NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei
Have you ever asked yourself how the stars manage to generate such a great amount of energy, or how nuclear power stations manage to produce electricity? The solution is in the minute nucleus of atoms, which, in spite of being almost a thousand times smaller than the atom itself, contains nearly all of its weight. Class 12 Physics Chapter 13 Nuclei presents the students with the fascinating field of nuclear physics, which includes such topics as atomic masses, nuclear binding energy, nuclear forces, radioactivity, and nuclear energy, which is fundamental to the field in both theory and practice.
After receiving their CBSE Class 12th admit card 2026, students can review the helpful advice listed below:
- Have a copy of your 2026 CBSE 12th admission card with you. You must have a photocopy of your admit card on hand in case you misplace the original.
- Students must review the information on their 2026 Class 12 CBSE admission card. Students must get in touch with the appropriate school official as soon as possible to resolve any discrepancies.
- Exam day instructions should be read as stated on the 12th-grade admit card on cbse.nic.
This Story also Contains
- NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei: Download PDF
- NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei: Exercise Questions
- NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei: Additional Questions
- NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei: Higher Order Thinking Skills (HOTS) Questions
- Nuclei NCERT Solutions: Topics
- Class 12 Physics Chapter 13 - Nuclei: Important Formulas
- How Can NCERT Solutions for Class 12 Physics Chapter 13 Help in Exam Preparation?
- Approach to Solve Questions of Class 12 Physics Chapter 13 - Nuclei
- Importance of NCERT Solutions for Class 12 Physics Chapter 13: Nuclei
- What Students Learn from NCERT Solutions for Class 12 Physics Chapter 13: Nuclei?
- What Extra Should Students Study Beyond NCERT for JEE/NEET?
- NCERT Solutions for Class 12 Physics Chapter-wise

NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei are specifically developed by the experts of the subject to explain all the exercises given in the textbook, the other practice questions, and the HOTS problems step by step. These NCERT solutions have made complex nuclear concepts simple to understand, and at the same time, derivations and solving of numerical problems are easy to understand. These NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei are available in free PDF and are highly useful in the preparation of the board exams of Class 12 of the CBSE, and are a powerful resource for candidates. Through these solutions, students will be able to improve their fundamentals, gain accuracy, and gain confidence in the exam.
NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei: Download PDF
The Class 12 Physics Chapter 13 - Nuclei Solutions presented the answers to all the questions with step-by-step explanations. These Class 12 physics chapter 13 Nuclei question answers will make the students grasp tricky topics without difficulty, and can be used both in the CBSE board exams as well as JEE and NEET exams. The PDF version can also be downloaded so that you can have an easy offline reference and a quick revision anytime.
NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei: Exercise Questions
Class 12 Physics Chapter 12 - Nuclei question answers (Exercise questions) offer step-by-step answers to all textbook questions, covering topics like nuclear structure, binding energy, and radioactivity. These Class 12 physics chapter 13 Nuclei question answers help students strengthen concepts, practice effectively, and prepare for board exams as well as competitive exams like JEE and NEET.
Answer:
$
\begin{aligned}
& \mathrm{m}_{\mathrm{n}}=1.00866 \mathrm{u} \\
& \mathrm{~m}_{\mathrm{p}}=1.00727 \mathrm{u}
\end{aligned}
$
Atomic mass of Nitrogen $m=14.00307 u$
Mass defect $\Delta m=7 \times m_n+7 \times m_p-m$
$
\Delta m=7 \times 1.00866+7 \times 1.00727-14.00307
$
$\Delta m=0.10844$
Now 1u is equivalent to 931.5 MeV
$
\begin{aligned}
& E_b=0.10844 \times 931.5 \\
& E_b=101.01186 \mathrm{MeV}
\end{aligned}
$
Therefore binding energy of a Nitrogen nucleus is 101.01186 MeV
$m (_{26}^{56}\textrm{Fe})=55.934939\; \; u$
Answer:
$
\begin{aligned}
& m_H=1.007825 u \\
& m_n=1.008665 u
\end{aligned}
$
Atomic mass of ${ }_{26}^{56} \mathrm{Fe}$ is $\mathrm{m}=55.934939 \mathrm{u}$
Mass defect
$
\begin{aligned}
& \Delta m=(56-26) \times m_H+26 \times m_p-m \\
& \Delta m=30 \times 1.008665+26 \times 1.007825-55.934939 \\
& \Delta m=0.528461
\end{aligned}
$
Now 1 u is equivalent to 931.5 MeV
$
\begin{aligned}
& E_b=0.528461 \times 931.5 \\
& E_b=492.2614215 \mathrm{MeV}
\end{aligned}
$
Therefore binding energy of a ${ }_{26}^{56} \mathrm{Fe}$ nucleus is 492.2614215 MeV .
Average binding energy
$
=\frac{492.26}{56} \mathrm{MeV}=8.79 \mathrm{MeV}
$
$m(_{83}^{209}\textrm{Bi})=208.980388\; \; u$
Answer:
$
m_H=1.007825 u
$
$
m_n=1.008665 u
$
Atomic mass of ${ }_{83}^{209} \mathrm{Bi}$ is $\mathrm{m}=208.980388 \mathrm{u}$
Mass defect
$
\begin{aligned}
& \Delta m=(209-83)+83 \times m_H-m \\
& \Delta m=126 \times 1.008665+83 \times 1.007825-208.980388 \\
& \Delta m=1.760877 u
\end{aligned}
$
Now 1u is equivalent to 931.5 MeV
$
\begin{aligned}
& E_b=1.760877 \times 931.5 \\
& E_b=1640.2569255 \mathrm{MeV}
\end{aligned}
$
Therefore binding energy of a ${ }_{83}^{209} \mathrm{Bi}$ nucleus is 1640.2569255 MeV .
Average binding energy $=\frac{1640.25}{208.98}=7.84 \mathrm{MeV}$
Answer:
Mass of the coin is $w=3 g$
Total number of Cu atoms in the coin is n
$
\begin{aligned}
n & =\frac{w \times N_A}{\text { Atomic Mass }} \\
n & =\frac{3 \times 6.023 \times 10^{23}}{62.92960} \\
\mathrm{n} & =2.871 \times 10^{22} \\
\mathrm{~m}_{\mathrm{H}} & =1.007825 \mathrm{u} \\
\mathrm{~m}_{\mathrm{n}} & =1.008665 \mathrm{u}
\end{aligned}
$
Atomic mass of ${ }_{29}^{63} \mathrm{Cu}$ is $\mathrm{m}=62.92960 \mathrm{u}$
Mass defect $\Delta m=(63-29) \times m_n+29 \times m_H-m$
$\Delta m=34 \times 1.008665+29 \times 1.007825-62.92960$
$\Delta \mathrm{m}=0.591935 \mathrm{u}$
Now 1u is equivalent to 931.5 MeV
$
E_b=0.591935 \times 931.5
$
$\mathrm{E}_{\mathrm{b}}=551.38745 \mathrm{MeV}$
Therefore binding energy of a ${ }_{29}^{63} \mathrm{Cu}$ nucleus is 551.38745 MeV .
The nuclear energy that would be required to separate all the neutrons and protons from each other is
$
\begin{aligned}
& n \times E_b=2.871 \times 10^{22} \times 551.38745 \\
& =1.5832 \times 10^{25} \mathrm{MeV} \\
& =1.5832 \times 10^{25} \times 1.6 \times 10^{-19} \times 10^6 \mathrm{~J} \\
& =2.5331 \times 10^9 \mathrm{~kJ}
\end{aligned}
$
Answer:
The nuclear radii are directly proportional to the cube root of the mass number.
The ratio of the radii of the given isotopes is
$\left ( \frac{197}{107} \right )^{1/3}=1.23$
$m(_{1}^{2}\textrm{H})=2.014102\; u$
$m(_{1}^{3}\textrm{H})=3.0016049\; u$
$m(_{6}^{12}\textrm{H})=12.000000\; u$
$m(_{10}^{20}\textrm{Ne})=19.992439\; u$
Answer:
$\\\Delta m=m(_{1}^{1}\textrm{H})+m(_{1}^{3}\textrm{H})-2m(_{1}^{2}\textrm{H})\\ $
$\Delta m=1.007825+3.0016049-2\times 2.014102\\ $
$\Delta m=-0.00433$
The above negative value of mass defect implies there will be a negative Q value, and therefore the reaction is endothermic
$ _{6}^{12}\textrm{C}+_{6}^{12}\textrm{C}\rightarrow _{10}^{20}\textrm{Ne}+_{2}^{4}\textrm{He}$
$m(_{1}^{2}\textrm{H})=2.014102\; u$
$m(_{1}^{3}\textrm{H})=3.0016049\; u$
$m(_{6}^{12}\textrm{H})=12.000000\; u$
$m(_{10}^{20}\textrm{Ne})=19.992439\; u$
Answer:
$\\\Delta m=2m(_{6}^{12}\textrm{C})-m(_{10}^{20}\textrm{Ne})-m(_{2}^{4}\textrm{He})\\ $
$\Delta m=2\times 12.00000-19.992439-4.002603\\ $
$\Delta m=0.004958$
The above positive value of mass defect implies the Q value would be positive and therefore the reaction is exothermic.
Answer:
The reaction will be $_{26}^{56}\textrm{Fe}\rightarrow _{13}^{28}\textrm{Al}+_{13}^{28}\textrm{Al}$
The mass defect of the reaction will be
$\\\Delta m=m(_{26}^{56}\textrm{Fe})-2m( _{13}^{28}\textrm{Al})\\ $
$\Delta m=55.93494-2\times 27.98191\\ $
$\Delta m=-0.02888u$
Since the mass defect is negative, the Q value will also be negative, and therefore, the fission is not energetically possible.
Answer:
Number of atoms present in $1 \mathrm{~kg}(\mathrm{w})$ of ${ }_{94}^{239} \mathrm{Pu}=\mathrm{n}$
$
\begin{aligned}
n & =\frac{w \times N_A}{\text { mass number of Pu }} \\
n & =\frac{1000 \times 6.023 \times 10^{23}}{239} \\
n & =2.52 \times 10^{24}
\end{aligned}
$
Energy per fission $(E)=180 \mathrm{MeV}$
Total Energy released if all the atoms in $1 \mathrm{~kg}{ }_{94}^{239} \mathrm{Pu}$ undergo fission $=\mathrm{E} \times \mathrm{n}$
$
\begin{aligned}
& =180 \times 2.52 \times 10^{24} \\
& =4.536 \times 10^{26} \mathrm{MeV}
\end{aligned}
$
Answer:
The energy liberated on the fusion of two atoms of deuterium = 3.27 MeV
Number of fusion reactions in 2 kg of deuterium = N A $\times$500
The energy liberated by the fusion of 2.0 kg of deuterium atoms E
$\\=3.27\times 10^{6}\times 1.6\times 10^{-19}\times 6.023\times 10^{23}\times 500\\=1.576\times 10^{14}\ J$
Power of lamp (P)= 100 W
The time the lamp would glow using E amount of energy is T =
$\\=\frac{E}{P}\\ =\frac{1.576\times 10^{14}}{100\times 3600\times 24\times 365}$
=4.99 $\times$ $10^4$ years
Answer:
For a head-on collision of two deuterons, the closest distances between their centres will be $d=2 \times r$
$
\begin{aligned}
& d=2 \times 2.0 \\
& d=4.0 \mathrm{fm} \\
& d=4 \times 10^{-15} \mathrm{~m}
\end{aligned}
$
charge on each deuteron $=$ charge of one proton $=\mathrm{q}=1.6 \times 10^{-19} \mathrm{C}$
The maximum electrostatic potential energy of the system during the head-on collision will be E
$
\begin{aligned}
& =\frac{q^2}{4 \pi \epsilon_0 d} \\
& =\frac{9 \times 10^9 \times\left(1.6 \times 10^{-19}\right)^2}{4 \times 10^{-15}} J \\
& =\frac{9 \times 10^9 \times\left(1.6 \times 10^{-19}\right)^2}{4 \times 10^{-15} \times 1.6 \times 10^{-19}} \mathrm{eV} \\
& =360 \mathrm{keV}
\end{aligned}
$
The above basically means that bringing two deuterons from infinity to each other would require 360 keV of work to be done or would require 360 keV of energy to be spent.
Answer:
The mass of an element with mass number A will be about A u. The density of its nucleus, therefore, would be
$
\begin{aligned}
& d=\frac{m}{v} \\
& d=\frac{A}{\frac{4 \pi}{3} R^3} \\
& d=\frac{A}{\frac{4 \pi}{3}\left(R_0 A^{1 / 3}\right)^3} \\
& d=\frac{3}{4 \pi R_0{ }^3}
\end{aligned}
$
As we can see, the above density comes out to be independent of mass number $A$ and $R_0$ is constant, so matter density is nearly constant
NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei: Additional Questions
Nuclei class 12 question answers (Additional Questions) are designed to enhance conceptual clarity and problem-solving skills beyond the NCERT textbook. These questions cover nuclear forces, energy, and decay processes, making them highly useful for board exams and entrance tests like JEE/NEET.
Answer:
Masses of the two stable isotopes and their respective abundances are $6.01512 \; u$ and $7.01600 \; u$ and $7.5\; ^{o}/_{o}$ and $92.5\; ^{o}/_{o}$.
$m=\frac{6.01512\times7.5+7.01600\times92.5}{100}$
m=6.940934 u
Answer:
The atomic mass of boron is 10.811 u
Masses of the two stable isotopes are $10.01294 u$ and $11.00931 u$ respectively
Let the two isotopes have abundances $\mathrm{x} \%$ and $(100-\mathrm{x}) \%$
$
\begin{aligned}
& 10.811=\frac{10.01294 \times x+11.00931 \times(100-x)}{100} \\
& x=19.89 \\
& 100-x=80.11
\end{aligned}
$
Therefore the abundance of ${ }_5^{10} \mathrm{~B}$ is $19.89 \%$ and that of ${ }_5^{11} \mathrm{~B}$ is $80.11 \%$
Answer:
The atomic masses of the three isotopes are $19.99 u\left(m_1\right), 20.99 u\left(m_2\right)$ and $21.99 u\left(m_3\right)$
Their respective abundances are $90.51 \%\left(p_1\right), 0.27 \%\left(p_2\right)$ and $9.22 \%\left(p_3\right)$
$
\begin{aligned}
& m=\frac{19.99 \times 90.51+20.99 \times 0.27+21.99 \times 9.22}{100} \\
& m=20.1771 u
\end{aligned}
$
The average atomic mass of neon is 20.1771 u.
3. (i) Write nuclear reaction equations for $\; \alpha -decay\; of\; _{88}^{226}\textrm{Ra}$
Answer:
The nuclear reaction equations for the given alpha decay
$_{88}^{226}\textrm{Ra}\rightarrow _{86}^{222}\textrm{Rn}+_{2}^{4}\textrm{He}$
3. (ii) Write nuclear reaction equations for $\; \alpha -decay\; of\; _{94}^{242}\textrm{Pu}$
Answer:
The nuclear reaction equations for the given alpha decay is
$_{94}^{242}\textrm{Pu}\rightarrow _{92}^{238}\textrm{U}+_{2}^{4}\textrm{He}$
3. (iii) Write nuclear reaction equations for $\; \beta ^{-} -\: decay\; of\; _{15}^{32}\textrm{P}$
Answer:
The nuclear reaction equations for the given beta minus decay is
$_{15}^{32}\textrm{P}\rightarrow _{16}^{32}\textrm{S}+e^{-}+\bar{\nu }$
3.(iv) Write nuclear reaction equations for $\; \beta ^{-} -\: decay\; of\; _{83}^{210}\textrm{Bi}$
Answer:
The nuclear reaction equation for the given beta minus decay is
$_{83}^{210}\textrm{Bi}\rightarrow _{84}^{210}\textrm{Po}+e^{-}+\bar{\nu }$
3.(v) Write nuclear reaction equations for $\; \beta ^{+} -\: decay\; of\; _{6}^{11}\textrm{C}$
Answer:
The nuclear reaction for the given beta plus decay will be
$_{6}^{11}\textrm{C}\rightarrow _{5}^{11}\textrm{P}+e^{+}+\nu$
13.6 (vi) Write nuclear reaction equations for $\; \beta ^{+} -\: decay\; of\; _{43}^{97}\textrm{Tc}$
Answer:
nuclear reaction equations for
$\beta ^{+} -\: decay\; of\; _{43}^{97}\textrm{Tc}\ is$
$_{43}^{97}\textrm{Tc}\rightarrow _{42}^{97}\textrm{Mo}+e^{+}+\nu$
3.(vii) Write nuclear reaction equations for Electron capture of $_{54}^{120}\textrm{Xe}$
Answer:
The nuclear reaction for electron capture of $_{54}^{120}\textrm{Xe}$ is
$_{54}^{120}\textrm{Xe}+e^{-}\rightarrow _{53}^{120}\textrm{I}+\nu$
Answer:
(a) The activity is proportional to the number of radioactive isotopes present
The number of half-lives in which the number of radioactive isotopes reduces to x% of its original value is n.
$n=log_{2}(\frac{100}{x})$
In this case
$n=log_{2}(\frac{100}{3.125})=log_{2}32=5$
It will take 5T years to reach 3.125% of the original activity.
(b) In this case
$n=log_{2}(\frac{100}{1})=log_{2}100=6.64$
It will take 6.64T years to reach 1% of the original activity.
Answer:
Since we know that activity is proportional to the number of radioactive isotopes present in the sample.
$
\frac{R}{R_0}=\frac{N}{N_0}=\frac{9}{15}=0.6
$
Also
$
\begin{aligned}
& N=N_0 e^{-\lambda t} \\
& t=-\frac{1}{\lambda} \ln \frac{N}{N_0} \\
& t=-\frac{1}{\lambda} \ln 0.6 \\
& t=\frac{0.51}{\lambda}
\end{aligned}
$
but $\lambda=\frac{0.693}{T_{1 / 2}}$
Therefore
$
\begin{aligned}
& t=0.51 \times \frac{T_{1 / 2}}{0.693} \\
& t=0.735 T_{1 / 2} \\
& t \approx 4217
\end{aligned}
$
The age of the Indus-Valley civilisation, calculated using the given specimen, is approximately 4217 years.
Answer:
Required activity $=8.0 \mathrm{mCi}$
$1 \mathrm{Ci}=3.7 \times 10^{10}$ decay s $\mathrm{s}^{-1}$
$8.0 \mathrm{mCi}=8 \times 10^{-3} \times 3.7 \times 10^{10}=2.96 \times 10^8$ decay s ${ }^{-1}$
$T_{1 / 2}=5.3$ years
$
\lambda=\frac{0.693}{T_{1 / 2}}
$
$
\lambda=\frac{0.693}{5.3 \times 365 \times 24 \times 3600}
$
$
\lambda=4.14 \times 10^{-9} s^{-1}
$
$
\frac{\mathrm{d} N}{\mathrm{~d} t}=-N \lambda
$
$
N=-\frac{\mathrm{d} N}{\mathrm{~d} t} \times \frac{1}{\lambda}
$
$
N=-\left(-2.96 \times 10^8\right) \times \frac{1}{4.14 \times 10^{-9}}
$
$
N=7.15 \times 10^{16} \text { atoms }
$
The mass of those many atoms of Cu will be
$
\begin{aligned}
& w=\frac{7.15 \times 10^{16} \times 60}{6.023 \times 10^{23}} \\
& w=7.12 \times 10^{-6} g
\end{aligned}
$
$7.12 \times 10^{-6} \mathrm{~g}$ of ${ }_{27}^{60} \mathrm{Co}$ is necessary to provide a radioactive source of 8.0 mCi strength.
Answer:
$T_{1 / 2}=28$ years
$
\begin{aligned}
\lambda & =\frac{0.693}{28 \times 365 \times 24 \times 3600} \\
\lambda & =7.85 \times 10^{-10} \text { decay } \mathrm{s}^{-1}
\end{aligned}
$
The number of atoms in 15 mg of ${ }_{38}^{90} \mathrm{Sr}$ is
$
\begin{aligned}
& N=\frac{15 \times 10^{-3} \times 6.023 \times 10^{23}}{90} \\
& N=1.0038 \times 10^{20}
\end{aligned}
$
The disintegration rate will be
$
\begin{aligned}
& \frac{\mathrm{d} N}{\mathrm{~d} t}=-N \lambda \\
& =-1.0038 \times 10^{20} \times 7.85 \times 10^{-10} \\
& =-7.88 \times 10^{10} \mathrm{~s}^{-1}
\end{aligned}
$
The disintegration rate is therefore $7.88 \times 10^{10}$ decay $\mathrm{s}^{-1}$.
Given $m(_{88}^{226}\textrm{Ra})=226.02540\; u,$ $m(_{86}^{222}\textrm{Rn})=222.01750\; u,$
$m(_{86}^{222}\textrm{Rn})=220.01137\; u,$ $m(_{84}^{216}\textrm{Po})=216.00189\; u,$
Answer:
Mass defect is $\Delta m$
$
\begin{aligned}
& \Delta m=m\left({ }_{88}^{226} \mathrm{Ra}\right)-m\left({ }_{86}^{222} \mathrm{Rn}\right)-m\left({ }_2^4 \mathrm{He}\right) \\
& \Delta \mathrm{m}=226.02540-222.0175-4.002603 \\
& \Delta \mathrm{~m}=0.005297 \mathrm{u} \\
& 1 \mathrm{u}=931.5 \mathrm{MeV} / \mathrm{c}^2 \\
& \text { Q-value }=\Delta \mathrm{m} \times 931.5 \\
& =4.934515 \mathrm{MeV}
\end{aligned}
$
By using Linear Momentum Conservation and Energy Conservation
Kinetic energy of an alpha particle =
$
\begin{aligned}
& \frac{\text { mass of nucleus after decay }}{\text { mass of nucleus before decay }} \times Q-\text {value } \\
& =\frac{222.01750}{226.0254} \times 4.934515 \\
& =4.847 \mathrm{MeV}
\end{aligned}
$
Given $m(_{88}^{226}\textrm{Ra})=226.02540\; u,$ $m(_{86}^{222}\textrm{Rn})=222.01750\; u,$
$m(_{86}^{222}\textrm{Rn})=220.01137\; u,$ $m(_{84}^{216}\textrm{Po})=216.00189\; u,$
Answer:
Mass defect is $\Delta m$
$
\begin{aligned}
& \Delta m=m\left({ }_{88}^{226} \mathrm{Ra}\right)-m\left({ }_{86}^{222} \mathrm{Rn}\right)-m\left({ }_2^4 \mathrm{He}\right) \\
& \Delta \mathrm{m}=226.02540-222.0175-4.002603 \\
& \Delta \mathrm{~m}=0.005297 \mathrm{u} \\
& 1 \mathrm{u}=931.5 \mathrm{MeV} / \mathrm{c}^2 \\
& \text { Q-value }=\Delta \mathrm{m} \times 931.5 \\
& =4.934515 \mathrm{MeV}
\end{aligned}
$
By using Linear Momentum Conservation and Energy Conservation
Kinetic energy of an alpha particle =
$
\begin{aligned}
& \frac{\text { mass of nucleus after decay }}{\text { mass of nucleus before decay }} \times Q-\text { value } \\
& =\frac{222.01750}{226.0254} \times 4.934515 \\
& =4.847 \mathrm{MeV}
\end{aligned}
$
Answer:
If we use atomic masses
$\\\Delta m=m(_{6}^{11}\textrm{C})-m(_{5}^{11}\textrm{B})-2m_{e}\\ $
$\Delta m=11.011434-11.009305-2\times 0.000548\\ $
$\Delta m=0.001033u$
Q-value= 0.001033 $\times$ 931.5=0.9622 MeV, which is comparable with the maximum energy of the emitted positron.
$(i) m (_{10}^{23}\textrm{Ne} ) = 22.994466 \; u$
$(ii) m (_{11}^{23}\textrm{Na} ) = 22.089770 \; u$
Answer:
The $\beta$ decay equation is
$_{10}^{23}\textrm{Ne}\rightarrow _{11}^{23}\textrm{Na}+e^{-}+\bar{\nu }+Q$
$\\\Delta m=m(_{10}^{23}\textrm{Ne})-_{11}^{23}\textrm{Na}-m_{e}\\ $
$\Delta m=22.994466-22.989770\\ $
$\Delta m=0.004696u$
(We did not subtract the mass of the electron, as it is cancelled because of the presence of one more electron in the sodium atom)
Q=0.004696 $\times$ 931.5
Q=4.3743 eV
The emitted nucleus is way heavier than the $\beta$ particle, and the energy of the antineutrino is also negligible; therefore, the maximum energy of the emitted electron is equal to the Q value.
Answer:
The amount of energy liberated on fission of 1 $_{92}^{235}\textrm{U}$ atom is 200 MeV.
The amount of energy liberated on fission of 1g $_{92}^{235}\textrm{U}$
$\\=\frac{200\times 10^{6} \times 1.6\times 10^{-19}\times 6.023\times 10^{23}}{235}\\=8.2\times 10^{10}\ Jg^{-1}$
Total Energy produced in the reactor in 5 years
$\\=1000\times 10^{6}\times 0.8\times 5\times 365\times 24\times 3600\\ =1.261\times 10^{17}\ J$
Mass of $_{92}^{235}\textrm{U}$ which underwent fission, m
$=\frac{1.261\times 10^{17}}{8.2\times 10^{10}}$
=1537.8 kg
The amount present initially in the reactor = 2m
=2 $\times$ 1537.8
=3075.6 kg
$e^{+}+_{z}^{A}\textrm{X}\rightarrow _{Z-1}^{A}\textrm{Y}+v$
Answer:
For the electron capture, the reaction would be
$_{Z}^{A}\textrm{X}+e^{-}\rightarrow _{Z-1}^{A}\textrm{Y}+\nu +Q_{1}$
The mass defect and q value of the above reaction would be
$\\\Delta m_{1}=m(_{Z}^{A}\textrm{X})+m_{e}-m(_{Z-1}^{A}\textrm{Y})\\ Q_{1}=([m(_{Z}^{A}\textrm{X})-m(_{Z-1}^{A}\textrm{Y})]+m_{e})c^{2}$
where m N $(_{Z}^{A}\textrm{X})$ and m N $(_{Z-1}^{A}\textrm{Y})$ are the nuclear masses of elements X and Y respectively
For positron emission, the reaction would be
$_{Z}^{A}\textrm{X}\rightarrow _{Z-1}^{A}\textrm{Y}+e^{+}+\bar{\nu }+Q_{2}$
The mass defect and q value for the above reaction would be
$\\\Delta m_{2}=m(_{Z}^{A}\textrm{X})-m(_{Z-1}^{A}\textrm{Y})-m_{e}\\ Q_{2}=([m(_{Z}^{A}\textrm{X})-m(_{Z-1}^{A}\textrm{Y})]-m_{e})c^{2}$
From the above values, we can see that if Q2 is positive, Q1 will also be positive, but Q1 being positive does not imply that Q2 will also be positive.
Answer:
Let the abundances of $_{12}^{25}\textrm{Mg}$ and $_{12}^{26}\textrm{Mg}$ be x and y respectively.
x+y+78.99=100
y=21.01-x
The average atomic mass of Mg is 24.312 u
$\begin{aligned} & 24.312=\frac{78.99 \times 23.98504+x \times 24.98584+(100-x) \times 25.98259}{100} \\ & x \approx 9.3 \\ & y=21.01-x \\ & y=21.01-9.3 \\ & y=11.71\end{aligned}$
The abundances of $_{12}^{25}\textrm{Mg}$ and $_{12}^{26}\textrm{Mg}$ are 9.3% and 11.71% respectively
$m(_{20}^{40}\textrm{Ca})=39.962591\; u$
$m(_{20}^{41}\textrm{Ca})=40.962278 \; u$
$m(_{13}^{26}\textrm{Al})=25.986895 \; u$
$m(_{13}^{27}\textrm{Al})=26.981541 \; u$
Answer:
The reaction showing the neutron separation is
$_{20}^{41}\textrm{Ca}+E\rightarrow _{20}^{40}\textrm{Ca}+_{0}^{1}\textrm{n}$
$\\E=(m(_{20}^{40}\textrm{Ca})+m(_{0}^{1}\textrm{n})-m(_{20}^{41}\textrm{Ca}))c^{2}\\ $
$E=(39.962591+1.008665-40.962278)c^{2}\\ $
$E=(0.008978)u\times c^{2}$
But 1u=931.5 MeV/$c^{2}$
Therefore E=(0.008978) $\times$ 931.5
E=8.363007 MeV
Therefore to remove a neutron from the $_{20}^{41}\textrm{Ca}$ nucleus 8.363007 MeV of energy is required
$m(_{20}^{40}\textrm{Ca})=39.962591\; u$
$m(_{20}^{41}\textrm{Ca})=40.962278 \; u$
$m(_{13}^{26}\textrm{Al})=25.986895 \; u$
$m(_{13}^{27}\textrm{Al})=26.981541 \; u$
Answer:
The reaction showing the neutron separation is
$_{13}^{27}\textrm{Al}+E\rightarrow _{13}^{26}\textrm{Al}+_{0}^{1}\textrm{n}$
$\\E=(m(_{13}^{26}\textrm{Ca})+m(_{0}^{1}\textrm{n})-m(_{13}^{27}\textrm{Ca}))c^{2}\\ $
$\\E=(25.986895+1.008665-26.981541)c^{2} \\$
$\\E=(0.014019)u\times c^{2}$
But 1u=931.5 MeV/$c^{2}$
Therefore E=(0.014019) $\times$ 931.5
E=13.059 MeV
Therefore to remove a neutron from the $_{13}^{27}\textrm{Al}$ nucleus 13.059 MeV of energy is required
Answer:
Let initially there be $\mathrm{N}_1$ atoms of ${ }_{15}^{32} \mathrm{P}$ and $\mathrm{N}_2$ atoms of ${ }_{15}^{33} \mathrm{P}$ and let their decay constants be $\lambda_1$ and $\lambda_2$ respectively Since initially the activity of ${ }_{15}^{33} \mathrm{P}$ is $1 / 9$ times that of ${ }_{15}^{32} \mathrm{P}$ we have
$
N_1 \lambda_1=\frac{N_2 \lambda_2}{9}
$
Let after time $t$ the activity of ${ }_{15}^{33} \mathrm{P}$ be 9 times that of ${ }_{15}^{32} \mathrm{P}$
$
N_1 \lambda_1 e^{-\lambda_1 t}=9 N_2 \lambda_2 e^{-\lambda_2 t}
$
Dividing equation (ii) by (i) and taking the natural $\log$ of both sides, we get
$
\begin{aligned}
& -\lambda_1 t=\ln 81-\lambda_2 t \\
& t=\frac{\ln 81}{\lambda_2-\lambda_1}
\end{aligned}
$
where $\lambda_2=0.048 /$ day and $\lambda_1=0.027 /$ day
't' comes out to be 208.5 days
16. Under certain circumstances, a nucleus can decay by emitting a particle more massive than an $\alpha$ -particle. Consider the following decay processes:
$_{88}^{223}\textrm{Ra}\rightarrow _{82}^{209}\textrm{Pb}+_{6}^{14}\textrm{C}$
$_{88}^{223}\textrm{Ra}\rightarrow _{86}^{219}\textrm{Rn}+_{2}^{4}\textrm{He}$
Calculate the Q-values for these decays and determine that both are energetically allowed.
Answer:
$_{88}^{223}\textrm{Ra}\rightarrow _{82}^{209}\textrm{Pb}+_{6}^{14}\textrm{C}$
$\\\Delta m=m(_{88}^{223}\textrm{Ra})-m(_{82}^{209}\textrm{Pb})-m(_{6}^{14}\textrm{C})\\ =223.01850-208.98107-14.00324 \\=0.03419u$
1 u = 931.5 MeV/$c^{2}$
Q=0.03419 $\times$ 931.5
=31.848 MeV
As the Q value is positive, the reaction is energetically allowed
$_{88}^{223}\textrm{Ra}\rightarrow _{86}^{219}\textrm{Rn}+_{2}^{4}\textrm{He}$
$\\\Delta m=m(_{88}^{223}\textrm{Ra})-m(_{86}^{219}\textrm{Rn})-m(_{2}^{4}\textrm{He})\\ =223.01850-219.00948-4.00260 \\=0.00642u$
1 u = 931.5 MeV/$c^{2}$
Q=0.00642 $\times$ 931.5
=5.98 MeV
As the Q value is positive, the reaction is energetically allowed
$m(_{92}^{238}\textrm{U})=238.05079\; u$
$m(_{58}^{140}\textrm{Ce})=139.90543\; u$
$m(_{44}^{99}\textrm{Ru})= 98.90594\; u$
Answer:
The fission reaction given in the question can be written as
$_{92}^{238}\textrm{U}+_{0}^{1}\textrm{n}\rightarrow _{58}^{140}\textrm{Ce}+_{44}^{99}\textrm{Ru}+10e^{-}$
The mass defect for the above reaction would be
$\Delta m=m_{N}(_{92}^{238}\textrm{U})+m(_{0}^{1}\textrm{n})-m_{N}(_{58}^{140}\textrm{Ce})-m_{N}(_{44}^{99}\textrm{Ce})-10m_{e}$
In the above equation, mN represents nuclear masses
$\\\Delta m=m(_{92}^{238}\textrm{U})-92m_{e}+m(_{0}^{1}\textrm{n})-m(_{58}^{140}\textrm{Ce})+58m_{e}-m(_{44}^{99}\textrm{Ru})+44m_{e}-10m_{e} \\\Delta m=m(_{92}^{238}\textrm{U})+m(_{0}^{1}\textrm{n})-m(_{58}^{140}\textrm{Ce})-m(_{44}^{99}\textrm{Ru})\\ \Delta m=238.05079+1.008665-139.90543-98.90594\\ \Delta m=0.247995u$
but 1u =931.5 MeV/$c^{2}$
Q=0.247995 $\times$ 931.5
Q=231.007 MeV
Q value of the fission process is 231.007 MeV
$m(_{1}^{2}\textrm{H})=2.014102\; u$
$m(_{1}^{3}\textrm{H})=3.016049\; u$
Answer:
The mass defect of the reaction is
$\\\Delta m=m(_{1}^{2}\textrm{H})+m(_{1}^{3}\textrm{H})-m(_{2}^{4}\textrm{He})-m(_{0}^{1}\textrm{n})\\ $
$\Delta m=2.014102+3.016049-4.002603-1.008665\\ $
$\Delta m=0.018883u$
1u = 931.5 MeV/$c^{2}$
Q=0.018883 $\times$ 931.5=17.59 MeV
18. (b) Consider the D–T reaction (deuterium-tritium fusion) $_{1}^{2}\textrm{H}+_{1}^{3}\textrm{H}\rightarrow _{2}^{4}\textrm{He}+n$. Consider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction? (Hint: Kinetic energy required for one fusion event =average thermal kinetic energy available with the interacting particles $= 2(3kT/2)$; k = Boltzmann’s constant, T = absolute temperature.)
Answer:
To initiate the reaction, both nuclei would have to come into contact with each other.
Just before the reaction, the distance between their centres would be 4.0 fm.
The electrostatic potential energy of the system at that point would be
$\\U=\frac{q^{2}}{4\pi \epsilon _{0}d}\\ $
$U=\frac{9\times 10^{9}(1.6\times 10^{-19})^{2}}{4\times 10^{-15}}\\$
$U=5.76\times 10^{-14}J$
The same amount of Kinetic Energy, K, would be required to overcome the electrostatic forces of repulsion to initiate the reaction
It is given that $K=2\times \frac{3kT}{2}$
Therefore, the temperature required to initiate the reaction is
$\\T=\frac{K}{3k}\\ =\frac{5.76\times 10^{-14}}{3\times 1.38\times 10^{-23}}\\=1.39\times 10^{9}\ K$
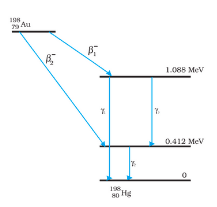
Answer:
$\gamma _{1}$ decays from 1.088 MeV to 0 V
Frequency of $\gamma _{1}$ is
$\\\nu _{1}=\frac{1.088\times 10^{6}\times 1.6\times 10^{-19}}{6.62\times 10^{-34}}\\ $
$\nu _{1}=2.637\times 10^{20}\ Hz$
Plank's constant, h=6.62 $\times$ $10 ^{-34}$ Js,
$E=h\nu$
Similarly, we can calculate frequencies of $\gamma _{2}$ and $\gamma _{3}$
$\\\nu _{2}=9.988\times 10^{19}\ Hz\\ $
$\nu _{3}=1.639\times 10^{20}\ Hz$
The energy of the highest level would be equal to the energy released after the decay
Mass defect is
$\\\Delta m=m(_{79}^{196}\textrm{U})-m(_{80}^{196}\textrm{Hg})\\ $
$\Delta m=197.968233-197.966760\\$
$\Delta m=0.001473u$
We know 1u = 931.5 MeV/$c^{2}$
Q value= 0.001473 $\times$ 931.5=1.3721 MeV
The maximum Kinetic energy of $\beta _{1}^{-}$ would be 1.3721-1.088=0.2841 MeV
The maximum Kinetic energy of $\beta _{2}^{-}$ would be 1.3721-0.412=0.9601 MeV
Answer:
(a) ${ }_1^1 \mathrm{H}_1^1 \mathrm{H}+{ }_1^1 \mathrm{H}+{ }_1^1 \mathrm{H}+{ }_1^1 \mathrm{H} \rightarrow{ }_2^4 \mathrm{He}$
The above fusion reaction releases the energy of 26 MeV
Number of Hydrogen atoms in 1.0 kg of Hydrogen is $1000 \mathrm{~N}_{\mathrm{A}}$
Therefore $250 \mathrm{~N}_{\mathrm{A}}$ such reactions would take place
The energy released in the whole process is $\mathrm{E}_1$
$
\begin{aligned}
& =250 \times 6.023 \times 10^{23} \times 26 \times 10^6 \times 1.6 \times 10^{-19} \\
& =6.2639 \times 10^{14} J
\end{aligned}
$
(b) The energy released in fission of one ${ }_{92}^{235} \mathrm{U}$ atom is 200 MeV
Number of ${ }_{92}^{235} \mathrm{U}$ atoms present in 1 kg of ${ }_{92}^{235} \mathrm{U}$ is N
$
\begin{aligned}
& N=\frac{1000 \times 6.023 \times 10^{23}}{235} \\
& N=2.562 \times 10^{24}
\end{aligned}
$
The energy released on fission of N atoms is $\mathrm{E}_2$
$
\begin{aligned}
& E=2.562 \times 10^{24} \times 200 \times 10^6 \times 1.6 \times 10^{-19} \\
& E=8.198 \times 10^{13} J \\
& \frac{E_1}{E_2}=\frac{6.2639 \times 10^{14}}{8.198 \times 10^{13}} \approx 8
\end{aligned}
$
Answer:
Let the amount of energy produced using nuclear power per year in 2020 be E
$E=\frac{200000\times 10^{6}\times 0.1\times 365\times 24\times 3600}{0.25}\ J$
(Only 10% of the required electrical energy is to be produced by Nuclear power, and only 25% of therm-nuclear is successfully converted into electrical energy)
The amount of Uranium required to produce this much energy is M
$=\frac{200000\times 10^{6}\times 0.1\times 365\times 24\times 3600\times 235}{0.25\times 200\times 10^{6}\times 1.6\times 10^{-19}\times 6.023\times 10^{23}\times 1000}$ (N A =6.023 $\times$ $10^{ 23}$ , Atomic mass of Uranium is 235 g)
=3.076 $\times$ $10^{4}$ kg
NCERT Solutions for Class 12 Physics Chapter 13 - Nuclei: Higher Order Thinking Skills (HOTS) Questions
Class 12 Physics Chapter 13 - Nuclei: Higher Order Thinking Skills (HOTS) Questions encourage students to apply concepts like nuclear binding energy, radioactivity, and nuclear reactions in complex scenarios. These advanced problems help in developing analytical skills and are very useful for competitive exam preparation.
Q1:
The energy released in the fusion of $2 \mathrm{~kg}$ of hydrogen deep in the sun is $\mathrm{E}_{\mathrm{H}}$ and the energy released in the fission of $2 \mathrm{~kg}$ of ${ }^{235} \mathrm{U}$ is $E_U$. The ratio $\frac{E_H}{E_U}$ is approximately :
(Consider the fusion reaction as $4{ }_1^1 \mathrm{H}+2 \mathrm{e}^{-} \rightarrow{ }_2^4 \mathrm{He}+2 \mathrm{v}+6 \gamma+26.7 \mathrm{MeV}$, energy released in the fission reaction of ${ }^{235} \mathrm{U}$ is $200 \mathrm{MeV}$ per fission nucleus and $\mathrm{N}_{\mathrm{A}}=6.023 \times 10^{23}$ )
Answer:
In each fusion reaction, $4{ }_1^1 \mathrm{H}$ nuclei are used.
Energy released per Nuclei of ${ }_1^1 \mathrm{H}=\frac{26.7}{4} \mathrm{MeV}$
$\therefore$ Energy released by $2 \mathrm{~kg}$ hydrogen $\left(\mathrm{E}_{\mathrm{H}}\right.$ )
$=\frac{2000}{1} \times \mathrm{N}_{\mathrm{A}} \times \frac{26.7}{4} \mathrm{MeV}$
$\therefore$ Energy released by $2 \mathrm{~kg}$ Cranium $\left(\mathrm{E}_{\mathrm{v}}\right)$
$=\frac{2000}{235} \times \mathrm{N}_{\mathrm{A}} \times 200 \mathrm{MeV}$
So,
$\frac{E_H}{E_V}=235 \times \frac{26.7}{4 \times 200}=7.84$
$\therefore$ Approximately close to 7.62
Q2:
The disintegration energy $Q$ for the nuclear fission of ${ }^{235} \mathrm{U} \rightarrow{ }^{140} \mathrm{Ce}+{ }^{94} \mathrm{Zr}+\mathrm{n}$ is _____ $\qquad$ $\mathrm{MeV}$.
Given atomic masses of
$\begin{aligned}
& { }^{235} \mathrm{U}: 235.0439 \mathrm{u} ;{ }^{140} \mathrm{Ce} ; 139.9054 \mathrm{u}, \\
& { }^{94} \mathrm{Zr}: 93.9063 \mathrm{u} ; \mathrm{n}: 1.0086 \mathrm{u},
\end{aligned}
$
Value of $\mathrm{c}^2=931 \mathrm{MeV} / \mathrm{u}$
Answer:
${ }^{235} \mathrm{U} \rightarrow{ }^{140} \mathrm{Ce}+{ }^{94} \mathrm{Zr}+\mathrm{n}$
Disintegration energy
$\begin{aligned}
\mathrm{Q} & =\left(\mathrm{m}_{\mathrm{R}}-\mathrm{m}_{\mathrm{p}}\right) \cdot \mathrm{c}^2 \\
\mathrm{~m}_{\mathrm{R}} & =235.0439 \mathrm{u} \\
\mathrm{m}_{\mathrm{p}} & =139.9054 \mathrm{u}+93.9063 \mathrm{u}+1.0086 \mathrm{u} \\
& =234.8203 \mathrm{u} \\
\mathrm{Q} & =(235.0439 \mathrm{u}-234.8203 \mathrm{u}) \mathrm{c}^2 \\
& =0.2236 \mathrm{c}^2 \\
& =0.2236 \times 931 \\
\mathrm{Q} & =208.1716
\end{aligned}
$
Q3:
The half-life of a radioactive isotope is 5.5 h. If there are initially $48 \times 12^{32}$ atoms of this isotope, the number of atoms of the isotope remaining after 22 h is -
Answer:
Use, $\mathrm{t}_{1 / 2}=\frac{\mathrm{t}}{\log \left(\frac{1}{2}\right)\left[\frac{\mathrm{N}(\mathrm{t})}{\mathrm{N}_0}\right]}$
$
\begin{aligned}
& 5.5=\frac{22}{\log \left(\frac{1}{2}\right) \frac{x}{48 \times 10^{32}}} \\
& \left(\frac{1}{2}\right) \frac{x}{48 \times 10^{32}}=\frac{1}{10000} \\
& \frac{x}{48 \times 10^{32}}=\frac{1}{4} \\
& x=12 \times 10^{32}
\end{aligned}
$
Q4:
The $\mathrm{k}_{\alpha}$ radiation of $\mathrm{M}_0(\mathrm{z}=42)$ has a wavelength of $0.71 \dot{\mathrm{~A}}$, the wave length of the corresponding radiation of $\mathrm{Cu}(z=29)$
Answer:
From Moseley's law for $\mathrm{K}_\alpha$ - line, we have
$
\begin{gathered}
\frac{1}{\lambda} \alpha(z-1)^2 \\
\therefore \frac{\lambda_{c u}}{\lambda m_0}=\frac{\left(z_{m_0}-1\right)^2}{\left(z_{c u}-1\right)^2}=\frac{(41)^2}{(28)^2} \\
\lambda m_0=0.71Å, \\
\therefore \lambda_{c u}=(0.71 Å) \times \frac{(41)^2}{(28)^2} \\
=1.52 Å
\end{gathered}
$
Q5:
The count rate from $100 \mathrm{~cm}^2$ of a radioactive liquid is c. Some of the liquid is now discarded. The count rate of the remaining liquid is found to be $\left(\frac{\mathrm{c}}{10}\right)$ after three half-lives. The volume of the remaining liquid in $\mathrm{cm}^2$ is:-
Answer:
Initial count rate (C R) for $1 \mathrm{~cm}^3$ of
$
\text { liquid }=\frac{c}{100}
$
After 3 half -lives, langle $R$ for $1 \mathrm{~cm}^3$ of liquid
$
=\frac{1}{8} \times \frac{\mathrm{c}}{100}
$
If $u$ is the volume of remaining liquid, then
$
\begin{aligned}
\mathrm{v} \times \frac{\mathrm{c}}{800} & =\frac{c}{10} \\
\therefore \quad \frac{\mathrm{v}}{80} & =1 \\
\mathrm{v} & =80
\end{aligned}
$
Nuclei NCERT Solutions: Topics
Class 12 Physics Chapter 13 - Nuclei deals with the study of the atomic nucleus, its properties, and the forces that hold it together. The topics cover atomic masses, binding energy, nuclear force, radioactivity, and nuclear energy, which are crucial for understanding both natural processes and modern technology
- Atomic Masses And Composition Of Nucleus
- Size Of The Nucleus
- Mass-Energy And Nuclear Binding Energy
- Nuclear Force
- Radioactivity
- Nuclear Energy
Class 12 Physics Chapter 13 - Nuclei: Important Formulas
Class 12 Physics Chapter 13 - Nuclei formulas include key equations related to nuclear radius, binding energy, decay laws, half-life, and activity. These formulas are essential for solving numerical problems in board exams as well as JEE and NEET.
1. Atomic Masses and Composition of Nucleus
- Nucleus $=$ Protons + Neutrons (Nucleons)
- Atomic number (Z), Mass number (A), Neutrons = A-Z
- 1 atomic mass unit $(\mathrm{u})=1.66 \times 10^{-27} \mathrm{~kg}$
- Isotopes: Same Z, different A
2. Size of the Nucleus
- Radius: $R=R_0 A^{1 / 3}, R_0 \approx 1.2 \times 10^{-15} \mathrm{~m}$
- Nuclear density is constant and very high ( $\sim 2.3 \times 10^{17} \mathrm{~kg} / \mathrm{m}^3$ )
3. Mass-Energy and Binding Energy
- Mass defect: Actual mass < sum of nucleons
- $E=m c^2$: Mass converts to energy
- Binding energy = energy needed to break the nucleus
- Higher binding energy = more stable nucleus
4. Nuclear Force
- Strong, short-range, attractive force
- Acts between all nucleons
- Charge-independent and repulsive at very short distances
5. Radioactivity
- Spontaneous decay of unstable nuclei
- Types: Alpha (He nucleus), Beta (electron/positron), Gamma (photons)
- Law: $N(t)=N_0 e^{-\lambda t}$
- Half-life: $T_{1 / 2}=0.693 / \lambda$
6. Nuclear Energy
- Fission: Heavy nucleus splits (used in reactors)
- Fusion: Light nuclei combine (in stars)
- Both release huge amounts of energy due to binding energy changes
How Can NCERT Solutions for Class 12 Physics Chapter 13 Help in Exam Preparation?
The Nuclei class 12 question answers help students to study physics to pass exams by simplifying the concepts of nucleus of atoms, nuclear forces, mass defect, binding energy and radioactivity. These Nuclei class 12 question answers offer explanations and step-by-step derivations, which make difficult concepts such as nuclear fission and fusion easy in board and entrance examinations. With the help of such NCERT solutions, the students can reinforce their clarity in concepts and their ability to solve numerical problems. Important formulas, definitions, and past exam questions are also emphasised in the Class 12 physics Nuclei question answers, making the students concentrate on high-weightage areas. In addition, the NCERT-based explanations are well-matched with the CBSE and competitive exam patterns, and hence, it is best to revise the information quickly and gain confidence before the final exam.
Approach to Solve Questions of Class 12 Physics Chapter 13 - Nuclei
In answering the questions of this chapter, attention should be drawn to developing the clarity of the stated problem, determining the correct physical principles, and systematically using formulas. Students are advised to be keen on derivations, units, and methods of approximation commonly examined in examinations. An organised approach to solving problems is not only time saving, but also minimises mistakes made when taking board as well as competitive exams, such as JEE and NEET.
-
Understand fundamental nuclear terms:
-
Atomic number (Z), Mass number (A), Neutrons (N = A–Z)
-
Nucleus structure: Protons and neutrons (nucleons)
- Understand the nuclear size formula:
Radius of nucleus: $R=R_0 A^{1 / 3}$, where $R_0 \approx 1.2 \times 10^{-15} \mathrm{~m}$ - Apply mass-energy equivalence:
E=mc2, where mass difference is utilised to generate energy - Calculate binding energy:
- Binding Energy (B.E) =Δm×931MeV
- Δm = mass defect = (total mass of nucleons – nucleus mass)
- Understand the stability of nuclei:
- More B.E per nucleon ⇒ stable nucleus
- See the graph for binding energy per nucleon versus mass number
- Learn about types of radioactive decay:
- Alpha decay: Mass number goes down by 4, atomic number goes down by 2
- Beta decay: Neutron → Proton (or vice versa), atomic number ±1
- Gamma decay: No change of mass or atomic number, only release of energy.
- Apply the radioactive decay law:
- $N=N_0 e^{-\lambda t}$, where $\lambda$ is decay constant
- Half-life: $T_{1 / 2}=\frac{0.693}{\lambda}$
- Activity: $A=\lambda N$
- Use appropriate units and convert them:
Energy in MeV or joules, mass in u, time in seconds - Learn nuclear reactions:
Balance the equations so that they retain mass number and atomic number. - Practice numerical + conceptual questions
- Emphasise half-life, decay, reaction equations, and energy calculations
Importance of NCERT Solutions for Class 12 Physics Chapter 13: Nuclei
NCERT Solutions for Class 12 Physics Chapter 13: Nuclei are essential for understanding the structure, stability, and energy aspects of atomic nuclei. The chapter presents some of the major concepts of nuclear physics, which are not only conceptually interesting but also numerically significant.
- Helps students know the composition of nuclei, protons, neutrons and nuclear forces.
- Gives clear reasoning and formulae to explain the concepts of nuclear size, mass defect and binding energy.
- Develops an effective conceptual understanding of radioactivity, the alpha, beta, and gamma decay.
- Strengthens understanding of nuclear reactions, fission, and fusion with real-world relevance.
- Plays a vital role in CBSE board exams, where derivations and numericals from this chapter are frequently asked.
- Highly useful for competitive exams like JEE and NEET, especially questions related to binding energy and decay laws.
- Enhances skills in numerical solving of problems step by step using decay constants and half-life.
- Shows the students how physics can be applied in real life in areas like nuclear energy, medical imaging and radiation safety.
What Students Learn from NCERT Solutions for Class 12 Physics Chapter 13: Nuclei?
The chapter Nuclei presents students with the inner world of the atom, in which huge energy is concentrated into a small nucleus. With the help of NCERT Solutions of Class 12 Physics Chapter 13, students can learn the explanations of the nuclear properties, stability, and transformations in simple terms that are easy to memorise when taking exams, with the help of the physically defined laws and calculations.
- Understand how the nuclei are made up of protons and neutrons and how the nuclear forces are formed.
- Know the size and radius of the nucleus and their dependence on the mass number.
- Learn about the concept of mass defect and how it is related to binding energy, which is the explanation of nuclear stability.
- Get acquainted with the various forms of radioactive decay (alpha, beta and gamma) and their characteristics.
- Understand the radioactive decay law, half-life, mean life, and decay constant.
- Study nuclear reactions such as fission and fusion and release of their energy.
- Study the real-life applications of nuclear physics in energy generation, healthcare, and industry.
- Develop strong numerical problem-solving skills through step-by-step solutions.
- Build conceptual clarity required for CBSE board exams and competitive exams like JEE and NEET
What Extra Should Students Study Beyond NCERT for JEE/NEET?
Beyond the NCERT, students preparing for JEE/NEET should focus on advanced concepts of the Nuclei chapter, such as mass defect and binding energy curve analysis, nuclear reactions (fission & fusion), Q-value calculations, and detailed radioactive decay series. These topics strengthen problem-solving ability and are frequently tested in competitive exams.
NCERT Solutions for Class 12 Physics Chapter-wise
The NCERT Solutions for Class 12 Physics provide step-by-step, detailed answers to all chapters, helping students strengthen their conceptual understanding and problem-solving skills. These chapter-wise solutions are designed as per the latest CBSE curriculum and are equally useful for JEE, NEET, and other competitive exams. With structured explanations and solved examples, these solutions make learning easier and revision more effective.
Also Check:
- NCERT Books Class 12 Physics
- NCERT Syllabus Class 12 Physics
- NCERT Books Class 12
- NCERT Syllabus Class 12
NCERT solutions subject-wise
- NCERT solutions for class 12 mathematics
- NCERT solutions for class 12 chemistry
- NCERT solutions for class 12 physics
- NCERT solutions for class 12 biology
Also, check NCERT Exemplar Class 12 Solutions
Frequently Asked Questions (FAQs)
For CBSE board exam from NCERT class, 12 chapters 13 around 4 to 6 marks questions can be expected. All topics of the NCERT syllabus for the chapter Nuclei should be covered for the CBSE board exam.
Yes the NCERT chapter Nuclei are important for both the exams. Both in NEET and JEE main syllabus the chapter Nuclei is present and 1 or 2 questions from the chapter can be expected for the exams. The questions discussed in the NCERT Solutions for the chapter Nuclei will give a better idea on how to use the formulas and give a better understanding of the concepts discussed.
The nucleus is made up of protons, which are positively charged particles, and neutrons, which are neutral particles.
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons in their nuclei.
NCERT solutions are important for the Board exam as they provide clear explanations, help in solving questions, cover all important topics, provide a structured approach to solving problems, and are designed with the exam pattern in mind, helping in exam-oriented preparation.
Common mistakes include missing units, failing to balance the mass and atomic numbers, and leaving out emission products like neutrinos. Correct NCERT solutions avoid these mistakes by clearly demonstrating the conservation of mass and charge as well as balancing both sides of the equation.
Common questions consist of
- Decay laws and half-life relationship derivations
- Finding the binding energy of each nucleon
- Neutron separation energy and radioactive decay numericals
- Balancing alpha and beta decay with nuclear processes.
The most common are numerical problems on half-life and decay law, concept-based questions on binding energy, and short-answer questions on radioactive decay.
The use of radioisotopes in medical imaging, cancer treatment and diagnosis is attributed to their regulated radiation release.
The most common are numerical problems on half-life and decay law, concept-based questions on binding energy, and short-answer questions on radioactive decay.
Questions related to CBSE Class 12th
On Question asked by student community
Hello
You will be able to download the CBSE Previous Year Board Question Papers from our official website, careers360, by using the link given below.
https://school.careers360.com/boards/cbse/cbse-previous-year-question-papers
I hope this information helps you.
Thank you.
Hello
You will be able to download the CBSE Pre-Board Class 12 Question Paper 2025-26 from our official website by using the link which is given below.
https://school.careers360.com/boards/cbse/cbse-pre-board-class-12-question-paper-2025-26
I hope this information helps you.
Thank you.
Hello,
Yes, it's completely fine to skip this year's 12th board exams and give them next year as a reporter or private candidate, allowing you to prepare better; the process involves contacting your current school or board to register as a private candidate or for improvement exams during the specified
HELLO,
Yes i am giving you the link below through which you will be able to download the Class 12th Maths Book PDF
Here is the link :- https://school.careers360.com/ncert/ncert-book-for-class-12-maths
Hope this will help you!
Failing in pre-board or selection tests does NOT automatically stop you from sitting in the CBSE Class 12 board exams. Pre-boards are conducted by schools only to check preparation and push students to improve; CBSE itself does not consider pre-board marks. What actually matters is whether your school issues your
Popular CBSE Class 12th Questions
Applications for Admissions are open.
As per latest syllabus. Physics formulas, equations, & laws of class 11 & 12th chapters
JEE Main Important Chemistry formulas
Get nowAs per latest syllabus. Chemistry formulas, equations, & laws of class 11 & 12th chapters
JEE Main high scoring chapters and topics
Get nowAs per latest 2024 syllabus. Study 40% syllabus and score upto 100% marks in JEE
JEE Main Important Mathematics Formulas
Get nowAs per latest syllabus. Maths formulas, equations, & theorems of class 11 & 12th chapters
