}=\frac{1}{2}, \frac{r_A}{r_B}=2$ and $\frac{\mathrm{K}_A}{K_B}=\frac{1}{2}$. The free ends of rods A and B are maintained at $400 \mathrm{~K}, 200 \mathrm{~K}$, respectively. The temperature of rods interface is _____ K, when equilibrium is established.
Answer:

$\begin{aligned} & \mathrm{R}_1=\frac{\ell_1}{\mathrm{~K}_1 \mathrm{~A}_1}, \mathrm{R}_2=\frac{\ell_2}{\mathrm{~K}_2 \mathrm{~A}_2} \\ & \frac{\mathrm{dQ}}{\mathrm{dt}}=\frac{\Delta \mathrm{T}}{\mathrm{R}} \\ & \left(\frac{\mathrm{dQ}}{\mathrm{dt}}\right)_1=\left(\frac{\mathrm{dQ}}{dt}\right)_2 \\ & \frac{400-T}{R_1}=\frac{T-200}{R_2} \\ & \frac{400-\mathrm{T}}{\mathrm{T}-200}=\frac{\mathrm{R}_1}{\mathrm{R}_2}=\left(\frac{\ell_1}{\ell_2}\right)\left(\frac{\mathrm{r}_2}{\mathrm{r}_1}\right)^2 \times \frac{\mathrm{K}_2}{\mathrm{~K}_1} =\frac{1}{2} \times\left(\frac{1}{2}\right)^2 \times 2 =\left(\frac{1}{4}\right) \\ & \frac{400-\mathrm{T}}{\mathrm{T}-200}=\frac{1}{4} \\ & 1600-4 \mathrm{~T}=\mathrm{T}-200 \\ & 5 \mathrm{~T}=1800 \\ & \mathrm{~T}=360 \mathrm{~K}\end{aligned}$
Hence, the answer is 360.
Q3:
Consider a rectangular sheet of solid material of length $/=9 \mathrm{~cm}$ and width $d=4 \mathrm{~cm}$. The coefficient of linear expansion is $\alpha=3.1 \times 10^{-5} \mathrm{~K}^{-1}$ at room temperature and one atmospheric pressure. The mass of sheet $m=0.1 \mathrm{~kg}$ and the specific heat capacity $C_v=900 \mathrm{~J} \mathrm{~kg}^{-1} \mathrm{~K}^{-1}$. If the amount of heat supplied to the material is $8.1 \times 10^2 \mathrm{~J}$, then the change in area of the rectangular sheet is
Answer:
$\begin{aligned} & Q=m c \Delta T \\ & 8.1 \times 10^2=900 \times 0.1 \times \Delta T \\ & \Delta T=9 \mathrm{~K} \\ & \Delta A=A \cdot 2 \propto \Delta T \\ & =36 \times 2 \times 3.1 \times 10^{-5} \times 9 \times 10^{-4} \\ & =2 \times 10^{-6} \mathrm{~m}^2\end{aligned}$
Q4: The temperature of a body in air falls from $40^{\circ} \mathrm{C}$ to $24^{\circ} \mathrm{C}$ in 4 minutes. The temperature of the air is $16^{\circ} \mathrm{C}$. The temperature of the body in the next 4 minutes will be :
Answer:
$\begin{aligned} & \frac{\mathrm{T}_2-\mathrm{T}_1}{\mathrm{t}}=\mathrm{K}\left[\mathrm{T}_{\text {avg }}-\mathrm{T}_{\mathrm{s}}\right] \\ & \mathrm{T}_1=24^{\circ} \mathrm{C} ; \mathrm{T}_2=40^{\circ} \mathrm{C}, \mathrm{t}=4, \mathrm{~T}_{\mathrm{s}}=16^{\circ} \mathrm{C} \\ & \frac{40-24}{4}=\mathrm{K}[32-16] \\ & \mathrm{K}=\frac{4}{16}=\frac{1}{4} \\ & \text { Now } \frac{24-\mathrm{T}}{4}=\mathrm{K}\left[\frac{\mathrm{T}+24}{2}-16\right] \\ & 24-\mathrm{T}=\frac{\mathrm{T}-16}{2}+16 \\ & \frac{3 \mathrm{~T}}{2}=28 \\ & \mathrm{~T}=\frac{56}{3} {}^\circ\mathrm{C}\end{aligned}$
Q5: A cup of coffee cools from $90^{\circ} \mathrm{C}$ to $80^{\circ} \mathrm{C}$ in t minutes when the room temperature is $20^{\circ} \mathrm{C}$. The time taken by a similar cup of coffee to cool from $80^{\circ} \mathrm{C}$ to $60^{\circ} \mathrm{C}$ at the same room temperature is :
Answer:
By using average form of Newton's law of cooling
$
\begin{aligned}
& \frac{90-80}{\mathrm{t}}=\mathrm{k}\left(\frac{90+80}{2}-20\right) \\
& \frac{80-60}{\mathrm{t}^{\prime}}=\mathrm{k}\left(\frac{80+60}{2}-20\right)
\end{aligned}
$
$
\begin{aligned}
& \text { (i)/(ii) } \\
& \frac{10 \times \mathrm{t}^{\prime}}{\mathrm{t} \times 20}=\frac{65}{50} \\
& \mathrm{t}^{\prime}=\frac{65}{50} \times 2 \mathrm{t}=\frac{65}{25} \mathrm{t}=\frac{13}{5}t
\end{aligned}
$
How to Master Class 11 Physics Chapter 10 Thermal properties of Matter?
In order to study the Thermal Properties of Matter, you need to have knowledge of how various materials react to the addition or removal of heat. This chapter provides a solid conceptual basis using such concepts as thermal expansion, heat capacity, calorimetry, and heat transfer. The majority of questions require the practical application of formulas to real-life scenarios, which is why a clear understanding of concepts and regular practice with numericals are critical. This chapter is scoring and very intuitive once you are going through a structured approach.
- The best place to start is to have a clear understanding of the fundamental concepts of heat, temperature, and thermal equilibrium to make all other concepts easier to relate.
- Study the formula of linear, area, and volume expansion and practise the small numericals to ensure that you are comfortable with the expansion of materials under heating.
- Learn heat transfer processes: conduction, convection, radiation, studying real-life examples, which will enable you to remember the concepts easily during exams.
- Develop a good command of specific heat capacity and calorimetry to solve problems of mixture and change of phase involving heat gained and lost.
- Learn the latent heat properly, and drill yourself on heating/cooling curve problems so that you are never confused when the temperature is kept constant during a change in state.
- Revise important laws such as the law of cooling of Newton and the Stefan-Boltzmann law, and use them in short conceptual numerical problems to better remember.
- Solved all NCERT and Exemplar numericals since most of the board and competitive questions are direct applications of the same.
- Prepare a clean formula sheet, including expansion, calorimetry, and radiation formula and update it on a regular basis to aid in strengthening memory.
- Draw simple diagrams such as heating curves, conduction setups, and bimetallic strips, because visual learning improves understanding.
- Write brief notes with definitions, major formulas and common values in order to revise before tests and exams in a few seconds.
Importance of Thermal Properties of Matter Class 11 Notes
NCERT notes Class 11 Physics Chapter 10 Thermal Properties of Matter are necessary to create a solid conceptual base. The Thermal Properties of Matter Class 11 Physics notes assist the students to understand the major subjects, such as the transfer of heat, thermal expansion, and specific heat, in a simple and fast manner. The notes are especially handy when revising, taking board exams, and competitive exams such as JEE and NEET.
- Understanding Heat Transfer Mechanisms - In the notes, the concepts of conduction, convection, and radiation have been explained. Students will be able to relate these concepts to real-life examples such as the reasons cooking vessels heat, the formation of the sea breezes and the transfer of energy from the Sun to the planet.
- Applications in Thermal Expansion: These notes discuss the thermal expansion of solids, liquids, and gases. Real-world examples like expansion joints in bridges, bimetallic strips in thermostats, and separations in the railway lines are described, and thus, concept learning becomes simpler.
- Specific and Latent Heat Calculations: There are formulae and derivations in the notes that allow one to easily calculate the amount of heat taken in or given out by a substance. This makes it effective in solving numerical problems on temperature change, phase change and heat energy.
- Newton's Law of Cooling: The notes elaborate on how objects cool down over time, with derivations and example problems. This can be connected to real-life situations, such as the cooling of hot tea by students, and this helps them to develop reasoning and problem-solving abilities.
- Association with Real-Life Applications: The concepts are easy to memorise and understand as the notes connect the theory with the real-world applications, such as mercury expansion in thermometers, ice melting, and thermal insulation.
- Exam-Oriented revision tool: These notes are a collection of all vital points, formulae, and plans, and thus, they are useful in both board exams preparation (CBSE Class 11) and also in competitive exams such as JEE Main, NEET, and Olympiads.
- Problem-Solving Practice: Solved problems and step-by-step directions to the common numerical problems enhance intellectual clarity and confidence in approaching both theoretical and practical questions.
- Quick Prep in Case of Last-minute Revision: Clearly organised points, formula highlights, as well as diagrams, enable quick revision prior to the exams without missing the vital concepts.
How to Use NCERT Class 11 Physics Chapter 10: Thermal Properties of Matter Notes Effectively?
The concepts of heat and temperature introduced in the Class 11 Physics chapter 11 Thermal Properties of Matter Notes for this chapter are introduced in a simple and understandable format, which makes students understand the concepts of thermal expansion and heat transfer without any confusion. These Thermal Properties of Matter NCERT Notes facilitate easier learning and make revision faster when done in a good manner.
- When you start with reading the Thermal Properties of Matter NCERT Notes, it is advisable to have an initial understanding of the chapter and then read through the detailed explanations.
- Pay attention to the study of concepts like temperature, heat, thermal expansion, specific heat capacity, and heat transfer methods using the assistance of simple examples.
- Write down all the key formulas individually and practice them regularly in order to recall them when taking the exams.
- After reading the Thermal Properties of Matter Class 10 Notes, solve numerical questions to enhance your skills in the application of the formulas.
- Notes have diagrams and examples, which will be useful in visualising the thermal processes.
- It is good to revise the Class 11 Thermal Properties of Matter Notes often, particularly before the tests and exams, in order to keep all the concepts in your mind.
- In case any of the topics appears to be confusing, consult the textbook rather than leaving the uncertainty unsolved.
NCERT Class 11 Notes Chapter-Wise
Get well-structured and easy-to-understand NCERT Class 11 Physics Notes Chapter-Wise to strengthen your exam preparation. These notes cover all important concepts, formulas, and derivations as per the latest CBSE syllabus, making them helpful for board exams and competitive exams like JEE & NEET
Subject-Wise NCERT Exemplar Solutions
NCERT Exemplar Solutions for Class 11 provide additional practice through higher-order and application-based questions. The subject-wise links help students strengthen conceptual clarity and improve problem-solving skills for exams and competitive tests.
Subject-Wise NCERT Solutions
NCERT Solutions for Class 11 offer detailed, step-by-step answers to all textbook questions across subjects. These subject-wise links make learning organised and help students prepare effectively for school exams.
NCERT Books and Syllabus
NCERT Books and the NCERT Syllabus for Class 11 form the backbone of the academic curriculum. Easy access to these links helps students understand the prescribed course structure and study systematically as per the latest guidelines.
Frequently Asked Questions (FAQs)
Vaporization :-Vaporization is the process of changing from a liquid to a gas.
The transition from a liquid to a vapour (or gas) is known as vaporisation.
Temperature is the measurement of a body's degree of hotness or coolness.
Example:-
A cup of hot soup or an ice cream scoop
Thermal Equilibrium: During this state, there is no heat loss or gain.
Sublimation:-Sublimation is the transformation of a solid into a gas.
Both the solid and vapour states of a substance coexist in thermal equilibrium throughout the sublimation (solid converts to vapour without going through liquid state) process.
No, the NCERT notes for Class 11 Physics chapter 10 do not include all of the important derivations. This NCERT note summarises the chapter's important points and equations and can be used to review the chapter's Thermal Properties of Matter.
From the notes for Class 11 Physics chapter 10, students should expect 4 to 6 mark questions, and they can use this note for quick revision to help them improve their grades.
Concept clusters are used to organize revision notes, which begin with basic qualities like temperature and heat, proceed to heat transmission mechanisms, essential laws and formulas, and conclude with application-based concepts. They offer brief summaries of each part, crucial formulas, and simplified main topics.
Popular Questions
Courses After 12th
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
